Abstract
Previous studies have consistently suggested that the ε4 allele of apolipoprotein E (APOE) gene is a major risk factor for Alzheimer’s disease (AD). However, whether the ε2 allele, a possible protective factor for AD, will express its protective effect in terms of cortical thickness in healthy elderly carriers is unclear. The goal of this study is to clarify the effects of APOE genotypes on cortical thickness in nondemented elderly subjects. We used 164 healthy, cognitively normal, elderly subjects, who were grouped into ε2 carriers, ε3 homozygotes, and ε4 carriers respectively. The APOE ε2 carriers had a significant thicker (corrected p < 0.05) cortical thickness in the superior temporal cortex compared with the ε3 homozygotes. In addition to this area, the APOE ε2 carriers had a significantly thicker region in the dorsolateral prefrontal cortex (corrected p < 0.05) than did the ε4 carriers. These findings suggest that the different alleles of the APOE gene have distinct neuroanatomic effects in elderly healthy subjects and may play specific roles in the development of AD.
Keywords: Cortical thickness, Apolipoprotein E, Alzheimer’s disease
Alzheimer’s disease (AD), the most common neurocognitive disorder, is characterized by a progressive loss of neurons and synapses, with cerebral deposits of amyloid-β (Aβ) senile plaques [21] and neurofibrillary tangles (NFTs) [18]. The apolipoprotein E (APOE) polymorphism, specifically the ε4 allele, is widely accepted as the most robust genetic risk factor for late onset Alzheimer’s disease (LOAD) [4,33]. APOE ε4 has been reported as being related to a lower level of Aβ42 in normal elderly adults [25,28] and a high level of tau in AD [17,34,35]. Both of these appear to contribute to the development of AD [20,31].
A growing number of studies have indicated that APOE ε4 modulates brain morphology specifically by a loss in hippocampal volume [6,24,29] and by causing abnormal white matter integrity [27]. The correlation between APOE ε4 and cortical thickness has also been increasingly reported. For example, healthy adults with the ε4 allele showed age-related reductions in cortical thickness in the medial prefrontal and pericentral cortex [9]. Thinner cortical thickness was also observed in the entorhinal cortex and the subiculum in healthy adults who had the ε4 allele [2].
Just as the APOE ε4 allele has been identified as a major risk factor for LOAD, other studies have found that the ε2 allele may have some protective qualities, since carriers appear to have a lower risk of developing Alzheimer’s disease [10,37]. One study [32] observed increased cortical thickness in healthy children and adolescents who were ε2 carriers. Other researchers [6], however, did not find any effect of ε2 on brain volume in healthy elderly subjects.
Since APOE has been identified as major risk factor in LOAD, finding the effects of this gene on cortical thickness in elderly adults is important. Our goal was, therefore, to examine the cortical thickness across the entire cortex of healthy, elderly adults to see if it correlates with the various APOE genotypes, especially with the ε2 allele. We expected that we would find increased cortical thickness for the ε2 allele carriers and decreased cortical thickness for the ε4 allele carriers, each compared to the ε3 homozygotes.
Data used in this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). The initial goal of ADNI was to recruit 800 adults, ages 55–90, to participate in the research—approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information see www.adni-info.org.
Our downloaded data initially included 225 baseline NC scans. We excluded 5 subjects because they converted to MCI or AD in 36 months. To ensure the accuracy of the segmentation, we manually checked the interface between the grey matter and white matter as well as the one between the white matter and the cerebrospinal fluid (CSF), and on this basis excluded 46 additional subjects due to segmentation errors. The resulting data for the cortical thickness analysis included 164 healthy, cognitively normal, elderly subjects, all collected from ADNI sites. The individuals were grouped into ε2 carriers (21), ε3 homozygotes (103) and ε4 carriers (37), with ε2ε4 individuals excluded from consideration. The Mini Mental State Examination (MMSE) and the education level had also been recorded as baseline data. Sample characteristics are presented in Table 1. In addition, those individuals in this study group for whom an intact record of their CSF biomarkers, including total tau (t-tau), hyperphosphorylated tau (p-tau), and Aβ42, was available were separately grouped into APOE ε2 carriers (14), ε3 homozygotes (50) and ε4 carriers (14).
Table 1.
Sample demographic characteristics.
| Genotype |
p-Value | |||
|---|---|---|---|---|
| APOE ε2+ (n = 21) | APOE ε3ε3 (n = 103) | APOE ε4+ (n = 37) | ||
| Age (years) | 74.45 ± 5.97 | 75.94 ± 5.06 | 75.83 ± 4.80 | 0.483a |
| Gender (f/m) | 11/9 | 55/47 | 18/19 | 0.710b |
| Education (years) | 14.2 ± 3.31 | 16.12 ± 2.80 | 16.16 ± 2.79 | 0.081a |
| MMSEa | 28.51 ± 0.32 | 28.6 ± 4 0.21 | 29.13 ± 0.91 | 0.170a |
| Race (Asian/African American/White) | 0/3/18 | 1/7/95 | 1/0/36 | 0.222b |
f/m: female and male; MMSE: Mini Mental State Examination.
The p-value was obtained by ANOVA.
The p-value was obtained by Pearson Chi-square.
The data sets included standard T1-weighted MR images acquired sagittally using volumetric 3D MPRAGE with 1.25 mm × 1.25 mm in-plane spatial resolution, TE = 3.9 ms, TR = 90 ms and 1.2 mm thick sagittal slices (8° flip angle). All of the images were obtained using 1.5 T scanners. The MRI scans were acquired at multiple sites using a GE, Siemens or Philips 1.5 T system. The images were preprocessed using a number of steps detailed on the ADNI website. This preprocessing was done to correct for differences across scanners used at various ADNI sites.
We processed each scan using FreeSurfer [5,12] (http://surfer.nmr.mgh.harvard.edu/) with its volume and surface pipeline. Starting with the segmentation of white matter and the tessellation of the grey/white matter boundary, an initial surface was obtained after an automated topological correction. We used this surface as the initial shape for a deformable model that was used to reconstruct the pial surface.
When all the surfaces had been reconstructed, the cortical thickness was computed. Each subject’s cortical thickness was measured in native space [11]. Surface based registration [13] was used to construct an average template, and all the individual reconstructed cortical surfaces were aligned to it. The cortical thickness measurements at the interconnection between the two hemispheres were masked because there is very little grey matter there. Finally, we smoothed the thickness using a heat kernel [3] 30 mm wide to increase the signal-to-noise ratio and improve the ability to detect morphometric variations.
We performed an Analysis of Covariance (ANCOVA) using SPSS 13.0 statistical software to assess the effects of APOE genotypes on biomarker levels (tau, Aβ42), controlling for age and gender. After that, a post hoc multiple comparison using Fisher’s LSD was conducted when the ANCOVAs were significant. In all tests, results with probability values less than 0.05 were considered statistically significant.
We used SurfStat (http://www.math.mcgill.ca/keith/surfstat/) toolbox for Matlab (R2007a, The Mathworks, Natick, MA, USA) to perform statistical analyses of the cortical thicknesses. Statistical tests were performed at every unmasked point on the pial surface. To test for variability in the thickness of the cortex, we applied a general liner model to check point-wise thickness differences using the APOE genotypes as fixed factor, and age and gender as covariates. The ensuing p-values were adjusted for multiple comparisons on the cortical surface to control for the false positive rates by the random field theory [39]. We discarded statistically significant clusters containing fewer than 50 points, in order to reduce the possible influence of noise.
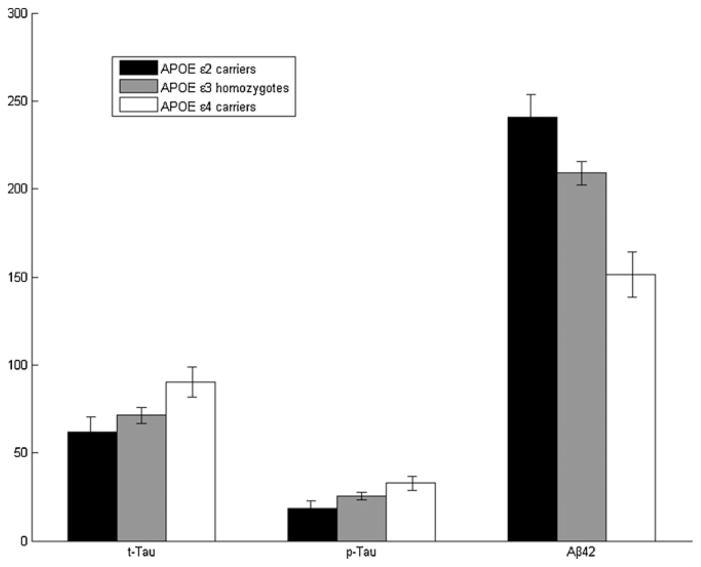
We tested the effects of the APOE genotypes on the CSF biomarkers t-tau, p-tau and Aβ42 level on a smaller subset of 78 samples. The ANCOVA showed a significant effect of APOE on Aβ42 level (p < 10−4). In addition, a post hoc comparison showed a statistically significant stepwise trend toward lower Aβ42 levels, with ε2 being the highest, ε4 the lowest, and ε3 homozygotes occupying an intermediate position (p = 0.034 for the step from ε2 to ε3, and p < 10 e–4 for the one from ε3 to ε4) (Fig. 1). In contrast to Aβ42, the effects of APOE on CSF t-tau or p-tau were only a trend (p = 0.063 and p = 0.054 respectively), and this was limited to comparisons of APOE ε4 and ε2 carriers (p = 0.024 for t-tau, and p = 0.016 for p-tau) (Fig. 1). No significant association between the APOE genotype and the MMSE score was observed (p = 0.170).
Fig. 1.
Mean biomarker levels (t-tau, p-tau and Aβ42) for the APOE genotype groups. The APOE ε2 carriers are represented in black, the ε3 homozygotes in grey and the ε4 carriers in white. The CSF Aβ42 levels show a significant stepwise trend downward, from ε2 carriers to ε3 homozygotes to ε4 carriers; whereas the t-tau and the p-tau levels show the opposite trend.
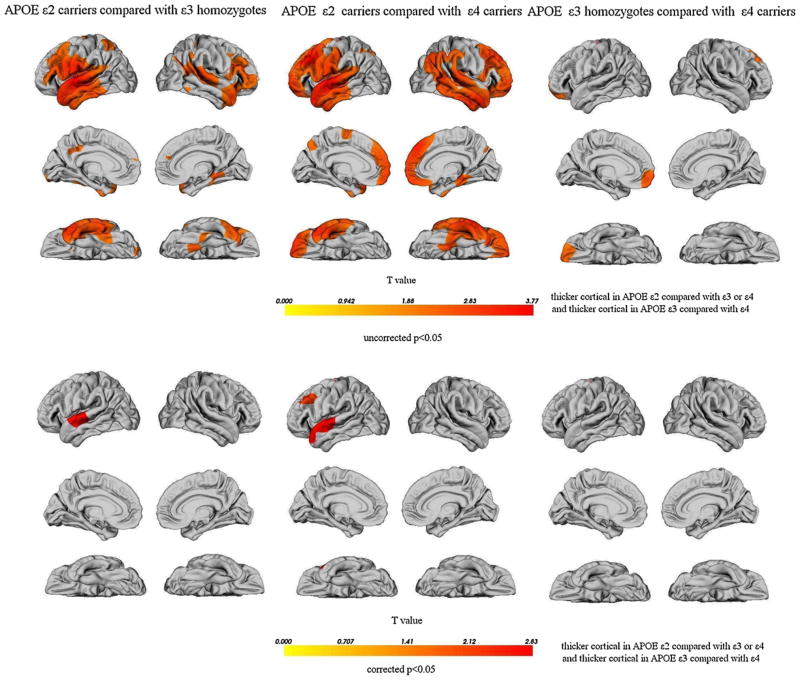
The results of the group comparisons of cortical thickness are displayed in Fig. 2. No increases, only decreases, in cortical thickness were found in any region in the APOE ε4 carriers and the ε3 homozygotes when compared with the same regions in the ε2 carriers. This was also true in the APOE ε4 carriers when they were compared with the ε3 homozygotes. The APOE ε2 allele carriers had significantly increased cortical thickness primarily in the bilateral superior temporal cortices, bilateral dorsolateral prefrontal cortices, left supramarginal gyrus, left precentral and postcentral gyri, left parietal operculum and right parahippocampal region compared to the APOE ε3ε3 subjects. The APOE ε2 carriers also had a significantly thicker cortex primarily in the bilateral lateral and medial frontal regions, the right parahippocampal cortex, right temporoparietal cortex and bilateral temporal regions than did subjects who were ε4 allele carriers. The APOE ε4 carriers showed significant atrophy in the left medial prefrontal cortex and left orbitofrontal cortices compared with the ε3 homozygotes. After performing multiple comparison corrections using random field theory, we determined that the APOE ε2 carriers had significantly thicker cortical thickness in the left superior temporal cortex compared with the ε3 homozygotes. Moreover, the cortices in the left superior temporal and left dorsolateral prefrontal region were thicker in the APOE ε2 carriers than in the ε4 carriers. However, no significant differences remained when comparing the APOE ε3 homozygotes with the ε4 carriers after applying the multiple comparison correction.
Fig. 2.
Cortical thinning maps (in percentage). Only points with statistically significant differences (p < 0.05) are displayed. The top shows uncorrected p < 0.05, and the bottom shows p < 0.05 corrected. The color bar displays the T value of the cortical thickness differences in millimeters.
In this study, we used a surface-based approach to quantify the local cortical thickness in healthy nondemented adults with different APOE genotypes. We observed a stepwise lower Aβ42 level for different APOE genotypes, and thicker cortical thickness when comparing APOE ε2 carriers to ε4 carriers or ε3 homozygotes in specific regions.
We examined the effect of APOE ε2 on brain morphology by comparing subjects with this genotype with ε3 homozygotes and ε4 carriers. Interestingly, when we compared the others with the ε2 carriers, the ε3 homozygotes and the ε4 carriers had a similar thinner cortical area, which was primarily located in the bilateral lateral prefrontal cortices, the bilateral temporal cortices, and the right parahippocampal region. The differences in the left superior temporal gyrus, which is associated with speech production [22,30], remained significant after a multiple comparison correction. A loss of the speech production function is a symptom in the development of AD [16]. Moreover, we observed a significant stepwise lowering of the Aβ42 levels across the APOE genotypes and a significantly higher t-tau or p-tau level when comparing APOE ε2–ε4 carriers. Importantly, the accumulation of Aβ peptide and tau in neurons has been implicated in the development of AD [20,31]. Aβ peptide deposition [8] and neurofibrillary tangle development [36] have been found to occur early in the development of AD. Thus, the same biological markers, that is, those for tau and Aβ42, which contribute to the Aβ peptide and neurofibrillary tangles could also induce a metabolic decline leading to neuronal loss and thus perhaps cortical thinning [19].
In addition to thinning in the superior temporal cortex, the ε4 allele was associated with greater atrophy in the bilateral pre-frontal cortex when compared with the ε2 allele. Moreover, after applying a multiple comparison correction, the differences in the left dorsolateral prefrontal cortex, which has been suggested as being correlated with cognition control [26] and long term memory formation [1], remained significant. Again, the failure of these functions is characteristics of AD [15]. Thus, we can conclude that the finding that APOE ε2 allele carriers possess a thicker left dorsolateral prefrontal cortex may indicate a specific protective role against possible memory loss in the development of AD.
We also found that APOE ε4 carriers displayed atrophy (uncorrected p < 0.05) in the left prefrontal cortex and the left orbitofrontal cortex compared to ε3 homozygotes. Although when comparing ε3 with ε4 individuals we did not observe atrophy in the parahippocampal cortex, which is the earliest region affected in AD [23], atrophy was present in this region in the ε3 homozygotes or ε4 carriers when compared with the APOE ε2 carriers (uncorrected p < 0.05). Published studies about APOE ε4s effect on brain morphology, especially in the parahippocampal region, are controversial. Several studies have suggested that APOE ε4 is related to hippocampal volume loss [6,24,29], but one recent study [29] which also used ADNI data found no effect of APOE ε4 on hippocampal volume loss in the normal control group. Because most published studies have subdivided APOE genotypes into APOE ε4 non-carriers and carriers, the inclusion of the ε2 carriers may have caused a continuum of thicknesses that caused any e4-related thinning of the parahippocampal cortex to be lost in the data.
The results of the present study may also indicate that APOE genotypes affect cognitive function in normal aging. A large meta-analysis study [38] investigated the effects of the APOE genotype on cognition in the nondemented population. Specifically, in the non-demented population the APOE ε4 allele carrier often had impaired cognitive functioning, and ε2 allele carriers showed better performance on episodic memory. Moreover, increased cortical thickness, including that in the frontal and temporal regions, was associated with higher cognitive performance in older healthy adults [14]. Our results are compatible with these findings. In this study, we did not find a significant relationship between the APOE genotypes and the MMSE scores. One of major reasons may be a ceiling effect that appeared because the healthy nondemented individuals had high scores that were similar to those of the subjects on these measures. However, in a previous study, APOE ε4 carriers were observed to have a greater rate of cognitive decline as shown by their MMSE scores using nondemented individuals [7]. Thus, the effects of APOE genotypes on cognitive decline in normal aging require further investigation.
In conclusion, the APOE ε2 allele may have a specific protective role in the development of AD. To our knowledge, this is the first study to explore the cortical thickness pattern between APOE genotypes in nondemented elderly subjects. Further studies are needed to clarify the exact mechanism and role of the APOE genotypes in the cognitive decline associated with normal aging as well as in the development of AD.
Acknowledgments
This work was supported by the National Key Basic Research and Development Program (973), Grant No. 2007CB512305, the National High Technology Program (863) Grant No. 2009AA02Z302, and National Natural Science Foundation of China, Grant Nos. 30730035 and 60903101. We thank the Computing Center, Gansu province, China for the data processing. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. We thank Edmund F. and Rhoda E. Perozzi for reviewing the English in this paper.
References
- 1.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage. 2008;41:1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. Cortical thickness analysis in autism with heat kernel smoothing. NeuroImage. 2005;25:1256–1265. doi: 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 5.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 6.den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- 7.Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, Deeg DJ. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 8.Duyckaerts C, Bennecib M, Grignon Y, Uchihara T, He Y, Piette F, Hauw JJ. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging. 1997;18:267–273. doi: 10.1016/s0197-4580(97)80306-5. [DOI] [PubMed] [Google Scholar]
- 9.Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 11.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 13.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM. Selective increase of cortical thickness in high-performing elderly-structural indices of optimal cognitive aging. NeuroImage. 2006;29:984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Forstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249:288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- 16.Frank EM. Effect of Alzheimer’s disease on communication function. JSC Med Assoc. 1994;90:417–423. [PubMed] [Google Scholar]
- 17.Glodzik-Sobanska L, Pirraglia E, Brys M, de Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis L, Sadowski MJ, Martiniuk F, Mehta P, Pratico D, Zinkowski RP, Blennow K, de Leon MJ. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Neurobiol Aging. 2009;30:672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goedert M, Spillantini MG, Crowther RA. Tau proteins and neurofibrillary degeneration. Brain Pathol (Zurich, Switzerland) 1991;1:279–286. doi: 10.1111/j.1750-3639.1991.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 19.Grignon Y, Duyckaerts C, Bennecib M, Hauw JJ. Cytoarchitectonic alterations in the supramarginal gyrus of late onset Alzheimer’s disease. Acta Neuropathol. 1998;95:395–406. doi: 10.1007/s004010050816. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 22.Hickok G, Erhard P, Kassubek J, Helms-Tillery AK, Naeve-Velguth S, Strupp JP, Strick PL, Ugurbil K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neurosci Lett. 2000;287:156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- 23.Huesgen CT, Burger PC, Crain BJ, Johnson GA. In vitro MR microscopy of the hippocampus in Alzheimer’s disease. Neurology. 1993;43:145–152. doi: 10.1212/wnl.43.1_part_1.145. [DOI] [PubMed] [Google Scholar]
- 24.Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23:382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kester MI, Blankenstein MA, Bouwman FH, van Elk EJ, Scheltens P, van der Flier WM. CSF biomarkers in Alzheimer’s disease and controls: associations with APOE genotype are modified by age. J Alzheimers Dis. 2009;16:601–607. doi: 10.3233/JAD-2009-0999. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 27.Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. NeuroReport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- 28.Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Farlow MR, DeCarli C, Raskind MA, Schellenberg GD, Lee VM, Galasko DR. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- 29.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 32.Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 33.Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tapiola T, Lehtovirta M, Ramberg J, Helisalmi S, Linnaranta K, Riekkinen P, Sr, Soininen H. CSF tau is related to apolipoprotein E genotype in early Alzheimer’s disease. Neurology. 1998;50:169–174. doi: 10.1212/wnl.50.1.169. [DOI] [PubMed] [Google Scholar]
- 35.Tapiola T, Pirttila T, Mehta PD, Alafuzofff I, Lehtovirta M, Soininen H. Relationship between apoE genotype and CSF beta-amyloid (1–42) and tau in patients with probable and definite Alzheimer’s disease. Neurobiol Aging. 2000;21:735–740. doi: 10.1016/s0197-4580(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 36.Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RS, Bienias JL, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73:672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.02.003. in press. [DOI] [PubMed] [Google Scholar]
- 39.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]