Abstract
Background: Rates of mental illness in children are increasing throughout the world. Observational studies of depression, anxiety, and attention-deficit hyperactivity disorder suggest that zinc is an alternative treatment.
Objective: We examined the effect of zinc supplementation on the mental health of school-age children in Guatemala.
Design: From January to October 2006, we conducted a 6-mo randomized, double-blind, controlled trial comparing zinc supplementation (10 mg ZnO/d for 5 d/wk) with a placebo (10 mg glucose) in 674 Guatemalan children in grades 1–4. Outcome measures included internalizing (ie, depression and anxiety) and externalizing (ie, hyperactivity and conduct disorder) problem behaviors, positive behaviors (ie, socialization and leadership), and serum zinc concentrations.
Results: Zinc and placebo groups did not differ significantly in any behavioral measures at baseline or at follow-up. At baseline, 21.4% of children had serum zinc concentrations <65 μg/dL. At follow-up, both groups improved significantly, and zinc concentrations were higher in the zinc group. Increases in serum zinc concentrations were inversely associated with decreases in depressive symptoms (estimate: −0.01 points per μg Zn/dL; P = 0.01), anxiety (estimate: −0.012 points per μg Zn/dL; P = 0.02), internalizing symptoms (estimate: −0.021 points per μg Zn/dL; P = 0.02), and social skills (estimate: −0.019 points per μg Zn/dL; P = 0.01) in adjusted models that were controlled for child age, sex, socioeconomic status, household, and treatment group.
Conclusions: Six months of zinc supplementation did not induce differences in mental health outcomes between zinc and placebo groups. However, increases in serum zinc concentrations were associated with decreases in internalizing symptoms (ie, depression and anxiety) in a community-based sample of children at risk of zinc deficiency. This trial was registered at clinicaltrials.gov as NCT00283660.
INTRODUCTION
It is estimated that the worldwide prevalence of child and adolescent mental disorders is 20% (1, 2). The World Health Organization predicts that childhood neuropsychiatric disorders will increase worldwide by >50% by 2020, and these disorders will be one of the 5 most common causes of morbidity, mortality, and disability in children (2). This burden of disease remains poorly addressed even in resource-rich nations. For example, in the United States, 1 in 10 children and adolescents suffers from a mental illness severe enough to significantly impair functioning (eg, anxiety disorder, depression, conduct disorder, and hyperactivity) (3–5), but ≈1 in 5 affected children currently receive appropriate treatment of their disorders (3). The situation in developing countries may be worse (2). Many children in Latin America confront substantial risk factors (ie, poverty, drug use, crime, violence, and social and political unrest), with very few mental health resources available (6). Psychotherapy is costly, requires time and commitment from children and parents, and is often not available or acceptable. Pharmacotherapy is often unavailable and poses risks of side effects (7). Thus, preventive strategies that are readily accessible and acceptable to populations nationally and internationally are needed to prevent unnecessary suffering in these children.
Ensuring adequate micronutrient intakes over the life span may be one such strategy. Compelling evidence for the role of micronutrients in mental health comes from studies that focused on the role of zinc in depression, anxiety, and attention-deficit hyperactivity disorder (ADHD), which are disorders that are common in children. Low concentrations of zinc have been associated with symptoms of depression in animals and adult humans (8–11). Zinc status is inversely related to anxiety in animals and young children (12, 13). Low zinc concentrations have also been associated with ADHD symptoms (14–16), and zinc may enhance the effects of traditional treatments for ADHD (17, 18).
However, most of these studies are observational and have focused on clinical patient samples rather than community samples (13, 17, 19). A few community-based studies have focused on the role of zinc in activity levels, infant behavior, and psychosocial and behavioral functioning, with mixed results (20). There is a paucity of well-designed controlled trials that evaluated the potential of zinc to enhance psychological functioning and improve or prevent mental health problems in community-based samples of children. Furthermore, only a few studies have examined the potential nutrition-environment interactions that may affect relations with psychological and behavioral outcomes (21, 22). For example, low tryptophan concentrations have been associated with negative mood and lower energy, especially after exposure to adverse events (21). In designing interventions, it is important to examine existing individual and environmental conditions that might modify the effect of any intervention (23). We studied school-age children in Guatemala, who are a population at risk of zinc deficiency (24), to assess the effects of zinc supplementation on mental health. We also examined whether 3 conditions at baseline (ie, serum zinc concentration, mental health status, and level of environmental stress) modified these effects.
SUBJECTS AND METHODS
Study design
We conducted a randomized, placebo-controlled, double-blind trial in 5 public schools in a low-income community in Guatemala City (www.clinicaltrials.gov, ID no. NCT00283660). The study was a collaborative effort between the Hubert Department of Global Health at the Rollins School of Public Health (RSPH), Emory University, Atlanta, GA, and the Institute of Nutrition of Central America and Panama (INCAP) in Guatemala City. The study protocol was reviewed and approved by the ethics committees of both institutions. The Data Safety Monitoring Committee (DSMC) included 2 nutritional epidemiologists from US-based universities and a pediatrician from a local hospital in Guatemala; none of the DSMC members were coauthors on the study.
Study population, eligibility, and consent
Children in grades 1–4 (ages 6–11 y) were eligible and recruited for the study. Permission was obtained from the Municipality Office of the Ministry of Education and school directors; teachers were then informed of the study and requested to cooperate. We conducted informational meetings at each school. Interviewers scheduled an appointment for an initial screening with parents who expressed interest in having their child participate. Recruitment occurred in January–March 2006. Children were screened for any known severe illness shown to affect zinc status such as sickle cell disease, cystic fibrosis, renal or liver disease, severe burns, or acrodermatitis enteropathica (25); no children were identified as having any of these conditions. In addition, children were screened for any other severe or chronic illness not necessarily linked to zinc status (eg, cancer, diabetes, or seizures). If a child was eligible on the basis of the screening, parents provided written informed consent and children provided verbal assent to participate in the study after the study had been explained individually to them either in their home or at school. Multiple children within the same family were allowed to participate.
Randomization, blinding, and intervention
Chewable zinc (10 mg ZnO) and placebo (10 mg glucose) tablets were developed, packaged, and donated by Laboratorios Zerboni SA (Mexico City, Mexico). The supplement dosage was based on the criteria of both efficacy and safety from review of previous studies (26–30) and was approved by the DSMC before beginning the intervention. The zinc and placebo tablets were divided into color-coded vials (2 colors assigned to zinc; 2 colors assigned to placebo) by a staff member at INCAP who was not involved in the study. Individual children within each classroom were randomly assigned by using a computer-generated list on the basis of a 1:1:1:1 allocation ratio without blocking constraints (31). This was done to ensure a balance between the zinc and placebo groups within a classroom and, thus, to achieve a more efficient design that improved power and controlled for potential effects of teachers or classes on study measures. Individuals who administered the supplements (n = 7) received a list of all children in their classroom enrolled in the study and their assigned color group. All of the tablets for each child were kept in a small plastic container with a label that had the child's name and a colored circle that corresponded to the color of his or her assigned group.
The first school began supplementation in late February 2006, and the fifth school began supplementation in early April 2006. Children were supplemented daily (5 d/wk) for ≈5.8 mo (≈125 d) in their classrooms. The zinc supplement or placebo was given in the form of chewable tablets with a fruit flavoring during the morning hours (ie, between 0830 and 1000) and was consumed in front of the study staff who administered the supplement and recorded this information. No food was given with the supplement. The placebo was similar in taste and appearance to the zinc tablet. In cases where a child was expected to be absent from school the next day (eg, school was not in session, or the child had an appointment), the person giving the supplement gave the child a tablet to consume at home the next day and followed up on the child's return to school to assess if the child had consumed the supplement; this information was recorded. Adherence was defined as the number of days the supplement was consumed (observed and unobserved) out of the total number of days of supplementation. The denominator for calculating adherence was based on the date the class began supplementation and the date of the last dose. These dates were the same for all children in a classroom because supplementation began right after all of the children in the classroom had completed the baseline measures; the exact number of days varied slightly by class but did not vary by individuals within the classroom (range: 100–128 d). All study participants and members of the study team were blinded to the treatment code, which was maintained in sealed envelopes at INCAP and Rollins School of Public Health. The envelopes were opened at the end of the study after preliminary data analyses had been completed.
Unanticipated simultaneous local intervention in schools
At approximately the time our study began, the local government in the study community implemented a school-based fortified milk program. Children in 4 out of the 5 schools received 200 mL whole milk/d fortified with zinc amino chelate to a target concentration of 2.6 mg elemental Zn/200 mL. Analyses in our laboratory measured that the actual concentration was 1.6 mg/200 mL. The milk also contained 4.7 mg iron (actual amount was 1.12 mg iron on the basis of laboratory analysis), 200 μg vitamin A, 0.8 μg vitamin B-12, and 133 μg folic acid. A tablet of folic acid was also distributed weekly in the schools, although not consistently. Children in the fifth school (with the smallest number of study participants [n = 47]) did not receive the fortified milk but instead received Incaparina (Alimentos SA, Guatamala City, Guatamala) daily, a food supplement that contained 2.1 mg zinc, 3 mg Fe, 35 μg folic acid, and 56 mg Ca (32). We did not learn about this program until our fieldwork was well underway and, thus, did not collect information on the actual consumption by the children.
Data-collection procedures and study variables
Procedures
Baseline data collection occurred from February to early April 2006, supplementation occurred from late February to early October 2006, and follow-up occurred from August to early October 2006. Information on sociodemographic characteristics (eg, age, sex, school, and grade of child; age, marital status, education, employment, and language of the mother and father or partner; number of children in the household; and characteristics of the home where the child lives) and the health and developmental histories of children were obtained by interview.
We conducted child assessments in 2 separate buildings in the community (one assessment for the psychological data and one assessment for the health and nutrition data), with each building a short driving distance from the various schools. There were several, private, enclosed spaces in which to conduct the various assessments. Groups of children were transported to one of the 2 sites. On the first day, children completed a series of psychological questionnaires and cognitive tests that lasted ≈2 h. These were administered by trained study staff in a quiet, controlled environment. On the next day, a study physician examined each child, and a trained nurse drew 7 mL venous blood by using standard procedures into trace element–free Vacutainers (Becton Dickinson, Franklin Lakes, NJ). Hemoglobin concentrations were measured on site (Hemacue Inc, Mission Viejo, CA) to assess for anemia (ie, moderate to severe anemia = hemoglobin concentrations <10.5 g/dL; mild anemia = hemoglobin concentrations from 10.5 to ≤11.4 g/dL; adjusted for altitude) (33). Parents were informed of any results requiring medical treatment. Anthropometric measurements (ie, weight, standing height, sitting height, knee-heel length, head circumference, arm circumference, abdominal circumference, calf circumference, triceps skinfold thickness, and subscapular skinfold thickness) were taken for each child. Serum zinc concentrations were assessed with flame atomic absorption spectroscopy (34) at the laboratories of the National Institute of Public Health in Mexico; serum ferritin concentrations and C-reactive protein (CRP) concentrations were also assessed at the National Institute of Public Health in Mexico. Finally, trained interviewers visited each child's home and conducted an interview with the child's mother (93.5% of children at baseline; 93.3% of children at follow-up) or primary caregiver on her own psychological status, her child's psychological and behavioral symptoms, and child dietary information.
Study variables
Outcome variables include child self-reported measures of symptoms of depression and anxiety and parental reports on a variety of behavioral symptoms and are listed below. The Spanish versions of the measures were piloted, and the items were cognitively tested in this community of children and parents in Guatemala. Measures of internal consistency (coefficient α) were calculated for the measures and subscales (see supplemental Table 1 under “Supplemental data” in the online issue).
Child-reported depressive symptoms. Child reports of depressive symptoms were measured with the Spanish version of the Children's Depression Inventory (CDI) (35). The CDI is a self-report inventory developed for children aged 7–17 y that contains 27 items that assess cognitive, affective, and behavioral signs of depression. The instrument uses a 3-alternative forced-choice format and provides a total continuous score (range: 0–54). The CDI is designed to screen for symptoms of depression, assess the severity of depressive symptoms, and monitor clinical improvement, but it is not designed to provide a diagnosis of clinical depression, although a cutoff of 19 has been used to designate a child at risk in nonclinical populations (35, 36). Previous studies reported an internal consistency reliability of 0.86 and an r = 0.55 (P < 0.001) between the CDI and clinicians’ independent global ratings of depression (37), with adequate internal consistency observed in the current study (coefficient α = 0.75).
Child-reported anxiety. Child-reported anxiety was measured by the Spanish version of the Revised Children's Manifest Anxiety Scale (RCMAS) (38). The RCMAS is used with children aged 6–19 y and includes 28 items that assess symptoms of anxiety in a yes or no response format and produces a total anxiety score (range: 0–37); a cutoff of 19 has been used to indicate children at risk (39). The RCMAS has shown good reliability and validity across diverse cultural and ethnic groups, including the use of the Spanish version, Lo Que Pienso y Siento (40–42). The RCMAS showed an adequate internal consistency in the current study (coefficient α = 0.82).
Parent-reported child behavioral symptoms. Parent reports of children's behavioral symptoms were measured with the Behavior Assessment System for Children (BASC) (43). The BASC evaluates positive and negative dimensions of the behaviors of children aged 2–18 y. Information from the Parent Rating Scale used in the current analysis included items related to the clinical scales of anxiety, depression, attention problems, and learning problems, as well as aggression, hyperactivity, conduct problems, somatization, atypicality, and withdrawal, and items related to adaptive behavior. Items were scored on a 4-point Likert scale ranging from never (0) to almost always (3), and scale and composite scores were available. The Parent Rating Scale has been shown to have fairly good reliability with internal consistency and test-retest for the scales ranging from 0.70 to the low 0.90s and slightly higher for composites. The measure has been shown to correlate highly with several other commonly used behavioral checklists (43). The Spanish version of the instrument, which was used and validated with populations in Colombia (44, 45), was used in the current study. Only the internalizing (sum of anxiety, depression, and somatization scales) and externalizing (sum of hyperactivity, aggression, and conduct problems scales) composite scales and the subscales of hyperactivity, aggression, conduct problems, anxiety, depression, somatization, social skills, and leadership were used in the current analyses on the basis of internal consistency criteria (coefficient α range: 0.61–0.86). Because normative data for T scores and percentiles were not based on the current Guatemalan population, raw scores were used in the analyses.
Additional study variables
Three potential effect modifiers were included in the analyses as follows: baseline serum zinc concentration, baseline mental health status, and baseline level of environmental stress. Being at risk of zinc deficiency was defined as zinc concentrations <65 μg/dL on the basis of cutoffs for a nonfasting sample collected in the morning (ie, between 0900 and 1200) for children (46). We created 4 dichotomous variables for baseline mental health status by using the variables of child self-reported depressive and anxious symptoms (on the basis of available cutoffs noted previously) and parent-reported internalizing and externalizing disorders (on the basis of median values).
Specific environmental stressors (ie, familial, school, and neighborhood) were measured by both child and parent reports of the frequency and difficulty of certain stressful situations. Formative work (ie, in-depth interviews and focus groups) was conducted to obtain information on the most frequently occurring and difficult situations experienced by children in this age group in Guatemala. On the basis of this information, we developed a child-report measure (which consisted of 36 items; coefficient α = 0.90 for frequency and 0.92 for difficulty) and a parent-report measure (which consisted of 39 items; coefficient α = 0.88 for frequency and 0.88 for difficulty) that assessed the frequency (1 = never; 4 = almost always) and difficulty (1 = not at all; 4 = a lot) of various stressors potentially experienced by the child and family.
In addition, maternal depressive symptoms were measured with the Spanish version of the Center for Epidemiologic Studies Depression Scale (CES-D) (47), which is a 20-item checklist of symptoms of depressive affect. Several studies adapted and used this measure with Spanish-speaking populations and confirmed the validity and reliability of this measure in these populations (48, 49). The CES-D showed adequate internal consistency in the current study (coefficient α = 0.89). A total continuous score was provided along with a cutoff of ≥16 points to signify maternal distress (47).
For the current analyses, we created a dichotomous variable of environmental stress experienced by the child, which was defined as meeting ≥2 of the following criteria: 1) maternal or caregiver depressive symptoms at or above the cutoff on the CES-D, 2) child-reported stress above the median for either frequency or difficulty, or 3) parent-reported stress above the median for either frequency or difficulty. Children who lived in a high-stress environment were exposed to a higher frequency or difficulty of stressors related to school and friends (eg, not feeling accepted or being bullied by children at school; lack of teacher attention because of too many children in the classroom), family (eg, fighting between parents; father migrating to the United States), and neighborhood (eg, drugs, violence, and theft in the neighborhood) or to living with a primary caregiver in emotional distress. Children who lived in a low-stress environment were exposed to a lower frequency or difficulty of these particular stressful situations.
Covariates
Additional variables used in the current analyses include child age (in y), sex, grade, height-for-age z score, body mass index z score, stunting [defined as a height-for-age z score less than −2 by using the World Health Organization 2007 reference (50)], hemoglobin concentrations (in g/dL), anemia, CRP concentrations (in mg/L), evidence of inflammation (defined as >5 mg CRP/L), ferritin concentrations (in μg/L), iron deficiency (defined as <15 μg ferritin/L excluding any children with elevated CRP), maternal education (in y), and socioeconomic status (SES). SES was estimated by using principal components analysis to construct a wealth index on the basis of participants’ household possessions (eg, radio, iron, bicycle, microwave, refrigerator, and television) and housing construction materials (eg, floor, roof, and walls) by using a method previously used in Guatemala (51).
Statistical analyses
We computed means and SDs for continuous variables and frequencies for categorical variables. The internal consistency (coefficient α) was calculated for each of the mental health outcome variables, and only those measures and scales with a coefficient α > 0.60 were retained in the analyses (see supplemental Table 1 under “Supplemental data” in the online issue).
We compared control and intervention groups on selected baseline demographic characteristics to evaluate the effectiveness of randomization. We compared the baseline and follow-up zinc concentrations and the prevalence of serum zinc concentrations <65 μg/dL by treatment groups. Student's t test was used for normally distributed continuous variables, and chi-square tests were used for categorical variables. All analyses were adjusted for household to account for clustering of children within the same household.
We calculated whether the difference in scores on the mental health outcomes between the zinc and placebo groups changed over time. To assess the effect of supplementation on each mental health measurement we used the SAS MIXED procedure (SAS version 9.1; SAS Institute, Cary, NC) to fit linear mixed models to account for repeated measurements and clustering of children within the same household. Given the occurrence of the unanticipated local school intervention in the placebo and zinc groups and the resulting increases in mean serum zinc concentrations in both groups, we assessed the association between changes in serum zinc concentration and changes in mental health outcomes over time. We used the generalized estimating equations approach (52) with the pooled sample to adjust for the correlation of siblings clustered within families with the change in serum zinc concentrations as a predictor and with adjustment for treatment group, household, child sex, age at measurement, and any variables that differed between treatment groups at baseline (ie, household SES).
To address specific aims related to potential effect modifiers, we tested for heterogeneity by baseline serum zinc concentrations and environmental stress on all outcomes by using statistical interactions. We tested for heterogeneity by baseline mental health status for child-reported symptoms of depression and anxiety and parent-reported internalizing and externalizing symptoms on their respective mental health outcomes. We also estimated models stratified by treatment group, baseline zinc status, environmental stress, and mental health to assess interactions between these variables and changes in zinc concentrations over time for each of the mental health outcome variables.
SAS version 9.1 (SAS Institute) was used for all analyses. We used P < 0.05 as the criterion of statistical significance.
RESULTS
Characteristics of the study population
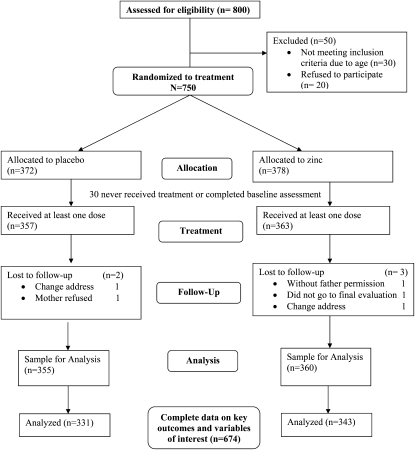
A total of 800 children were screened of whom 30 (3.7%) children did not meet inclusion criteria and a further 20 (2.5%) children refused to participate, which left a sample of 750 eligible children, aged 6–11 y, at baseline who were randomly assigned (Figure 1). Of the 750 children, 30 (4.0%) children never received treatment or completed the baseline assessment. Of the 720 children who received at least one tablet, 5 (<1.0%) children were lost to follow-up (eg, because of a change of address or parent refusal), which left a sample of 715 children. The final sample for analysis included 674 children with complete data on all outcome variables and serum zinc concentration and environmental stress at baseline to allow for comparison among the outcomes and different methods of analyses (Figure 1). For the 674 children, there were 530 families. A total of 138 (26%) families had more than one child in the study (132 families with 2 children in the study; 6 families with 3 children in the study). The group of children with incomplete data (n = 41) had a higher prevalence of stunting and higher levels of parent-reported stress than the sample of children with complete data; no other differences on baseline sociodemographic characteristics were observed (data not shown). No significant difference was observed for the distribution of these children between zinc and placebo groups.
FIGURE 1.
Participant flow [on the basis of guidelines for randomized controlled trials of the CONSORT (CONsolidated Standards of Reporting Trials) statement].
The mean (±SD) age of the children at baseline was 8.96 ± 1.16 y, and 50.0% of the children were boys. At baseline, 14.2% of the children in the sample were stunted, and 21.4% of the children had serum zinc concentrations <65 μg/dL. No children met the criteria for moderate to severe anemia; only one child met the criteria for mild anemia, and very few (<3%) children met criteria for iron deficiency. At baseline, 11.3% and 19.1% of children indicated high levels of symptoms of depression and anxiety, respectively. There were no significant differences in baseline demographic, nutrition, or environmental characteristics between treatment and control groups, with the exception of SES; children in the zinc group came from families with significantly lower SES than children in the control group (P = 0.03; Table 1). There were no differences in distribution of children between placebo and zinc groups within any of the schools. In the 674 children, all but 11 children attended school for ≥100 d during the intervention period. These 663 children received the study intervention for 65–118 d for an adherence rate of 86.4% (range: 63.1–95.9%) which did not differ between zinc and placebo groups (t test P = 0.95).
TABLE 1.
Baseline demographic, nutritional, and environmental characteristics (n = 674)1
| Variables | Placebo (n = 331) | Zinc (n = 343) | P2 |
| Age (y) | 8.9 ± 1.23 | 9.0 ± 1.2 | 0.58 |
| Sex (% male) | 49.2 | 50.7 | 0.70 |
| Grade (y) | 2.5 ± 1.0 | 2.5 ± 1.0 | 0.75 |
| SES | 0.1 ± 1.0 | −0.1 ± 1.0 | 0.03 |
| Maternal schooling (y) | 4.6 ± 3.3 | 4.6 ± 3.4 | 0.85 |
| BMI z score | 0.3 ± 1.0 | 0.4 ± 1.0 | 0.26 |
| Height-for-age z score | −1.2 ± 0.9 | −1.2 ± 0.9 | 0.45 |
| Stunted (%)4 | 14.5 | 14.0 | 0.85 |
| Hemoglobin (g/dL) | 13.8 ± 0.7 | 13.8 ± 0.8 | 0.75 |
| Ferritin (μg/L) | 46.9 (33.0, 63.0)5 | 47.1 (34.2, 62.7) | 0.59 |
| Iron deficiency (%)6 | 2.0 | 3.2 | 0.34 |
| C-reactive protein (mg/L) | 0.6 (0.3, 1.6) | 0.6 (0.3, 1.7) | 0.84 |
| Evidence of inflammation (%)7 | 7.3 | 7.9 | 0.76 |
| Child-reported stress frequency | 82.2 ± 20.4 | 80.2 ± 18.4 | 0.18 |
| Child-reported stress difficulty | 104.8 ± 20.4 | 105.4 ± 20.5 | 0.69 |
| CES-D total | 16.6 ± 11.6 | 16.9 ± 11.5 | 0.77 |
| Maternal distress (%)8 | 44.1 | 46.1 | 0.61 |
| Parent-reported stress frequency | 89.8 ± 15.9 | 89.5 ± 16.9 | 0.81 |
| Parent-reported stress difficulty | 130.4 ± 13.8 | 130.6 ± 14.7 | 0.87 |
| Baseline high environmental stress (%)9 | 69.2 | 65.6 | 0.32 |
SES, socioeconomic status; CES-D, Center for Epidemiologic Studies–Depression Scale.
t tests were used for comparison of means, and chi-square tests were used for comparison of proportions unless otherwise noted.
Mean ± SD (all such values).
Defined as height-for-age z score of less than −2 by using the World Health Organization 2007 reference (50).
Median; interquartile range in parentheses (all such values); P values based on nonparametric Wilcoxon's test.
Defined as ferritin concentrations <15 μg/L; the sample excluded any children with elevated C-reactive protein concentrations (ie, >5 mg/L); n = 307 for the placebo group and 316 for the zinc group.
C-reactive protein concentrations >5 mg/L.
Maternal depression total score ≥16.
Defined as above the cutoff for any 2 of the following variables: maternal depression (≥16), child stress (above the median for frequency or difficulty), and parent stress (above the median for frequency or difficulty).
Baseline zinc concentrations did not differ significantly between the 2 groups (Table 2); 19.6% and 23.0% of children in the placebo and zinc groups, respectively, were at risk of zinc deficiency. At follow-up, both zinc and placebo groups exhibited significantly increased serum zinc concentrations, with zinc concentrations in the zinc group significantly greater than those in the placebo group (P = 0.02; Table 2). The net difference for zinc concentrations was 6.37 μg/dL relative to a pooled SD of 22.02 μg/dL, which gave an effect size of 0.29.
TABLE 2.
Descriptive statistics of zinc status by treatment group (n = 674)
| Variables | Placebo1 | Zinc2 | P3 | P4 |
| Baseline zinc (μg/dL) | 75.6 ± 12.65 | 75.0 ± 13.0 | 0.54 | 0.01 |
| Follow-up zinc (μg/dL) | 105.9 ± 31.3 | 111.7 ± 31.2 | 0.02 | |
| Baseline zinc deficient (%)6 | 19.6 | 23.0 | 0.28 | 0.01 |
| Follow-up zinc deficient (%)6 | 4.4 | 1.2 | 0.01 |
n = 331 at baseline and 318 at follow-up for the placebo group.
n = 343 at baseline and 328 at follow-up for the zinc group.
t test for comparison of means; chi-square test for comparison of proportions.
Derived by repeated-measurements analysis to test the interaction between treatment and time and accounting for clustering of children within the same household.
Mean ± SD (all such values).
Zinc concentrations <65 μg/dL (46).
Effect of zinc supplementation on mental health outcomes
The zinc and placebo groups did not differ significantly on any of the mental health measures at baseline or follow-up, and there were not any differences between groups in repeated measures analyses that examined changes in scores over time (Table 3). Both groups showed decreases in scores over time for most of the measures of problem behaviors. Controls for household, child age, sex, and SES did not alter these inferences (data not shown).
TABLE 3.
Mental health outcome variables by treatment group (n = 674)1
| Variables | Baseline |
Follow-up |
P2 | P3 | ||||
| Placebo | Zinc | P1 | Placebo | Zinc | P1 | |||
| CDI | 10.5 ± 6.2 | 10.1 ± 6.4 | 0.50 | 9.6 ± 6.1 | 9.5 ± 6.0 | 0.78 | 0.02 | 0.77 |
| RCMAS | 13.8 ± 5.8 | 13.2 ± 5.5 | 0.19 | 12.8 ± 5.5 | 12.9 ± 5.3 | 0.80 | 0.02 | 0.27 |
| BASC | ||||||||
| Hyperactivity | 20.8 ± 4.8 | 20.3 ± 5.3 | 0.25 | 19.4 ± 4.4 | 19.8 ± 5.2 | 0.35 | 0.01 | 0.14 |
| Aggression | 21.8 ± 5.4 | 21.7 ± 5.5 | 0.89 | 21.2 ± 5.3 | 21.2 ± 5.4 | 0.97 | 0.06 | 0.94 |
| Conduct | 14.8 ± 2.9 | 14.8 ± 2.8 | 1.00 | 14.7 ± 2.5 | 14.9 ± 2.9 | 0.37 | 0.70 | 0.54 |
| Anxiety | 23.7 ± 5.3 | 24.0 ± 4.9 | 0.37 | 23.2 ± 4.8 | 23.9 ± 4.9 | 0.08 | 0.25 | 0.57 |
| Depression | 19.2 ± 3.8 | 19.4 ± 3.8 | 0.57 | 18.8 ± 3.6 | 18.8 ± 3.8 | 0.99 | 0.03 | 0.68 |
| Somatization | 18.7 ± 4.3 | 18.2 ± 3.8 | 0.08 | 18.2 ± 4.4 | 17.8 ± 3.8 | 0.27 | 0.04 | 0.66 |
| Social skills | 38.2 ± 7.2 | 37.4 ± 7.2 | 0.18 | 37.3 ± 7.6 | 36.5 ± 7.2 | 0.17 | 0.03 | 0.97 |
| Leadership | 26.5 ± 5.5 | 26.2 ± 5.5 | 0.46 | 26.3,5.9 | 25.9 ± 5.8 | 0.46 | 0.47 | 0.98 |
| Internalizing2 | 61.6 ± 10.1 | 61.6 ± 8.9 | 0.98 | 60.2,9.2 | 60.5 ± 8.9 | 0.66 | 0.02 | 0.75 |
| Externalizing3 | 57.3 ± 11.2 | 56.8 ± 11.6 | 0.56 | 55.4,10.2 | 55.9 ± 11.6 | 0.54 | 0.02 | 0.40 |
All values are means ± SDs. CDI, Children's Depression Inventory; RCMAS, Revised Children's Manifest Anxiety Scale; BASC, Behavioral Assessment System for Children (parent report). Participants had data on all outcome variables. P1, P values derived by t test to compare mean differences between treatment groups at baseline and follow-up; P2, P values derived by repeated-measurements analysis to test the main effect of time and accounting for clustering of children within the same household; P3, P values derived by repeated-measurements analysis to test the interaction between treatment and time and accounting for clustering of children within the same household.
Internalizing problems: sum of anxiety, depression, and somatization scales.
Externalizing problems: sum of hyperactivity, aggression, and conduct scales.
Associations between changes in zinc concentrations and changes in mental health outcomes
An increase in serum zinc concentrations was inversely associated with a decrease in parent-reported child anxiety (estimate: −0.012 points per μg Zn/dL; P = 0.02), depressive symptoms (estimate: −0.01 points per μg Zn/dL; P = 0.01), social skills (estimate: −0.019 points per μg Zn/dL; P = 0.01), and internalizing symptoms (estimate: −0.021 points per μg Zn/dL; P = 0.02) in adjusted models that were controlled for child age, sex, SES, household, and treatment group (Table 4). Changes in the other outcomes were not significantly associated with changes in zinc concentration. There was no evidence of heterogeneity by treatment group for these estimates (see supplemental Table 2 under “Supplemental data” in the online issue).
TABLE 4.
Associations between changes in zinc concentrations and mental health outcomes in school-age children in Guatemala (n = 674)1
| Estimate (95% CI) | P2 | |
| CDI | 0.011 (−0.004, 0.026) | 0.17 |
| RCMAS | −0.002 (−0.014, 0.010) | 0.75 |
| BASC | ||
| Hyperactivity | 0.003 (−0.006, 0.013) | 0.52 |
| Aggression | 0.005 (−0.005, 0.016) | 0.30 |
| Conduct | 0.001 (−0.004, 0.007) | 0.64 |
| Anxiety | −0.012 (−0.023, −0.002) | 0.02 |
| Depression | −0.01 (−0.019, −0.002) | 0.01 |
| Somatization | 0.002 (−0.006, 0.010) | 0.60 |
| Social skills | −0.019 (−0.034, −0.004) | 0.01 |
| Leadership | −0.005 (−0.016, 0.007) | 0.40 |
| Internalizing3 | −0.021 (−0.038, −0.003) | 0.02 |
| Externalizing4 | 0.01 (−0.009, 0.029) | 0.31 |
All values are associations between changes in zinc concentrations and changes in mental health outcomes. CDI, Children's Depression Inventory; RCMAS, Revised Children's Manifest Anxiety Scale; BASC, Behavioral Assessment System for Children (parent report).
Derived by using a generalized estimating equations approach to examine the associations between changes in zinc concentrations and changes in mental health outcomes that were adjusted for treatment group, child sex, age at measurement, and socioeconomic status and accounting for clustering of children within the same household.
Internalizing problems: sum of anxiety, depression, and somatization scales.
Externalizing problems: sum of hyperactivity, aggression, and conduct scales.
Stratification by baseline zinc deficiency, environmental stress, and mental health status
There was no evidence of heterogeneity by baseline zinc deficiency, level of environmental stress, or baseline mental health on the effect of treatment allocation (data not shown). There was a marginally significant interaction (P = 0.06) between the levels of environmental stress at baseline and changes in zinc for parent-reported symptoms of anxiety; an inverse association (estimate: −0.022 points; P < 0.01) was observed in the low–environmental stress group; no significant association was seen in the high–environmental stress group (estimate: −0.007 points, P = 0.35). No other evidence of heterogeneity by level of environmental stress was shown. There was no evidence of heterogeneity by baseline zinc deficiency or mental health status on the association of changes in zinc concentration with changes in mental health status (data not shown).
DISCUSSION
We examined the effects of zinc supplementation on child- and parent-reported mental health of school-age children in a country where the prevalence of zinc deficiency is high. Zinc supplementation produced significant increases in serum zinc concentrations, with an effect size of 0.29. However, we did not observe significant differences at follow-up on any of the mental health outcomes between the zinc supplemented and placebo groups. These results are consistent with previous studies in the effects of zinc supplementation or fortification on the psychosocial functioning and behavior of Mexican school-age children exposed to lead (53) or of adolescents in the United States (54).
A number of untested hypotheses may explain this lack of effect. These include the possibility that 6 mo was not long enough for some of the functional changes in behaviors and mental health symptoms to take effect. An alternative is the possibility that zinc is most effective in patients with more severe, diagnosed, mental health conditions used in conjunction with and to enhance traditional pharmacotherapy, as noted in several studies of patients with major depressive disorder and ADHD (17, 19). We did not find evidence of heterogeneity by baseline mental health status; however, our measures reflected elevated levels of mental health symptoms and were not intended as clinical diagnostic tools. By using cutoffs suggested from previous studies (35, 39), our sample of children in Guatemala showed a fairly similar prevalence of high depressive and anxious symptoms (11.3% and 19.1%, respectively) to the reported worldwide prevalence of child and adolescent mental disorders of 20% (1, 2). However, these data may need to be interpreted with caution because they are based on cutoffs developed for a US-based population.
A further alternative hypothesis is that the absence of significant differences between zinc and placebo groups in mental health outcomes at follow-up may be due to the zinc-fortified milk and Incaparina (Alimentos SA) provided by the local schools. Both groups showed increases in serum zinc concentrations over time, and both groups showed improvements in many of the mental health outcomes. Furthermore, some of the other nutrients received through the school-based intervention (eg, folate) may have had the potential to improve mental health (55). Although we were not able to quantify the other nutrients received, all children received them, and the randomized controlled trial design of the study makes it very unlikely that this biased our results, as there is no reason to believe that one group received more of these nutrients than the other group. The amount of zinc available in the fortified milk was low (ie, 1.6 mg Zn/200 mL) relative to our intervention of 10 mg Zn/d, and we found it inadequate to explain the increase in the serum zinc concentration in the placebo group. In fact, a recent study in Mexico showed no effect of fortified milk on serum zinc at a much higher concentration, although these children were much younger than those in our study (ie, 10–30 mo of age) (56). There was an effect of study-specific zinc supplementation on zinc concentrations despite the local school intervention; however, this may have been too little to show any further improvement in these children's functional mental health outcomes.
Because both groups received supplemental zinc, there was no evident treatment effect, and both groups showed increases in zinc concentrations, we examined the effect on changes in mental health symptoms of changes in zinc concentration over time. Changes in zinc concentration were significantly and inversely associated with changes in parent-reported symptoms of depression, anxiety, and internalizing problems in these children. These findings confirm results from studies that noted the relation between low amounts of zinc and symptoms of depression and anxiety (11, 13). Again, these results suggest that zinc may play a role in depression and anxiety. However, increases in zinc concentrations were also associated with decreases in social skills, which is a finding in contrast to results from a previous study (57). Given the large number of comparisons and mental health outcomes examined, we cannot rule out the possibility that some of these findings may have occurred by chance. Additional randomized controlled studies are needed to better understand the role of zinc in child mental health outcomes, particularly for internalizing disorders such as depression and anxiety.
We explored possible interactions between treatment and baseline zinc status, levels of environmental stress, and mental health status to test the hypothesis that children who were more at risk at baseline may be affected differently by zinc supplementation. Contrary to our expectations, no evidence of heterogeneity of effects was noted. In children who experienced low levels of environmental stress at baseline, an increase in zinc concentrations was associated with a decrease in parent-reported symptoms of child anxiety; this relation did not exist in children who experienced high levels of stress at baseline. Perhaps for anxiety, zinc is most influential in situations where stress is more manageable and the child is not overwhelmed by external environmental stressors, which is a possibility that needs further exploration. However, because this interaction was marginally significant and was one of many interactions examined, we suggest interpreting this result with caution.
The current study has 2 potential limitations. First, the decision of the local government to provide zinc-fortified milk and Incaparina (Alimentos SA) within the schools at the time the study was being conducted, although likely beneficial to the children, may have limited the current study's ability to detect differences between the zinc-supplemented and placebo groups because the placebo group was no longer a true placebo group. Serum zinc concentrations increased for both groups, and the resulting between-group difference in zinc nutriture may not have been large enough to lead to observable differences on functional mental health outcomes. Nevertheless, the results from the pooled analyses suggest a relation between changes in zinc concentrations and several mental health outcomes (ie, depression and anxiety), despite the apparent lack of association with treatment, which is consistent with previous reports in the literature (10, 11, 13).
Second, a lower coefficient α on several of the BASC subscales suggested the possibility that these measures may not have adequately measured these mental health constructs in this sample of Guatemalan children. Ideally, we would have liked internal consistency coefficients ≥0.70–0.80. We focused on those behavioral measures with reasonable internal consistency (ie, coefficient α ≥ 0.60), and the internal consistency of the 2 composite scales on the BASC, internalizing and externalizing behaviors, had coefficient αs of 0.77 and 0.86, respectively.
The study has several strengths. This was a carefully controlled trial of zinc supplementation with effective randomization at the individual child level and high rates of retention and follow-up that resulted in a balance between groups for many potential confounding factors. Although children with incomplete data not included in the current study showed slightly higher percentages of stunting and levels of parent-reported stress, this group was very small (n = 41) and balanced by treatment group; thus it is highly unlikely that the exclusion of these children from the study biased the results. The study used validated measures for assessing child mental health problems from both child and parent reports.
In conclusion, the current study does not provide evidence that supplementing elementary school–age children with zinc leads to improved psychological functioning. However, the study provides some evidence that positive changes in zinc concentrations may be associated with decreases in internalizing symptoms such as those associated with depression and anxiety in children.
Supplementary Material
Acknowledgments
We acknowledge the valuable input into the psychological component of the study from Yetilu de Baessa (University of Francisco Marroquín, Guatemala City, Guatemala) and V Nelly Salgado de Snyder (National Institute of Public Health, Cuernavaca, Mexico). We also thank Clara Zuleta at INCAP for all of her oversight and direction of the study in the field. Finally, we thank all of the children and parents who participated in the study for their time and input.
The authors’ responsibilities were as follows—AMD, RF-A, RM, LMN, UR, DS, MMB, and ADS: study conception and design; AMD, MR-Z, MW, and RF-A: acquisition of data; AMD, MR-Z, MW, RF-A, RM, LMN, UR, DS, and ADS: analysis and interpretation of data and statistical analyses; AMD, ADS, and MW: drafting of the manuscript; and AMD, MR-Z, RF-A, RM, LMN, UR, DS, MMB, and ADS: critical revision of the manuscript for important intellectual content. All authors had access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analyses and final report. None of the authors had any conflicts of interest.
REFERENCES
- 1.Belfer ML, Saxena S. WHO Child Atlas project. Lancet 2006;367:551–2 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization The World Health Report 2001: mental health: new understanding, new hope. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 3.US Public Health Service Report of the Surgeon General's conference on children's mental health: a national action agenda. Washington, DC: US Department of Health and Human Services, 2000 [PubMed] [Google Scholar]
- 4.The National Advisory Mental Health Council Workgroup on Child and Adolescent Mental Health Intervention Development and Deployment Blueprint for change: research on child and adolescent mental health. Washington, DC: National Institute of Mental Health, 2001 [Google Scholar]
- 5.Burns BJ, Costello EJ, Angold A, et al. Children's mental health service use across service sectors. Health Aff (Millwood) 1995;14:147–59 [DOI] [PubMed] [Google Scholar]
- 6.Belfer ML, Rohde LA. Child and adolescent mental health in Latin America and the Caribbean: problems, progress, and policy research. Rev Panam Salud Publica 2005;18:359–65 [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Brent DA, Benson RS. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1998;37:1234–8 [DOI] [PubMed] [Google Scholar]
- 8.Hesse GW, Hesse KA, Catalanotto FA. Behavioral characteristics of rats experiencing chronic zinc deficiency. Physiol Behav 1979;22:211–5 [DOI] [PubMed] [Google Scholar]
- 9.Golub MS, Takeuchi PT, Keen CL, Hendrickx AG, Gershwin ME. Activity and attention in zinc-deprived adolescent monkeys. Am J Clin Nutr 1996;64:908–15 [DOI] [PubMed] [Google Scholar]
- 10.McLoughlin IJ, Hodge JS. Zinc in depressive disorder. Acta Psychiatr Scand 1990;82:451–3 [DOI] [PubMed] [Google Scholar]
- 11.Maes M, De Vos N, Demedts P, Wauters A, Neels H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord 1999;56:189–94 [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Tamano H, Kan F, Itoh H, Oku N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav Brain Res 2007;177:1–6 [DOI] [PubMed] [Google Scholar]
- 13.Hubbs-Tait L, Kennedy TS, Droke EA, Belanger DM, Parker JR. Zinc, iron, and lead: relations to head start children's cognitive scores and teachers’ ratings of behavior. J Am Diet Assoc 2007;107:128–33 [DOI] [PubMed] [Google Scholar]
- 14.Toren P, Eldar S, Sela BA, et al. Zinc deficiency in attention-deficit hyperactivity disorder. Biol Psychiatry 1996;40:1308–10 [DOI] [PubMed] [Google Scholar]
- 15.Arnold LE, Bozzolo H, Hollway J, et al. Serum zinc correlates with parent- and teacher- rated inattention in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2005;15:628–36 [DOI] [PubMed] [Google Scholar]
- 16.Arnold LE, DiSilvestro RA. Zinc in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2005;15:619–27 [DOI] [PubMed] [Google Scholar]
- 17.Akhondzadeh S, Mohammadi MR, Khademi M. Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial [ISRCTN64132371]. BMC Psychiatry 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold LE, Pinkham SM, Votolato N. Does zinc moderate essential fatty acid and amphetamine treatment of attention-deficit/hyperactivity disorder? J Child Adolesc Psychopharmacol 2000;10:111–7 [DOI] [PubMed] [Google Scholar]
- 19.Nowak G, Siwek M, Dudek D, Zieba A, Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol J Pharmacol 2003;55:1143–7 [PubMed] [Google Scholar]
- 20.DiGirolamo AM, Ramirez-Zea M. Role of zinc in maternal and child mental health. Am J Clin Nutr 2009;89:940S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyton M, Young SN, Pihl RO, et al. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology 2000;22:52–63 [DOI] [PubMed] [Google Scholar]
- 22.Grantham-McGregor SM, Chang S, Walker SP. Evaluation of school feeding programs: Some Jamaican examples. Am J Clin Nutr 1998;67:785S–9S [DOI] [PubMed] [Google Scholar]
- 23.Wachs T. The nature and nurture of child development. Food Nutr Bull 1999;20:7–22 [Google Scholar]
- 24.Mora J, Mora OL. Micronutrient deficiencies in Latin America and the Caribbean: Iodine, calcium and zinc. Washington, DC: USAID/WHO, 1998 [Google Scholar]
- 25.Gibson R. Assessment of trace-element status. Principles of nutritional assessment. New York, NY: Oxford University Press, 1990:511–76 [Google Scholar]
- 26.Ruel MT, Rivera JA, Santizo MC, Lönnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics 1997;99:808–13 [DOI] [PubMed] [Google Scholar]
- 27.Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK. Effect of zinc supplementation on observed activity in low socioecnomic Indian preschool children. Pediatrics 1996;98:1132–7 [PubMed] [Google Scholar]
- 28.Sandstead HH, Penland JG, Alcock NW, et al. Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am J Clin Nutr 1998;68(suppl):470S–5S [DOI] [PubMed] [Google Scholar]
- 29.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes of vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2002 [Google Scholar]
- 30.International Zinc Nutrition Consultative Group (IZiNCG) Assesment of the risk of zinc deficiency in populations and option for its control. Food Nutr Bull 2004;25:S91–204 [PubMed] [Google Scholar]
- 31.Meinert C. Clinical trials: design, conduct, and analysis. New York, NY: Oxford University Press, 1986 [Google Scholar]
- 32.Incaparina INCAPARINA: Nutritional data. Available from: http://www.incaparina.com/datosnutricionales.php (cited 31 March 2010) (in Spanish)
- 33.DeMaeyer E, Dallman P, Gurney J, Hallberg L, Sood S, Srikantia SG. Preventing and controlling iron deficiency anaemia through primary health care: a guide for health administrators and programme managers. Geneva, Switzerland: World Health Organization, 1989 [Google Scholar]
- 34.Makino T, Takahara K. Direct determination of plasma copper and zinc in infants by atomic absorption with discrete nebulization. Clin Chem 1981;27:1445–7 [PubMed] [Google Scholar]
- 35.Kovacs M. Children's Depression Inventory (CDI) manual. North Tonawanda, NY: Multi-Health Systems, 1992;2001 [Google Scholar]
- 36.Sitarenios G, Kovacs M. Use of the Children's Depression Inventory. : Marvish M, The use of psychological testing for treatment planning and outcomes assessment. 2nd ed Mahwah, NJ: Lawrence Erlbaum Associates Inc Publishers, 1999:267–98 [Google Scholar]
- 37.Reynolds WM, Anderson G, Bartell N. Measuring depression in children: a multimethod assessment investigation. J Abnorm Child Psychol 1985;13:513–26 [DOI] [PubMed] [Google Scholar]
- 38.Reynolds CR, Richmond BO. What I think and feel: a revised measure of children's manifest anxiety. J Abnorm Child Psychol 1978;6:271–80 [DOI] [PubMed] [Google Scholar]
- 39.Stallard P, Velleman R, Langsford J, Baldwin S. Coping and psychological distress in children involved in road traffic accidents. Br J Clin Psychol 2001;40:197–208 [DOI] [PubMed] [Google Scholar]
- 40.Ferrando PJ. Factorial structure of the Revised Children's Manifest Anxiety Scale in a Spanish sample: relations with Eysenck personality dimensions. Pers Individ Dif 1994;16:693–9 [Google Scholar]
- 41.Richmond BO, Rodrigo G, de Rodrigo M. Factor structure of a Spanish version of the Revised Children's Manifest Anxiety Scale in Uruguay. J Pers Assess 1988;52:165–70 [DOI] [PubMed] [Google Scholar]
- 42.Reynolds C, Richmond BO. Revised Children's Manifest Anxiety Scale (Spanish version). [Escala de ansiedad manifiesta en ninos (revisada) CMAS-R]. Mexico City, Mexico: Editorial El Manual Moderno, 1985 [Google Scholar]
- 43.Reynolds C, Kamphaus R. Behavior Assessment System for Children (BASC). Circle Pines, MN: American Guidance Service Inc, 1992 [Google Scholar]
- 44.Pineda DA, Kamphaus RW, Mora O, et al. [Use of multidimensional scale for parents of children aged 6 to 11 for the diagnosis of attention deficit with hyperactivity.] Rev Neurol 1999;28:952–9 (in Spanish) [PubMed] [Google Scholar]
- 45.Pineda DA, Kamphaus RW, Mora O, et al. [A system of multidimensional behavior assessment. A scale for parents of children from 6 to 11 years of age. Colombian version.] Rev Neurol 1999;28:672–81 (in Spanish) [PubMed] [Google Scholar]
- 46.International Zinc Nutrition Consultative Group (IZiNCG) Brown KH, Rivera JA, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25:S94–203 [PubMed] [Google Scholar]
- 47.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 48.Soler J, Pérez-Sola V, Puigdemont D, Pérez-Blanco J, Figueres M, Alvarez E. [Validation study of the Center for Epidemiological Studies-Depression of a Spanish population of patients with affective disorders]. Actas Luso Esp Neurol Psiquiatr Cienc Afines 1997;25:243–9(in Spanish) [PubMed] [Google Scholar]
- 49.Masten WG, Caldwell-Colbert AT, Alcala ST, Mijares BE. Reliability and validity of the Center for Epidemiological Studies Depression Scale. Hispanic J Behav Sci 1986;8:77–84 [Google Scholar]
- 50.DeOnis M, Blossner M. WHO global database on child growth and malnutrition. Available from: http://www.who.int/nutgrowthdb/about/introduction/en/index5.html (cited 31 March 31 2010.)
- 51.Maluccio JA, Murphy A, Yount KM. Research note: a socioeconomic index for the INCAP longitudinal study 1969-77. Food Nutr Bull 2005;26:S120–4 [DOI] [PubMed] [Google Scholar]
- 52.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 53.Kordas K, Stoltzfus RJ, Lopez P, Rico JA, Rosado JL. Iron and zinc supplementation does not improve parent or teacher ratings of behavior in first grade Mexican children exposed to lead. J Pediatr 2005;147:632–9 [DOI] [PubMed] [Google Scholar]
- 54.Penland JG, Lukaski HC, Gray JS. Zinc affects cognition and psychosocial function of middle school children. FASEB J 2005;19:A973.(abstr) [Google Scholar]
- 55.Taylor MJ, Geddes J. Folic acid as ultimate in disease prevention: folate also improves mental health. BMJ 2004;328:768–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villalpando S, Shamah T, Rivera JA, Lara Y, Monterrubio E. Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers. J Nutr 2006;136:2633–7 [DOI] [PubMed] [Google Scholar]
- 57.Wachs TD, Bishry Z, Moussa W, et al. Nutritional intake and context as predictors of cognition and adaptive behaviour of Egyptian school-age children. Int J Behav Dev 1995;18:425–50 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.