Abstract
Background: The effect of caffeine intake during pregnancy on the risk of preterm delivery has been studied for the past 3 decades with inconsistent results.
Objective: We performed a meta-analysis examining the association between caffeine consumption during pregnancy and risk of preterm birth.
Design: We searched MEDLINE and EMBASE articles published between 1966 and July 2010, cross-referenced reference lists of the retrieved articles, and identified 15 cohort and 7 case-control studies that met inclusion criteria for this meta-analysis.
Results: The combined odds ratios (ORs) obtained by using fixed-effects models for cohort studies were 1.11 (95% CI: 0.96, 1.28), 1.10 (95% CI: 1.01, 1.19), and 1.08 (95% CI: 0.93, 1.27) for risk of preterm birth comparing the highest with the lowest level of caffeine intake (or no intake) (mg/d) during the first, second, and third trimesters, respectively. Results for the case-control studies yielded no associations for the first (OR: 1.07; 95% CI: 0.84, 1.37), second (OR: 1.17; 95% CI: 0.94, 1.45), or third (OR: 0.94; 95% CI: 0.79, 1.12) trimesters. No overall heterogeneity was found by region, publication decade, exposure and outcome assessment, caffeine sources, or adjustment for confounding, which was largely driven by individual studies.
Conclusion: In this meta-analysis, we observed no important association between caffeine intake during pregnancy and the risk of preterm birth for cohort and case-control studies.
INTRODUCTION
Preterm birth is the onset of spontaneous labor before 37 wk of gestation. Despite screening for fetal distress and advancement of medical interventions, preterm birth remains an important public health problem. Approximately 12–13% of births in the United States and 5–9% of births in Europe are preterm (1). Preterm birth, a leading cause of neonatal mortality, is associated with an increased risk of neurodevelopmental, respiratory, and gastrointestinal complications (2); hypertension; and reduced insulin concentrations in later life (3, 4). Several pathways, including inflammatory response pathways, have been proposed to explain the early onset of mechanisms involved in normal labor (5).
One of the prenatal exposures examined for association with preterm birth has been caffeine consumption by pregnant women. Caffeine (1,3,7-trimethylxanthine), a plant alkaloid found in coffee, tea, cocoa, and cola soft drinks, is one of the most frequently consumed substances (6). Studies suggest an increased risk of growth restriction, cardiovascular abnormalities, and skeletal abnormalities in children of women with high caffeine intake during pregnancy (7). Because many women continue to consume coffee and caffeine-containing beverages during pregnancy, a possible relation of caffeine intake to perinatal morbidities is a concern (8–10). During pregnancy, the rate of caffeine metabolism decreases progressively from the first to third trimester, with a doubling of the half-life of caffeine. Caffeine has been detected in uterine secretions and amniotic fluid, which suggests that caffeine can be transported across the placenta (11, 12). Delayed clearance leading to higher concentrations in the fetus (13) and a higher half-life of caffeine in neonates than in adults are of concern (11). Whether maternal caffeine intake during pregnancy is associated with preterm birth has been examined during the past 30 y with inconsistent results (6, 14). Other reviews have only qualitatively summarized the data and have not explored sources of heterogeneity between studies (15, 16). A recent meta-analysis by Santos et al (14) found a positive risk estimate for results combined across 8 studies but with considerable heterogeneity between studies. Here, we systematically reviewed all available epidemiologic evidence and conducted a meta-analysis on the association between maternal caffeine consumption during pregnancy and the risk of preterm birth.
METHODS
We followed the MOOSE consensus statement for conducting a meta-analysis of observational studies (17).
Search strategy
We searched PubMed (www.ncbi.nlm.nih.gov/pubmed) and EMBASE (www.embase.com/) databases from 1966 through July 2010 for English and non-English studies using Medical Subject Headings (MeSH) terms or key words, including caffeine and coffee with premature birth and infant, premature. Our search included variations of caffeine such as coffein, calcium caffeine, caffeine calcium complex, anhydrous caffeine, cafeine, animine, and caffein and variations of the outcome using premature infant, preterm, and preterm birth. Reference lists of the retrieved articles were examined for additional relevant studies.
Study selection
Inclusion criteria
We included case-control and cohort studies with coffee, tea, cocoa/chocolate, and cola or soda drinks as the sources of caffeine exposure. The outcome was defined as birth before 37 wk of gestation.
Exclusion criteria
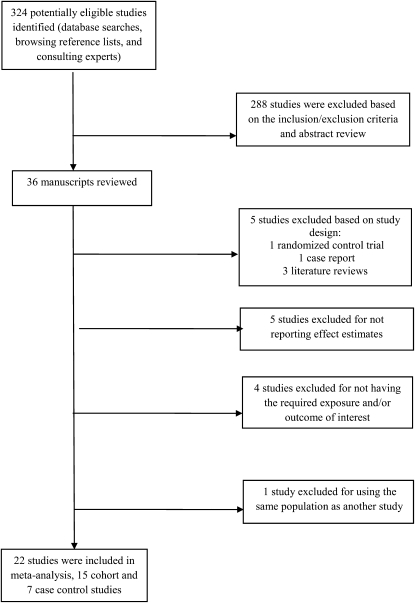
Literature reviews, case reports, animal studies, or studies examining only low birth weight or specific obstetric complications were excluded. Also excluded were studies with inadequate information on amount or source of caffeine intake (18–21), no control subjects (20), or no measures of association that could be extracted or derived (20–24) (Figure 1).
FIGURE 1.
Literature search results for publications related to caffeine consumption during pregnancy and the risk of preterm delivery. Studies were excluded on the basis of study design, absence of control subjects, no measures of association, and no definition or reporting of the required exposure (caffeine intake) outcome of interest.
Data extraction
We extracted information on study design, participant population, study period, measurement of caffeine consumption, measurement of outcomes, adjustment for potential confounders, and estimates of association. Effect estimates [odds ratios (ORs) and relative risks] and corresponding 95% CIs in subgroups according to exposure duration, timing (trimester), and beverage types were extracted. All data extraction was performed independently by 2 investigators, and discrepancies were resolved by a third investigator. If the publication did not provide effect estimates, we manually calculated the crude effect estimates and the 95% CIs.
Statistical analysis
We used logistic regression models to calculate the combined effect estimate of the risk of preterm birth in case-control and cohort studies and modeled the log-OR of preterm delivery as the dependent variable using fixed-effects estimates. The fixed-effects model assumes that a single common (or “fixed”) effect underlies every study in the meta-analysis and uses inverse variance weighting of the sampling error variance to weight the individual studies (25). We conducted separate meta-analyses comparing different levels of consumption with the lowest level/no caffeine intake and by trimester. If the study did not specify a trimester but estimated the effect estimate for the full duration of the pregnancy, the individual effect estimates were included in each trimester under the assumption that caffeine intake remained constant across trimesters (23, 26). If consumption was reported as cups/d, we converted the values and estimated mg/d based on conversion factors provided by the study or by consulting the US Department of Agriculture National Nutrient Database for Standard Reference (27), the Health Canada website, and the European Food Information Council, as appropriate. We classified exposure according to 5 categories based on the provided or converted consumption levels. For example, the lowest category (reference category) contained the lowest intake reported by the studies, ranging from 0 to <400 mg/d, and the highest category included the highest intake reported, ranging from any caffeine consumption to ≥1330 mg/d. Because we used the categories reported by the studies, these categories were not mutually exclusive. Given a high variability across categories of caffeine intake, we conducted sensitivity analyses, limiting the highest category of caffeine consumption to ≥300 mg/d.
For the subanalyses with coffee only or tea only, exposure was defined as cups/d, converted from mg/d if required, and described above. Each beverage was also categorized according to 5 categories. For coffee, the lowest (reference) category contained 0 cups/d, which included reports of 0–3 cups/d; the highest category included >1 to ≥10 cups/d. For tea, the lowest category contained 0 cups/d, which included 0–3 cups/d; the highest category included ≥1 to ≥8 cups/d. We used STATA (version 10; StataCorp LP, College Station, TX) for all statistical analyses. Statistical significance was defined at the 0.05 level.
Quality assessment
To examine potential publication bias, we used funnel plots and tested for symmetry, as suggested by Egger et al (28). To estimate whether publication bias would explain the observed associations, we calculated the fail-safe number of studies of average precision needed to reverse the observed significance.
To examine sources of heterogeneity, we conducted separate meta-regression analyses with independent variables: study design, region (North America, Europe, and South America), publication decade, exposure assessment (interview or questionnaire), outcome assessment (medical records or other), sources of caffeine (coffee only; coffee and tea; coffee, tea, and chocolate; or all sources), and whether studies adjusted for confounders [none; age, socioeconomic status, race, smoking, parity, and body mass index (BMI)]. We included the original studies' effect estimates from multivariate models or univariate effect estimates as available. We also examined the combined risk of preterm birth excluding studies for which we calculated the crude effect estimates (29–31), limiting the combined risk estimate to results provided directly by the studies.
RESULTS
The PubMed and EMBASE database searches as well as the reference lists of publications yielded 324 studies. We examined the abstracts of these publications and their reference lists and found 36 potentially eligible manuscripts. Detailed review of these for inclusion and exclusion criteria (Figure 1) yielded a total of 22 epidemiologic studies, including 15 cohort studies (8, 9, 29–41) and 7 case-control studies (26, 42–47) (Tables 1 and 2). One of the case-control studies (47) reported effect estimates for both coffee and caffeinated soda consumption. Assuming that the primary caffeine source came from coffee consumption and to avoid undue influence on the combined OR, we reported the results using the coffee effect estimates only. Because of the limited number of studies in various exposure categories, we compared only the lowest and highest intake categories of caffeine across trimesters.
TABLE 1.
Cohort studies of caffeine consumption and risk of preterm birth1
| Author, publication year, and country | No. of cases/total n | Intake measurement | Trimester considered | Preterm definition | Comparisons made by authors | Risk estimate (95% CI) | Comparisons made in meta-analysis | Adjusted confounders |
| Bakker et al, 2010, Netherlands (32) | 337/7083 | Coffee (including decaffeinated) and tea (including caffeinated, decaffeinated, herbal, and green tea); intakes were considered in units/d with 1 unit = 1 cup (90 mg) caffeine | Third | Gestational age <37 wk | <2 units/d 2–3.9 units/d 4–5.9 units/d ≥6 units/d |
1.00 0.92 (0.72, 1.18) 1.12 (0.71, 1.73) 1.35 (0.58, 3.15) |
<180 mg/d ≥540 mg/d |
Gestational age at visit, maternal age, educational level, ethnicity, parity, smoking habits, alcohol consumption, height, BMI at intake, nutritional intake (total energy, total carbohydrate, total fat, and total protein), folic acid supplement use, maternal pregnancy complications (pregnancy-induced hypertension, preeclampsia, and gestational diabetes), and fetal sex |
| Mikkelsen et al, 2008, Denmark (41) | 1543/35,530 | Coffee consumption (cups/d) | Second | Gestational age <37 wk (<35 and 35–36 wk were also considered) | ≥3 cups/d ≤2 cups/d |
1.00 0.85 (0.75, 0.98) |
≤200 mg/d ≥300 mg/d2 |
Mediterranean diet criteria, parity, BMI, maternal height, socioeconomic status, and cohabitant status |
| Haugen et al, 2008, Norway (40) | 1184/40,817 | Coffee (including brewed, instant, coffee latte/cappuccino, espresso intake) | Second | Gestational age <37 wk (<35 and 35–36 wk were also considered) | ≥3 cups/d ≤2 cups/d |
1.00 1.14 (0.94, 1.37) |
≤200 mg/d ≥300 mg/d3 |
Mediterranean diet criteria, parity, BMI, maternal height, socioeconomic status, and cohabitant status |
| Santos et al,2005, Brazil (38) | 413/5168 | Maté tea (d/wk) | Throughout pregnancy | Singleton live birth before 37 wk | 0 d/wk 1–6 d/wk 7 d/wk |
1.00 1.0 (0.8, 1.3) 1.0 (0.8, 1.2) |
0 mg/d 300 mg/d |
Mother's and partner's education, monthly income, mother's skin color, previous fetal death, and maternal smoking during the third trimester of gestation, parity, previous abortion, and previous low birth weight |
| Bracken et al, 2003, USA (33) | 160/2292 and 134/2157 for first and third trimesters, respectively | Coffee, tea, and soda consumption (serving size/preparation methods); authors computed daily caffeine intake (mg/d) | First and third | Singleton live birth before 37 wk | First trimester: 0 mg/d 1–149 mg/d 150–299 mg/d ≥300 mg/d Third trimester: 0 mg/d 1–149 mg/d 150–299 mg/d ≥300 mg/d |
First trimester: 1.00 1.20 (0.82, 1.76) 1.74 (0.93, 3.27) 1.67 (0.74, 3.81) Third trimester: 1.00 0.84 (0.56, 1.24) 1.19 (0.56, 2.53) 1.79 (0.54, 6.00) |
First trimester: 0 mg/d ≥300 mg/d Third trimester: 0 mg/d ≥300 mg/d |
Age, parity, number of prior pregnancies,marital status, race, education, height, smoking during the third trimester, and weight |
| Eskenazi et al, 1999, USA (34) | 636/7855 | Decaffeinated coffee and caffeinated beverages, including coffee, tea, and cola; authors reported coffee consumption (yes or no) | Second | Gestational duration <37 completed weeks | No coffee Caffeinated coffee only |
1.0 1.3 (1.0, 1.7) |
0 mg/d >1 mg/d |
Age, education, cigarette smoking during the third trimester, height, parity, adequacy of prenatal care, tea and cola consumption, and race |
| Wisborg et al, 1996, Denmark (31) | 148/3464 | Coffee, tea, chocolate, and cola (servings in cups and bottles); authors computed daily caffeine intake (mg/d) | Second | Delivery before 37 wk gestation | <400 mg/d ≥400 mg/d |
1.04 1.16 (0.84, 1.61) |
<400 mg/d ≥400 mg/d |
NA |
| Peacock et al, 1995, UK (29) | 113/1513 | Caffeine; authors did not specify source (mg/wk) | Second | <37 wk of gestation | 0 mg/wk 1–1400 mg/wk 1401–2800 mg/wk ≥2801 mg/wk |
1.04 1.15 (0.29, 4.6) 0.87 (0.22, 3.46) 0.97 (0.24, 3.87) |
0 mg/d ≥400 mg/d |
NA |
| Fortier et al, 1993, Canada (9) | 394/7025 | Caffeinated coffee, tea, colas, and chocolate (serving size/preparation methods); authors computed daily caffeine intake (mg/d) | Throughout pregnancy | Gestational age of <37 wk | 0–10 mg/d 11–150 mg/d 151–300 mg/d >300 mg/d |
1.0 1.08 (0.83, 1.42) 1.04 (0.71, 1.53) 0.84 (0.46, 1.54) |
0–10 mg/d >300 mg/d |
Cigarette consumption (0, 1–5, 6–15, and ≥16 cigarettes/d), number of previous preterm newborns (0, 1, and ≥2), family income, and parity (0, ≥1) |
| McDonald et al, 1992, Canada (36) | 2803/40,445 | Coffee consumption (cups/d) | Throughout pregnancy | <37 wk of gestation | 0 cups/d 1–2 cups/d 3–4 cups/d 5–9 cups/d ≥10 cups/d |
1.00 1.00 (0.92, 1.09) 1.08 (0.94, 1.24) 1.06 (0.86, 1.30) 1.24 (0.86, 1.79) |
0 mg/d ≥1330 mg/d |
Age, race, education, employment, pregnancy order, previous spontaneous abortions, previous low birth weight infants, prepregnancy weight, cigarette, and alcohol consumption |
| Olsen et al, 1991, Denmark (37) | 370/11550 | Coffee and tea separately (cups/d); authors estimated 100 mg/cup for filtered coffee | Average of first and second | <37 wk of gestation | 0–3 cups/d 4–7 cups/d ≥8 cups/d |
1.0 1.1 (0.9, 1.4) 1.2 (0.8, 1.7) |
0–300 mg/d ≥800 mg/d |
Social status, smoking, alcohol, and parity |
| Fenster et al, 1991, USA (8) | NA/1230 | Caffeinated coffee, tea, and soft drinks (cups or cans); authors computed average daily consumption (mg/d) | First | Gestational age <37 wk | 0 mg/d >300 mg/d |
1.00 1.31 (0.63, 2.69) |
0 mg/d >300 mg/d |
Education(less than high school, high school graduate, some college, college graduate), race, hypertension, cigarettes, and alcohol |
| Teitelman et al, 1990, USA (39) | NA/3797 | Caffeine; authors did not specify source (mg/d) | First | <37 wk of gestation | ≤300 mg/d >300 mg/d |
1.00 1.14 (0.42, 3.13) |
≤300 mg/d >300 mg/d |
Sedentary job, standing job, nulliparity, cigarettes, education, marijuana use, black, interviewed at >12 wk, and unmarried |
| Martin and Bracken 1987, USA (35) | NA/3891 | Caffeinated coffee, tea, colas, and drugs (servings/wk); authors computed daily caffeine intake (mg/d) | Throughout pregnancy | Delivery before 37 wk gestation | 0 mg/d >300 mg/d |
1.0 1.4 (0.8, 2.2) |
0 mg/d >300 mg/d |
None |
| van den Berg, 1977, USA (30) | 470/8040 | Coffee (cups/d) | Throughout pregnancy | Delivery before 37 wk gestation | ≤1 cup/d 2–6 cups/d ≥7 cups/d |
1.04 1.3 (1.0, 1.7) 1.8 (1.7, 2.0) |
≤80 mg/d ≥560 mg/d |
NA |
NA, not available.
Because the categories were reversed, the odds ratio was recalculated as 1.18 (95% CI: 1.02, 1.33).
Because the categories were reversed, the odds ratio was recalculated as 0.88 (95% CI: 0.73, 1.06).
Effect estimates were not given in the publication; therefore, we calculated the univariate effect estimates for this meta-analysis based on figures provided in the publication.
TABLE 2.
Case-control studies of caffeine consumption and risk of preterm birth1
| Author, publication year, and country | No. of cases/no. of controls | Intake measurement | Trimester considered | Preterm definition | Comparisons made by authors | Risk estimate (95% CI) | Comparisons made in meta-analysis | Adjusted confounders |
| Chiaffarino et al, 2006, Italy (42) | 502/1966 | Coffee, tea, and cola (cups or glasses/d) | Third and throughout pregnancy | 28–37 wk gestation | 0 cups/d | 1.0 | 0 mg/d | Age, education, parity, smoking during the first trimester of pregnancy, gestational hypertension, and history of preterm births |
| 1 cup (90 mg)/d | 0.9 (0.6, 1.2) | |||||||
| ≥2 cups/d | 0.9 (0.7, 1.3) | ≥150 mg/d | ||||||
| de Souza and Sichieri, 2005, Brazil (45) | 140/162 | Coffee, tea, maté, and powdered chocolate (servings in mL or g); authors computed daily caffeine intake (mg/d) | Throughout pregnancy | <37 wk of gestation | <50 mg/d | 1.00 | <50 mg/d | NA |
| 50–99 mg/d | 1.58 (0.88, 2.84) | |||||||
| ≥100 mg/d | 1.35 (0.48, 3.80) | ≥100 mg/d | ||||||
| Tough et al, 2003, Canada (47) | 323/664 | Coffee | Throughout pregnancy | Live born singleton infant at <37 wk of gestation | <1 cup/d | 1.0 | <100 mg/d | Prior conception experiences, emotional health, interpregnancy intervals, pregnancy complications, maternal complications during pregnancy |
| 1.38 (1.01, 1.88) | ≥100 mg/d | |||||||
| Caffeinated soft drinks | ≥1 cup/d | 1.0 | <27 mg/d | |||||
| 1.22 (0.89, 1.72) | ≥27 mg/d | |||||||
| Bicalho and Filho, 2002, Brazil (46) | 182/354 | Coffee, soft drinks, and tea (servings in mL); authors computed daily caffeine intake (mg/d) | Throughout pregnancy | Delivery before 37 wk gestation | 0 mg/d | 1.0 | 0 mg/d | Mother's age, schooling, income, marital status, skin color, parity, smoking, previous low-birth-weight children, mother's prepregnancy weight, employment status, interval between pregnancies, prenatal care, and high blood pressure |
| <300 mg/d | 0.59 (0.32, 1.09) | |||||||
| ≥300mg/d | 0.32 (0.15, 0.72) | ≥300 mg/d | ||||||
| Pastore and Savitz 1995, USA (43) | 408/490 | Caffeinated coffee, tea, cola soft drinks, and non-cola caffeinated soft drinks (servings and preparation method); authors computed daily caffeine intake (mg/d) | Second and third | <37 wk of gestation | Second trimester: 0 mg/d >1 mg/d Third trimester: 0 mg/d >1 mg/d |
Second trimester: 1.0 1.5 (1.0, 2.3) Third trimester: 1.0 0.8 (0.6, 1.2) |
Second trimester: 0 mg/d >1 mg/d Third trimester: 0 mg/d >1 mg/d |
None |
| Williams et al, 1992, USA (44) | 488/2252 | Coffee (cups/d) | First | Delivery before 37 wk gestation without premature rupture of membranes | 0 cups/d | 1.0 | 0 mg/d | Race, education, maternal age, welfare status, marijuana and alcohol use during pregnancy, parity, previous spontaneous or induced abortion, cervical incompetence, bleeding during pregnancy, prepregnancy BMI, and cigarette smoking |
| 1–2 cups/d | 1.0 (0.8, 1.3) | |||||||
| 3 cups/d | 1.4 (0.9, 2.3) | |||||||
| 4 cups/d | 1.8 (0.9, 3.4) | |||||||
| ≥5 cups/d | 1.1 (0.5, 2.1) | >400 mg/d | ||||||
| Berkowitz et al, 1982, USA (26) | 166/299 | Coffee and tea, including iced coffee and iced tea, separately (cups/d) | First | Singleton live birth before 37 wkof gestation not preceded by spontaneous labor or rupture of membranes | 0 cups/d | 1.0 | 0 mg/d | Race, socioeconomic status, infertility history, first trimester spotting or light bleeding, pregravid weight, maternal weight gain, previous induced abortion, leisure-time physical activity, and attitude toward pregnancy |
| 1 cup/d | 0.7 (0.4, 1.3) | |||||||
| 2 cups/d | 0.9 (0.5, 1.6) | |||||||
| 3 cups/d | 1.6 (0.8, 3.3) | |||||||
| ≥4 cups/d | 0.6 (0.3, 1.4) | ≥320 mg/d | ||||||
| Second | 0 cups/d | 1.0 | 0 mg/d | |||||
| 1 cup/d | 0.6 (0.4, 1.1) | |||||||
| 2 cups/d | 0.8 (0.4, 1.4) | |||||||
| 3 cups/d | 1.3 (0.7, 2.8) | |||||||
| ≥4 cups/d | 0.6 (0.3, 1.3) | ≥320 mg/d | ||||||
| Third | 0 cups/d | 1.0 | 0 mg/d | |||||
| 1 cup/d | 0.6 (0.4, 1.1) | |||||||
| 2 cups/d | 1.1 (0.6, 2.0) | |||||||
| 3 cups/d | 1.5 (0.7, 3.0) | |||||||
| ≥4 cups/d | 0.5 (0.2, 1.1) | ≥320 mg/d |
NA, not available.
Quality assessment
We examined the Egger test estimates for the individual trimesters separately for all cohort and case-control studies. No evidence of significant publication bias was found for either the cohort or case-control studies (see Supplementary Figures S1–3 under “Supplemental data” in the online issue).
Heterogeneity assessment
We found significant heterogeneity between study designs (P < 0.0001). All subsequent analyses were conducted separately for the cohort and case-control studies. On initial testing, we found significant heterogeneity for all trimesters for the cohort studies (P < 0.0001) and for the case-control studies (P < 0.03). To examine this heterogeneity, we conducted separate meta-regression analyses for each trimester with independent variables: region, publication decade, exposure assessment outcome assessment, sources of caffeine, and whether the studies adjusted for confounders. For cohort studies, significant heterogeneity was found for geographic region (second trimesters), decade of publication (all trimesters), outcome assessment (first and third trimesters), and adjustment for different confounders (first and third trimesters).
The observed heterogeneity for the cohort studies was largely due to the study by van den Berg et al (30). On removal of this study, the heterogeneity for all cohort study analyses became nonsignificant. By creating broad categories of caffeine intake, the van den Berg study exerted undue influence on the effect estimate. In addition, the van den Berg study did not adjust for important confounding variables. Therefore, we removed this study from the combined analysis. For case-control studies, the heterogeneity was driven by the Tough et al study (47). After this study was removed, heterogeneity was found only for the second trimester and was driven by studies that adjusted for different confounders. No significant heterogeneity was found for sources of caffeine, outcome, or exposure assessment. The inclusion of both caffeine sources from the study by Tough et al (47) generated significant heterogeneity by region, exposure assessment, and adjustment for confounding for the third trimester only. The region-specific ORs were 1.27 (95% CI: 1.05, 1.54) (26, 43, 47) for North America and 0.54 (95% CI: 0.29, 1.01) (45, 46) for Brazil.
In case-control studies that assessed the exposure through phone interview, the combined OR was 0.81 (95% CI: 0.65, 1.00) (42, 43, 45, 46), whereas in studies that used questionnaire-based assessment, the combined OR was 1.22 (95% CI: 0.98, 1.52) (26, 47).
Studies adjusted for different confounding variables. When combining case-control studies that did not adjust for any confounders (43, 45), we found a weak inverse combined OR (0.84; 95% CI: 0.61, 1.17). A stronger inverse association was found for studies that adjusted for age, socioeconomic status, race, parity, smoking, and BMI (0.39; 95% CI: 0.22, 0.70) (26, 46), whereas the combined OR for the 2 caffeine sources in the Tough et al study (47), which adjusted for other variables (prior conception experiences, emotional health, interpregnancy intervals, pregnancy complications, and maternal complications during pregnancy), was 1.30 (95% CI: 1.04, 1.63).
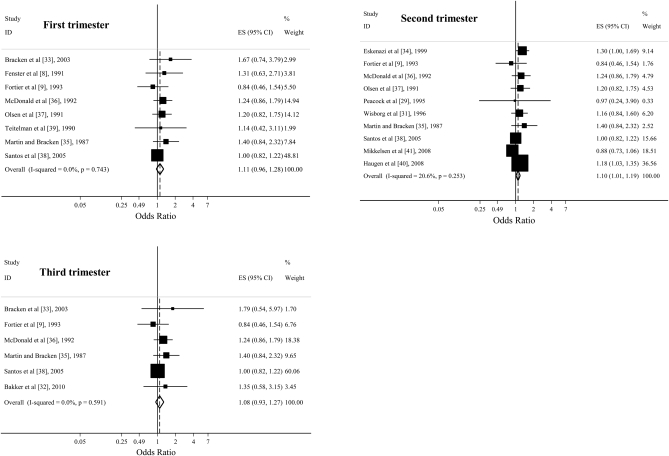
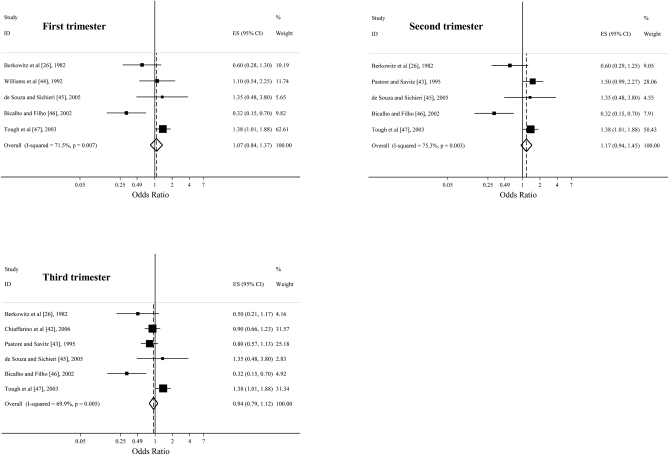
The overall effect estimates from a comparison of the highest caffeine consumption category with the lowest category in the first, second, and third trimesters in cohort studies were 1.11 (95% CI: 0.96, 1.28; P = 0.15), 1.10 (95% CI: 1.01, 1.19; P = 0.02), and 1.08 (95% CI: 0.93, 1.27; P = 0.32) (Figure 2), respectively. The combined effect estimates in case-control studies during the first, second, and third trimesters were 1.04 (95% CI: 0.84, 1.37; P = 0.60), 1.17 (95% CI: 0.94, 1.45; P = 0.17), and 0.94 (95% CI: 0.79, 1.12; P = 0.46), respectively (Figure 3.). Limiting the analysis to studies that directly provided the effect estimates did not substantially change the results.
FIGURE 2.
Odds ratios from cohort studies estimating the association between prenatal caffeine consumption (highest compared with lowest intake) in the first, second, and third trimesters and preterm birth. Squares indicate study-specific estimates, horizontal lines indicate the 95% CIs, and diamonds indicate the summary estimate of the odds ratio with its corresponding 95% CI. ES, effect size; ID, identification.
FIGURE 3.
Odds ratios from case-control studies estimating the association between prenatal caffeine consumption (highest compared with lowest intake) in the first, second, and third trimesters and preterm birth. Squares indicate study-specific estimates, horizontal lines indicate the 95% CIs, and diamonds indicate the summary estimate of the odds ratio with its corresponding 95% CI. ES, effect size; ID, identification.
Subgroup analysis
A subanalysis that examined caffeine intake as a dichotomous variable (none compared with any) strengthened the effect estimate among cohort studies (1.12; 95% CI: 1.02, 1.23). Only 2 case-control studies included a dichotomized exposure, with a combined effect estimate of 1.00 (95% CI: 0.79, 1.27) (43, 47). Analyses that limited the highest intake to ≥300 mg/d (8, 9, 29, 31, 33, 35–39) resulted in combined effect estimates for the cohort studies that were similar to those observed in the original analysis. For case-control studies, limiting the highest intake to ≥300 mg/d (26, 44–46) shifted the association in the inverse direction for all trimesters: first (0.70; 95% CI: 0.47, 1.04), second (0.57; 95% CI: 0.35, 0.91), and third (0.53; 95% CI: 0.32, 0.87).
Results from the subanalysis using only coffee intake were similar to the results from the main caffeine analysis. The estimates for the first (1.22; 95% CI: 1.00, 1.49), second (1.12; 95% CI: 1.02, 1.22), and third (1.22; 95% CI: 0.95, 1.57) trimesters in the cohort studies and for the third (0.88; 95% CI: 0.73, 1.07) trimester estimates in case-control studies were strengthened. No important association was found for tea drinking during pregnancy.
DISCUSSION
In this meta-analysis of 15 cohort and 7 case-control studies, we found no important association between maternal caffeine consumption during pregnancy and the risk of preterm birth. This association has been examined for >30 y with inconsistent results (6, 14). After reviewing the literature, we found 8 studies with either positive (26, 30, 32, 40, 43) or inverse (41, 42, 46) associations, whereas most of the studies suggested no association (8, 9, 29, 31–37, 39, 44, 45, 47). A recent randomized, double-blind, controlled trial conducted in pregnant women who normally drank ≥3 cups coffee/d randomly assigned the subjects to drink their usual amounts of either caffeinated or decaffeinated coffee. The trial found no significant difference in mean length of gestation (49).
The present meta-analysis is the most comprehensive quantitative review of the available evidence; compared with the most recent reviews (14–16), we included more studies, half of which were published within the past 10 y (33, 38, 40–42, 45–47). Our initial cohort results were strongly weighted by the van den Berg (30) study, which was published in the 1977 Proceedings of a Symposium on the Epidemiology of Prematurity at the National Institute of Child Health and Human Development. Although this study suggested a positive relation between caffeine intake and preterm birth, the authors did not use modern methods of multivariate adjustment. Its failure to adjust for confounding by maternal characteristics likely led to the observed positive association and heterogeneity. Furthermore, this study used the broad reference of ≤1 cup/d rather than 0 cups/d, which was used in most cohort studies. The resulting low variance of comparison with the highest intake gave this study undue weight in the combined analysis, and its removal generated an overall null result except for the borderline significant second trimester. This study's inclusion in past meta-analyses may have led to an upward bias of the combined effect estimates. Although our results were based on a considerable number of studies, the estimates were sensitive to adjustment for cofounders, and adjustment of important preterm birth predictors should be carefully considered in future analyses.
The results for the case-control studies were weighted by the recent study by Tough et al (47), which generated an overall null association for all 3 trimesters. Its influence was predominant for the first and second trimesters, where fewer studies were present. For most of the case-control studies, an inverse association was observed. This may be attributed to their inherent limitations, particularly recall and selection bias. Mothers with preterm infants may underreport caffeine intake to avoid blame, which leads to an erroneous protective association. Furthermore, most of the case-control studies used hospital controls, who may not represent the underlying population from which cases were derived because of referral patterns. Whereas many studies selected a valid control group, including matching on age, sex, race, or day or time of the week of delivery (42, 43, 45, 47) and excluding transfer patients (26), no single study took all these precautions; therefore, we cannot exclude the possibility of selection bias. Duration of exposure and changes in consumption patterns during pregnancy may have influenced the results, especially because cases were exposed to caffeine for a shorter period of time than were the controls. Caffeine consumption was shown to be fairly constant for the entire pregnancy duration (23, 26); hence, the scope for such bias is limited.
The presence of heterogeneity among the included cohort studies was limited after the exclusion of the van den Berg study (30). Minor heterogeneity remained only for outcome assessment. We also examined studies that based their exposure on a variety of caffeine sources. Some studies included multiple beverages, whereas others included only coffee and tea. We assumed that the main source of caffeine was coffee and that differences between coffee blends were minimal (50). We were unable to consistently consider alternate sources of caffeine, although some studies included cold remedies, pain medications, and chocolate (9, 35, 45). Subanalyses were conducted on coffee and tea, and no significant heterogeneity was found. The results were similar to those from the caffeine analysis, except for the case-control studies, which showed an inverse association for all 3 trimesters. These results were affected by the absence of the study by Tough et al (47), which weighted the caffeine analysis. The inclusion of both coffee and soda as sources of caffeine for this study generated heterogeneity across region, exposure assessment, and adjustment for confounding. It is difficult to establish whether this observed heterogeneity represented true differences across the covariates or reflected excessive influence by the Tough et al study.
Many preterm births are due to premature rupture of membranes (PROM) (51), which may be caused by infection and poor prenatal care. We included studies that defined preterm birth as a general category or classified with or without PROM [16, 43, 44], which possibly led to a discrepancy of outcome definition. Two studies defined preterm birth without PROM (26, 44), whereas another had 3 categories: PROM, medically induced labor, and idiopathic preterm labor (44). We used the effect estimate for idiopathic preterm labor because it had a more specific outcome definition. The results for PROM and medically induced labor were similar for this study, although, in another study (44), the effect estimates for PROM were higher than for non-PROM. Therefore, predicting the effect of the inclusion of PROM cases on the combined effect estimate is difficult.
Preterm birth has numerous risk factors, including previous preterm births, maternal smoking (52), parity (53), low BMI (54), and ethnicity (55). Only 4 studies stratified on smoking, and we could not evaluate this interaction. Two studies suggested an adverse association among smokers who were heavy coffee drinkers (31, 33), although other studies found no association (37, 44). One study found an almost double, though nonsignificant, risk among primiparous as compared with multiparous women (37). Caffeine intake across BMI categories indicated little difference (9, 36). We could not stratify on these factors because most studies did not provide sufficient data. Using our data, we found that, whereas caffeine intake was adversely associated with preterm births in North America, there was no association in South America and Europe (data not shown). A strong inverse association was found for the third trimester among studies conducted in Brazil. However, this was limited to case-control studies and was explained by recall and selection bias. Variation in caffeine metabolism between individuals and between populations (56–58) may be explained by genetic polymorphisms that affect CYP1A2 activity—the primary enzyme associated with caffeine clearance. Most studies were conducted with white participants (9, 26, 30, 31, 33, 35–37, 39–42, 44, 47). Only 3 studies included a large proportion of minority populations (8, 34, 43). Research on discrepancies between ethnicities is warranted for public health efforts. In particular, future observational prospective studies or clinical trials may consider examining genetic or epigenetic consequences related to prenatal caffeine exposures in different populations.
The strengths of our analyses included searching multiple databases and including non-English studies [Portuguese (45, 46) and German (19)]. The search found publications over the past 30 y, incorporating the trends of caffeine-containing beverage consumption. We also examined the potential associations of different exposure levels compared with no exposure, which were similar; thus, we reported only the comparison of the highest with the lowest levels. In addition, we compared the associations for both coffee and tea consumption, which may be more relevant for public health recommendations. We found no evidence of existing publication bias.
The current recommendation is to either eliminate caffeine during pregnancy or limit intake to <300 mg/d (6). Whereas these recommendations may be sensible with respect to other pregnancy outcomes, such as low birth weight, where risk may be increased even with low caffeine intake (59), the risk of preterm birth does not seem to be affected by caffeine consumption. However, we were unable to draw conclusions regarding caffeine intakes >300–400 mg/d as most studies used this as their upper limit. Higher intakes of caffeine are especially important in light of new caffeine sources such as bottled water, energy drinks, and herbal supplements, which often do not report caffeine content and may therefore covertly increase caffeine consumption during pregnancy (10). Blood caffeine concentrations and/or careful consideration of the dietary sources could resolve this discrepancy and should be examined in future studies.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—EM, SB, and S-WL: reviewed the literature on the caffeine intake and preterm births and extracted relevant information; EM: conducted the statistical analyses; EM, SB, and S-WL: wrote the manuscript; and KBM: contributed advice on analyses and revisions of the manuscript. None of the authors had any personal or financial conflicts of interest.
REFERENCES
- 1.Hamilton BE, Martin JA, Ventura SJ. 14 May 14 2009. Available from: www.cdc.gov/nchs/products/pubs/pubd/hestats/prelimbirths05/prelimbirths05.htm (cited 14 May 2009.)
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9 [DOI] [PubMed] [Google Scholar]
- 3.Irving RJ, Belton NR, Elton RA, Walker BR. Adult cardiovascular risk factors in premature babies. Lancet 2000;355:2135–6 [DOI] [PubMed] [Google Scholar]
- 4.Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med 2004;351:2179–86 [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113(suppl 3):17–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam 2003;20:1–30 [DOI] [PubMed] [Google Scholar]
- 7.Golding J. Reproduction and caffeine consumption–a literature review. Early Hum Dev 1995;43:1–14 [DOI] [PubMed] [Google Scholar]
- 8.Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and fetal growth. Am J Public Health 1991;81:458–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortier I, Marcoux S, Beaulac-Baillargeon L. Relation of caffeine intake during pregnancy to intrauterine growth retardation and preterm birth. Am J Epidemiol 1993;137:931–40 [DOI] [PubMed] [Google Scholar]
- 10.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 2005;105:110–3 [DOI] [PubMed] [Google Scholar]
- 11.Soyka LF. Caffeine ingestion during pregnancy: in utero exposure and possible effects. Semin Perinatol 1981;5:305–9 [PubMed] [Google Scholar]
- 12.Goldstein A, Warren R. Passage of caffeine into human gonadal and fetal tissue. Biochem Pharmacol 1962;11:166–8 [DOI] [PubMed] [Google Scholar]
- 13.Aldridge A, Aranda JV, Neims AH. Caffeine metabolism in the newborn. Clin Pharmacol Ther 1979;25:447–53 [DOI] [PubMed] [Google Scholar]
- 14.Santos IS, Victora CG, Huttly S, Morris S. Caffeine intake and pregnancy outcomes: a meta-analytic review. Cad Saude Publica 1998;14:523–30 [DOI] [PubMed] [Google Scholar]
- 15.Peck JD, Leviton A, Cowan LD. A review of the epidemiologic evidence concerning the reproductive health effects of caffeine consumption: a 2000-2009 update. Food Chem Toxicol; (Epub ahead of print 15 June 2010) [DOI] [PubMed] [Google Scholar]
- 16.Pacheco AH, Barreiros NS, Santos IS, Kac G. Caffeine consumption during pregnancy and prevalence of low birth weight and prematurity: a systematic review. Cad Saude Publica 2007;23:2807–19 [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 18.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol 2008;27:604–15 [DOI] [PubMed] [Google Scholar]
- 19.Mau G, Netter P. [Are coffee and alcohol consumption risk factors in pregnancy? ] Geburtshilfe Frauenheilkd 1974;34:1018–22 (in German) [PubMed] [Google Scholar]
- 20.Obaid-ur-Rahman M, Ahmed T, Rahman S, Atiqur R. Effects of socioeconomic factors, psychological stress, smoking, alcohol and caffeine on preterm delivery. Pak J Pharm Sci 1998;11:35–9 [PubMed] [Google Scholar]
- 21.Teles TP, Barros H, da Silva MV. [Risk or pre-term labor. A cross-sectional study of its determinant factors.] Acta Med Port 1992;5:247–50 (in Portuguese) [PubMed] [Google Scholar]
- 22.Furuhashi N, Sato S, Suzuki M, Hiruta M, Tanaka M, Takahashi T. Effects of caffeine ingestion during pregnancy. Gynecol Obstet Invest 1985;19:187–91 [DOI] [PubMed] [Google Scholar]
- 23.Watkinson B, Fried PA. Maternal caffeine use before, during and after pregnancy and effects upon offspring. Neurobehav Toxicol Teratol 1985;7:9–17 [PubMed] [Google Scholar]
- 24.Weathersbee PS, Olsen LK, Lodge JR. Caffeine and pregnancy. A retrospective survey. Postgrad Med 1977;62:64–9 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol 2009;62:97–128 [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz GS, Holford TR, Berkowitz RL. Effects of cigarette smoking, alcohol, coffee and tea consumption on preterm delivery. Early Hum Dev 1982;7:239–50 [DOI] [PubMed] [Google Scholar]
- 27.USDA National Nutrient Database for Standard Reference Release 21. Washington, DC: US Department of Agriculture, Agricultural Research Service, 2008 [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peacock JL, Bland JM, Anderson HR. Preterm delivery: effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ 1995;311:531–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Berg BJ. Epidemiological observations of prematurity: effects of tobacco, coffee and alcohol. : Reed DM, Stainley FJ, The epidemiology of prematurity. Baltimore, MD: Urban & Schwarzenberg, 1977:157–77 [Google Scholar]
- 31.Wisborg K, Henriksen TB, Hedegaard M, Secher NJ. Smoking during pregnancy and preterm birth. Br J Obstet Gynaecol 1996;103:800–5 [DOI] [PubMed] [Google Scholar]
- 32.Bakker R, Steegers EA, Obradov A, Raat H, Hofman A, Jaddoe VW. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am J Clin Nutr 2010;91:1691–8 [DOI] [PubMed] [Google Scholar]
- 33.Bracken MB, Triche EW, Belanger K, Hellenbrand K, Leaderer BP. Association of maternal caffeine consumption with decrements in fetal growth. Am J Epidemiol 2003;157:456–66 [DOI] [PubMed] [Google Scholar]
- 34.Eskenazi B, Stapleton AL, Kharrazi M, Chee WY. Associations between maternal decaffeinated and caffeinated coffee consumption and fetal growth and gestational duration. Epidemiology 1999;10:242–9 [PubMed] [Google Scholar]
- 35.Martin TR, Bracken MB. The association between low birth weight and caffeine consumption during pregnancy. Am J Epidemiol 1987;126:813–21 [DOI] [PubMed] [Google Scholar]
- 36.McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. Am J Public Health 1992;82:87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen J, Overvad K, Frische G. Coffee consumption, birthweight, and reproductive failures. Epidemiology 1991;2:370–4 [DOI] [PubMed] [Google Scholar]
- 38.Santos IS, Matijasevich A, Valle NCJ. Mate drinking during pregnancy and risk of preterm and small for gestational age birth. J Nutr 2005;135:1120–3 [DOI] [PubMed] [Google Scholar]
- 39.Teitelman AM, Welch LS, Hellenbrand KG, Bracken MB. Effect of maternal work activity on preterm birth and low birth weight. Am J Epidemiol 1990;131:104–13 [DOI] [PubMed] [Google Scholar]
- 40.Haugen M, Meltzer HM, Brantsaeter AL, et al. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:319–24 [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen TB, Osterdala ML, Knudsena VK, et al. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:325–30 [DOI] [PubMed] [Google Scholar]
- 42.Chiaffarino F, Parazzini F, Chatenoud L, et al. Coffee drinking and risk of preterm birth. Eur J Clin Nutr 2006;60:610–3 [DOI] [PubMed] [Google Scholar]
- 43.Pastore LM, Savitz DA. Case-control study of caffeinated beverages and preterm delivery. Am J Epidemiol 1995;141:61–9 [DOI] [PubMed] [Google Scholar]
- 44.Williams MA, Mittendorf R, Stubblefield PG, Lieberman E, Schoenbaum SC, Monson RR. Cigarettes, coffee, and preterm premature rupture of the membranes. Am J Epidemiol 1992;135:895–903 [DOI] [PubMed] [Google Scholar]
- 45.de Souza RA, Sichieri R. [Caffeine intake and food sources of caffeine and prematurity: a case-control study.] Cad Saude Publica 2005;21:1919–28(in Portuguese) [DOI] [PubMed] [Google Scholar]
- 46.Bicalho GG, Barros Filho Ade A. [Birthweight and caffeine consumption.] Rev Saude Publica 2002;36:180–7 (in Portuguese) [DOI] [PubMed] [Google Scholar]
- 47.Tough SC, Newburn-Cook CV, White DE, et al. Do maternal characteristic and past pregnancy experiences predict delivery among women aged 20 to 34? J Obstet Gynaecol Can 2003;25:656–66 [DOI] [PubMed] [Google Scholar]
- 48.Weinberg BA, Bealer BK. The world of caffeine: the science and culture of the world's most popular drug. New York, NY: Routledge, 2002 [Google Scholar]
- 49.Bech BH, Obel C, Henriksen TB, Olsen J. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ 2007;334:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casal S, Oliveira MBPP, Alves MR, Ferreira MA. Discriminate analysis of roasted coffee varieties for trigonelline, nicotinic acid, and caffeine content. J Agric Food Chem 2000;48:3420–4 [DOI] [PubMed] [Google Scholar]
- 51.ACOG Premature rupture of membranes. Washington, DC: American College of Obstetricians and Gynecologists, 2007 [Google Scholar]
- 52.Cnattingius S, Granath F, Petersson G, Harlow BL. The influence of gestational age and smoking habits on the risk of subsequent preterm deliveries. N Engl J Med 1999;341:943–8 [DOI] [PubMed] [Google Scholar]
- 53.Fuentes-Afflick E, Hessol NA. Interpregnancy interval and the risk of premature infants. Obstet Gynecol 2000;95:383–90 [DOI] [PubMed] [Google Scholar]
- 54.Mercer BM, Macpherson CA, Goldenberg RL, et al. Are women with recurrent spontaneous preterm births different from those without such history? Am J Obstet Gynecol 2006;194:1176–84, discussion 84–5 [DOI] [PubMed] [Google Scholar]
- 55.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA 2000;283:1591–6 [DOI] [PubMed] [Google Scholar]
- 56.Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5′-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem 1999;125:803–8 [DOI] [PubMed] [Google Scholar]
- 57.Han XM, Ou-Yang DS, Lu PX, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734A polymorphisms of human CYP1A2. Pharmacogenetics 2001;11:429–35 [DOI] [PubMed] [Google Scholar]
- 58.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 1999;47:445–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod 2008;23:168–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.