Abstract
Secretory IgA (SIgA), the predominant class of antibody in intestinal secretions, serves as the first line of defense against enteric infections. SIgA has also been proposed to function in immune surveillance, given that both SIgA and SIgA-antigen complexes are actively transported by Peyer’s patch M cells from the intestinal lumen to sub-epithelial dendritic cells (DCs). The goal of the present study was to identify the receptor(s) potentially utilized by mucosal DCs to recognize and internalize SIgA. We demonstrate that human colostral SIgA is recognized by purified recombinant human DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN) in a solid phase binding assay, as well as by DC-SIGN ectopically expressed on the surface of Chinese hamster ovary (CHO-S) cells. The interaction between SIgA and DC-SIGN was specific, given that it was Ca2+-dependent and inhibited by mannan. Moreover, SIgA bound to, and was internalized by, endogenous DC-SIGN expressed on THP-1 cells following monocyte to macrophage-like cell differentiation by stimulation with phorbol ester and interleukin-4. These data identify DC-SIGN as a putative receptor for SIgA, and reveal a mechanism by which DCs could collaborate with M cells in immune surveillance at mucosal surfaces.
Keywords: Dendritic cells, Secretory IgA, M cells
1. Introduction
As the largest continuous mucosal surface in the human body, the intestinal epithelium is constantly being exposed to potentially toxic environmental antigens, pathogenic food- and water-borne microorganisms, and commensal microflora [1]. To cope with the antigen barrage, the intestinal mucosa is endowed a local network of organized lymphoid follicles, commonly referred to as the mucosal immune system [2]. These organized lymphoid follicles, such as the Peyer’s patches in the small intestine, contain germinal centers whose activity (i.e., B cell differentiation and somatic cell hypermutation) is driven in response to antigens present in the intestinal lumen [3,4]. In the intestinal mucosa, the vast majority of plasma cells secrete dimeric IgA, which is then vectorally transported across the intestinal epithelium into the gut lumen by the polymeric immunoglobulin receptor (pIgR). A fragment of pIgR, known as secretory component (SC), remains covalently associated with IgA after the antibody is released on the luminal face of the epithelium, to form secretory IgA (SIgA) [3,5]. Once in the intestinal lumen, SIgA serves as an immunological barrier capable of preventing toxins and enteric pathogens from attaching to and penetrating the intestinal epithelium [5–7]. SIgA is heavily N- and O-glycosylated, a property integral of the antibody’s function in intestinal secretions [8–11].
While the primary function of SIgA appears to be promoting exclusion of antigens and pathogens, there is evidence that a fraction of secreted antibody is actually transported “retrograde,” back into the mucosa [12]. Specifically, we recently documented the selective adhesion and transepithelial transport of SIgA by Peyer’s patch M cells [13]. M cells are specialized epithelial cells found exclusively within the epithelium that overlies organized mucosa-associated lymphoid tissues [2]. The primary function of M cells is the uptake and transepithelial transport of the intestinal epithelium is antigens, including viruses, bacteria, and parasites, from the lumen to an underlying network of B cells, T cells, macrophages, and dendritic cells (DCs) [2]. However, we and other have shown that M cells also mediate the transport of SIgA (but not IgG or IgM) and SIgA-antigen complexes from the intestinal lumen to the sub-epithelial compartment [13–15]. Following M cell transepithelial transport, SIgA and SIgA immune complexes associate primarily with DCs [14]. It has been postulated that retrograde transport of SIgA by M cells constitutes a mechanism by which sub-epithelial DCs can survey the antigenic status of the intestinal lumen [2,12].
While both mouse- and human-derived DCs are capable of binding and internalizing SIgA, the specific IgA receptor(s) on DCs involved in immunoglobulin A recognition have not been identified. Heystek and colleagues demonstrated that the interaction of SIgA with human monocyte-derived DCs (MoDCs) was not diminished by the addition of anti-CD89 antibody, thus indicating that the interaction is not mediated by the one known human FcαR [16]. In contrast, the binding was abrogated by the addition of mannose or fucose, suggesting that the N- and/or O-linked oligosaccharides on SIgA are being recognized by amember(s) of the C-type lectin family of receptors known to be expressed on DCs. This family includes the mannose receptor (MR) and DC-specific ICAM-3 grabbing non-integrin (DC-SIGN) [17,18]. Because antibodies against the MR only marginally reduced the interaction of SIgA with MoDCs [16], we sought to identify a role for DC-SIGN in this interaction. In this study, we report that DC-SIGN, in recombinant form or expressed on the surfaces of CHO-S cells, selectively and specifically binds to human SIgA. Moreover, we present evidence that SIgA is endocytosed following association with DC-SIGN on the cell surface. Based on these results we propose that DC-SIGN may serve as the receptor on mucosal DCs involved in the recognition and internalization of SIgA, and possibly SIgA-antigen complexes.
2. Materials and methods
2.1. Chemicals, reagents, buffers and antibodies
Purified human colostral IgA, bovine serum albumin (BSA), ovalbumin (OVA), mannan from Saccharomyces cerevisiae, and streptavidin conjugated to horseradish peroxidase (SA-HRP), were purchased from Sigma–Aldrich (St. Louis, MO). Purified human plasma Igs (IgA, IgM, and IgG) and myeloma proteins (IgA1 and IgA2) were obtained from EMD Biosciences (San Diego, CA). Human SC was obtained from Nordic Immunology (Tilburg, The Netherlands). The lectin RCA-II was obtained from Vector Labs (Burlingame, CA). Recombinant mouse macrophage mannose receptor (CD206), recombinant human DC-SIGN-Fc chimera [19], and anti-human DC-SIGN mouse monoclonal antibody conjugated to phycoerythrin (PE) were obtained from R&D Systems (Minneapolis, MN). EZ-Link sulfo-NHS-LC-biotin, biotin-LC-hydrazide and fluorescein isothiocyanate (FITC) were purchased from Thermo Fisher Scientific (Waltham, MA). Biotinylation was performed in phosphate buffered saline (PBS; pH 7.4), whereas FITC-conjugation was performed in bicarbonate buffer (0.1Mcarbonate, 0.1M bicarbonate pH 9.0). Tween-20 was obtained from BioRad (Torrance, CA), and paraformaldehyde (16%) was purchased from Electron Microscopy Sciences (Fort Washington, PA). Dialysis was performed with a Slide-a-Lyzer (10,000 molecular weight cut-off) purchased from Thermo Fisher Scientific. The following buffers were prepared by the media facility at the Wadsworth Center: Dulbecco’s PBS (DPBS), calcium magnesium-free (CMF)-DPBS supplemented with EDTA (1–5 mM), Hanks’ balanced salt solution (HBSS), CMF-HBSS, and enhanced HBBS (E-HBSS) supplemented with 10mM CaCl2.
2.2. Solid phase binding assays
Lyophilized recombinant human DC-SIGN and recombinant mouse MR were dissolved in PBS (pH 7.4) to a concentration of 8 nM and then used to coat wells (100 µL per well) of Nunc MaxiSorb™ 96 well plates (Thermo Fisher Scientific). The plates were incubated in a humidified chamber at 4 °C for 24 h, and then washed with PBST (0.05%) and blocked with Ig-free BSA (2%, w/v in PBS). Biotinylated Ig or control proteins were diluted into E-HBSS or CMF-HBSS to a final concentration of 2 nM and then applied to the plates and incubated for 30 min incubation period at 37 °C. The plates were developed with SA-HRP (1.0 µg/mL) and one-component tetramethylbenzidine (TMB) colorimetric substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Plates were read at 450nm on a SpectraMax 250 microtiter plate reader (Molecular Devices, Sunnyvale, CA) with accompanying Softmax software.
2.3. Cell culture
CHO-S cells were obtained from Invitrogen (Carlsbad, CA). CHO-S cell lines stably transfected with DC-SIGN or another C-type lectin were obtained from the laboratory of Dr. Ralph M. Steinman (The Rockefeller University, New York, NY). CHO-S cells were maintained in DMEM with high glucose (4500 mg/L) supplemented with fetal bovine serum (10%, v/v) and non-essential amino acids (Invitrogen). When necessary, G418 (Sigma–Aldrich) was added to the medium to a final concentration of 0.5–1.5 mg/mL. All cell culture media were prepared by the Wadsworth Center media facility. Cells were routinely maintained in a humidified incubator at 37 °C with 5% CO2.
The human monocytic leukemia cell line THP-1 was obtained from ATCC (Manassas, VA). The cells were grown in RPMI 1640 supplemented with 10% FBS. Differentiation was induced by treatment with phorbol 12-myristate 13-acetate (PMA;EMD Biosciences) and recombinant human interleukin 4 (IL-4; EMD Biosciences), as previously described [20]. Cells were seeded at ~5×105 cells/mL in a T75 cm2 tissue culture flask and then treated with PMA (10 ng/mL) for 24 h. The culture medium was then further supplemented with IL-4 (200 ng/mL) for an additional 72 h before the cells were collected for analysis by flow cytometry or microscopy. Control cells were treated in parallel with 0.1% BSA instead of PMA and IL-4.
2.4. Flow cytometry
CHO-S cells and C-type lectin-transfected derivatives were detached from the surfaces of cell culture flasks by treatment with CMF-DPBS containing 5mM EDTA for ~30 min on ice, collected by centrifugation, washed with serum-free DMEM, and then adjusted to ~1–5×106 cells/mL in E-HBSS or CMF-HBSS. The cells were then treated with FITC-labeled ligand (e.g., SIgA, IgA1, IgA2, IgG, OVA) for 30 min at 4 °C or 37°C. For immunolabeling, the cells were suspended in HBSS and then incubated with fluorophore-conjugated antibodies, at concentrations recommended by the manufacturer, for 60 min at 37 °C. The cells were washed three times to remove unbound ligands or antibodies, and fixed with 1% paraformaldehyde for 10 min at room temperature. Following fixation, the cells were suspended in PBS containing 1mM NaN3 and 2% goat serum (Invitrogen), and were then subjected to flow cytometry using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ). A minimum of 10,000 cells were analyzed per sample.
2.5. Fluorescence microscopy
CHO-S cells and C-type lectin-transfected derivatives were detached from cell culture flasks by treatment with trypsin, collected by centrifugation, washed with serum-free DMEM, and then adjusted to ~1–5×106 cells/mL. The cells were then seeded onto sterile, poly-l-lysine coated glass cover slips placed in 6 well cell culture plates (Costar) and incubated overnight at 37 °C with 5% CO2. The following morning, the cells were treated with FITC-coupled ligands for 30 min at 4 °C. To assess ligand endocytosis, the cells were then transferred to 37 °C for a additional 30 min incubation. The cells were fixed with 4% PFA, and then mounted on microscope slides using VectaShield (Vector Labs). The cells were visualized using a Zeiss Axioskop 2 fluorescence microscope or a Leica TCS SP5 confocal laser scanning microscope.
3. Results
3.1. Recognition of SIgA by DC-SIGN in a solid phase binding assay
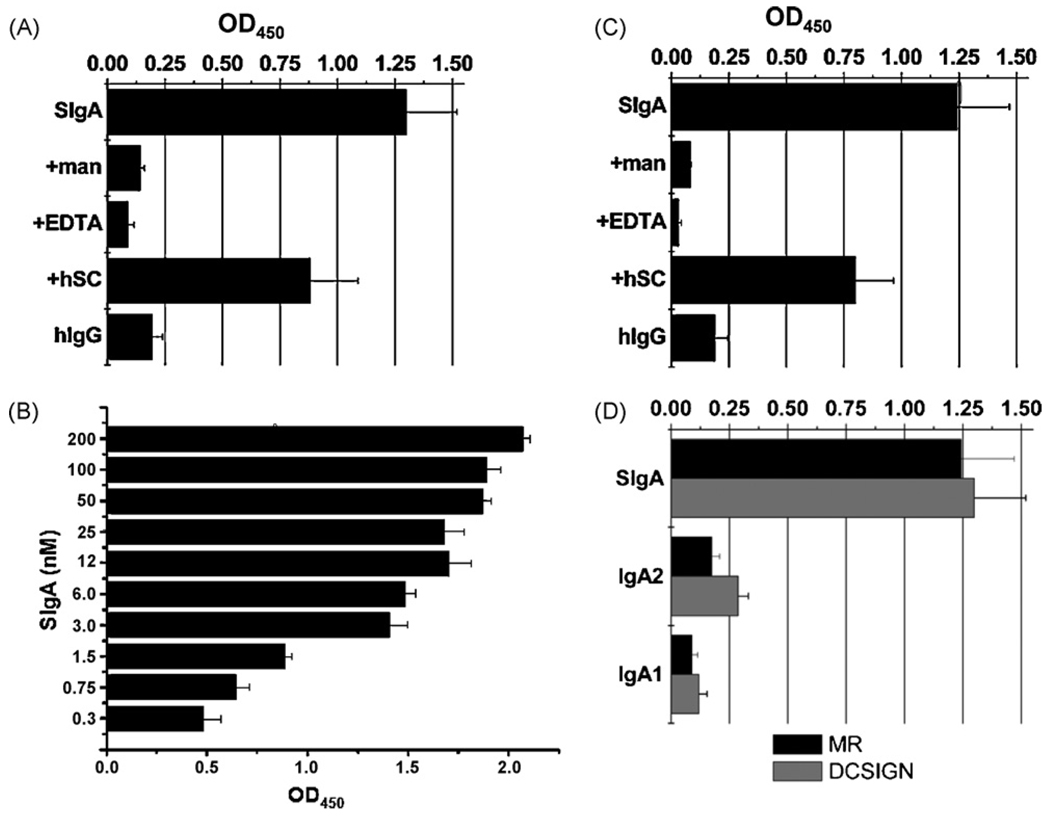
We used a solid phase binding assay in amicrotiter plate format to investigate a possible interaction between SIgA and DC-SIGN. Microtiter plates were coated with recombinant human DC-SIGN and then probed with biotinylated human colostral SIgA (2 nM) or, as a control, human serum IgG (2 nM). The biotin-labeled immunoglobulin preparations used for these studies were free of any detectable contaminating proteins. For example, SDS-PAGE and Western blot analysis of SIgA samples revealed three distinct protein species with apparent molecular masses of 25 kDa, 55 kDa, and 70 kDa, corresponding to light chain (LC), heavy chain (HC), and SC, respectively (data not shown). J chain (15-kDa) was not detected, although we have previously identified it in these preparations using J-chain specific antibodies [21]. Analysis of the IgG preparation by similar methods revealed two distinct protein species corresponding to LC and HC. Using these samples, we found that biotin-SIgA adhered strongly to wells coated with DC-SIGN, whereas human serum IgG did not (Fig. 1A). The binding of SIgA to DC-SIGN was abrogated by the addition of mannan (1 mg/mL) or EDTA (1 mM), demonstrating that the observed interaction is both carbohydrate-mediated and Ca2+-dependent. A titration of SIgA (0.3–200 nM) revealed dose-dependent interaction between SIgA and DC-SIGN (Fig. 1B). For comparative purposes, we also examined a possible interaction between SIgA and the MR. SIgA bound to MR-coated microtiter wells, and this interaction was blocked by mannan (1 mg/mL) or EDTA (1mM) (Fig. 1C). These data demonstrate that both DC-SIGN and the MR are capable of recognizing human SIgA in a mannose- and Ca2+-dependent manner.
Fig. 1.
Recognition of SIgA by recombinant DC-SIGN and MR. Ninety-six well microtiter plates were coated with purified, recombinant DC-SIGN (A, B) or MR (C). In panels A and C, the plates were probed with biotinylated human IgG (2 nM) or biotinylated human SIgA (2 nM), either alone or coadministered with mannan (man), EDTA (5 mM), or human SC (4 nM). The plates were overlaid with streptavidin-HRP and developed using TMB (see Section 2). (Panel B) Microtiter plates coated with DC-SIGN were probed with SIgA at indicated concentrations. (Panel D) Relative binding of different IgA subclasses with human DC-SIGN and MR. Microtiter plates were coated with DC-SIGN (black) or MR (shaded), probed with biotinylated SIgA, human myeloma IgA1, or human IgA2, and then developed as described for panels A–B. For each panel, the bars represent the average (with SD) of a single experiment done in triplicate.
To determine whether DC-SIGN and the MR recognized the carbohydrate side chains on IgA and/or those on SC, microtiter plates were probed with purified, biotinylated human myeloma IgA1 and IgA2. Neither C-type lectin bound the IgA myelomas (Fig. 1D), even though we have previously demonstrated that these immunoglobulin preparations are glycosylated [21]. On the other hand, the binding of biotin-labeled SIgA to plate-bound DC-SIGN and MR was reduced by ~30% when premixed with an equimolar amount of unlabeled SC (Fig. 1A, C). These data suggest that DC-SIGN and the MR preferentially recognize carbohydrate side chains on SC.
3.2. SIgA is recognized by surface-expressed DC-SIGN
While the solid phase binding assays revealed that both DC-SIGN and the MR are capable of recognizing SIgA, we chose to focus our attention on DC-SIGN for two reasons. First, DC-SIGN is expressed on myeloid DCs in the SED, whereas the MR is not [22,23]. This population of DCs is likely involved in the sampling of SIgA-antigen complexes following transepithelial transport by M cells. Second, Heystek and colleagues [16] previously reported that the interaction of SIgA with human monocyte-derived DCs is only partially inhibited by antibodies against the MR, indicating that other DC-specific receptors contribute to SIgA recognition.
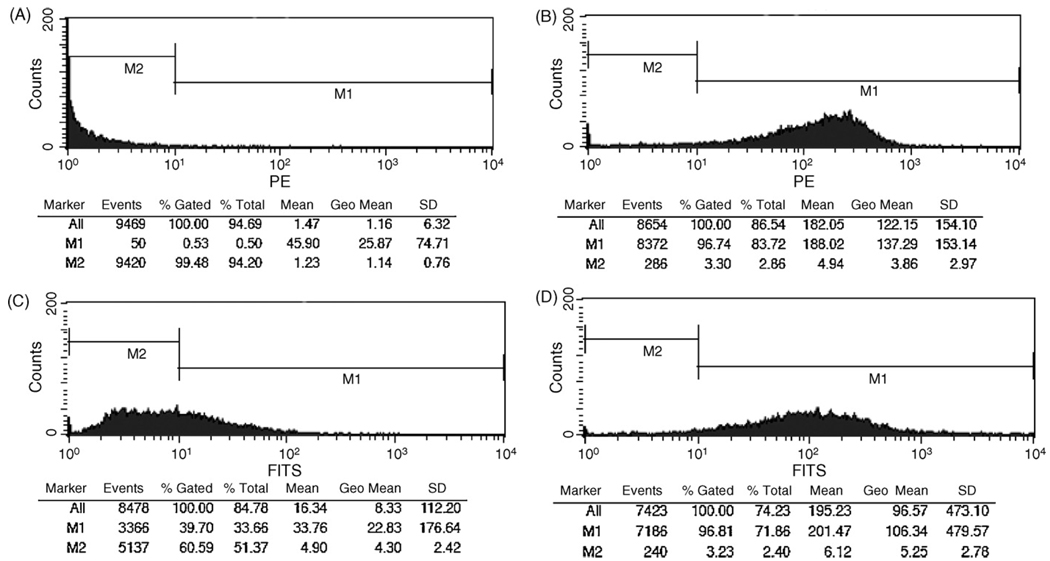
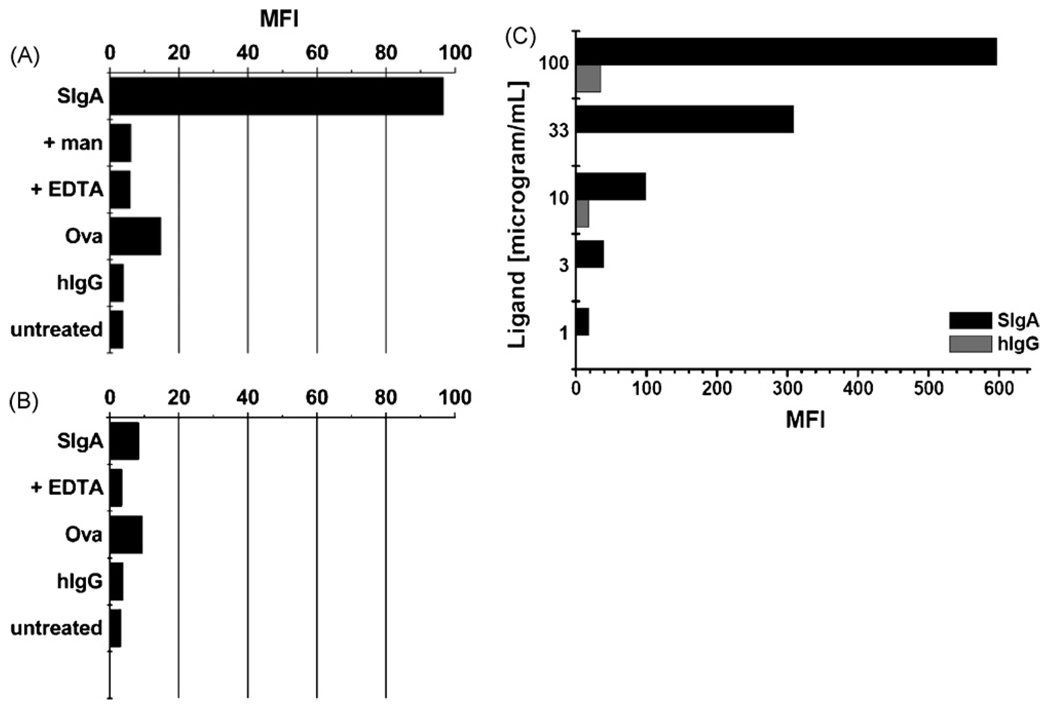
To determine whether SIgA is recognized by surface-expressed DC-SIGN, CHO-S cells stably expressing human DC-SIGN were probed with FITC-labeled SIgA and then subjected to flow cytometry. Direct labeling with a PE-conjugated anti-DC-SIGN monoclonal antibody verified high levels of DC-SIGN on the transfected cells (MFI >120), as compared to control, untransfected CHO-S cells (MFI < 2) (Fig. 2A, B). When probed with FITC-SIgA, we found that SIgA bound to >95% of the DC-SIGN transfected cells, with an overall MFI of 96, but did not associate with control CHO-S cells (MFI < 10) (Figs. 2C, D; 3A, B). The interaction between SIgA and DC-SIGN was dose-dependent (Fig. 3C) and specific, as evidenced by the fact that binding was abrogated by the addition of mannan (1 mg/mL) or EDTA (5mM) (Fig. 3A). Neither FITC-labeled human IgG nor OVA bound to DC-SIGN expressing cells (Fig. 3A, C).
Fig. 2.
Association of SIgA with DC-SIGN expressed on the surface of CHO-S cells. (Panels A, B) The relative levels of DC-SIGN expression on CHO-S cells was determined by flow cytometry. CHO-S control cells (A) or DC-SIGN transfected cells (B) were detached from tissue culture flasks and then stained at 4 °C with an anti-human DC-SIGN monoclonal antibody conjugated PE, and then analyzed by flow cytometry. (Panels C, D) SIgA-binding to control or DC-SIGN transfected cells. Control (C) or DC-SIGN transfected (D) cells were incubated with SIgA-FITC (2 nM) for 30 min on ice, and then subjected to flow cytometry.
Fig. 3.
Specific association of SIgA with surface-expressed DC-SIGN. DC-SIGN transfected- (panel A) or control CHO-S cells (panel B) were incubated with FITC-labeled OVA (2 nM), human IgG (2 nM), SIgA (2 nM; equivalent to ~3 µg/mL), either alone or with mannan (man) or EDTA (5 mM), for 30 min on ice, and then subjected to flow cytometry. (Panel C) DC-SIGN transfected CHO cells were incubated SIgA-FITC (solid bars) and IgG-FITC (shaded bars) at the indicated concentrations and then subjected to flow cytometry. Each experiment was repeated at least three times with identical results. The results of a single experiment are shown.
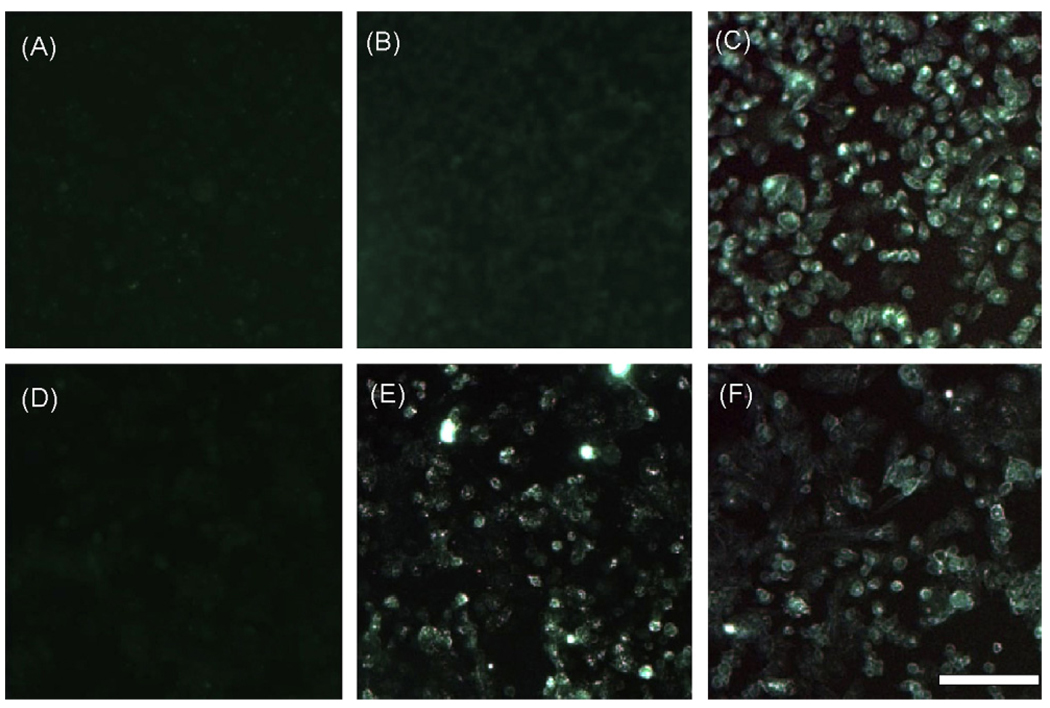
The interaction of SIgA with surface-expressed DC-SIGN was further analyzed by fluorescence microscopy. CHO-S control or DC-SIGN-transfected cells were grown on glass coverslips, and then incubated for 30 min at 4 °C with FITC-conjugated human IgG, colostral SIgA, or the lectin RCA-II (Fig. 4). There was no detectable labeling of DC-SIGN-transfected cells treated with FITC-IgG (panels A, D), whereas FITC-SIgA bound to DC-SIGN transfected cells, but not control CHO-S cells (panels B, E). FITC-RCA-II, which served as a positive control for these experiments, labeled transfected and non-transfected cell types with equal intensities (panels C, F). RCA-II recognizes terminal galactose-containing glycolipids and glycoproteins on all cell types [24]. The data confirm that cell surface-expressed DC-SIGN is sufficient to bind SIgA.
Fig. 4.
Adhesion of SIgA to cells expressing DC-SIGN. CHO-S control (panels A–C) or DC-SIGN transfected (panels D–F) cells were grown on glass cover slips and treated with FITC-labeled human IgG (A, D), SIgA (B, E), or the lectin RCA-II (C, F) for 30 min at 4 °C. The cover slips were then washed thoroughly to remove unbound ligand, fixed with 4% paraformaldehyde, and visualized by fluorescence microscopy. Scale bar ~100 µm.
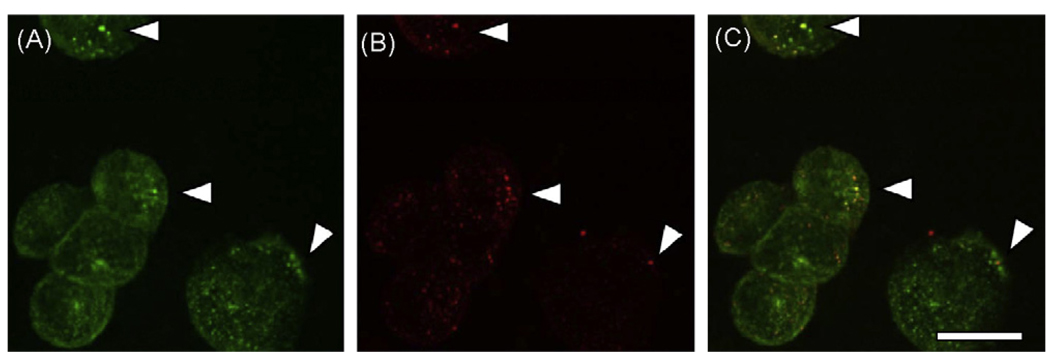
We next used CLSM to evaluate the possibility that SIgA is selectively internalized by DC-SIGN. DC-SIGN-transfected CHO-S cells grown on glass coverslips were incubated for 30 min at 4 °C with FITC-conjugated SIgA, to permit ligand-lectin interaction. These cells were then labeled with PE-conjugated anti-human DC-SIGN and transferred to a cell chamber set at 37 °C; they were then incubated for 30 min to permit endocytosis. CLSM analysis revealed that SIgA was present in vesicular bodies, as evidenced by punctate FITC staining throughout the cell cytoplasm (Fig. 5A). DC-SIGN was detected within similar vesicular bodies, and in some instances, it co-localized with SIgA (Fig. 5B, C). However, co-localization of SIgA with DC-SIGN was relatively rare (i.e., ~10% of the SIgA positive vesicular bodies were DC-SIGN positive), which could reflect either a low rate of SIgA-mediated endocytosis by DC-SIGN, or rapid recycling of DC-SIGN back to the cell surface following SIgA internalization.
Fig. 5.
Co-localization of SIgA and DC-SIGN. DC-SIGN transfected cells were grown on glass cover slips and treated with FITC-labeled human SIgA and anti-human DC-SIGN monoclonal antibody-conjugated to PE for 30 min at 4 °C, as described in Section 2. The cover slips were then washed thoroughly and transferred to 37 °C incubator for 30 min, to allow endocytosis of ligands bound to the cell surface. The cells were then fixed and visualized by confocal laser scanning microscopy. Panels: A, SIgA-FITC; B, Anti-DC-SIGN-PE; (C) Merge of panels A and B. The arrowheads indicate regions in which the distribution of SIgA and DC-SIGN are coincident. Scale bar 20 µm.
3.3. Mannose- and calcium-dependent binding of SIgA to differentiated THP-1 cells
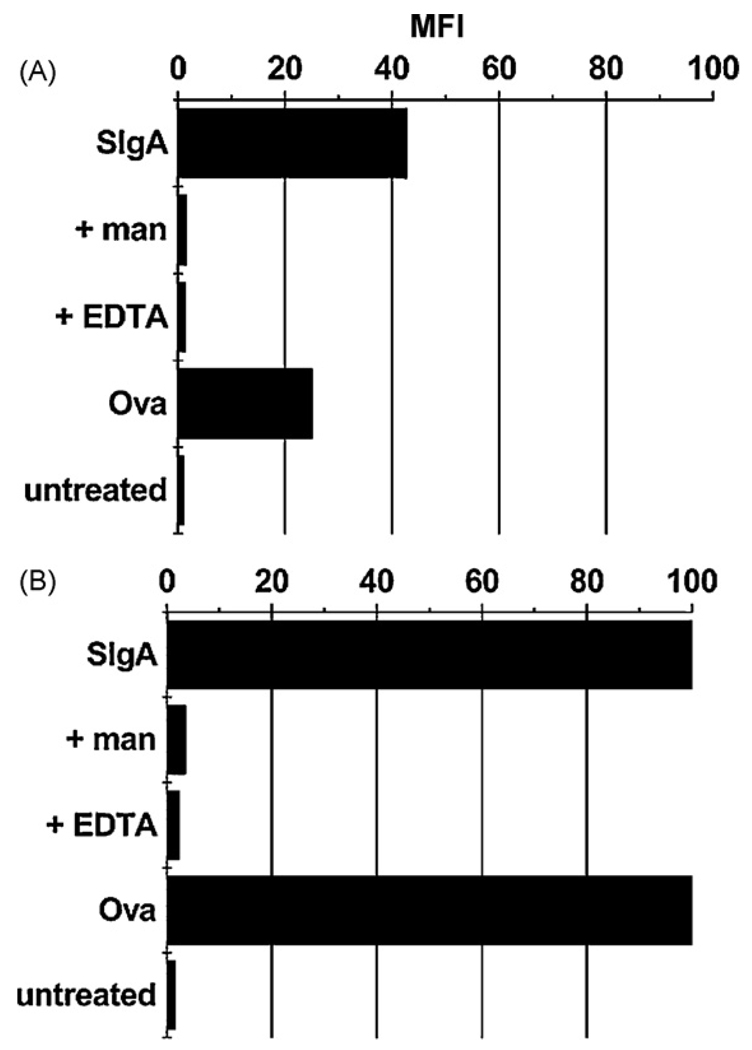
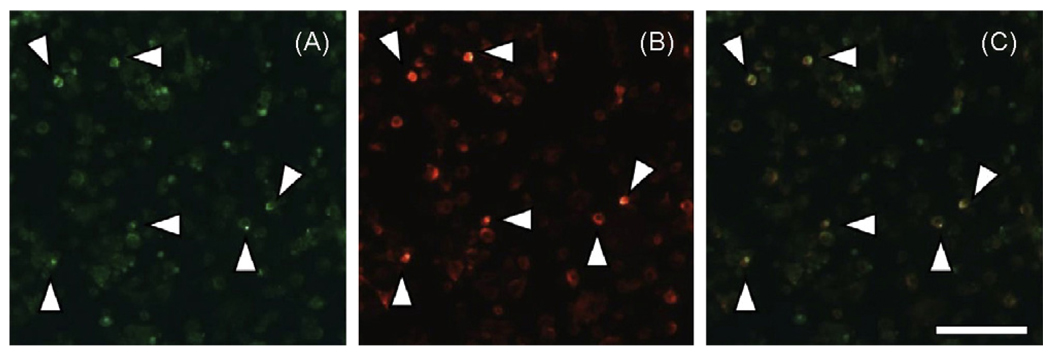
While SIgA selectively interacted with DC-SIGN in a solid phase binding assay, as well on the surfaces transfected CHO cells, we wished to determine whether it was also able to associate with native, endogenously expressed DC-SIGN. We used the human monocytic leukemia cell line THP-1 as a model system to address this question. Puig-Kroger and colleagues have recently shown that THP-1 cells express high levels of DC-SIGN following treatment with PMA and IL-4 [20]. Indeed, we verified this finding by flow cytometric analysis of THP-1 cells labeled with a PE-conjugated anti-DC-SIGN MAb before or after PMA and IL-4 treatment. Untreated cells had an MFI of ~37, whereas cells treated PMA and IL-4 had an MFI >450 (data not shown). We next treated these cells with FITC-SIgA. SIgA adhered to THP-1 cells with an MFI of~45, and to PMA and IL-4 treated TPH-1 cells with an MFI of~100 (Fig. 6). The association of SIgA with these cells was specific, because binding was inhibited by the addition of mannan(1 mg/mL)or EDTA (5 mM). Moreover, fluorescence microscopy analysis revealed a co-localization of SIgA and DC-SIGN on the surface of PMA and IL-4 treated THP-1 cells (Fig. 7). Interestingly, it should be noted that OVA also bound to THP-1 cells and this binding was augmented following PMA and IL-4 treatment. While OVA is not a ligand for DC-SIGN it is known to be recognized with other members of the C-type lectin family [25]. Based on this finding, we cannot exclude the possibility that another C-type lectin, in addition to DC-SIGN, is expressed on the surface of THP-1 cells and may contribute to SIgA binding.
Fig. 6.
SIgA adheres to THP-1 cells expressing high levels of DC-SIGN. THP-1 cells (A) before or (B) after treatment with PMA and IL-4 were incubated with FITC-labeled OVA (2 nM), or SIgA (2 nM), either alone or with mannan (man) or EDTA (5 mM), for 30 min on ice, and then subjected to flow cytometry. This experiment was repeated three times. The results of a single representative experiment are shown.
Fig. 7.
Co-localization of SIgA and DC-SIGN on the surfaces of THP-1 cells. THP-1 cells treated with PMA and IL-4 were incubated with FITC-labeled human SIgA and anti-human DC-SIGN monoclonal antibody conjugated PE for 30 min at 4 °C and then transferred to 37 °C incubator for 30 min to allow endocytosis of ligands bound to the cell surface. The cells were then washed thoroughly and spotted onto glass microscope slides by cytospin and visualized by confocal laser scanning microscopy. Panels: A, SIgA-FITC; B, Anti-DC-SIGN-PE; (C) Merge of panels A and B. The arrowheads indicate regions in which the distribution of SIgA and DC-SIGN overlapped. Scale bar ~100 µm.
4. Discussion
In this study, we demonstrated that DC-SIGN selectively binds human SIgA. The interaction between DC-SIGN and SIgA was specific because it was Ca2+-dependent, and could be inhibited by the addition of mannan. Moreover, fluorescence microscopy revealed the occasional co-localization of SIgA and DC-SIGN within intracellular vesicles, suggesting that SIgA is in fact endocytosed following association with DC-SIGN on cell surfaces. DC-SIGN was first described as a DC-specific adhesion molecule that binds to ICAM-3 [26]. Sequence analysis subsequently revealed that DC-SIGN was in fact a previously identified C-type lectin that had been shown to be capable of binding the envelope glycoprotein gp120 from HIV-1 [27,28]. It is now well-established that DC-SIGN has dual roles on the surface of DCs; mediating contact with T cells, via ICAM-3, and promoting the uptake of HIV-1, and other pathogens, including hepatitis C virus, Mycobacterium tuberculosis, Schistosoma mansoni [29]. Based on the results of this current study, we now propose that DC-SIGN has yet a third function; recognition and internalization of SIgA, and possibly SIgA-antigen complexes, by mucosal DCs. DC-SIGN is expressed on a population of DCs located within the sub-epithelial dome region of human Peyer’s patches [22,30]. These cells are that uniquely situated to sample SIgA-antigen complexes following transepithelial transport by M cells. We speculate that DC-SIGN-mediated uptake of SIgA-antigen complexes by DCs could serve as an immune surveillance mechanism important in the maintenance of mucosal immunity and intestinal homeostasis.
DC-SIGN recognizes a range of oligosaccharide ligands, including mannan, complex high mannose-containing glycoconjugates, and asialyated Lewis blood group antigens [19,31]. Therefore, it is not surprising that DC-SIGN recognizes SIgA. SIgA is decorated withN- andO-linked oligosaccharides, including high mannose and Lewis antigen structures [8–11]. Oligosaccharides account for >10% of the molecular mass of human IgA [11], and >20% of the mass of SC [10,32,33]. In contrast, glycans constitute only about 3% of the molecular mass of IgG [34]. The diversity of the glycoconjugate side chains on SIgA is staggering; Royle and colleagues identified over 50 different O-glycan structures alone [10]. These oligosaccharide side chains are an integral feature of SIgA, in that they protect the immunoglobulin heavy chains from intestinal proteases, promote antibody association with mucus, and serve as “decoys” for lectin-like receptors expressed by pathogenic toxins, viruses and bacteria [21,33,35,36].
It is interesting that DC-SIGN, when tested in a solid phase binding assay, bound to SIgA, but not to purified, monomeric forms of IgA1 or IgA2. The fact that neither IgA1 nor IgA2 was capable of blocking the interaction of SIgA with DC-SIGN agrees with results presented by Heysteck and colleagues. Those investigators reported that human MoDCs bound SIgA, but not serum IgA [16]. A number of factors could explain these observations. For example, glycosylation patterns differ between monomeric and polymeric serum-derived forms of IgA [37]. Monomeric forms of IgA may lack oligomannose side chains, which would be predicted to serve as effective ligands for DC-SIGN. Alternatively, SC may constitute the primary component of SIgA that is recognized by DC-SIGN. This is not inconceivable, considering that SC has seven N-linked oligosaccharide side chains, which collectively form a carbohydrate “shield” around the Fc regions of dimeric IgA [10,32]. Other have shown that certain bacteria-derived lectins preferentially recognize the carbohydrate side chains on SC more than those on IgA [33]. A third possibility to explain the preferential association of DC-SIGN with SIgA relates to ligand density and receptor clustering. Mitchell and colleagues demonstrated that the carbohydrate recognition domains (CRDs) of DC-SIGN form tetramers that act cooperatively to bind oliogosaccharides [38]. In the case of SIgA, oligosaccharides may be spatially distributed in such a manner as to be optimally recognized by DC-SIGN. While further studies are needed to uncover the molecular basis of this interaction, it is interesting to speculate that the preferential association of DC-SIGN with SIgA serves as a means to enable DCs to sample IgA derived from mucosal secretions, rather than the form of IgA antibody found in serum and interstitial fluids.
DCs could potentially encounter SIgA-antigen complexes at two distinct locations in the intestinal mucosa. As discussed above, the first is within the SED regions of organized lymphoid follicles present in Peyer’s patches [22,39]. The SED is the primary depot for antigens and SIgA-antigen complexes following M cells transcytosis [13–15]. The second site where DC-SIGN+ DCs could potentially encounter SIgA is in the intestinal lumen. In mice, it has been documented that lamina propria DCs in certain segments of the small intestine can extend their dendrites into the intestinal lumen as a means of surveying the gut for antigens [40,41]. If this is a genuine phenomenon, then we would expect that such periscope-like dendrites to also encounter SIgA and SIgA-antigen complexes. DC-SIGN+ DCs are numerous in the lamina propria [22].
It should be stressed that the immunological significance of sampling of SIgA-antigen complexes by DC-SIGN+ DCs is unknown [12]. SIgA is by itself not sufficient to promote maturation or activation of immature MoDCs [16]. However, to our knowledge, the effect of SIgA-antigen complexes on DC maturation/activation has not been determined. We speculate that the uptake of SIgA is a surveillance mechanism involving both M cells and DCs to assess luminal antigens, and possibly to promote tolerance against both commensal bacteria and dietary components. It is interesting to note that in humans, a significant proportion of the commensal microflora is coated with SIgA [42].
Acknowledgements
We gratefully acknowledge Emily Gage, Elizabeth McCarthy, and Joanne O’Hara for their technical assistance with cell culture and flow cytometry. We extend our special thanks to Dr. Richard Cole of the Advanced Light Microscopy Core facility at the Wadsworth Center for assistance with fluorescence microscopy and live cell imaging, and Dr. Ralph M. Steinman (The Rockefeller University) for providing us with CHO-S cells transfected with DC-SIGN and other C-type lectins. We also thank Dr. Blaise Corthésy for critically reading this manuscript. This work was supported by grants from the NIH (R21AI081053) and from the Northeast Biodefense Center (U54-AI057158-Lipkin) to NJM.
References
- 1.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neutra M, Mantis N, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissue. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 5.Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. 2008;76:4137–4144. doi: 10.1128/IAI.00416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74:3455–3462. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestecky J, Moro I, Kerr MA, Woof JM. Mucosal immunoglobulins. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. Burlington, MA: Academic Press; 2005. pp. 153–181. [Google Scholar]
- 9.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 10.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 11.Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976;13:325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- 12.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 14.Kadaoui KA, Corthesy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 15.Rey J, Garin N, Spertini F, Corthesy B. Targeting of secretory IgA to Peyer’s patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172:3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 16.Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol. 2002;168:102–107. doi: 10.4049/jimmunol.168.1.102. [DOI] [PubMed] [Google Scholar]
- 17.McGreal EP, Martinez-Pomares L, Gordon S. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol Immunol. 2004;41:1109–1121. doi: 10.1016/j.molimm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 18.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 19.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 20.Puig-Kroger A, Serrano-Gomez D, Caparros E, Dominguez-Soto A, Relloso M, Colmenares M, et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 21.Mantis NJ, Farrant SA, Mehta S. Oligosaccharide side chains on human secretory IgA serve as receptors for ricin. J Immunol. 2004;172:6838–6845. doi: 10.4049/jimmunol.172.11.6838. [DOI] [PubMed] [Google Scholar]
- 22.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linehan SA, Martinez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- 25.Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol. 2004;16:819–829. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- 26.Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 27.Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 29.Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 30.Salim SY, Silva MA, Keita AV, Larsson M, Andersson P, Magnusson KE, et al. CD83+CCR7− dendritic cells accumulate in the subepithelial dome and internalize translocated Escherichia coli HB101 in the Peyer’s patches of ileal Crohn’s disease. Am J Pathol. 2009;174:82–90. doi: 10.2353/ajpath.2009.080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 32.Hughes GJ, Reason AJ, Savoy L, Jaton J, Frutiger-Hughes S. Carbohydrate moieties in human secretory component. Biochim Biophys Acta. 1999;1434:86–93. doi: 10.1016/s0167-4838(99)00168-5. [DOI] [PubMed] [Google Scholar]
- 33.Perrier C, Sprenger N, Corthesy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281:14280–14287. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- 34.Jefferis R, Lund J. Glycosylation of antibody molecules: structural and functional significance. Chem Immunol. 1997;65:111–128. [PubMed] [Google Scholar]
- 35.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 36.Wold AE, Mestecky J, Tomona M, Kobata A, Ohbayashi H, Endo T, et al. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam JW, et al. Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol. 2006;17:3529–3539. doi: 10.1681/ASN.2006040388. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 39.Kelsall B, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 42.van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]