Abstract
The electron transport chains in the membranes of bacteria and organelles generate proton-motive force essential for ATP production. The c-type cytochromes, defined by the covalent attachment of heme to a CXXCH motif, are key electron carriers in these energy-transducing membranes. In mitochondria, cytochromes c and c1 are assembled by the cytochrome c heme lyases (CCHL and CC1HL) and by Cyc2p, a putative redox protein. A cytochrome c1 mutant with a CAPCH heme-binding site instead of the wild-type CAACH is strictly dependent upon Cyc2p for assembly. In this context, we found that overexpression of CC1HL, as well as mutations of the proline in the CAPCH site to H, L, S, or T residues, can bypass the absence of Cyc2p. The P mutation was postulated to shift the CXXCH motif to an oxidized form, which must be reduced in a Cyc2p-dependent reaction before heme ligation. However, measurement of the redox midpoint potential of apocytochrome c1 indicates that neither the P nor the T residues impact the thermodynamic propensity of the CXXCH motif to occur in a disulfide vs. dithiol form. We show instead that the identity of the second intervening residue in the CXXCH motif is key in determining the CCHL-dependent vs. CC1HL-dependent assembly of holocytochrome c1. We also provide evidence that Cyc2p is dedicated to the CCHL pathway and is not required for the CC1HL-dependent assembly of cytochrome c1.
THE c-type cytochromes, also referred to as cytochrome c, represent a universal class of heme-containing proteins that function as electron carriers in the energy-transducing pathways of bacteria, plastids, and mitochondria (Thöny-Meyer 1997; Nakamoto et al. 2000; Bonnard et al. 2010). Because cytochromes c carry a heme covalently attached to a CXXCH motif, they constitute an attractive object of study to address the question of cofactor protein assembly. The biochemical requirements for cytochrome c assembly were deduced from in vivo and in vitro studies, and the conclusion is that both apocytochromes c and heme are transported independently across at least one biological membrane and maintained as reduced prior to catalysis of the heme attachment reaction (Allen et al. 2003; Hamel et al. 2009; Kranz et al. 2009; Sanders et al. 2010). Bacterial cytochromes c are assembled in the periplasmic space, a compartment where cysteine pairs in proteins form disulfide bonds in reactions catalyzed by dedicated enzymes (Inaba 2009; Kadokura and Beckwith 2010). The current thinking holds that a c-type apocytochrome is a substrate of the disulfide bond-forming pathway, which introduces an intramolecular disulfide between the two cysteines of the CXXCH sequence (Allen et al. 2003; Sanders et al. 2010). This disulfide needs to be reduced to a dithiol to provide free sulfhydryls for the heme ligation. Consistent with this view is the fact that groups of specific oxido-reductases that constitute a transmembrane dithiol-disulfide relay from the cytosol to the periplasmic space have been shown to function as c-type cytochrome assembly factors (Allen et al. 2003; Kadokura et al. 2003; Mapller and Hederstedt 2006; Sanders et al. 2010). The proposal that the components of this pathway control the in vivo redox status of the CXXCH sulfhydryls has been inferred from the presence of motifs in their protein sequences that are consistent with a function in redox chemistry and also from the demonstration that their recombinant forms participate in dithiol–disulfide exchange reactions (Monika et al. 1997; Setterdahl et al. 2000). Moreover, the ability of exogenous thiol compounds to bypass the lack of these factors in vivo substantiates the view that the redox components have a disulfide-reducing activity in the pathway (e.g., Sambongi and Ferguson 1994; Fabianek et al. 1998; Beckett et al. 2000; Deshmukh et al. 2000; Bardischewsky and Friedrich 2001; Erlendsson and Hederstedt 2002; Erlendsson et al. 2003; Feissner et al. 2005; Turkarslan et al. 2008).
While the role of these pathways is well established in bacteria, much less is known about the components that catalyze thiol/disulfide chemistry in the mitochondrial intermembrane space (IMS), which is topologically equivalent to the bacterial periplasm. By analogy with the bacterial pathways, the participation of redox-active factors that catalyze thiol formation is expected, as the mitochondrial IMS houses two c-type cytochromes, the soluble cytochrome c and the membrane-bound cytochrome c1, both of which function in respiration. In fungi, heme attachment to apocytochromes c and c1 is dependent upon the IMS resident cytochrome c and c1 heme lyases, CCHL and CC1HL, although the exact role of these lyases in the assembly process is still unclear (Dumont et al. 1987; Zollner et al. 1992). Conversion of apocytochrome to holocytochrome c depends only on CCHL, while apocytochrome c1 can be acted upon by both CCHL and CC1HL (Matner and Sherman 1982; Dumont et al. 1987; Stuart et al. 1990; Zollner et al. 1992; Bernard et al. 2003). In animals, apoforms of cytochromes c and c1 are assembled by a unique heme lyase, HCCS, which carries both the CCHL and CC1HL activities (Prakash et al. 2002; Schwarz and Cox 2002; Bernard et al. 2003).
Cyc2p, a component first described as a mitochondrial biogenesis factor in yeast (Matner and Sherman 1982; Dumont et al. 1993; Pearce et al. 1998; Sanchez et al. 2001), was recently rediscovered in the context of cytochrome c1 maturation (Bernard et al. 2003). Cyc2p is located at the mitochondrial inner membrane with its C-terminal domain containing a non-covalently bound FAD exposed to the IMS (Bernard et al. 2005). A redox function for Cyc2p is likely based on the finding that a recombinant form of the molecule exhibits a NAD(P)H-dependent reductase activity (Bernard et al. 2005). However, as Cyc2p activity is not essential for the maturation process, a functional redundancy was postulated based on the fact that a cyc2-null mutant still assembles holoforms of cytochromes c and c1 (Bernard et al. 2005). The absolute requirement of Cyc2p was revealed via genetic analysis of the cyc2-null cyt1-34 combination that displays a synthetic respiratory-deficient phenotype with loss of holocytochrome c1 assembly (Bernard et al. 2005). The cyt1-34 mutation maps to the gene encoding cytochrome c1 and results in a CAPCH heme-binding site replacing the wild-type CAACH site (Bernard et al. 2005). The synthetic interaction is specific for the cyt1-34 allele carrying the A-to-P mutation and is not observed in a cyc2-null cyt1-48 strain carrying an A-to-D mutation at the heme-binding site of apocytochrome c1 (Bernard et al. 2005). The fact that Cyc2p becomes essential when the cytochrome c1 heme-binding site carries an A-to-P mutation suggests that the CXXCH motif could be the target of Cyc2p action in vivo. One possible interpretation for this observation is that the P residue alters the reactivity of the cysteinyl thiols to redox chemistry so that the apocytochrome c1 CAPCH heme-binding site occurs in an oxidized (disulfide) form, which must be reduced in a Cyc2p-dependent reaction before heme attachment can occur.
In this article, we have undertaken a genetic approach to elucidate this pathway and searched for suppressors that alleviate the respiratory deficiency of the cyc2-null cyt1-34 strain. Either overexpression of CC1HL or replacement of the P mutation in the heme-binding site by H, L, S, or T residues restore the assembly of holocytochrome c1. In vitro measurement of redox potential of apoforms of CA(A/P/T)CH cytochrome c1 indicates that there is no change in the thermodynamic stability of the disulfide at the CXXCH motif that could account for the Cyc2p-dependent assembly of cytochrome c1. Genetic studies reveal that the replacement of the second A residue at the CAACH motif by H, L, P, S, and T residues is key in determining the conversion of apocytochrome c1 to its corresponding holoform via the CCHL and/or CC1HL-dependent pathway. We also demonstrate that Cyc2p is a component dedicated to the CCHL pathway and is not required for the CC1HL-dependent assembly of cytochrome c1. We propose that the CAPCH cytochrome c1 is strictly dependent upon CCHL and Cyc2p for its assembly but becomes a substrate of CC1HL upon overexpression of CC1HL or in the presence of H, L, S, or T mutations.
MATERIALS AND METHODS
Construction, manipulation, and growth of yeast strains:
All of the yeast strains used in the course of this study are listed in Table 1. YDM1 and YDM2 strains carry the cyt1∷kan allele and originate from the parental SMY1 (MATa) and YCT1-11C (MATα) Δcc1hl strains, respectively. PCR amplification of the cyt1∷kan cassette was performed using oligonucleotide sequences (5′-AACTGGATCCATAGACTATCTAAG-3′ and 5′-GACACTATTGAAGTGAGACG-3′) and the genomic DNA of the cyt1∷kan strain from the ResGen knockout collection. Yeast cells were transformed by the lithium acetate procedure (Schiestl and Gietz 1989) or by the one-step technique (Chen et al. 1992). Genetic crosses were performed as described in Dujardin et al. (1980). Media used for Saccharomyces cerevisiae contain glucose or galactose as fermentable substrates and glycerol, ethanol, ethanol/glycerol, or lactate as respiratory substrates and were described elsewhere (Dujardin et al. 1980; Hamel et al. 1998; Saint-Georges et al. 2002).
TABLE 1.
Genotypes and sources of yeast strains
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 | R. Rothsteina |
| W303-1B | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 | R. Rothsteina |
| PHT3 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1∷LEU2 | Hamel et al. (1998) |
| YPH71-14B | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-34 | Bernard et al. (2005) |
| YPH10-8A | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-48 | Bernard et al. (2005) |
| YPH1 | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyc2∷hph | Bernard et al. (2003) |
| YPH6-9C | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-34 cyc2∷hph | Bernard et al. (2005) |
| YDB8 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-48, cyc2∷hph | Bernard et al. (2005) |
| SMY1 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt2∷his5+ | Bernard et al. (2003) |
| YCT1-11C | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt2∷his5+ | Bernard et al. (2003) |
| UV34 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-34 cyt2∷his5+ | Bernard et al. (2003) |
| UV48 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-48 cyt2∷his5+ | Bernard et al. (2003) |
| SSP4,5,8,10 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-35 cyc2∷hph | This study |
| SSP6 | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100, cyt1-36 cyc2∷hph | This study |
| SSP1,2 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-37 cyc2∷hph | This study |
| SSP3,7,9,11,12 | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-38 cyc2∷hph | This study |
| YDM1 | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1∷kan cyt2∷his5+ | This study |
| YDM2 | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1∷kan cyt2∷his5+ | This study |
| YDM3-2B | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-35 | This studyb |
| YDM2-2C | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-36 | This studyc |
| YDM1-2A | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-37 | This studyd |
| YDM4-2A | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-38 | This studye |
| YDM3-1X | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-35 cyt2∷his5+ | This studyf |
| YDM2-1X | aade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-36 cyt2∷his5+ | This studyg |
| YDM1-1B | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-37 cyt2∷his5+ | This studyd |
| YDM4-1B | α ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 cyt1-38 cyt2∷his5+ | This studye |
Department of Human Genetics, Columbia University.
Segregant of diploid created by crossing YDM2 with SSP10.
Segregant of diploid created by crossing YDM1 with SSP6.
Segregant of diploid created by crossing YDM2 with SSP2.
Segregant of diploid created by crossing YDM1 with SSP12.
Created by inactivation of CYT2 in YDM3-2B.
Created by inactivation of CYT2 in YDM2-2C.
Isolation of multicopy and genetic suppressors:
The multicopy wild-type genomic libraries constructed in the URA3-based pFL44L vector (Bach et al. 1979) (a generous gift from F. Lacroute) and in the LEU2-based Yep24 vector (Carlson and Botstein 1982) (a generous gift from M. Carlson) were used to search for suppressor genes able to alleviate the respiratory deficiency of the cyc2-null cyt1-34 strain. Transformants were plated on medium lacking uracil or leucine, replicated on lactate medium to select for respiratory proficiency, and incubated for 15 days at 28°. Plasmids retrieved through the multicopy suppressor screen were extracted from yeast transformants and propagated in Escherichia coli strains (Hoffman and Winston 1987). Independent spontaneous suppressors bypassing the respiratory deficiency of the Δcyc2cyt1-34 strain were isolated on lactate medium. Cells were grown to stationary phase and plated on respiratory medium, and suppressed strains were isolated after 10–15 days incubation at 28°.
Overexpression and purification of apocytochromes c1:
The CYT1 gene encoding the soluble form (amino acids 62–263) of wild-type apocytochrome c1 was amplified by PCR from yeast genomic DNA and cloned into the NheI/XhoI sites of the hexahistidinyl tag plasmid pET-24b(+) (Novagen). The cysteines (C188 and C258) were replaced by serines by site-directed mutagenesis. Mutations in the heme-binding motif (CAPCH and CATCH) were also engineered by site-directed mutagenesis. Apocytochromes c1 were overexpressed in E. coli BL21(DE3) strain in the presence of 1 mm isopropyl-β-d-thiogalactopyranoside for 5 hr at 30°. All recombinant apocytochromes c1 (wild-type and mutants) carry a C-terminal (His)6-tag, and as a result of the C188,258S substitutions, the only cysteines present are the two associated with the heme-binding site. Purification of His-tagged proteins was performed under denaturing conditions. Cells were harvested, resuspended in a HEPES buffer (100 mm HEPES, 100 mm NaCl, 0.01% Tween 20, pH 8.0) containing 8 m urea and 50 mm imidazole and incubated at room temperature for 1 hr. The lysate was clarified by centrifugation at 10,000 × g for 20 min at room temperature. The supernatant was then applied to the Ni-NTA resin (Qiagen). Protein renaturation and refolding were carried out directly on the column by gradient buffer exchange to eliminate urea. The resin was washed with the HEPES buffer containing 100 mm imidazole, and the proteins were eluted with 250 mm imidazole. Samples were then dialyzed to remove imidazole and concentrated by using a Centriprep filter unit (Millipore). Purity was assessed by SDS–PAGE, and the concentration of the purified protein was measured by recording the absorbance at 260 nm.
Measurement of midpoint redox potentials of apocytochromes c1:
Redox titrations of disulfide/dithiol couples were performed using thiol labeling with 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS; Invitrogen) (Motohashi and Hisabori 2006). Wild-type and CAP/TCH apocytochromes c1 recombinant proteins (5 μg) were incubated separately under anaerobic conditions at 25° for 5 or 16 hr in 100 mm HEPES (pH 7.0) and various concentrations of reduced and oxidized DTT at a total DTT concentration of 5 mm. Ambient potential (Eh) values for different DTTred/DTTox ratios were calculated from the two-electron Nernst equation, using the literature value for the midpoint potential of DTT at pH 7.0 (Hutchison and Ort 1995). Incubations in the presence of 50 μm of CuCl2 or 5 mm reduced DTT served to produce fully oxidized or fully reduced protein controls, respectively. After incubation, samples were treated with 10% trichloroacetic acid (TCA) and allowed to stand on ice for 30 min. Protein precipitates were washed with 1% TCA and then with ice-cold acetone. Samples were resuspended in buffer containing 50 mm HEPES (pH 7.0), 2.5% SDS, and 10 mm AMS and incubated at 37° for 1 hr in an argon atmosphere. Reduced (AMS-labeled) and oxidized (nonlabeled) forms of apocytochromes c1 were separated by nonreducing SDS–PAGE on a 16% gel, stained with silver nitrate (Rabilloud et al. 1994), and quantified using Image J software (National Institutes of Health). Data were plotted and fitted to the Nernst equation for a single redox couple by iteration, using the solver of Excel (Microsoft). The midpoint potential (Em) was defined as a variable, and its value was taken after the target cell, containing the average quadratic variation between the theoretical curve and the experimental data, converged to its minimum value. Best fits were obtained for an n-value of 2 (i.e., for a two-electron redox reaction). All Em values reported correspond to the average of three independent titrations.
Mitochondrial protein preparation and analysis:
Mitochondria were purified from yeast strains grown in galactose medium as previously described (Bernard et al. 2005), and the protein concentration was measured using the Bradford reagent (Bio-Rad). For heme staining of mitochondrial c-type cytochromes, protein samples were reduced with 50 mm DTT on ice for 1 hr and separated at 4° by lithium dodecyl sulfate (LDS)–PAGE (Dutta and Henry 1990). Detection of holocytochromes c and c1 was performed on polyvinylidene difluoride membrane (PVDF; 0.45 μm) by the enhanced chemiluminescence (ECL) method (SuperSignal West Pico, Pierce) using the heme-associated peroxidase activity of holocytochromes c (Vargas et al. 1993). For immunoblotting, mitochondrial proteins were separated by SDS–PAGE and subsequently immobilized by electro-transfer to PVDF membranes. Polyclonal antibodies raised against cytochrome c (C. Koehler, University of California, Los Angeles), cytochrome c1 (C. Lemaire, Gif-sur-Yvette, France), CCHL (Bernard et al. 2005), CC1HL (Zollner et al. 1992), Cyc2p (Bernard et al. 2005), and Coq1 (Gin and Clarke 2005) were used for immunodetection. Bound antibodies were detected by horseradish peroxidase-conjugated secondary antibody (Bio-Rad) and ECL reagent (SuperSignal West Dura, Pierce).
RESULTS
A multicopy suppressor screen of the Δcyc2 cyt1-CAPCH mutant identifies CC1HL:
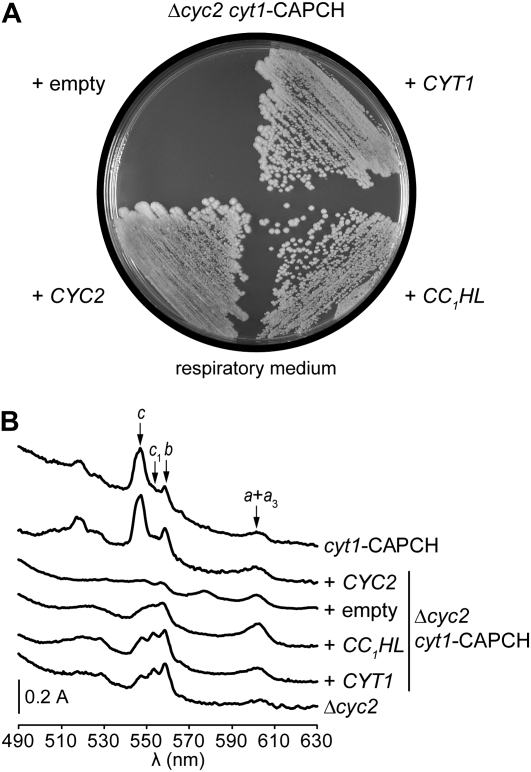
For convenience, the cyc2-null cyt1-34 mutant will be referred to as Δcyc2cyt1-CAPCH throughout the entire manuscript. To gain further insight into the function of Cyc2p, we undertook a search for multicopy suppressors able to alleviate the respiratory deficiency of the Δcyc2cyt1-CAPCH mutant. The respiratory-deficient phenotype of the Δcyc2cyt1-CAPCH strain is tight and characterized by a dual deficiency in holoforms of cytochrome c and c1 (Figures 1 and 2). Of 500,000 primary transformants, 87 respiratory-competent colonies were selected after a 10-day incubation on medium containing a respiratory substrate. Extended incubation gave rise to colonies in which respiratory proficiency was independent of the presence of the multicopy plasmid and probably caused by suppressor mutations. Diagnostic PCR amplification using CYC2- and CYT1-specific primers showed that of the 87 transformants, 44 carried a plasmid containing the CYC2 gene, whereas 36 harbored a plasmid with the cytochrome c1-encoding gene. Both genes were expected to be recovered, as the Δcyc2cyt1-CAPCH respiratory-deficient phenotype is synthetic (Figures 1A, 2A, and 3A). The remaining 7 transformants displayed a slower growth phenotype, and PCR amplification failed to reveal the presence of CYC2 or CYT1 genes in the transforming plasmids. Sequencing of the insert ends in one multicopy plasmid and subsequent diagnostic PCR revealed that all slow-growing transformants carry the gene encoding CC1HL. Spectral analysis showed that overexpression of CC1HL is able to partially restore holocytochromes c and c1 assembly (Figure 1B). This result is consistent with the restoration of the respiratory growth as both cytochromes c and c1 are required for respiration (Figure 1A). As expected, overexpression of CYT1 in Δcyc2cyt1-CAPCH resulted in levels of c-type cytochromes identical to that of a Δcyc2 strain while overexpression of CYC2 yielded a spectral phenotype identical to the one of the cyt1-CAPCH mutant (Figure 1B). We also demonstrated that overexpression of either CCHL- or apocytochrome c-encoding genes in Δcyc2cyt1-CAPCH does not restore cytochrome c or c1 assembly (not shown). We concluded that CC1HL acts as a multicopy suppressor that can partially compensate for the dual deficiency of the holocytochromes c and c1 in the Δcyc2cyt1-CAPCH mutant.
Figure 1.—
The CC1HL gene is a multicopy suppressor of the Δcyc2 cyt1-CAPCH mutant. (A) Respiratory growth of the Δcyc2 cyt1-CAPCH transformants. The cyc2-null cyt1-CAPCH transformants (YPH6-9C) carrying pFL44L (empty), pFL44L-CYT1 (+ CYT1), pFL44L-CYC2 (+ CYC2), and pFL44L-CC1HL (+ CC1HL) plasmids were grown on respiratory media containing ethanol/glycerol at 28° for 3 days. The synthetic respiratory-deficient phenotype of the Δcyc2 cyt1-CAPCH mutant cannot be suppressed by overexpression of the CYC1 or CYC7 genes encoding the isoforms 1 and 2 of cytochrome c, respectively, or by the CCHL gene (not shown). (B) Cytochrome absorption spectra of the Δcyc2 cyt1-CAPCH transformants. Low-temperature absorption spectra of cells grown in galactose were recorded with a Cary 400 spectrophotometer as previously described (Claisse et al. 1970). The arrows indicate the absorption maxima of the α-bands of cytochromes c (546 nm), c1 (552 nm), b (558 nm), and a + a3 (602 nm).
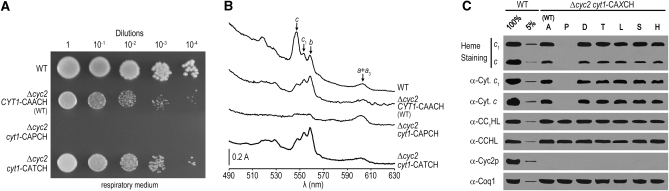
Figure 2.—
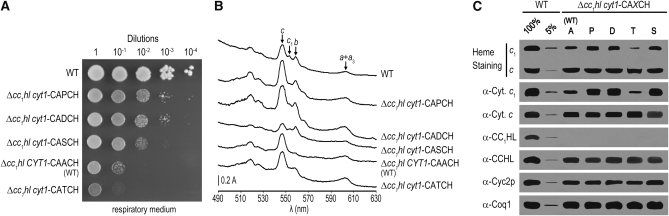
Mutations in cytochrome c1 heme-binding site suppress the Δcyc2 cyt1-CAPCH mutant. (A) Respiratory growth of the cyc2-null mutants. Ten-fold serial dilutions of wild-type (WT) (W303-1A), Δcyc2 CYT1-CAACH (YPH1), Δcyc2 cyt1-CAPCH (YPH6-9C), and Δcyc2 cyt1-CATCH (SSP2) strains were grown on ethanol/glycerol as respiratory substrates and incubated at 28° for 3 days. The respiratory phenotypes of Δcyc2 cyt1-CAXCH strains carrying the H, L, and S mutations in the cytochrome c1 heme-binding motif are equivalent to that of a Δcyc2 cyt1-CATCH strain (data not shown). (B) Cytochrome absorption spectra of the cyc2-null mutants. Spectrophotometric maxima of cytochromes c, c1, b, and a + a3 were detected as previously indicated (see Figure 1B). The spectral profiles of Δcyc2 strains carrying the cyt1 suppressor mutations D, H, L, or S are identical to that of a Δcyc2 cyt1-CATCH strain (data not shown). (C) Heme stain and immunodetection of mitochondrial c-type cytochromes and assembly proteins in the cyc2-null mutants. Mitochondrial proteins (85 μg) purified from cells grown in galactose medium were separated by LDS–PAGE for heme staining or by SDS–PAGE for immunoblotting as described in materials and methods. The cyt1-CADCH and cyt1-CAPCH were selected as suppressor mutations of the absence of CC1HL (Bernard et al. 2003). The Δcyc2 cyt1-CADCH, unlike Δcyc2 cyt1-CAPCH, does not display a respiratory-deficient phenotype (Bernard et al. 2005). Coq1 immunodetection serves as a loading control.
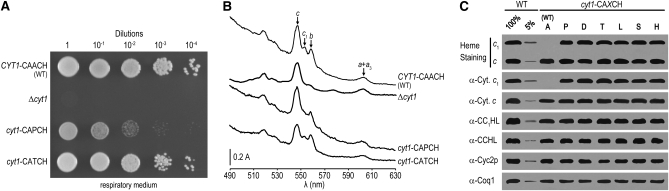
Figure 3.—
The suppressor mutations at the heme-binding motif do not impact cytochrome c1 maturation. (A) Respiratory growth of the cyt1 mutants. Ten-fold serial dilutions of a wild-type (WT) strain carrying the CYT1-CAACH allele (W303-1A), Δcyt1 (PHT3), cyt1-CAPCH (YPH71-14B), and cyt1-CATCH (YDM1-2A) strains were grown on respiratory medium containing ethanol/glycerol as described in Figure 2A. The respiratory phenotype of strains harboring cyt1-CA(D, L, S, or H)CH mutations is indistinguishable from the cyt1-CATCH or wild-type strains (data not shown). (B) Cytochrome absorbance spectra of the cyt1 mutants. The absorption maxima of cytochromes c, c1, b, and a + a3 were measured as previously mentioned (see Figure 1B). Absorbance spectra profiles for strains carrying cyt1-CA(D, L, S, or H)CH mutations are comparable to the cyt1-CATCH strain (data not shown). (C) Heme stain and immunodetection of mitochondrial c-type cytochromes and assembly factors in the cyt1 mutants. Mitochondrial proteins were prepared, separated, and visualized for heme staining and immunoblotting as described in materials and methods. Immunoblotting against Coq1 served as a loading control.
Suppressor mutations of the Δcyc2 cyt1-CAPCH mutant map to the cytochrome c1 heme-binding site:
We observed that Δcyc2cyt1-CAPCH could revert to respiratory proficiency, allowing us to isolate SSP (for Suppressor of Synthetic Phenotype) strains. Spontaneous independent suppressors (SSP1–SSP14) of the Δcyc2cyt1-CAPCH were isolated and further characterized. All SSP strains were restored to the same extent of respiratory growth (Figure 2A). Genetic analysis indicated that the suppressor mutation is monogenic and linked to the original mutations. Because CYC2 and CYT1 are genetically linked and the Δcyc2 allele corresponds to a complete deletion of the CYC2 gene, we reasoned that the suppressor mutation mapped to the cytochrome c1-encoding gene (CYT1). Sequencing of the CYT1 gene in the different SSP strains showed that a single-nucleotide change resulted in the alteration of the proline in the CAPCH heme-binding site to A, H, L, S, or T residues (Table 2). Note that SSP13 and SSP14 have reverted to a wild-type heme-binding site (CAACH), an expected finding as assembly of wild-type cytochrome c1 does not require Cyc2p (Bernard et al. 2005) (Figure 2, B and C). Analysis of the holoforms of c-type cytochromes of representative SSP strains carrying the CAHCH, CALCH, CASCH, and CATCH heme-binding sites reveals that holocytochrome c1 accumulation is restored to the wild-type level while the level of holocytochrome c is similar to that of the Δcyc2 mutant (Figure 2, B and C).
TABLE 2.
Sequences of cytochrome c1 heme-binding sites
| Strain | DNA sequence | Heme-binding motif |
|---|---|---|
| Wild type | TGT GCC GCC TGC CAT | CAACH |
| Δcyc2 cyt1-34 | TGT GCC CCC TGC CAT | CAPCH |
| SSP1,2 | TGT GCC ACC TGC CAT | CATCH |
| SSP4,5,8,10 | TGT GCC CAC TGC CAT | CAHCH |
| SSP6 | TGT GCC CTC TGC CAT | CALCH |
| SSP3,7,9,11,12 | TGT GCC TCC TGC CAT | CASCH |
| SSP13,14 | TGT GCC GCC TGC CAT | CAACH |
The suppressor mutations do not affect cytochrome c1 assembly:
The observation that the A-to-P mutation results in a slow respiratory growth associated with a decrease in holocytochrome c1 accumulation (Bernard et al. 2005) (Figure 3) prompted us to assess the impact of the cyt1-CAHCH, -CALCH, -CASCH, and -CATCH alleles in an otherwise wild-type background. Strains carrying cytochrome c1 with a CAHCH, CALCH, CASCH, or CATCH heme-binding site were constructed by genetic crosses and examined for respiratory competence and holocytochrome c assembly. All strains were found to have respiratory growth rates similar to that of the wild type (Figure 3A). Spectral and heme stain analyses showed that they were indistinguishable from wild type as far as holocytochrome c and c1 assembly is concerned (Figure 3, B and C). We concluded that, unlike the A-to-P mutation, the A-to -H, -L, -S, and -T substitutions in the heme-binding site have no impact on holocytochrome c1 assembly.
Redox titrations of apocytochromes c1 do not indicate a change in the midpoint potential of cysteines at the heme-binding site:
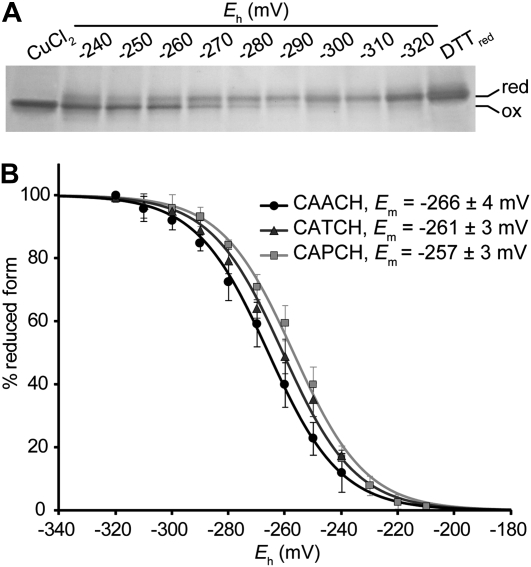
One possible scenario to account for the fact that CAPCH cytochrome c1 assembly is dependent upon Cyc2p is that the A-to-P mutation alters the redox chemistry of the cysteinyl thiols so that the apocytochrome c1 heme-binding site is present in a predominantly oxidized, disulfide form. In such a scenario, the cysteine thiols necessary for heme attachment are either not present or present only to a limited extent. To assess whether mutations in the heme-binding motif of cytochrome c1 might affect the redox chemistry of the cysteinyl thiols of the heme-binding motif in a manner that would make the oxidized disulfide state thermodynamically more stable, we chose to measure the midpoint redox potential (Em) of recombinant wild-type (CAACH) and mutant (CAPCH and CATCH) apocytochromes c1. This is the best quantitative measure of the thermodynamic propensity of a cysteine pair to be either in a reduced or in an oxidized state (Sevier and Kaiser 2002; Ortenberg and Beckwith 2003). It originally appeared likely that CAPCH apocytochrome c1 might be characterized by a midpoint potential value that was substantially more negative than that of the wild-type form, given our prediction that the A-to-P mutation produces a higher level of the oxidized form of the apoprotein. To test this hypothesis, redox titrations were carried out using recombinant forms of the apocytochrome c1 that corresponded to the soluble domain of the protein and did not contain cysteine residues other than the pair present at the heme-binding site. Measurements of redox potentials were carried out using DTTred/DTTox buffers to poise samples at defined ambient potential (Eh) values, prior to their treatment with the thiol modifier AMS (Motohashi and Hisabori 2006). AMS increases the molecular weight of a protein by 0.5 kDa per thiol modified, allowing the separation of the doubly modified, reduced form from the unmodified oxidized form by electrophoresis on a nonreducing SDS gel (Figure 4A). All titrations gave excellent fits to the Nernst equation for a single two-electron redox component, with Em values of −266 ± 4 mV, −261 ± 3 mV, and −257 ± 3 mV for the apocytochromes c1 carrying the CAACH (wild type), CATCH, and CAPCH variations of the heme-binding motif, respectively (Figure 4B). Because there was little difference between the midpoint potentials determined for these three forms of the apocytochrome c1, we concluded that the A, P, and T residues did not significantly impact the thermodynamic propensity of the heme-binding site to occur in a disulfide vs. dithiol form.
Figure 4.—
Determination of the redox midpoint potential of wild-type and mutant apocytochromes c1. Redox titrations of the dithiol/disulfide couple in apocytochrome c1 were carried out using DTT redox buffer as described in materials and methods. Redox equilibration (5 μg of apocytochromes c1 per reaction) was performed under anaerobic conditions prior to the AMS labeling. (A) Separation of oxidized and reduced forms of CAACH apocytochrome c1 under nonreducing SDS–PAGE. Oxidized (non-modified) and reduced (AMS modified) forms of wild-type proteins carrying a CAACH heme-binding motif were separated on a 16% nonreducing SDS gel and visualized by silver staining. As a control for fully oxidized or fully reduced forms, apocytochrome c1 was incubated in the presence of either 50 μm CuCl2 or 5 mm DTTred, respectively. (B) Oxidation-reduction titrations of wild-type and mutant apocytochromes c1. Oxidized (unmodified) and reduced (AMS modified) fractions of apoproteins for different ambient potentials were quantified by using Image J software. Percentages of the reduced form of apocytochrome c1 with a CAACH (wild type, solid circles), CATCH (darkly shaded triangles), and CAPCH (lightly shaded squares) heme-binding motif were plotted as a function of ambient potential (Eh). Lines represent fits of the data to a two-electron Nernst curve. Error bars correspond to the standard deviation calculated from three independent experiments.
The CASCH mutation suppresses the absence of CC1HL:
The cyt1-CAPCH allele and the cyt1-CADCH mutation were originally isolated in a screen for mutations enhancing the activity of CCHL toward apocytochrome c1 in the absence of its cognate lyase CC1HL (Bernard et al. 2003). To test whether H, L, S, and T mutations increase the activity of CCHL toward apocytochrome c1, we generated the Δcc1hl cyt1-CAHCH, -CALCH, -CASCH, and -CATCH strains. In such strains, CC1HL is absent, and apocytochrome c1 conversion to its corresponding holoform is completely dependent upon CCHL. While cytochrome c1 with a CASCH heme-binding domain could restore the respiratory growth in a Δcc1hl strain, cytochrome c1 with a CAHCH, CALCH, or CATCH heme-binding domain further diminished the ability of the Δcc1hl strain to grow on a respiratory substrate (Figure 5A). Consistent with the respiratory growth phenotype, the Δcc1hl cyt1-CASCH strain exhibits enhanced level of holocytochrome c1 while the Δcc1hl cyt1-CATCH strain accumulates lower level of holocytochrome c1 than the Δcc1hl strain (Figure 5, B and C). Note that the Δcc1hl strain displays a reduced level of respiratory growth that is attributed to the weak activity of CCHL toward apocytochrome c1 (Bernard et al. 2003). We concluded that the S mutation in the heme-binding site increased the activity of CCHL toward apocytochrome c1 while the H, L, and T residues decreased the activity of CCHL toward its noncognate substrate.
Figure 5.—
Impact of the mutations at the cytochrome c1 heme-binding motif in the absence of CC1HL. (A) Respiratory growth of the Δcc1hl mutants. Serial dilutions of wild-type (WT) (W303-1A), Δcc1hl cyt1-CAPCH (UV34), Δcc1hl, cyt1-CADCH (UV48), Δcc1hl cyt1-CASCH (YDM4-1B), Δcc1hl carrying the wild-type allele CAACH (SMY1), and Δcc1hl cyt1-CATCH (YDM1-1B) strains were grown on ethanol/glycerol as respiratory substrates as mentioned in Figure 2A. In a cc1hl-null context, the cyt1 mutants carrying the P, D (Bernard et al. 2003), or S (this work) mutations at the heme-binding motif are restored for respiratory growth when compared to a Δcc1hl. Δcc1hl cyt1-CATCH, -CAHCH, and -CALCH all display the same respiratory growth (not shown). (B) Cytochrome absorbance spectra of the Δcc1hl mutants. The absorption spectra of cytochromes c, c1, b, and a + a3 were detected as previously described (see Figure 1B). (C) Heme stain and immunodetection of mitochondrial c-type cytochromes and assembly proteins in the Δcc1hl mutants. Heme staining and protein immunodetection were performed as indicated in materials and methods. Coq1 immunodetection was used as a loading control.
Cyc2p is not required for CC1HL-dependent assembly of cytochrome c1:
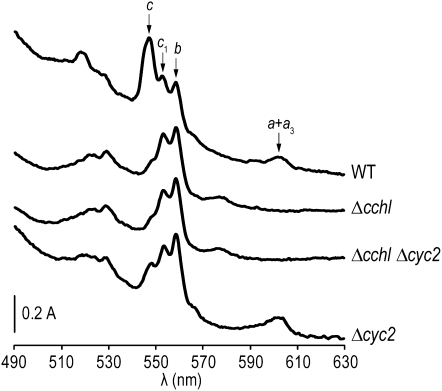
Earlier studies have established that the assembly of cytochrome c1 proceeds normally in the absence of CCHL, an indication that holocytochrome c1 is synthesized independently via its cognate heme lyase, CC1HL (Matner and Sherman 1982; Dumont et al. 1987; Stuart et al. 1990; Bernard et al. 2003). To assess whether Cyc2p function was also required for the assembly of cytochrome c1 via CC1HL, we constructed a cchl-null cyc2-null mutant and monitored the level of c-type cytochromes by spectra (Figure 6). As expected, the cchl-null cyc2-null strain is deficient in holocytochrome c due to the lack of its cognate heme lyase but retains a wild-type level of cytochrome c1, suggesting that CC1HL does not require the activity of Cyc2p for conversion of apocytochrome c1 to its corresponding holoform.
Figure 6.—
Cyc2p is not required for the CC1HL-dependent assembly of cytochrome c1. Whole-cell absorption spectra of wild type (WT) (W303-1A), Δcchl (SMY4), Δcchl Δcyc2 (YCT2-14C), and Δcyc2 (YPH1) strains were recorded as mentioned in Figure 1B. Note the absence of spectrally detectable cytochromes a + a3 in the Δcchl and Δcchl Δcyc2 mutants. This phenotype is not a specific trait of the cchl-null mutants but results from the loss of cytochrome oxidase assembly as a secondary effect due to the absence of holocytochrome c (Sherman et al. 1965; Pearce and Sherman 1995).
The function of Cyc2p is clearly redundant based on the fact that a cyc2-null mutant is not completely deficient in cytochrome c (Figure 2, B and C). Functional redundancy does not involve Mcr1p and Cbr1p, two proteins with mitochondrial localization that exhibit similarity to Cyc2p (Haucke et al. 1997; Sickmann et al. 2003; Bernard et al. 2005) (not shown).
DISCUSSION
Previous studies have identified Cyc2p, a pyridine nucleotide-dependent flavoprotein, as a redox component controlling the maturation of mitochondrial c-type cytochromes (Bernard et al. 2003, 2005). Genetic analyses revealed that Cyc2p is not absolutely required for c-type cytochrome maturation but becomes essential when the cytochrome c1 heme-binding site is modified from CAACH to CAPCH, an indication that the CXXCH motif could be the relevant target of Cyc2p action in vivo (Bernard et al. 2005). Our initial interpretation of this observation was that the A-to-P mutation altered the redox chemistry of the cysteinyl thiols so that the apocytochrome c1 CAPCH heme-binding site exists largely in an oxidized form (i.e., the cysteines form an intramolecular disulfide bond) and is no longer able to participate in the heme attachment without Cyc2p intervention. It is now well recognized that the nature of the residues between the cysteines at a CXXC redox motif influences the propensity of this motif to be in the oxidized (disulfide) or reduced form (dithiol). Examples come from the documented changes in midpoint potential caused by mutations of the residues between the two cysteines at the redox active site of thioredoxin (Krause et al. 1991; Lundstrom et al. 1992; Mossner et al. 1999), protein disulfide isomerase (Chivers et al. 1996) and DsbA oxido-reductase (Grauschopf et al. 1995). To better understand why Cyc2p is required for the assembly of cytochrome c1 with a CAPCH heme-binding site, we isolated secondary mutations that enable Cyc2p-independent assembly of cytochrome c1. All the bypass mutations that we isolated change the proline at the cytochrome c1 heme-binding motif to A (wild-type), H, L, S, or T residues and restored a wild-type level of holocytochrome c1 assembly (Figure 2). As stated above, we had originally interpreted this result in the context of a change in thiol chemistry at the heme-binding site and postulated that cytochrome c1 with a CAHCH, CALCH, CASCH, or CATCH is able to be assembled because the presence of a H, L, S, and T residue instead of P results in a shift of the equilibrium of the CXXCH heme-binding site toward the reduced form (i.e., dithiol) that is competent for the heme ligation reaction. Measurements of midpoint potentials reveal that there is no significant difference between wild-type, CAPCH, and CATCH cytochromes c1, an indication that, at redox equilibrium, the thermodynamic stability of the disulfide bond at the heme-binding site is not influenced by the P and T mutations (Figure 4). Hence it is unlikely that the Cyc2p-dependent assembly of CAPCH cytochrome c1 can be explained by a change in the equilibrium dithiol–disulfide ratio of the apocytochrome c1 heme-binding site. However, because the midpoint potential reflects only the redox state at thermodynamic equilibrium (Sevier and Kaiser 2002), it is conceivable that the P residue has altered the reactivity of cysteines in the CXXCH motif so that Cyc2p activity is required for the heme attachment to apocytochrome c1.
We had previously shown that apocytochrome c1 is a substrate of both CC1HL and CCHL (Bernard et al. 2003). While we demonstrate in this work that Cyc2p is not required for the CC1HL-dependent assembly of apocytochrome c1, its activity is absolutely essential for the CCHL-dependent assembly of apocytochrome c1 (Bernard et al. 2003). In the Δcyc2cyt1-CAPCH mutant, the holocytochrome c1-deficient phenotype can be explained by the fact that both CCHL- and CC1HL-dependent assembly pathways for apocytochrome c1 are inactive. Whereas the absence of Cyc2p can account for the lack of cytochrome c1 assembly via the CCHL pathway, we also envision that CC1HL-dependent assembly of cytochrome c1 is abolished in the Δcyc2cyt1-CAPCH mutant due to the inability of CC1HL to interact with CAPCH apocytochrome c1. This is supported by the fact that the level of assembled CAPCH holocytochrome c1 is not influenced by the presence or the absence of CC1HL (Figures 3 and 5), an indication that CAPCH holocytochrome c1 assembly is dependent only on CCHL. Because the A-to-P modification in cytochrome c1 was recovered in a search for mutations that suppress the absence of CC1HL (Bernard et al. 2003), it is conceivable that the P residue enhances the activity of CCHL toward apocytochrome c1 while simultaneously preventing the CC1HL from acting on its cognate substrate. The finding that the H, L, S, and T mutations (Figure 2) and CC1HL overexpression (Figure 1) suppress the cytochrome c1 defect in a Δcyc2cyt1-CAPCH context can be explained by a restoration of the CC1HL-dependent assembly of cytochrome c1, presumably via enhanced interaction between CC1HL and its cognate apocytochrome c1 substrate.
The observation that the Δcyc2cyt1-CAPCH mutant also displays a cytochrome c deficiency suggests that holocytochrome c assembly is also blocked as a result of an A-to-P mutation in the heme-binding site of cytochrome c1. As apocytochrome c can be acted upon only by CCHL, we postulate that CCHL activity toward apocytochrome c is inactivated in the Δcyc2cyt1-CAPCH strain. Two possible scenarios can be envisioned to account for this phenotype. In one model, CCHL can no longer act on apocytochrome c because CAPCH apocytochrome c1 is trapped in a complex with the lyase. However, we view this model as unlikely on the basis of our observation that overexpression of CCHL in the Δcyc2cyt1-CAPCH strain does not relieve the block in holocytochrome c assembly (not shown). The observation that the apoform of the molecule is not immunodetected in the Δcyc2cyt1-CAPCH strain suggests that it has undergone proteolytic degradation, which does not appear consistent with a model in which apocytochrome c1 is trapped in a stable CCHL complex. Instead, we favor an alternative scenario in which, in the absence of Cyc2p, CAPCH apocytochrome c1 is engaged in a transient interaction with CCHL resulting in a deactivated enzyme that cannot assemble apocytochrome c. Note that the Δcyc2 Δcc1hl strain also displays a dual deficiency in cytochromes c and c1, similarly to the Δcyc2cyt1-CAPCH mutant (Bernard et al. 2005). Hence, we postulate that this deactivation is Cyc2p-dependent and takes places in situations where apocytochrome c1 maturation is dependent only on CCHL (i.e., when the apocytochrome c1 heme-binding site carries the A-to-P mutation or in the absence of CC1HL).
The proposal that the H, L, S, and T residues restore the CC1HL-dependent assembly of cytochrome c1 further underscores the critical role of this residue in the maturation process. Interestingly, while the S residue at this position enhances CCHL-dependent assembly of cytochrome c1, the presence of H, L, or T further decreases the intrinsic activity of CCHL toward apocytochrome c1 (Figure 5). This suggests that the identity of the second intervening amino acid in the CXXCH motif is crucial in modulating the CCHL-dependent assembly of cytochrome c1. The fact that CAPCH apocytochrome c1 has become dependent on CCHL alone for its assembly indicates that the P residue is essential in determining the CCHL- vs. CC1HL-dependent assembly of cytochrome c1. It is likely that the nature of the residue is a determinant for enzyme–substrate interaction, but it is also possible that CCHL- or CC1HL-catalyzed assembly requires a specific residue at this position for the enzymatic reaction.
Acknowledgments
We thank R. Lamb for grammatical and stylistic suggestions. This work is supported by the Muscular Dystrophy Association (grant 4727) and the National Science Foundation (grant MCB-0920062) to P.H. and by a grant from the Robert A. Welch Foundation (D-0710) to D.B.K. V.C. is supported by an American Heart Association post-doctoral fellowship. D.G.B. was supported by a Ministère de l'Education Nationale, de la Recherche et de la Technologie Fellowship. This work is sponsored by the “action CNRS–US CNRS 2008-2010” grant from Centre National de la Recherche Scientifique.
References
- Allen, J. W., O. Daltrop, J. M. Stevens and S. J. Ferguson, 2003. C-type cytochromes: diverse structures and biogenesis systems pose evolutionary problems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, M. L., F. Lacroute and D. Botstein, 1979. Evidence for transcriptional regulation of orotidine-5′-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc. Natl. Acad. Sci. USA 76 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardischewsky, F., and C. G. Friedrich, 2001. Identification of ccdA in Paracoccus pantotrophus GB17: disruption of ccdA causes complete deficiency in c-type cytochromes. J. Bacteriol. 183 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett, C. S., J. A. Loughman, K. A. Karberg, G. M. Donato, W. E. Goldman et al., 2000. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol. Microbiol. 38 465–481. [DOI] [PubMed] [Google Scholar]
- Bernard, D. G., S. T. Gabilly, G. Dujardin, S. Merchant and P. P. Hamel, 2003. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 278 49732–49742. [DOI] [PubMed] [Google Scholar]
- Bernard, D. G., S. Quevillon-Cheruel, S. Merchant, B. Guiard and P. P. Hamel, 2005. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J. Biol. Chem. 280 39852–39859. [DOI] [PubMed] [Google Scholar]
- Bonnard, G., V. Corvest, E. H. Meyer and P. Hamel, 2010. Redox processes controlling the biogenesis of c-type cytochromes. Antioxid. Redox Signal. (in press). [DOI] [PubMed]
- Carlson, M., and D. Botstein, 1982. Two differentially regulated mRNAs with different 5′ ends encode secreted with intracellular forms of yeast invertase. Cell 28 145–154. [DOI] [PubMed] [Google Scholar]
- Chen, D. C., B. C. Yang and T. T. Kuo, 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21 83–84. [DOI] [PubMed] [Google Scholar]
- Chivers, P. T., M. C. Laboissiere and R. T. Raines, 1996. The CXXC motif: imperatives for the formation of native disulfide bonds in the cell. EMBO J. 15 2659–2667. [PMC free article] [PubMed] [Google Scholar]
- Claisse, M. L., G. A. Pere-Aubert, L. P. Clavilier and P. P. Slonimski, 1970. Method for the determination of cytochrome concentrations in whole yeast cells Eur. J. Biochem. 16 430–438 (in French). [DOI] [PubMed] [Google Scholar]
- Deshmukh, M., G. Brasseur and F. Daldal, 2000. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol. Microbiol. 35 123–138. [DOI] [PubMed] [Google Scholar]
- Dujardin, G., P. Pajot, O. Groudinsky and P. P. Slonimski, 1980. Long range control circuits within mitochondria and between nucleus and mitochondria. I. Methodology and phenomenology of suppressors. Mol. Gen. Genet. 179 469–482. [DOI] [PubMed] [Google Scholar]
- Dumont, M. E., J. F. Ernst, D. M. Hampsey and F. Sherman, 1987. Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 6 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont, M. E., J. B. Schlichter, T. S. Cardillo, M. K. Hayes, G. Bethlendy et al., 1993. CYC2 encodes a factor involved in mitochondrial import of yeast cytochrome c. Mol. Cell. Biol. 13 6442–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, C., and H. L. Henry, 1990. Detection of hemoprotein peroxidase activity on polyvinylidene difluoride membrane. Anal. Biochem. 184 96–99. [DOI] [PubMed] [Google Scholar]
- Erlendsson, L. S., and L. Hederstedt, 2002. Mutations in the thiol-disulfide oxidoreductases BdbC and BdbD can suppress cytochrome c deficiency of CcdA-defective Bacillus subtilis cells. J. Bacteriol. 184 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlendsson, L. S., R. M. Acheson, L. Hederstedt and N. E. Le Brun, 2003. Bacillus subtilis ResA is a thiol-disulfide oxidoreductase involved in cytochrome c synthesis. J. Biol. Chem. 278 17852–17858. [DOI] [PubMed] [Google Scholar]
- Fabianek, R. A., H. Hennecke and L. Thöny-Meyer, 1998. The active-site cysteines of the periplasmic thioredoxin-like protein CcmG of Escherichia coli are important but not essential for cytochrome c maturation in vivo. J. Bacteriol. 180 1947–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feissner, R. E., C. S. Beckett, J. A. Loughman and R. G. Kranz, 2005. Mutations in cytochrome assembly and periplasmic redox pathways in Bordetella pertussis. J. Bacteriol. 187 3941–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin, P., and C. F. Clarke, 2005. Genetic evidence for a multi-subunit complex in coenzyme Q biosynthesis in yeast and the role of the Coq1 hexaprenyl diphosphate synthase. J. Biol. Chem. 280 2676–2681. [DOI] [PubMed] [Google Scholar]
- Grauschopf, U., J. R. Winther, P. Korber, T. Zander, P. Dallinger et al., 1995. Why is DsbA such an oxidizing disulfide catalyst? Cell 83 947–955. [DOI] [PubMed] [Google Scholar]
- Hamel, P., C. Lemaire, N. Bonnefoy, P. Brivet-Chevillotte and G. Dujardin, 1998. Mutations in the membrane anchor of yeast cytochrome c1 compensate for the absence of Oxa1p and generate carbonate-extractable forms of cytochrome c1. Genetics 150 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel, P., V. Corvest, P. Giege and G. Bonnard, 2009. Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim. Biophys. Acta 1793 125–138. [DOI] [PubMed] [Google Scholar]
- Haucke, V., C. S. Ocana, A. Honlinger, K. Tokatlidis, N. Pfanner et al., 1997. Analysis of the sorting signals directing NADH-cytochrome b5 reductase to two locations within yeast mitochondria. Mol. Cell. Biol. 17 4024–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., and F. Winston, 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57 267–272. [DOI] [PubMed] [Google Scholar]
- Hutchison, R. S., and D. R. Ort, 1995. Measurement of equilibrium midpoint potentials of thiol/disulfide regulatory groups on thioredoxin-activated chloroplast enzymes. Methods Enzymol. 252 220–228. [DOI] [PubMed] [Google Scholar]
- Inaba, K., 2009. Disulfide bond formation system in Escherichia coli. J. Biochem. 146 591–597. [DOI] [PubMed] [Google Scholar]
- Kadokura, H., and J. Beckwith, 2010. Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid. Redox Signal. (in press). [DOI] [PMC free article] [PubMed]
- Kadokura, H., F. Katzen and J. Beckwith, 2003. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 72 111–135. [DOI] [PubMed] [Google Scholar]
- Kranz, R. G., C. Richard-Fogal, J. S. Taylor and E. R. Frawley, 2009. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 73 510–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, G., J. Lundstrom, J. L. Barea, C. Pueyo de la Cuesta and A. Holmgren, 1991. Mimicking the active site of protein disulfide-isomerase by substitution of proline 34 in Escherichia coli thioredoxin. J. Biol. Chem. 266 9494–9500. [PubMed] [Google Scholar]
- Lundstrom, J., G. Krause and A. Holmgren, 1992. A Pro to His mutation in active site of thioredoxin increases its disulfide-isomerase activity 10-fold. New refolding systems for reduced or randomly oxidized ribonuclease. J. Biol. Chem. 267 9047–9052. [PubMed] [Google Scholar]
- Mapller, M., and L. Hederstedt, 2006. Role of membrane-bound thiol-disulfide oxidoreductases in endospore-forming bacteria. Antioxid. Redox Signal. 8 823–833. [DOI] [PubMed] [Google Scholar]
- Matner, R. R., and F. Sherman, 1982. Differential accumulation of two apo-iso-cytochromes c in processing mutants of yeast. J. Biol. Chem. 257 9811–9821. [PubMed] [Google Scholar]
- Monika, E. M., B. S. Goldman, D. L. Beckman and R. G. Kranz, 1997. A thioreduction pathway tethered to the membrane for periplasmic cytochromes c biogenesis: in vitro and in vivo studies. J. Mol. Biol. 271 679–692. [DOI] [PubMed] [Google Scholar]
- Mossner, E., M. Huber-Wunderlich, A. Rietsch, J. Beckwith, R. Glockshuber et al., 1999. Importance of redox potential for the in vivo function of the cytoplasmic disulfide reductant thioredoxin from Escherichia coli. J. Biol. Chem. 274 25254–25259. [DOI] [PubMed] [Google Scholar]
- Motohashi, K., and T. Hisabori, 2006. HCF164 receives reducing equivalents from stromal thioredoxinacross the thylakoid membrane and mediates reduction of target proteins in the thylakoid lumen. J. Biol. Chem. 281 35039–35047. [DOI] [PubMed] [Google Scholar]
- Nakamoto, S. S., P. Hamel and S. Merchant, 2000. Assembly of chloroplast cytochromes b and c. Biochimie 82 603–614. [DOI] [PubMed] [Google Scholar]
- Ortenberg, R., and J. Beckwith, 2003. Functions of thiol-disulfide oxidoreductases in E. coli: redox myths, realities, and practicalities. Antioxid. Redox Signal. 5 403–411. [DOI] [PubMed] [Google Scholar]
- Pearce, D. A., and F. Sherman, 1995. Degradation of cytochrome oxidase subunits in mutants of yeast lacking cytochrome c and suppression of the degradation by mutation of yme1. J. Biol. Chem. 270 20879–20882. [DOI] [PubMed] [Google Scholar]
- Pearce, D. A., T. S. Cardillo and F. Sherman, 1998. Cyc2p is required for maintaining ionic stability and efficient cytochrome c import and mitochondrial function in Saccharomyces cerevisiae. FEBS Lett. 439 307–311. [DOI] [PubMed] [Google Scholar]
- Prakash, S. K., T. A. Cormier, A. E. McCall, J. J. Garcia, R. Sierra et al., 2002. Loss of holocytochrome c-type synthetase causes the male lethality of X-linked dominant micro-phthalmia with linear skin defects (MLS) syndrome. Hum. Mol. Genet. 11 3237–3248. [DOI] [PubMed] [Google Scholar]
- Rabilloud, T., L. Vuillard, C. Gilly and J. J. Lawrence, 1994. Silver-staining of proteins in polyacrylamide gels: a general overview. Cell. Mol. Biol. (Noisy-le-grand) 40 57–75. [PubMed] [Google Scholar]
- Saint-Georges, Y., N. Bonnefoy, J. P. di Rago, S. Chiron and G. Dujardin, 2002. A pathogenic cytochrome b mutation reveals new interactions between subunits of the mitochondrial bc1 complex. J. Biol. Chem. 277 49397–49402. [DOI] [PubMed] [Google Scholar]
- Sambongi, Y., and S. J. Ferguson, 1994. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase-like protein. FEBS Lett. 353 235–238. [DOI] [PubMed] [Google Scholar]
- Sanchez, N. S., D. A. Pearce, T. S. Cardillo, S. Uribe and F. Sherman, 2001. Requirements of Cyc2p and the porin, Por1p, for ionic stability and mitochondrial integrity in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 392 326–332. [DOI] [PubMed] [Google Scholar]
- Sanders, C., S. Turkarslan, D.-W. Lee and F. Daldal, 2010. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 18 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., and R. D. Gietz, 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16 339–346. [DOI] [PubMed] [Google Scholar]
- Schwarz, Q. P., and T. C. Cox, 2002. Complementation of a yeast CYC3 deficiency identifies an X-linked mammalian activator of apocytochrome c. Genomics 79 51–57. [DOI] [PubMed] [Google Scholar]
- Setterdahl, A. T., B. S. Goldman, M. Hirasawa, P. Jacquot, A. J. Smith et al., 2000. Oxidation-reduction properties of disulfide-containing proteins of the Rhodobacter capsulatus cytochrome c biogenesis system. Biochemistry 39 10172–10176. [DOI] [PubMed] [Google Scholar]
- Sevier, C. S., and C. A. Kaiser, 2002. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 3 836–847. [DOI] [PubMed] [Google Scholar]
- Sherman, F., H. Taber and W. Campbell, 1965. Genetic determination of iso-cytochromes c in yeast. J. Mol. Biol. 13 21–39. [DOI] [PubMed] [Google Scholar]
- Sickmann, A., J. Reinders, Y. Wagner, C. Joppich, R. Zahedi et al., 2003. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA 100 13207–13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, R. A., D. W. Nicholson, U. Wienhues and W. Neupert, 1990. Import of apocytochrome c into the mitochondrial intermembrane space along a cytochrome c1 sorting pathway. J. Biol. Chem. 265 20210–20219. [PubMed] [Google Scholar]
- Thöny-Meyer, L., 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61 337–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkarslan, S., C. Sanders, S. Ekici and F. Daldal, 2008. Compensatory thio-redox interactions between DsbA, CcdA and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation. Mol. Microbiol. 70 652–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, C., A. G. McEwan and J. A. Downie, 1993. Detection of c-type cytochromes using enhanced chemiluminescence. Anal. Biochem. 209 323–326. [DOI] [PubMed] [Google Scholar]
- Zollner, A., G. Rodel and A. Haid, 1992. Molecular cloning and characterization of the Saccharomyces cerevisiae CYT2 gene encoding cytochrome-c1-heme lyase. Eur. J. Biochem. 207 1093–1100. [DOI] [PubMed] [Google Scholar]