Abstract
Background: Few studies have examined temporal trends in sodium intake in the US population. Collections of 24-h urine sodium excretions are reliable markers for dietary sodium intake.
Objective: We examined temporal trends in 24-h urine sodium excretions to estimate temporal trends in sodium intake in the US population.
Design: We performed a systematic search of English-language articles in MEDLINE for studies that reported collections of 24-h urine sodium excretions in the United States. We estimated mean urine sodium excretions over time for all studies and demographic subgroups.
Results: We analyzed 38 studies, which dated from 1957 to 2003, and estimated a mean (±SE) 24-h urine sodium excretion per person of 3526 ± 75 mg Na. In a multivariate random-effects model with study year, sex, age, and race, the study year was not associated with any significant change in sodium excretions (coefficient = 154 mg Na · 24 h−1 · 10 y−1; 95% CI: −140, 448 mg Na · 24 h−1 · 10 y−1). In subgroup analyses, there was no significant temporal trend seen in male, female, black, or white study participants.
Conclusion: Sodium intake in the US adult population appears to be well above current guidelines and does not appear to have decreased with time.
INTRODUCTION
See corresponding editorial on page 1005.
The prevalence of hypertension in the US population has increased over the past 20 y in men, women, blacks, and whites (1–3). Because greater dietary sodium intakes increase blood pressure and the risk of hypertension (4, 5), a reduction in sodium intake has been recommended for the US population (4–10). Two recently published computer-simulation models concluded that a population-wide reduction in sodium intake would decrease cardiovascular disease and death and associated health care costs (9, 11). However, some authors have questioned the basis for reduction in sodium intake because only a few randomized controlled trials with morbidity and mortality endpoints have been conducted (12, 13).
Because the large majority of sodium in the US diet is added in manufacturing and food services (14, 15), for many years the food industry has been urged to reduce sodium in processed foods (8). Whether sodium intake in the United States has changed over time is unclear. The calculated intake of sodium appears to have increased in the United States slightly over the last several decades (16–18), but it is possible that changes in food-composition databases have not fully captured changes in food processing. The collection of 24-h urine sodium excretion is the principal biochemical indicator used in epidemiologic studies for estimating daily sodium intake and reflects both changes in food choices and processing (19). Collections of 24-h urine excretions in subjects from the United Kingdom between 1984 and 2008 showed a narrow range of sodium excretion and no decline in intake (13). To our knowledge, there have been no nationally representative assessments of 24-h urinary sodium excretion in the United States over time. Therefore, to estimate temporal trends in sodium intake in the US population, we reviewed all published studies in which collections of 24-h urine sodium excretions were reported.
METHODS
We observed the preferred reporting items for systematic reviews and meta-analyses guidelines for this systematic review (20).
Studies used
We performed a search of peer-reviewed, published articles by using the MEDLINE electronic database (http://www.ncbi.nlm.nih.gov/pubmed). The search aimed to retrieve articles on urinary sodium (by using terms such as sodium, salt, urination, or urinalysis) and population trends in the United States (including terms such as trends, epidemiology, and population). No restriction on the dates of publication was imposed, but the language of publication was limited to English. The search was performed on 26 March 2009 (see supplemental material under “Supplemental data” in the online issue for search terms). Additional citations were found from discussions with experts in the fields of nutrition and epidemiology.
The primary search identified >1000 publications. Publications were selected for secondary review if they reported results of an observational study or clinical trial and if the study subjects were adults living in the United States. There was no restriction on disease status of, or medication use by, study participants. Because none of the voluntary study participants in any of the studies was acutely ill, and none of the participants had their medications abruptly discontinued, all participants were deemed to have reached a steady state of sodium balance where intake equals output (21).
All articles meeting secondary inclusion criteria were reviewed, as were their bibliographic references. Publications and bibliographic references were selected for data extraction if they reported 24-h urine sodium excretion amounts in text or tables or if they reported sufficient information in figures to estimate these amounts. Studies were excluded if we were unable to arrive at precise point estimates for sodium excretion per 24 h. Studies that reported the urine concentration of sodium (eg, mmol/L or mEq/L) were excluded, as were articles with overnight (8 or 9 h) urine samples. To reduce the bias in estimates of 24-h urine sodium excretions, articles that combined multiple collections of urine samples of several hours into one 24-h collection and articles that estimated 24-h urine sodium excretion by multiplying a collection of urine samples of shorter duration by a correction factor were also excluded. Spot urine samples were excluded, as were studies that reported only 24-h urine excretion amounts at the end of an experimental intervention. Recognizing that laboratory techniques for measuring urine sodium likely have evolved over the past 50 y, we did not restrict our analysis to one particular type of technique (eg, ion-electrode compared with flame photometry). There were 38 studies retrieved.
Data analyses
With the use of piloted forms, we extracted the following variables from the 38 studies: year of study (if the date of study was not available, then the date of submission of the manuscript was used; if this information was not available, then the year of the article publication was used; if the study was conducted over several years, then the study midpoint was used for our analysis), number of study participants, mean age of participants, percentage of participants who were women, percentage of participants who were black or African American, location of the study, mean plus SD of systolic blood pressure and diastolic blood pressure, and mean plus SD urinary sodium excretion per 24 h. Millimoles or milliequivalents of urine sodium were converted to milligrams (23 mg Na = 1 mmol Na or 1 mEq Na). For intervention trials, baseline values were used, and if these values were not available, then values for the run-in period or for the control group were used. SDs were converted to SEs (SE = SD divided by square root of the number of study participants). For studies that did not report an overall SE but only the SEs among study subgroups (eg, in men and women or blacks and whites), the overall study SE was calculated by using the following formula (22): SE for all groups in a particular study was equal to the square root of 1/(w1 + w2), where w1 = 1/(SE of subgroup 1)2 and w2 = 1/(SE of subgroup 2)2.
For each of the 38 studies, if a mean overall sodium excretion for all study participants was not reported, then we estimated it by multiplying the mean of each demographic subgroup (eg, blacks and whites or men and women) by the percentage contribution of the subgroup to the total number of study participants and summed up these values (thus, we weighted each subgroup by their number of study participants). We arrived at a summary estimate of the mean sodium excretion among all study participants from all 38 studies by summing the means from all 38 studies, with each study weighted by the square root of the number of study participants in each study. Similarly, we arrived at the mean sodium excretion for men, women, blacks, and whites and for studies with a mean age of participants <50 y and for studies with a mean age ≥50 y by summing the means in each of these subgroups, with each study weighted by the square root of number of participants in each subgroup. We estimated a 95% CI for the mean overall sodium excretion for all study participants by random-effects meta-analysis (meta function, STATA 10; StataCorp, College Station, TX).
We looked at the mean sodium excretion among all study participants and among demographic subgroups stratified by decade (before 1980, 1981–1990, 1991–2000, and after 2000). We tested for trends across time and calculated P values by using weighted linear regression models with sodium excretion as the continuous dependent variable and the year of the study as the continuous independent variable. We did this for all studies and for demographic subgroups, with each study weighted by the square root of the number of study participants. We estimated a power of 92% to detect a difference in urinary sodium excretion (an effect size) of 6 mg Na/24 h per year.
We further explored the association of sodium excretion with the year of the study by using a random-effects regression model with urine sodium as the continuous dependent variable and the year of study as the continuous independent variable (metareg function, STATA 10; StataCorp, College Station, TX). The random-effects model accounts for the SEs of individual studies and is used in meta-analysis to examine associations between pooled relative risks or odds ratios and independent study variables. We chose this random-effects model, rather than a fixed-effects model, because of the variability among studies in terms of design, date of participation, and demographic characteristics of study participants. We used the model to perform a meta-regression and explore the relation between the mean sodium excretion and demographic variables of studies. We fit separate univariate random-effects models with sodium excretion as the continuous dependent variable and age (a binary variable for each study that indicated if the mean age of the participants was ≥50 y), race (percentage of participants in each study who were black or African American), and sex (percentage of participants in each study who were men) as independent variables. We also fit a multivariate random-effects model with continuous sodium excretion as the dependent variable and the year of the study plus all demographic variables included as independent variables. Studies that did not report an SE and for which we could not derive one from the subgroups were not included in the meta-regression.
RESULTS
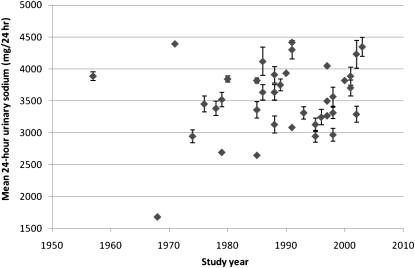
We located 38 studies that dated from 1957 to 2003, with analyzable data on sodium excretion from 26,271 study participants in populations living across the United States (Table 1) (23–61). Over this 46-y period, the overall mean 24-h urine sodium excretion was 3526 mg, with an SEM of 75 mg. The studies fell within a fairly narrow range as follows (Figure 1): the 25th percentile was 3266 mg Na/24 h and the 75th percentile was 3818 mg Na/24 h. With the use of a meta-analysis random-effects model, we calculated a mean 24-h urine sodium excretion of 3417 mg Na (95% CI: 3395, 3440 mg Na). One study from 1968 with only 5 participants reported a sodium excretion <2000 mg Na.
TABLE 1.
Publications analyzed for trends in 24-h urinary sodium excretion in the United States, 1957–20031
| Reference (study year) | Trial name | Study type | Location (US state postal abbreviation) | Participants | Age | Women | Black or African American | Systolic and diastolic blood pressure | Urine measurements | Urine sodium |
| n | y | % | % | mm Hg | n | mmol or mEq/24 h | ||||
| Nishizaka et al (23) (2000–2004)23 | NA | PC | AL | 265 | 56 ± 124 | 56 | 43 | Blacks: 160 ± 27 and 91 ± 18; whites: 160 ± 27and 87 ± 15 | 1 | Blacks: 172 ± 85; whites: 194 ± 74 |
| Nishizaka et al (24) (2003)35 | NA | PC | AL | 76 | 55 ± 12 | 59 | 59 | 163 ± 18 and 91 ± 14 | 1 | 189 ± 75 |
| Carbone et al (25) (2002)6 | NA | SV | TN | 89 | 647 | 100 | 44 | NR | 1 | 143 ± 64 |
| Calhoun et al (26) (2001–2002)3 | NA | PC | AL | 88 | 57 ± 12 | 62 | 49 | 159 ± 24 and 90 ± 16 | 1 | 169 ± 71 |
| Chapman et al (27) (2001)358 | NA | NRT | GA, MN | 505 | 487 | 52 | 44 | Men: 144 ± 13 and 97 ± 5; women: 149 ± 13 and 96 ± 5; whites: 142 ± 12 and 95 ± 5; blacks: 153 ± 14 and 97 ± 5 | 1 | Men: 182 ± 75; women: 146 ± 57; whites: 161 ± 66; blacks: 167 ± 62 |
| Taylor and Curhan (28) (1994–1999; 2001)9 | Health Professionals Follow-Up Study | PC | National | 1003 | 637 | 0 | 0 | NR | 3 | 176 |
| Taylor and Curhan (28) (1994–1999; 2001)9 | NHS | PC | National | 1286 | 657 | 100 | 0 | NR | 3 | 142 |
| Taylor and Curhan (28) (1994–1999; 2001)9 | NHS II | PC | National | 984 | 487 | 100 | 0 | NR | 3 | 152 |
| Powell et al (29) (2000) | NA | SV | National | 5942 | 43–4810 | 29 | NR | NR | 1 | Men: 180; women: 139 |
| Sacks et al (30) (1997–1999)311 | DASH-S | RCT | MA, NC, OR, MD, LA | 412 | 487 | 56 | 56 | Control; 135 ± 10 and 86 ± 4; DASH-S: 134 ± 10 and 86 ± 5 | 1 | 155 ± 75 |
| Zhou et al (31) (1997–1999) | INTERMAP | SV | IL, MS, HI, MN, TX, PA, MD | 2195 | 497 | 50 | NR | NR | NR | Men: 161 ± 51; women: 127 ± 40 |
| Ripley et al (32) (1998)358 | NA | NRT | VA | 18 | 447 | 78 | 50 | Baseline, supine: black, 150 ± 12 and 88 ± 5; whites, 144 ± 8 and 86 ± 9 | 1 | Blacks: 117 ± 51; whites: 140 ± 50 |
| McCarron et al (33) (1996)358 | NA | RCT | OR, IN, MI, SC, PA, AL | 99 | 52 ± 10 | 42 | 24 | 134 ± 11 and 86 ± 6 | 1 | 141 ± 62 |
| Appel et al (34) (1994–1996)311 | Dietary Approaches to Stop Hypertension | RCT | MA, NC, OR, MD, LA | 459 | 447 | 49 | 60 | Ambulatory: 132 ± 10 and 84 ± 4 | 1 | 136 ± 54 |
| Dawson-Hughes et al (35) (1995)5 | Sites Testing Osteoporosis Prevention/Intervention Trial | SV | NE, CT, MA | 914 | 717 | 73 | NR | NR | 1 | Men: 156 ± 58; women: 118 ± 44 |
| Appel et al (36) (1992–1994)3 | Trial of Nonpharmacologic Interventions in the Elderly | RCT | NJ, MD, NC | 639 | 66 ± 5 | 47 | 23 | 127 ± 9 and 71 ± 7 | 2 | Men: 161 ± 54; women: 126 ± 45 |
| Kumanyika et al (37) (1990–1992)12 | Trials of Hypertension Prevention, Phase II | RCT | MD, CA, MS, TN, NJ, OR, PA, MO, AL | 1138 | 447 | 34 | 17 | Men: 127 ± 6 and 86 ± 2; women: 128 ± 7 and 86 ± 2; whites: 127 ± 6 and 86 ± 2; blacks: 128 ± 7 and 86 ± 2 | 1 | Men: 203 ± 84; women: 155 ± 63; whites; 192 ± 73; blacks: 166 ± 132 |
| Smith et al (38) (1991)35813 | Piedmont Health Survey of the Elderly | RCT | NC | 21 | 66 ± 6 | 43 | 29 | 150 ± 5 and 86 ± 3 | 1–3 | 192 ± 50 |
| Davis et al (39) (1991)58 | Trial of Antihypertensive Interventions and Management | RCT | NY, AL, MS | 785 | 497 | 44 | NR | 145 and 94 | NR | 134 |
| Loria et al (40) (1990)14 | CARDIA | PC | AL, IL, MN, CA | 906 | 307 | 57 | 54 | NR | 3 | Men: 195; women: 156; blacks: 170; whites: 176 |
| Kumanyika et al (41), Trials of Hypertension Prevention Collaborative Research Group (42), and Whelton et al (43) (1987–1990)15 | Trials of Hypertension Prevention, Phase I | RCT | MD, AL, CA, MA, MS, TN, NJ, PA, OR, MO | 2182 | 437 | 30 | 15 | 125 and 84 | 2 | Men: 170 ± 68; women: 131 ± 54 |
| Alderman et al (44) (1981–1990)3816 | NA | PC | NY | 2937 | 537 | 35 | NR | Men: 150 ± 18 and 98 ± 9; women: 150 ± 18 and 94 ± 10 | 1 | Men: 12617; women: 9717 |
| Krishna et al (45) (1989)68 | NA | RCT | PA | 10 | 307 | 0 | 0 | 120 ± 2 and 76 ± 2 | 1 | 163 ± 47 |
| Weinberger et al (46) (1988)3 | NA | NRT | IN | 114 | 517 | 30 | 13 | NR | 3 | 170 ± 66 |
| Schmieder et al (47) (1988)35 | NA | SV | LA | 37 | NR | NR | NR | NR | 1 | 136 ± 69 |
| INTERSALT Cooperative Research Group (48, 49) (1986)18 | INTERSALT | SV | IL, MS, HI | 1150 | 20–59 | NR | NR | 118 ± 13 and 74 ± 9 | 1 | 158 ± 61 |
| Veterans Administration Cooperative Study Group on Antihypertensive Agents (50) (1986)358 | Veterans Administration Cooperative Study | RCT | MS, FL, TN, AL, DC | 623 | 497 | 0 | 65 | Whites: 145 ± 17 and 100 ± 6; blacks: 147 ± 17 and 101 ± 7 | 1 | Whites: 184 ± 108; blacks: 176 ± 117 |
| Langford et al (51) (1985)3 | Hypertension Detection and Follow-up Program | RCT | NY, AL, MS | 425 | 577 | NR | NR | NR | 1 | 146 ± 67 |
| Kaplan et al (52) (1985)19 | NA | RCT | TX | 16 | 497 | 62 | 81 | 131 ± 3 and 96 ± 1 | 1 | 166 ± 19 |
| Sullivan et al (53) (1980)68 | NA | NRT | TN | 27 | 297 | 30 | 15 | 111 and 82 | 1 | 167 ± 24 |
| Pietinen et al (54) (1979)68 | NA | SV | DC | 50 | 267 | 38 | 46 | 119 ± 10 and 77 ± 8 | 3 | 153 ± 59 |
| Schachter et al (55) (1979)5 | NA | SV | PA | 9 | NR | 78 | NR | NR | 3 | 117 |
| Connor et al (56) (1978–1979)20 | NA | NRT | OR | 352 | 367 | 52 | NR | Men: 116 ± 10 and 75 ± 8; women: 109 ± 14 and 70 ± 9 | 1 | Men: 168 ± 67; women: 127 ± 52 |
| Luft et al (57) (1974–1978)21 | NA | NRT | IN | 345 | 307 | NR | NR | NR | 1 | Whites: 153 ± 66; blacks: 136 ± 61 |
| Kilcoyne et al (58) (1974)3522 | NA | NRT | NY | 146 | 487 | 68 | 100 | 174 ± 29 and 111 ± 13 | 1 | 128 ± 50 |
| Gros et al (59) (1971)36 | NA | NRT | MI | 10 | NR | NR | NR | NR | NR | 191 |
| Veverbrants and Arky (60) (1968)523 | NA | NRT | MA | 5 | NR | NR | NR | NR | 3 | 73 ± 8 |
| Dahl (61) (1957)324 | NA | SV | NY | 9 | 42 ± 11 | 0 | NR | NR | 6–38 | 169 ± 34 |
NA, not applicable; PC, prospective cohort; SV, survey; NR, not reported; NRT, nonrandomized trial; NHS, Nurses' Health Study; DASH-S, Dietary Approaches to Stop Hypertension–Sodium; RCT, randomized controlled trial; INTERMAP, International Collaborative Study on Macronutrients, Micronutrients, and Blood Pressure; CARDIA, Coronary Artery Risk Development in Young Adults; INTERSALT, International Cooperative Study on the Relation of Blood Pressure to Electrolyte Excretion in Populations.
Total of 82% of patients on diuretics.
All or some participants had a history of high blood pressure.
Mean ± SD (all such values).
Date of study not reported; assumed date of submission.
Date of study not reported; assumed date of publication.
Mean.
No antihypertensive medications used or patients were normotensive.
Includes participants with high blood pressure and kidney stones and taking thiazide diuretics.
Range (all such values).
During run-in period for the trial, all patients had typical US diet (customary, self-selected diet), and patients were not then taking antihypertensive medications.
Participants were overweight and had prehypertension but were not taking antihypertensive medications; 1138 of 1159 patients provided urine.
No baseline values; used values of placebo group after 4 d.
Data from CARDIA study referenced in Loria et al (40) article (unpublished CARDIA data, 1990).
Patients had prehypertension but were not taking antihypertensive medications.
Participants receiving usual diet and not taking antihypertensive medications.
Median for 24-h urinary sodium.
Mean values from appendix II.
Used data from 4-wk control period.
Nineteen participants were taking antihypertensive medications, but authors noted that mean urine sodium was not different when these participants were excluded.
Twenty-four-hour urine samples from 345 of the 347 participants.
Patients with renal dysfunction and high blood pressure.
Five study participants in the “mean control” group provided 24-h urinary sodium.
Used data from all subjects in the “average salt” diet group.
FIGURE 1.
Mean (95% CI) 24-h urinary sodium excretion (mg/24 h) by study year.
We observed a trend toward a higher sodium excretion over time when we looked at all studies; however, the association did not reach statistical significance with the weighted regression model (Table 2). There was a trend of increased sodium excretion among those studies with a mean age of participants ≥50 y (P for trend: 0.02).
TABLE 2.
Urinary sodium excretion (mg/24 h) by decade1
| Group | 1957–20032 | Before 1980 | 1981–1990 | 1991–2000 | After 2000 | P for trend3 |
| All groups | 3526 (38) | 3319 (8) | 3418 (10) | 3499 (14) | 3849 (6) | 0.14 |
| Age | ||||||
| <50 y | 3616 (21) | 3356 (5) | 3758 (6) | 3548 (8) | 3792 (2) | 0.73 |
| ≥50 y | 3369 (13) | — | 2975 (3) | 3420 (6) | 3978 (4) | 0.02 |
| Sex | ||||||
| Male | 3911 (13) | 3868 (2) | 3548 (3) | 4052 (6) | 4149 (2) | 0.30 |
| Female | 3084 (12) | 2921 (1) | 2577 (2) | 3203 (7) | 3242 (2) | 0.16 |
| Race | ||||||
| Black or African American | 3645 (9) | 3021 (2) | 4048 (1) | 3618 (4) | 3889 (2) | 0.22 |
| White | 3801 (11) | 3519 (1) | 4232 (1) | 3755 (7) | 4024 (2) | 0.81 |
All values are means; number of studies in parentheses. For estimation of values of all groups, each study was weighted by the square root of the number of participants; for analysis of demographic subgroups, each study was weighted by the square root of the number of subgroup participants (eg, the number of blacks or whites in each study); 4 studies [ie, INTERSALT (International Cooperative Study on the Relation of Blood Pressure to Electrolyte Excretion in Populations) (48, 49), Schachter (55), Gros et al (59), and Veverbrants and Arky (60)] did not provide precise ages of study participants.
Difference in values between subgroups: <50 compared with ≥50 y (P = 0.002, ANOVA), men compared with women (P < 0.001, ANOVA), and blacks or African Americans compared with whites (P = 0.90, ANOVA).
Calculated with weighted linear regression model by using sodium excretion as a continuous dependent variable and the year of the study as a continuous independent variable, with each study weighted by the square root of the number of study participants or subgroup participants.
Over the entire 46-y observation period, we observed that black study participants did not have significantly different sodium amounts per 24 h than did white participants (3645 and 3801 mg Na, respectively, with P = 0.90 by analysis of variance). Studies with a mean age of participants ≥50 y had significantly lower excretion amounts than studies with a mean age of participants <50 y (3369 compared with 3616 mg Na/24 h; P = 0.002), and men had significantly higher excretion amounts than did women (3911 compared with 3084 mg Na/24 h; P <0.001).
In the univariate and multivariate random-effects models, we observed no significant association between year, race, or age and 24-h urinary sodium excretion (Table 3). Male sex was associated with higher sodium excretion in the univariate and multivariate models. The coefficient for age was negative in the univariate analysis but positive in multivariate analyses. On further examination, we observed that the age variable coefficient changed direction when either race or sex was added to the multivariate model but not when the study year was added. The coefficient for sex was significant in the univariate analysis and in the multivariate analysis, although in the multivariate analysis the 95% CI became wider. Upon further analysis, we observed that the 95% CI for sex widened when the variable for race was added to the regression model. We did not see evidence of strong collinearity between sex and race (Spearman's correlation: −0.32; variance inflation factor: 1.26 for sex and 1.18 for race). Thus, sex and race were both left in the multivariate model.
TABLE 3.
Random-effects models of urinary sodium excretion (mg/24 h) as a function of study year and study participant characteristics1
| Univariate model | P value | Multivariate model2 | P value | |
| Study year (10-y increments) | 146 (−44, 337) | 0.13 | 154 (−140, 448) | 0.29 |
| Mean age of study participants ≥50 y | −61 (−495, 372) | 0.78 | 248 (−239, 736) | 0.30 |
| Male sex3 | 879 (132, 1626) | 0.02 | 1281 (89, 2473) | 0.04 |
| Black or African American race4 | −415 (−1223, 393) | 0.30 | 234 (−781, 1250) | 0.63 |
All values are coefficients; 95% CIs in parentheses.
Covariates were study year, mean age of study participants (< or ≥50 y), sex (percentage of study participants who are men), and race (percentage of study participants who are black or African American).
Percentage of men (continuous variable; 1-unit increase in coefficient = 100%).
Percentage of blacks or African Americans (continuous variable; 1-unit increase in coefficient = 100%).
DISCUSSION
From 38 studies conducted in the United States between 1957 and 2003, the mean 24-h urine sodium excretion per person was 3526 mg Na (95% CI: 3380, 3672 mg Na). Because 95% of daily dietary sodium intake is excreted in the urine (62), this excretion amount corresponds to an intake of ≈3712 mg Na/d. We observed no suggestion of a decrease in sodium excretion over time.
The National Health and Nutrition Examination Survey (NHANES) regularly estimates the sodium intake of the US population by using 24-h dietary recalls. These data suggest that over the past 20 y (16, 17), and perhaps over the past 35 y (18), there has been an increase in sodium intake. However, dietary recall data may be biased because of errors in self-reporting and inaccurate or incomplete food databases (62). Although 24-h collections of urinary sodium excretions are also subject to error and bias (eg, because of individual sodium losses through sweat and feces and laboratory error), they have been shown to have a higher coefficient of reliability among repeated measures than do 24-h food recalls (62).
Our results are similar to those reported by McCarron et al (13) from the United Kingdom: the mean (±SD) in the United Kingdom from 1984 to 2008 was 150 ±7 mmol Na/24 h (= 3450 ± 161 mg Na/24 h), whereas in our analyses, the mean (±SE) was 153 ± 3 mmol Na/24 h (= 3526 ± 75 mg/24 h). As in our analyses, the UK study showed little variation in sodium excretion over time. Moreover, despite different food cultures, the mean observed in our study was similar to the mean observed worldwide in the International Cooperative Study on the Relation of Blood Pressure to Electrolyte Excretion in Populations (INTERSALT) study (mean ± 2 SD: 162 ± 22 mmol Na/24 h, which is equal to 3726 ± 506 mg Na/24 h) (13).
The mean amount of sodium excretion in our analysis appears to be well above levels recommended by the Institute of Medicine: 1500 mg Na/d for young adults, 1300 mg Na/d for adults aged 50–70 y, and 1200 mg Na/d for adults aged ≥71 y (63). Our estimates were also >2300 mg Na/d, which is the upper limit suggested by the American Heart Association for individuals who are not at an elevated risk of hypertension (5) and the amount recommended by the 2005 US Department of Agriculture's Dietary Guidelines (6). It has been suggested that sodium intake is physiologically set at the current intakes (13), but a set point would not explain why other populations have substantially different sodium intakes (48, 49).
Sodium intake is one of multiple etiologic factors in the development of hypertension, and therefore it is not surprising that the prevalence of hypertension is increasing in the US population despite the absence of a significant temporal rise in sodium intake. A recent study by Forman et al (64) suggested that the largest population attributable risks for hypertension were due to overweight and obesity, regular nonnarcotic analgesic use, physical inactivity, and not adhering to a low-sodium diet [ie, the Dietary Approaches to Stop Hypertension (DASH) diet]. Thus, despite the increase in processed foods in the US marketplace over the past 50 y, total caloric imbalance and the resultant epidemic of obesity may be a more important determinant of the increased prevalence of hypertension than sodium intake.
Our analyses were limited because the studies reviewed were not a random sample taken from across the United States. Not all studies reported sex or race, and although states from across the United States were represented, many studies took place in northeast or southern states. Yet despite these limitations, the mean values in our analysis were within a quite narrow range and approximate those calculated by the NHANES and in the United Kingdom. Moreover, although it is possible that sodium intake has decreased since 2003 because of changes to processed foods, it has been reported that more salt is now being added to poultry, meat, and fish (8, 14). Future research should track trends in 24-h urinary sodium intakes since 2003.
In conclusion, on the basis of studies conducted over a 46-y period, the sodium intake in the United States appears well above recommended intakes and without evidence of a temporal decrease.
Supplementary Material
Acknowledgments
We thank Rob van Dam for statistical support.
The authors' responsibilities were as follows—AMB and WCW: hypothesis generation, data collection and analysis, and manuscript preparation. Neither author had a conflict of interest.
REFERENCES
- 1.National Center for Health Statistics Health, United States, 2008, with chartbook. Hyattsville, MD: National Center for Health Statistics, 2009 [Google Scholar]
- 2.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004;44:398–404 [DOI] [PubMed] [Google Scholar]
- 3.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 2008;52:818–27 [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, Centers for Disease Control and Prevention Application of lower sodium intake recommendation to adults: United States, 1999-2006. MMWR Morb Mortal Wkly Rep 2009;58:281–83 [PubMed] [Google Scholar]
- 5.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308 [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services, US Department of Agriculture Dietary guidelines for Americans, 2005. 6th ed Washington, DC: US Government Printing Office, 2005 [Google Scholar]
- 7.Committee on Strategies to Reduce Sodium Intake, Food and Nutrition Board, Institute of Medicine Strategies to reduce sodium intake in the United States. Washington, DC: National Academy Press, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel LJ, Anderson CA. Compelling evidence for public health action to reduce salt intake. N Engl J Med 2010;362:650–2 [DOI] [PubMed] [Google Scholar]
- 9.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010;362:590–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frieden TR, Briss PA. We can reduce dietary sodium, save money, and save lives. Ann Intern Med 2010;152:526–7, W182 [DOI] [PubMed] [Google Scholar]
- 11.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med 2010;152:481–7, W170–3 [DOI] [PubMed] [Google Scholar]
- 12.Alderman MH. Reducing dietary sodium: the case for caution. JAMA 2010;303:448–9 [DOI] [PubMed] [Google Scholar]
- 13.McCarron DA, Geerling JC, Kazaks AG, Stern JS. Can dietary sodium intake be modified by public policy? Clin J Am Soc Nephrol 2009;4:1878–82 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson MF. Sodium content of processed foods: 1983-2004. Am J Clin Nutr 2005;81:941–2 [DOI] [PubMed] [Google Scholar]
- 15.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr 1991;10:383–93 [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Want CY, Kennedy-Stephenson J, Ervin RB. Dietary intake of ten key nutrients for public health, United States: 1999-2000. Advance data from vital and health statistics; no 334. Hyattsville, MD: National Center for Health Statistics, 2003 [PubMed] [Google Scholar]
- 17.Department of Health and Human Services Centers for Disease Control and Prevention Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988-94. Vital Health Stat 11 2002;245:1-158 [PubMed] [Google Scholar]
- 18.Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics Fats, cholesterol, and sodium intake in the diet of persons 1-74 years: United States. Washington, DC: US Government Printing Office, 1981 [Google Scholar]
- 19.Willett W. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998 [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64 [DOI] [PubMed] [Google Scholar]
- 21.Rose BDR, Helmut G. Renal pathophysiology—the essentials. Baltimore, MD: Williams & Wilkins, 1994 [Google Scholar]
- 22.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GV, Altman DG, eds Systematic reviews in health care: meta-analysis in context. London, United Kingdom: BMJ Publishing Group, 2001:292–3 [Google Scholar]
- 23.Nishizaka MK, Pratt-Ubunama M, Zaman MA, Cofield S, Calhoun DA. Validity of plasma aldosterone-to-renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens 2005;18:805–12 [DOI] [PubMed] [Google Scholar]
- 24.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003;16:925–30 [DOI] [PubMed] [Google Scholar]
- 25.Carbone LD, Bush AJ, Barrow KD, Kang AH. The relationship of sodium intake to calcium and sodium excretion and bone mineral density of the hip in postmenopausal African-American and Caucasian women. J Bone Miner Metab 2003;21:415–20 [DOI] [PubMed] [Google Scholar]
- 26.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002;40:892–6 [DOI] [PubMed] [Google Scholar]
- 27.Chapman AB, Schwartz GL, Boerwinkle E, Turner ST. Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int 2002;61:1047–55 [DOI] [PubMed] [Google Scholar]
- 28.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis 2006;48:905–15 [DOI] [PubMed] [Google Scholar]
- 29.Powell CR, Stoller ML, Schwartz BF, et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology 2000;55:825–30 [DOI] [PubMed] [Google Scholar]
- 30.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 31.Zhou BF, Stamler J, Dennis B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 2003;17:623–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripley E, King K, Sica DA. Racial differences in response to acute dosing with hydrochlorothiazide. Am J Hypertens 2000;13:157–64 [DOI] [PubMed] [Google Scholar]
- 33.McCarron DAWA, Egan BM, Krishna GG, Morris CD, Cohen M, Oparil S. Blood pressure and metabolic responses to moderate sodium restriction in isradipine-treated hypertensive patients. Am J Hypertens 1997;10:68–76 [DOI] [PubMed] [Google Scholar]
- 34.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B, Fowler SE, Dalsky G, Gallagher C. Sodium excretion influences calcium homeostasis in elderly men and women. J Nutr 1996;126:2107–12 [DOI] [PubMed] [Google Scholar]
- 36.Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001;161:685–93 [DOI] [PubMed] [Google Scholar]
- 37.Kumanyika SK, Cook NR, Cutler JA, et al. Sodium reduction for hypertension prevention in overweight adults: further results from the Trials of Hypertension Prevention Phase II. J Hum Hypertens 2005;19:33–45 [DOI] [PubMed] [Google Scholar]
- 38.Smith SR, Klotman PE, Svetkey LP. Potassium chloride lowers blood pressure and causes natriuresis in older patients with hypertension. J Am Soc Nephrol 1992;2:1302–9 [DOI] [PubMed] [Google Scholar]
- 39.Davis BR, Oberman A, Blaufox MD, et al. Effect of antihypertensive therapy on weight loss. The Trial of Antihypertensive Interventions and Management Research Group. Hypertension 1992;19:393–9 [DOI] [PubMed] [Google Scholar]
- 40.Loria CM, Obarzanek E, Ernst ND. Choose and prepare foods with less salt: dietary advice for all Americans. J Nutr 2001;131:536S–51S [DOI] [PubMed] [Google Scholar]
- 41.Kumanyika SK, Hebert PR, Cutler JA, et al. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Trials of Hypertension Prevention Collaborative Research Group. Hypertension 1993;22:502–12 [DOI] [PubMed] [Google Scholar]
- 42.The Trials of Hypertension Prevention Collaborative Research Group The effects of nonpharmacologic interv entions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992;267:1213–20 [DOI] [PubMed] [Google Scholar]
- 43.Whelton PK, Kumanyika SK, Cook NR, et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. Am J Clin Nutr 1997;65:652S–60S [DOI] [PubMed] [Google Scholar]
- 44.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 1995;25:1144–52 [DOI] [PubMed] [Google Scholar]
- 45.Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med 1989;320:1177–82 [DOI] [PubMed] [Google Scholar]
- 46.Weinberger MH, Cohen SJ, Miller JZ, Luft FC, Grim CE, Fineberg NS. Dietary sodium restriction as adjunctive treatment of hypertension. JAMA 1988;259:2561–5 [PubMed] [Google Scholar]
- 47.Schmieder RE, Messerli FH, Garavaglia GE, Nunez BD. Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation 1988;78:951–6 [DOI] [PubMed] [Google Scholar]
- 48.The INTERSALT Co-operative Research Group INTERSALT Study: an international co-operative study on the relation of blood pressure to electrolyte excretion in populations. I. Design and methods. J Hypertens 1986;4:781–7 [DOI] [PubMed] [Google Scholar]
- 49.The INTERSALT Co-operative Research Group Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988;297:319–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veterans Administration Cooperative Study Group on Anti-hypertensive Agents Urinary and serum electrolytes in untreated black and white hypertensives. J Chronic Dis 1987;40:839–47 [DOI] [PubMed] [Google Scholar]
- 51.Langford HG, Blaufox MD, Oberman A, et al. Dietary therapy slows the return of hypertension after stopping prolonged medication. JAMA 1985;253:657–64 [PubMed] [Google Scholar]
- 52.Kaplan NM, Carnegie A, Raskin P, Heller JA, Simmons M. Potassium supplementation in hypertensive patients with diuretic-induced hypokalemia. N Engl J Med 1985;312:746–9 [DOI] [PubMed] [Google Scholar]
- 53.Sullivan JM, Ratts TE, Taylor JC, et al. Hemodynamic effects of dietary sodium in man: a preliminary report. Hypertension 1980;2:506–14 [DOI] [PubMed] [Google Scholar]
- 54.Pietinen PI, Wong O, Altschul AM. Electrolyte output, blood pressure, and family history of hypertension. Am J Clin Nutr 1979;32:997–1005 [DOI] [PubMed] [Google Scholar]
- 55.Schachter J, Harper PH, Radin ME, Caggiula AW, McDonald RH, Diven WF. Comparison of sodium and potassium intake with excretion. Hypertension 1980;2:695–9 [DOI] [PubMed] [Google Scholar]
- 56.Connor SL, Connor WE, Henry H, Sexton G, Keenan EJ. The effects of familial relationships, age, body weight, and diet on blood pressure and the 24 hour urinary excretion of sodium, potassium, and creatinine in men, women, and children of randomly selected families. Circulation 1984;70:76–85 [DOI] [PubMed] [Google Scholar]
- 57.Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 1979;59:643–50 [DOI] [PubMed] [Google Scholar]
- 58.Kilcoyne MM, Thomson GE, Branche G, et al. Characteristics of hypertension in the black population. Circulation 1974;50:1006–13 [DOI] [PubMed] [Google Scholar]
- 59.Gros G, Weller JM, Hoobler SW. Relationship of sodium and potassium intake to blood pressure. Am J Clin Nutr 1971;24:605–8 [DOI] [PubMed] [Google Scholar]
- 60.Veverbrants E, Arky RA. Effects of fasting and refeeding. I. Studies on sodium, potassium and water excretion on a constant electrolyte and fluid intake. J Clin Endocrinol Metab 1969;29:55–62 [DOI] [PubMed] [Google Scholar]
- 61.Dahl LK. Evidence for an increased intake of sodium in hypertension based on urinary excretion of sodium. Proc Soc Exp Biol Med 1957;94:23–6 [DOI] [PubMed] [Google Scholar]
- 62.Espeland MA, Kumanyika S, Wilson AC, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol 2001;153:996–1006 [DOI] [PubMed] [Google Scholar]
- 63.Institute of Medicine Food and Nutrition Board Water, potassium, sodium, chloride, and sulfate. Dietary reference intakes. Washington, DC:National Academy Press, 2005:270 [Google Scholar]
- 64.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009;302:401–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.