Abstract
Replication of the Escherichia coli chromosome usually initiates at a single origin (oriC) under control of DnaA. Two forks are established and move away in opposite directions. Replication is completed when these meet in a broadly defined terminus area half way around the circular chromosome. RecG appears to consolidate this arrangement by unwinding D-loops and R-loops that PriA might otherwise exploit to initiate replication at other sites. It has been suggested that without RecG such replication generates 3′ flaps as the additional forks collide and displace nascent leading strands, providing yet more potential targets for PriA. Here we show that, to stay alive, cells must have either RecG or a 3′ single-stranded DNA (ssDNA) exonuclease, which can be exonuclease I, exonuclease VII, or SbcCD. Cells lacking all three nucleases are inviable without RecG. They also need RecA recombinase and a Holliday junction resolvase to survive rapid growth, but SOS induction, although elevated, is not required. Additional requirements for Rep and UvrD are identified and linked with defects in DNA mismatch repair and with the ability to cope with conflicts between replication and transcription, respectively. Eliminating PriA helicase activity removes the requirement for RecG. The data are consistent with RecG and ssDNA exonucleases acting to limit PriA-mediated re-replication of the chromosome and the consequent generation of linear DNA branches that provoke recombination and delay chromosome segregation.
REPLICATION of the Escherichia coli chromosome initiates at a single origin (oriC) under the control of DnaA (Messer 2002). Two forks are established, which then move round the circular chromosome in opposite directions. Duplication of the chromosome is achieved when they meet in a broadly defined terminus area flanked by polar sequences (ter) that when bound by the Tus terminator protein allow forks to enter but not leave this area (Mulcair et al. 2006; Duggin et al. 2008). Thus, the chromosome is divided into two replichores within each of which replication proceeds in a polar fashion from oriC toward ter. However, the two replisome complexes meeting within the terminus area may not be those assembled at oriC, but new complexes assembled following the rescue of stalled or damaged forks (Gabbai and Marians 2010).
Studies by Kogoma and co-workers showed that this highly evolved replichore arrangement is compromised when DnaA-independent stable DNA replication (SDR) is initiated via PriA-mediated DnaB loading and replisome assembly at sites other than oriC (Kogoma 1997). PriA facilitates DnaB loading at stalled forks, D-loops, and R-loops, potentially enabling replication to initiate wherever such structures arise (Marians 2000; Sandler and Marians 2000; Heller and Marians 2006b; Michel et al. 2007; Gabbai and Marians 2010). Kogoma and co-workers identified a constitutive form of SDR (cSDR), which they proposed to initiate at R-loops, and distinguished it from an inducible form (iSDR), which is triggered in cells exposed to genotoxic agents and characterized by its dependence on RecBCD enzyme (Kogoma 1997). cSDR is elevated in the absence of RecG or RNase HI (Asai and Kogoma 1994; Hong et al. 1995). These two proteins provide different ways of eliminating R-loops. RecG is a double-stranded DNA (dsDNA) translocase and dissociates the RNA from the structure by catalyzing branch migration whereas RNase HI digests the RNA from the RNA:DNA hybrid (Hong et al. 1995; Vincent et al. 1996; Fukuoh et al. 1997; McGlynn et al. 1997; Singleton et al. 2001). Strains lacking both proteins are inviable, indicating that excessive levels of SDR may be harmful (Hong et al. 1995). Following UV irradiation, which triggers iSDR, ΔrecG cells show a very extended and PriA helicase-dependent delay in chromosome segregation and cell division (Rudolph et al. 2009a). The majority of the DNA synthesis detected during this period is DnaA independent and associated with an increase in the number of replication forks traversing the chromosome. It can lead to replication of both origin and terminus areas of the chromosome and of all regions in between. However, it can also lead to disproportionate amplification of some chromosomal areas and to the accumulation of branched DNA resistant to cleavage by a Holliday junction resolvase (Rudolph et al. 2009a,b).
Although SDR disturbs the replichore arrangement, it is not obvious why this should have such dramatic effects in the absence of RecG. We have suggested that by increasing the number of replication fork collisions, SDR may trigger repeated cascades of chromosome re-replication and recombination and that by limiting SDR and dissociating recombination intermediates RecG reduces the likelihood of such pathology (Rudolph et al. 2009a).
Exactly what happens when forks meet in the terminus area is not known, although it is generally assumed that the replisome components dissociate as any remaining gaps are filled in and the nascent strands are finally sealed by DNA ligase (Figure 1A, i–iii). However, the priming of SDR on either strand at sites other than oriC means some forks will now meet outside of the normal termination zone (Figure 1B, i) (Kogoma 1997). Studies of DNA replication in vitro raised the possibility that without Tus to arrest forks at ter, the replisome of one fork might sometimes displace the 3′ end of the nascent leading strand of the fork coming in the other direction (Hiasa and Marians 1994). If such displacement were to occur in vivo, it would generate a 3′ flap (Figure 1B, ii). Unless removed, the exposed single-stranded DNA (ssDNA) might provide a template on which the RecFOR proteins could establish a RecA filament, thus provoking recombination (Umezu et al. 1993; Morimatsu and Kowalczykowski 2003). Alternatively, the branch point might provide a substrate that PriA could exploit with the aid of its helicase activity to load DnaB and initiate re-replication of the DNA, with leading strand synthesis primed perhaps via DnaB–DnaG interactions (Figure 1C, i–iii) (Heller and Marians 2006b). Depending on which fork had its leading strand displaced, the new fork would move either toward oriC or toward ter, generating DNA branches with duplex ends that provoke RecBCD-mediated recombination, thus establishing yet more new forks (Figure 1C, iv and v). Pathological cascades of this nature may explain the over-replication of DNA observed in vivo in the absence of Tus/ter control (Krabbe et al. 1997; Markovitz 2005).
Figure 1.—
Models depicting possible outcomes of replication fork collision [adapted from Rudolph et al (2009b)]. (A) Fork merging and nascent strand ligation. (B) Pathological replication resulting from unscheduled replication fork collisions or during normal termination: (B, i) Schematic of the E. coli chromosome showing normal replication from oriC and the presence of several additional replication forks initiated as a result of SDR induction. The opposed arrowheads indicate the positions of unscheduled fork collisions outside of the normal termination zone bounded by Tus-ter (for simplicity, only two ter sites are depicted). (B, ii) Nascent strand displacement following unscheduled collisions triggered by SDR or, at a lower frequency, in the absence of SDR. (C, i–v) Pathological, PriA helicase-dependent replication in the absence of RecG generates a dsDNA branch that can provoke recombination. (D) Termination achieved via a 5′ ssDNA exonuclease after RecG converts a 3′ flap to a 5′ flap. (E) Termination achieved via a 3′ ssDNA exonuclease. (F) Pathological, PriA helicase-independent replication in the presence of RecG.
A 3′ flap might be eliminated in wild-type cells by 3′–5′ ssDNA exonucleases or converted to a 5′ flap by branch migration and then eliminated by 5′–3′ ssDNA exonucleases (Figure 1, D and E). RecG is well suited to carry out the conversion as it has a particularly high affinity for 3′ flap structures and very efficiently unwinds the strand ending 5′ at the branch point (McGlynn and Lloyd 2001; Tanaka and Masai 2006). Without RecG, the initial number of SDR events initiated would be increased, leading to even higher levels of unscheduled fork collisions and therefore of 3′ flaps. In the absence of RecG, these flaps would also have a longer half-life, increasing the opportunity for their targeting by PriA or for the loading of RecA. Furthermore, without RecG, any D-loops established by subsequent recombination would be stabilized, further increasing the likelihood of perpetuating cycles of fork collisions and re-replication.
According to this scenario, 3′–5′ ssDNA exonucleases might be rather vital in the absence of RecG. In this article we show that the presence of exonuclease I (ExoI), exonuclease VII (ExoVII), or the SbcCD nuclease, all of which can digest ssDNA from 3′ ends, is needed to keep ΔrecG cells alive. This requirement can be overcome by eliminating the helicase activity of PriA, consistent with the idea that a major function of RecG is to curb pathological replication of the chromosome. The presence of at least one of these three enzymes is also needed to help keep rep and uvrD cells alive, but for different reasons consistent with ssDNA exonucleases having multiple roles in DNA replication and repair.
MATERIALS AND METHODS
Strains:
Bacterial strains are listed in supporting information, Table S1. All constructs used for synthetic lethality assays are based on E. coli K-12 MG1655 ΔlacIZYA strains carrying derivatives of pRC7 (Bernhardt and De Boer 2004). The quiescent rusA gene was activated to express the RusA Holliday junction resolvase using constructs carrying rus-2, an IS10 insertion upstream of the coding sequence (Mahdi et al. 1996). Chromosomal genes were inactivated using Tn10 or kan insertions, conferring resistance to tetracycline (Tcr) and kanamycin (Kmr), respectively, or with deletions tagged with sequences encoding resistance to chloramphenicol (Cmr; cat), kanamycin (kan), trimethoprim (Tmr; dhfr), apramycin (Aprar; apra), or spectinomycin (Spcr; spc) (Mahdi et al. 2006; Zhang et al. 2010). New deletion alleles of xseA (ΔxseA∷dhfr; ΔxseA∷cat), recA (ΔrecA∷spc; ΔrecA∷cat; ΔrecA∷kan), recQ (ΔrecQ∷apra), uvrD (ΔuvrD∷cat), and sbcCD (ΔsbcCD∷spc) were made using the one-step gene inactivation method of Datsenko and Wanner (2000). The ΔuvrD, ΔrecA, and ΔxseA alleles remove all but 39, 45, and 48 bp, respectively, from the 5′ and 3′ ends of the coding sequence. For the sbcCD deletion, 50 bp at the beginning of sbcD and 100 bp at the end of sbcC were retained.
Plasmids:
pRC7 is a low-copy-number, mini-F derivative of the lac+ construct pFZY1 (Bernhardt and De Boer 2004). pJJ100 (recG+), pAM375 (recB+), pAM390 (ruvABC+), and pAM409 (recG+ ruvABC+) are derivatives of pRC7 encoding the wild-type genes indicated. Their construction has been described elsewhere (Mahdi et al. 2006; Zhang et al. 2010). pAM401 (sbcCD+) and pAM490 (rnhA+) are also derivatives of pRC7. In each case, the indicated wild-type coding sequence was PCR amplified from MG1655 using 5′ and 3′ primers incorporating ApaI sites, and the product was cloned into the ApaI site within the lacIq gene of pRC7. The inserts are transcribed in the same orientation as the disrupted lacIq.
Media and general methods:
LB broth and 56/2 minimal salts media and methods for monitoring cell growth and for strain construction by P1vir-mediated transduction have been cited (Al-Deib et al. 1996; McGlynn and Lloyd 2000; Trautinger et al. 2005). The incidence of spontaneous resistance to rifampicin (rpoB mutants) was determined by spreading 100-μl samples of broth cultures grown to ∼2 × 109 cells/ml on LB agar plates supplemented with rifampicin at a final concentration of 50 μg/ml, which were then incubated overnight. Wild-type strain MG1655 typically yields <10 resistant colonies under these conditions whereas derivatives lacking a functional MutHLS system usually yield several hundred (mutator phenotype).
Measuring sensitivity to DNA damage:
Sensitivity to UV light and ionizing radiation was measured using exponential-phase cells grown to an A650 of 0.4 (1–2 × 108 cells/ml for strain MG1655). Samples of appropriate dilutions were irradiated on the surface of LB agar plates and survivors were scored after 18–24 hr incubation. Survival data are means from at least two, and usually three to six, independent experiments. Errors (SE) range between 5% and 15% of the mean. Sensitivity to mitomycin C was determined by growing cultures to an A650 of 0.4 and spotting 10 μl of serial 10-fold dilutions from 10−1 to 10−5 on LB agar with or without mitomycin C at a final concentration of 0.5 μg/ml and incubating at 37°. Plates were photographed after 24 hr incubation, unless stated otherwise. Sensitivity to 2-aminopurine (2-AP; Sigma) was determined by the same method, using LB agar containing 2-AP at a final concentration of 300 μg/ml.
SOS induction:
SOS induction was analyzed using sulA(sfiA)∷lacZ fusion strains. Cultures were grown in broth to an A650 of 0.3 and split in two before adding mitomycin C to one half to a final concentration of 1 μg/ml. Incubation was then continued for 1 hr, and samples were assayed for β-galactosidase activity as described (Miller 1972).
Synthetic lethality assays:
The rationale for synthetic lethality assays has been described (Bernhardt and De Boer 2004; Mahdi et al. 2006). Essentially, a wild-type gene of interest is cloned in pRC7, a lac+ mini-F plasmid that is rapidly lost, and used to cover a null mutation in the chromosome in a Δlac background. A mutation in another gene of interest is then introduced into the chromosome. If the double mutant is viable, the plasmid-free cells segregated during culture will form Lac− colonies on agar plates. If synthetically lethal, they will fail to grow and only Lac+ colonies formed by cells retaining the plasmid will be observed. When viability is reduced but not eliminated, the colonies formed by cells retaining the plasmid are notably larger than those formed by plasmid-free cells. To record the phenotype, cultures of strains carrying the relevant pRC7 derivatives were grown overnight in LB broth containing ampicillin to maintain plasmid selection, diluted 80-fold in LB broth, and grown without ampicillin selection to an A650 of 0.4 before spreading dilutions on LB agar or 56/2 glucose minimal salts agar supplemented with X-gal and IPTG. Plates were photographed and scored after 48 hr (LB agar) or 72 hr (56/2 agar) at 37°, unless stated otherwise. Plasmid-free cells forming small white colonies were re-streaked to see if they could be subcultured, and the streaked plates were photographed after incubation at 37° for 24–48 hr (LB agar) or 48–72 hr (56/2 glucose salts agar), as indicated. In certain specified cases where plasmid-free segregants (Lac− clones) form healthy colonies on 56/2 agar, but fail to appear on LB agar, sample colonies from the 56/2 agar plates were grown in 56/2 glucose minimal salts medium to an A650 of 0.4 and further tested to quantify the effect. Each culture was diluted in 10-fold steps from 10−1 to 10−5, and 10-μl aliquots were spotted on both LB and 56/2 glucose minimal agar. Colony-forming ability was recorded by photographing the plates after incubation for 24 hr (LB agar) or 48 hr (56/2 agar), unless specified otherwise.
Identification of 2-AP-resistant suppressors:
Samples from seven independent cultures of strain N7037, which is deleted for xonA, xseA, and sbcCD and therefore sensitive to 2-AP and mitomycin C, were spread on LB agar plates containing 2-aminopurine at a final concentration of 300 μg/ml. A few colonies of 2-AP-resistant derivatives were visible on each plate after 24 hr at 37°. Seven resistant clones, one from each of the original cultures, were purified for further analysis. Four exhibited a strong mutator phenotype but remained as sensitive to mitomycin C as the parent. The other three were not mutators but exhibited increased resistance to mitomycin C. In one of these three, strain N7050, the mutation responsible was identified as an allele of rpoC in the following way: The ΔsbcCD∷kan allele in N7050 was first replaced with a deletion tagged with resistance to spectinomycin (ΔsbcCD∷spc), and the resulting construct (N7683) was transduced with P1 phage grown on pools of cells carrying random kan insertions in the chromosome generated in strain MG1655 using the EZ-Tn5 <kan-2> Tnp Transposome system (Epicentre Technologies). The Kmr transductants were screened for those that were also sensitive to mitomycin C and 2-AP on the basis that such a clone would carry a kan insertion linked to the wild-type allele of the suppressor locus. One candidate was identified (N7704) and shown by PCR sequencing to carry an insertion in yijC at minute 89.64 of the genetic map. P1 phage from this clone was used to transduce N7683 to Kmr. Fifty-three percent of the transductants tested proved sensitive to mitomycin C and to 2-AP; i.e., they had lost the suppressor. P1 grown on a transductant retaining the suppressor phenotype (N7711) was used to transduce strain N7427, which is deleted for xonA, xseA, and sbcCD. In this case, 37% of the Kmr transductants selected acquired resistance to mitomycin C and to 2-AP; i.e., they had inherited the suppressor. These proved as resistant as the original isolate, N7050, from which we concluded that the mutation linked to yijC was the sole factor responsible for the suppression in that isolate. Further genetic analyses suggested a mutation in the vicinity of the rpoBC operon. PCR sequencing revealed a G-to-A transition at bp 2755 in rpoC, encoding an R919H substitution in RpoC, the β′-subunit of RNA polymerase.
RESULTS
The scenario outlined in Figure 1 predicts that ssDNA exonucleases might be vital in the absence of RecG. To investigate whether this is the case, we exploited a synthetic lethality assay based on a recG+ derivative of pRC7, a lac+ mini-F plasmid that is rapidly lost. The plasmid was used to cover ΔrecG in a strain also deleted for the lac operon and carrying additional mutations inactivating one or more of the enzymes with known ssDNA exonuclease activity. We tested ExoI (encoded by xonA), which attacks 3′ ends (Lehman and Nussbaum 1964); RecJ, which attacks 5′ ends (Lovett and Kolodner 1989); and ExoVII (encoded by xseA), which can target either end (Chase and Richardson 1974). We also tested the SbcCD enzyme, which has multiple nuclease activities, including the ability to remove 3′ overhangs from partial duplexes and to cut hairpin structures (Chalker et al. 1988; Connelly et al. 1998, 1999; Eykelenboom et al. 2008). Synthetic lethality between the covered and the uncovered mutations is revealed if the construct fails to show growth of plasmid-free Lac− clones (white colonies and white sectors within blue colonies) on agar plates supplemented with X-gal and IPTG (Mahdi et al. 2006). A reduction in viability is indicated when the colonies formed by plasmid-free cells are smaller than the blue/sectored colonies formed by those cells that retained the plasmid at the time of plating. In such cases, viability can be evaluated further by streaking samples of the colonies on the relevant agar media to see if they can be subcultured. A failure to subculture indicates that the colonies formed were the result of abortive growth following plasmid loss and dilution of the relevant plasmid-encoded gene product. The emergence of large colony variants indicates a viability defect that can be overcome by the acquisition of suppressors.
3′ ssDNA exonuclease activity is vital for cells lacking RecG:
The assays conducted revealed that ΔrecG cells lacking ExoI, ExoVII, and SbcCD are inviable on LB agar, showing no ability to form visible colonies without a covering recG+ plasmid (Figure 2A, i and ii; Figure S1, xiv). Some colonies of plasmid-free cells are detected on minimal salts agar, but these are tiny and tend to accumulate suppressors, as evident from the appearance of large colony variants (Figure 2A, iii and iv). Otherwise, ΔrecG cells lacking any one or two of ExoI, ExoVII, or SbcCD form colonies on both LB and minimal salts agar and can be subcultured on both types of media without acquisition of suppressors (Tables 1 and 2; Figure S1; data not shown). These observations reveal that ΔrecG cells need a 3′ exonuclease to stay alive and that this requirement can be satisfied by any one of ExoI, ExoVII, or SbcCD. However, SbcCD alone is able to do so efficiently only if RecJ is present. Without RecJ, the cells form small colonies on LB agar that take nearly 24 hr to become visible to the naked eye (Figure 2A, v; Figure 2C, strain N7317). ExoI and ExoVII show no such limitation (Figure S1, xv and xvi).
Figure 2.—
Maintenance of cell viability by the combined actions of DNA helicases and ssDNA exonucleases. (A) Effect of RecG. (B and C) Effect of RecJ. (D) Effects of RecQ, HelD, DinG, Rep, and UvrD. (A, B, and D) Synthetic lethality assays. These, and similar assays reported in subsequent figures, are described in detail in materials and methods. The relevant genotype of the construct used is shown above each photograph, with the strain number in parentheses. The fraction of white colonies is shown below with the number of white colonies/total colonies analyzed in parentheses. The spot assays in C are of cultures of the strains indicated as serially diluted in 10-fold steps from 10−1 to 10−5 before spotting 10-μl samples on the media indicated, as described in materials and methods.
TABLE 1.
Viability of exonuclease-deficient cells lacking RecG, RuvABC, RecA, Rep, UvrD, or RNaseHI
| Colony formation by plasmid-free segregants of synthetic lethality constructsa |
||||
|---|---|---|---|---|
| Other chromosomal mutation(s)b | pAM401 sbcCD+/c | pJJ100 recG+/ΔrecG | pAM390 ruv+/Δruv | pAM490 rnhA+/ΔrnhA |
| None, or any 1 or 2 of xonA, xseA, sbcCD, recJ | + | + | + | + |
| xonA xseA recJ | + | + | * | − |
| xonA sbcCD recJ | + | + | + | + |
| xseA sbcCD recJ | + | + | + | + |
| xonA xseA sbcCD | + | − | − | − |
| rep or uvrD plus any 1 or 2 of xonA, xseA, sbcCD | + | |||
| xonA xseA sbcCD recQ | + | |||
| xonA xseA sbcCD helD | + | |||
| xonA xseA sbcCD dinG | + | |||
| xonA xseA sbcCD recF | * | |||
| priA300 | + | + | + | + |
| xonA xseA sbcCD priA300 | + | + | +d | −e |
As determined by using a synthetic lethality assay (materials and methods). +, plasmid-free segregants form well-developed colonies on LB agar equal to or approaching in size those formed by cells retaining the plasmid (unless indicated otherwise in the text). They also account for 25–75% of the total colonies observed and can be subcultured without difficulty. −, colonies of plasmid-free segregants not detected on either LB or 56/2 minimal salts agar, except as indicated in the text. *, plasmid-free segregants account for >20% of the total colonies observed on LB agar but establish colonies that are much smaller than those formed by cells retaining the plasmid; they establish much stronger colonies on 56/2 minimal salts agar and can be subcultured without difficulty under these conditions. See text for additional details.
The mutations identified were deletions or in some cases (recA and recJ) Tn10 insertions (see Table S1), except for rus-2, which is an orf-56∷IS10 insertion activating rusA, and priA300, which is a base substitution encoding helicase-defective PriAK230R.
The chromosome carries sbcCD+, except as indicated in column 1.
The colonies formed on LB agar are smaller than those established by cells retaining the plasmid, but these two types of colonies are about equal in size on 56/2 minimal salts agar.
Small colonies of plasmid-free segregants detected on 56/2 minimal salts agar, but could not be subcultured.
TABLE 2.
Effect of RecG on UV sensitivity of exonuclease-depleted strains
| xonA, xseA, sbcCD, exoX, recJ genotype | Exonuclease deficiency (polarity) |
rec+ constructs |
ΔrecG constructs |
||
|---|---|---|---|---|---|
| Strain | Survivala | Strain | Survivala | ||
| Wild type | None | TB28 | 0.68 | N5742 | 0.14 |
| xonA | ExoI− (3′–5′) | N6946 | 0.67 | N7293 | 0.21 |
| xseA | ExoVII− (3′–5′ and 5′–3′) | N6951 | 0.68 | N7297 | 0.11 |
| sbcCD | SbcCD− (3′–5′) | N5281 | 0.59 | N7295 | 0.11 |
| recJ | RecJ− (5′–3′) | N4934 | 0.62 | N7294 | 0.044 |
| xonA xseA | ExoI− ExoVII− | N6954 | 0.58 | N7301 | 0.093 |
| xonA sbcCD | ExoI− SbcCD− | N6945 | 0.44 | N7299 | 0.059 |
| xonA recJ | ExoI− RecJ− | N7065 | 0.22 | N7298 | 0.028 |
| xseA sbcCD | ExoVII− SbcCD− | N6952 | 0.65 | N7303 | 0.13 |
| xseA recJ | ExoVII− RecJ− | N7066 | 0.32 | N7302 | 0.0028 |
| sbcCD exoX | SbcCD− ExoX− | N7004 | 0.80 | NCb | |
| sbcCD recJ | SbcCD− RecJ− | N7056 | 0.63 | N7300 | 0.0067 |
| xonA xseA sbcCD | ExoI− ExoVII− SbcCD− | N6953 | 0.33 | Inviablec | |
| N7037 | 0.30 | ||||
| xonA xseA recJ | ExoI− ExoVII− RecJ− | N7036 | 0.0072 | N7317d | 0.001d |
| xonA sbcCD exoX | ExoI− SbcCD− ExoX− | N7005 | 0.49 | NCb | |
| xonA sbcCD recJ | ExoI− SbcCD− RecJ− | N7063 | 0.17 | N7312 | 0.011 |
| xseA sbcCD exoX | ExoVII− SbcCD− ExoX− | N7006 | 0.79 | NCb | |
| xseA sbcCD recJ | ExoVII− SbcCD− RecJ− | N7064 | 0.21 | N7311 | 0.00025 |
| xonA xseA sbcCD exoX | ExoI− ExoVII− SbcCD− ExoX− | N7007 | 0.23 | NCb | |
| xonA xseA sbcCD recJ | ExoI− ExoVII− SbcCD− RecJ− | N7074d | 0.001d | inviablec | |
Survival was determined at a dose of 30 J/m2. Except where indicated otherwise, survival was determined by using cultures grown to exponential phase in LB broth and irradiated on the surface of LB agar plates. Values are means of three to seven experiments.
NC, not constructed.
As revealed by using synthetic lethality constructs carrying pJJ100 (recG+).
These strains cannot be subcultured on LB agar (N7074) or subculture poorly (N7317), but can be grown with no difficulty, and their UV survival determined, by using 56/2 glucose minimal salts media under otherwise identical conditions.
The only nuclease activity reported for ExoI is the ability to digest unpaired ssDNA from a 3′ end (Lehman and Nussbaum 1964). Therefore, the fact that this enzyme suffices to keep ΔrecG cells alive, and robustly so (Table 1; Figure S1, x), is highly informative. It shows that the viability of these cells can be maintained provided unpaired 3′ ssDNA can be degraded, indicating that the accumulation of such strands might have toxic consequences. ExoVII and SbcCD appear to provide the only other nucleases capable of eliminating this threat. None of the several other E. coli enzymes reported to attack ssDNA from 3′ ends, such as exonuclease III, exonuclease IX, and exonuclease X (ExoX) (Viswanathan and Lovett 1999; Lombardo et al. 2003; Centore et al. 2008), appears up to the task. However, the ability to digest 3′ ssDNA is vital only in the absence of RecG. With RecG present, cells lacking ExoI, ExoVII, and SbcCD remain viable (Figure 2A, i), which implies that ssDNA species with exposed 3′ termini either do not accumulate or can be dealt with by other means. This observation also argues against the idea that ExoI is needed to keep some essential protein complex intact. For example, ExoI is known to bind the ssDNA binding protein SSB, which itself interacts with a number of other proteins associated with genome replication and maintenance (Shereda et al. 2008).
Assays with constructs based on an sbcCD+ derivative of pRC7 revealed that ExoI− ExoVII− SbcCD− cells grow very slowly on LB agar if RecJ is eliminated. However, provided RecG is available, they form colonies on minimal salts agar that can be subcultured without difficulty (Figure 2B, i–iv; Figure 2C, strain N7074). From these data, and those in Table 1 and Figure S1, it seems that, whereas ΔrecG cells have a specific requirement for a 3′ ssDNA exonuclease, recG+ cells can be maintained by either a 3′ or a 5′ activity. However, the 3′ activity has to be ExoI, ExoVII, or SbcCD. No other nuclease present in E. coli seems to be able to maintain viability.
Rep and UvrD promote viability in rich media:
We extended our studies to investigate whether DNA helicases other than RecG are required to support growth of ExoI− ExoVII− SbcCD− cells. RecQ, HelD, and DinG proved dispensable (Figure 2D, i–iii; Table 1). However, Rep, a 3′–5′ DNA helicase considered to be involved with DNA replication (Yarranton and Gefter 1979), proved essential for colony formation on LB agar, although dispensable on minimal salts agar (Figure 2D, iv and v), which contrasts with the requirement for RecG under both conditions (cf. Figure 2D, iv and v, with Figure 2A, ii and iii). Likewise, UvrD, a 3′–5′ DNA helicase associated initially with DNA repair (Matson 1986), is required specifically for good growth of colonies, but only on LB agar (Figure 2D, vi and vii). As with cells lacking RecG, the presence of any one of ExoI, ExoVII, or SbcCD suffices to maintain robust viability on LB agar (Table 1 and data not shown). These observations indicate that ExoI− ExoVII− SbcCD− cells are likely to have multiples defects in DNA macromolecular metabolism.
ExoI− ExoVII− SbcCD− cells are sensitive to mitomycin C and 2-aminopurine:
The above data (Figure 3C; Table 1) demonstrate that any combination of ExoI, ExoVII, and SbcCD can be eliminated from wild-type cells without obvious loss of viability, as reported (Dermic 2006). The mutants form healthy colonies on LB agar and are almost as resistant to UV light and mitomycin C as the wild type (Figure 3, A and C; Table 2), with the exception of a construct lacking all three nucleases. Cells lacking ExoI, ExoVII, and SbcCD are fairly resistant to UV light but proved sensitive to ionizing radiation and mitomycin C, although not as sensitive as a recB strain defective in DNA double-strand break repair (Figure 3, B and C; data not shown). They are also sensitive to the base analog 2-AP (Figure 3C). A strain lacking ExoX in addition to ExoI, ExoVII, and SbcCD was made without difficulty (Table 2, strain N7007), but did not appear different from an ExoI− ExoVII− SbcCD− strain in terms of sensitivity to UV, mitomycin C, or 2-AP (Table 2 and data not shown). Reducing both 3′ and 5′ activities increases sensitivity to UV, as reported (Viswanathan and Lovett 1998; Dermic 2006), but especially if RecG is also absent (Table 2; Figure 3A).
Figure 3.—
Effect of DNA-damaging agents on strains depleted of 3′ ssDNA exonucleases. (A) Sensitivity to UV light. (B) Sensitivity to ionizing radiation. (C) Sensitivity to mitomycin C and 2-AP. (D) Effect of MutS on the sensitivity of an ExoI− ExoVII− SbcCD− strain to 2-AP and mitomycin C. (E) Synthetic lethality assays showing the effect of eliminating MutS on the viability of ExoI− ExoVII− SbcCD− strains lacking RecG, RecJ, Rep, or UvrD. (F and G) mutS derivatives of ExoI− ExoVII− SbcCD− cells lacking RecJ or Rep accumulate suppressors during growth on LB agar.
Samples of ExoI− ExoVII− SbcCD− cells spread on LB agar supplemented with 2-AP at 300 μg/ml give rise to colonies of resistant derivatives within 24–48 hr. These suppressors appear at a frequency of ∼1/106 initial cells plated. We isolated seven independent isolates for further analysis. Four of these proved strong mutators (see materials and methods), consistent with a defect in mismatch repair. A mutS construct confirmed that resistance to 2-AP is restored by inactivating the MutHLS-dependent and methyl-directed mismatch repair system (Figure 3D). These data indicate that mismatch repair is compromised in ExoI− ExoVII− SbcCD− cells and leads to inviability when mismatches are increased by exposure to 2-AP. They fit with previous studies demonstrating the involvement of ExoI and ExoVII in mismatch repair (Harris et al. 1998; Viswanathan and Lovett 1998; Burdett et al. 2001; Viswanathan et al. 2001) and suggest that SbcCD may also be engaged in this process. Eliminating MutS also improves the ability of ExoI− ExoVII− SbcCD− cells lacking UvrD to form colonies on LB agar (Figure 3E, i;Table 3). Thus, the poor growth seen with MutS present may be attributed in part to some consequence of abortive mismatch repair.
TABLE 3.
Suppression of the reduced viability of exonuclease-deficient cells lacking recombination/repair activities
| Other chromosomal mutation(s)b | Effect of mutS, rpoC[R919H], and priA300 mutations on colony formation by plasmid-free segregants of pAM401 sbcCD+/ΔsbcCD ΔxonA ΔxseA constructsa |
|||
|---|---|---|---|---|
| mut+ rpo+ pri+ | mutS | rpoC[R919H] | priA300 | |
| None | + | + | + | + |
| ΔrecG | − | −b | + | |
| recJ284 | * | *,c | + | −d |
| Δrep | −d | *,cd | + | |
| ΔuvrD | *,c | + | + | + |
| ΔruvABC | * | *,c | *,c | + |
| ΔruvABC rus-2 | + | |||
| ΔrecA or recA269 | −d | −bd | *,e | |
+, −, and * are defined in Table 1, footnote a.
Plasmid-free segregants for pin-prick colonies that cannot be subcultured.
The colonies observed develop outgrowths of suppressors that form large colonies on subculture.
Plasmid-free segregants establish robust colonies on 56/2 minimal salts agar where they account for >35% of the total and can be subcultured without difficulty using 56/2 salts media.
Plasmid-free segregants can be subcultured on LB agar.
Eliminating MutS from ExoI− ExoVII− SbcCD− cells does not restore resistance to mitomycin C (Figure 3D), nor does it eliminate the dependence on RecG, RecJ, and Rep for growth on LB agar (Figure 3E, ii–iv; Table 3). There is some improvement in the recovery of plasmid-free colonies of recJ and rep derivatives (Figure 3E, iii and iv), but this is largely attributable to the outgrowth of suppressors, which is not surprising, given the mutator phenotype of the mutS construct. The outgrowth of suppressors is very evident when cells subcultured in minimal salts medium are plated on LB agar (recJ derivative; Figure 3F) or when white colonies initially detected on minimal salts indicator plates are streaked directly on LB agar (rep derivative; Figure 3G).
One of the three suppressor strains that did not display a mutator phenotype proved strongly resistant to both 2-AP and mitomycin C (Figure 4A, strain N7050). This isolate carries an rpoC mutation encoding an R919H substitution in the β′-subunit of RNA polymerase. Genetic reconstructions established that this mutation alone is responsible for the suppression (materials and methods). In other work, a particular class of stringent RNA polymerase (RNAP) mutations (rpo*) was shown to act as partial suppressors of the DNA repair-deficient phenotype of ruv mutants (McGlynn and Lloyd 2000; Trautinger and Lloyd 2002). These rpo* mutations also promote growth of uvrD rep double mutants in rich media (Guy et al. 2009). They are thought to act by destabilizing RNAP transcription complexes, thereby reducing the likelihood of pathological consequences following collisions with replication forks (Trautinger et al. 2005; Rudolph et al. 2007). We examined three rpoB alleles in a strain lacking ExoI, ExoVII, and SbcCD and found that each conferred resistance to mitomycin C and, to a lesser extent, to 2-AP (Figure 4A). Thus, the sensitivity of the ExoI− ExoVII− SbcCD− strain may reflect in part a reduced ability to resolve conflicts between DNA replication and transcription, which are elevated when the template DNA is corrupted.
Figure 4.—
Mutations in subunits of RNAP modulate the phenotype of ExoI− ExoVII− SbcCD− strains. (A) Effect of RpoB and RpoC mutations on sensitivity to mitomycin C and 2-AP. (B) Synthetic lethality assays showing the effect of RpoCR919H on the viability of derivatives lacking RecG, RecJ, Rep, or UvrD. (B, ii, b) Abortive growth of a plasmid-free colony from B, ii, a.
However, none of the RNAP mutations tested eliminates the requirement for RecG to maintain viability. The synthetic lethality assays exploited revealed that some white colonies do appear on LB agar, but are tiny and cannot be subcultured without acquisition of suppressors (Figure 4B, i and ii; Table 3; data not shown). Therefore, although conflicts between replication and transcription most probably contribute to the sensitivity of ExoI− ExoVII− SbcCD− cells to mitomycin C and 2-AP, we suspect that such conflicts are not the primary reason for the inviability observed when the cells are also depleted of RecG. However, they might be the reason why the cells need Rep and UvrD to grow well on LB agar as the rpoC suppressor abolishes the requirement (Figure 4B, iii and iv; Table 3). They may also help to explain why the cells need RecJ, although such conflicts cannot be the only reason as the recJ rpoC construct still shows a growth defect relative to the recJ+ control (Figure 4B, cf. i and v).
3′ ssDNA exonucleases limit recombination:
Early genetic studies indicated that ExoI and SbcCD eliminate substrates that RecA protein might otherwise exploit to initiate homologous DNA pairing and strand exchange (Kushner et al. 1971; Lloyd and Buckman 1985; Kowalczykowski 2000). To determine whether such substrates accumulate in ExoI− ExoVII− SbcCD− cells, we examined a ΔruvABC derivative on the premise that the initiation of recombination might be toxic without an efficient system for resolving Holliday junctions. Synthetic lethality assays revealed that a ΔruvABC derivative of an ExoI− ExoVII− SbcCD− strain grows very poorly on LB agar, forming very small colonies without a covering plasmid, colonies that become overgrown with suppressors (Figure 5A, i and ii; Table 1; data not shown). Activation of the RusA Holliday junction resolvase (rus-2 insertion) largely abolishes this defect (Figure 5A, iii; Table 3), demonstrating that the poor growth is indeed most likely due to an accumulation of Holliday junctions. However, the ruv cells have no difficulty growing on minimal salts agar (Figure 5A, iv), indicating that the accumulation of Holliday junctions is a problem that arises during conditions permitting rapid growth. The rpoC[R919H] suppressor improves colony growth on LB agar, but the effect is marginal (Figure 5A, v; Table 3). Eliminating MutS also increases colony growth (Figure 5A, vi; Table 3), but much of this increase can be attributed to the accumulation of suppressors, triggered no doubt by the mutator phenotype (data not shown).
Figure 5.—
ExoI− ExoVII− SbcCD− cells need a Holliday junction resolvase to maintain rapid growth. (A) Synthetic lethality assays revealing the extremely poor growth on LB agar compared with minimal agar of ExoI− ExoVII− SbcCD− cells lacking RuvABC. Robust growth on LB agar is restored by activating the RusA resolvase (rus-2 mutation) but not by eliminating MutS, nor by expressing RpoCR919H. (B) Synthetic lethality assays demonstrating how the ability of SbcCD to maintain the viability of ΔruvABC cells depends on RecJ.
Any one of ExoI, ExoVII, or SbcCD is sufficient to maintain robust viability in cells lacking RuvABC (Figure 5A, i; Table 1). But, as we found with ΔrecG cells, RecJ is redundant, except when both ExoI and ExoVII are missing, in which case the cells grow very slowly, even more slowly than their ΔrecG counterparts (Figure 5B, i and ii). Thus, it seems that, under conditions promoting rapid growth, recombination is provoked specifically in cells lacking the 3′ ssDNA exonuclease activities of ExoI, ExoVII, and SbcCD and for reasons that have little to do with any defect in mismatch repair or with any conflict between replication and transcription.
Recombination is necessary in ExoI− ExoVII− SbcCD− cells:
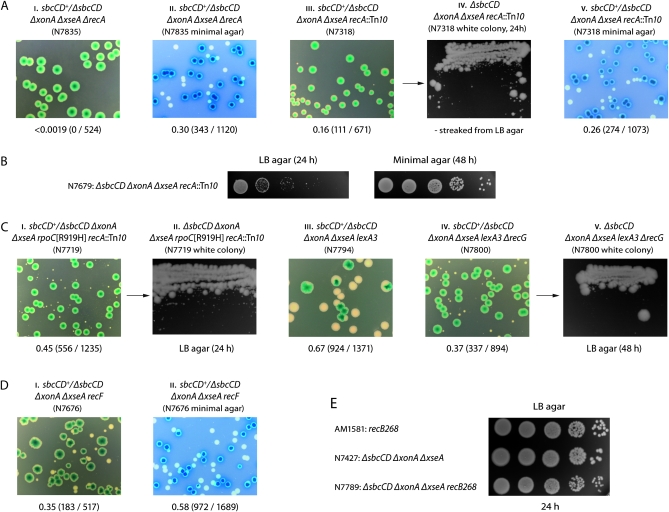
We investigated recA derivatives of ExoI− ExoVII− SbcCD− cells to see if the recombination detected using ruv constructs is needed to maintain viability. Synthetic lethality assays revealed that these cells do need RecA to form colonies on LB agar, although not on minimal salts agar (Figure 6, A and B; Table 3), which is consistent with the absence of any need for RuvABC under these conditions. A construct made using recA∷Tn10 yields plasmid-free colonies on LB agar, but these appear at a reduced frequency, grow very slowly, and reveal large colony variants on subculture (Figure 6A, iii and iv). These variants proved to be recA+ derivatives resulting from excision of Tn10 (data not shown). Their accumulation under these conditions emphasizes the need for RecA to maintain viability. The rpoC[R919H] suppressor does little to eliminate this requirement. Plasmid-free colonies are more frequent, but still very small, and the emergence of large colony variants remains a major feature (Figure 6C, i and ii; Table 3).
Figure 6.—
The recombinase activity of RecA is needed to maintain rapid growth of ExoI− ExoVII− SbcCD− cells. (A and B) Synthetic lethality and spot dilution assays showing how ExoI− ExoVII− SbcCD− cells lacking RecA grow well on minimal agar but are inviable on LB agar. (C–E) Synthetic lethality and spot dilution assays showing the effects of RpoCR919H, lexA3, RecF, and RecB on the viability of ExoI− ExoVII− SbcCD− cells.
We made a lexA3 construct to investigate whether RecA might be needed to induce the SOS repair response. SOS is induced when RecA is assembled on ssDNA exposed during replication of damaged DNA or following DNA breakage and triggers autocleavage of LexA protein, the SOS repressor (Sassanfar and Roberts 1990). The lexA3 allele encodes a mutant repressor resistant to autocleavage. Consequently, lexA3 cells, like recA cells, cannot induce SOS (Sassanfar and Roberts 1990). The construct made revealed that lexA3 does not prevent ExoI− ExoVII− SbcCD− cells from growing on LB agar. Indeed, they form fairly robust colonies (Figure 6C, iii). Furthermore, they still need RecG to do so (Figure 6C, iv) as well as RecA, Rep, and UvrD (data not shown). Some small white colonies are seen with the construct lacking RecG, but these are full of suppressors (Figure 6C, v).
From these data we conclude that excessive SOS expression is not the main reason why the viability of ExoI− ExoVII− SbcCD− cells is so reduced in the absence of RecG, Rep, or UvrD. The data also enable us to conclude that these cells rely on the recombinase activity of RecA to survive growth in rich media rather than on its ability to promote SOS induction. However, the recombinase activity requires assembly of a RecA filament on ssDNA, in which case it might be expected to cause some increase in the expression of SOS genes. We investigated this possibility and found that the basal level of expression is increased quite substantially. This is clear from the approximately fivefold higher levels of β-galactosidase seen in ExoI− ExoVII− SbcCD− cells carrying the lacZ+ gene fused to the LexA-regulated sulA (sfiA) promoter (Table 4). It is also clear that the presence of any one of ExoI, ExoVII, or SbcCD curbs this increase, whereas the rpoC[R919] suppressor does not. The SOS response is induced strongly in every case following exposure to mitomycin C.
TABLE 4.
Effect of ssDNA exonuclease deficiency on SOS expression
| β-Galactosidase activitya |
||||
|---|---|---|---|---|
| Exonuclease deficiency (additional genotype) | Strain no. | No. of experiments | −MMC | +MMC |
| None | N7843 | 10 | 77 ± 8 | 1474 ± 70 |
| ExoI− | N7844 | 7 | 94 ± 4 | 1591 ± 96 |
| ExoVII− | N7859 | 4 | 83 ± 12 | 1343 ± 60 |
| SbcCD− | N7845 | 4 | 65 ± 13 | 1384 ± 61 |
| ExoI− ExoVII− | N7860 | 4 | 136 ± 6 | 1542 ± 95 |
| ExoI− SbcCD− | N7846 | 4 | 102 ± 5 | 1354 ± 82 |
| ExoVII− SbcCD− | N7847 | 4 | 109 ± 7 | 1309 ± 47 |
| ExoI− ExoVII− SbcCD− | N7837 | 7 | 398 ± 28 | 1962 ± 52 |
| None (rpoC[R919H]) | N7888 | 4 | 135 ± 7 | 2520 ± 42 |
| ExoI− ExoVII− SbcCD− (rpoC[R919H]) | N7889 | 4 | 476 ± 40 | 2365 ± 75 |
Measured in ΔlacIZYA strains carrying a sulA∷ lacZ+ fusion as described in materials and methods and expressed as Miller Units. Values are means ± SE. MMC = mitomycin C.
These data demonstrate that ssDNA must be exposed and in a form that not only is vulnerable to attack by ExoI, ExoVII, or SbcCD but also is amenable to RecA loading. Two pathways have been identified by which a stable RecA filament can be assembled on ssDNA: one mediated by RecBCD enzyme and the other by the RecFOR proteins. RecBCD unwinds and resects duplex DNA ends and loads RecA at a 3′ ssDNA overhang created after the enzyme activity has been modulated at a χ-sequence. The 3′ end of the exposed strand may be held within the RecBCD complex, protecting it from other nucleases, while RecB's helicase activity may strip away any SSB protein (Kowalczykowski 2000; Amundsen and Smith 2003; Singleton et al. 2004). The RecFOR proteins normally load RecA at ssDNA gaps. They enable RecA to displace SSB protein, which has a higher affinity for ssDNA (Umezu et al. 1993; Morimatsu and Kowalczykowski 2003; Cox 2007). However, RecFOR can also assemble RecA at ssDNA ends generated independently of RecBCD, although normally such DNA would be vulnerable to exonuclease digestion.
It is therefore significant that even after 48 hr incubation, ExoI− ExoVII− SbcCD− cells lacking RecF form small and sickly colonies on LB agar, although they grow well enough on minimal salts agar (Figure 6D, i and ii; Table 1), whereas those lacking RecB are quite viable, forming colonies on both types of media as efficiently as the recB+ parent. Unlike the recF derivative, the colonies formed on LB agar are well developed after only 24 hr incubation (Figure 6E; data not shown). The relevant recB construct was made both with and without the aid of a covering plasmid, but any covering plasmid used is eliminated with such a high frequency under nonselective conditions that it prohibits use of our standard assay for synthetic lethality. However, this itself emphasizes the viability of the plasmid-free cells (Table S1, strains N7783 and N7789). We conclude that at least some of the ssDNA on which RecA is loaded to initiate recombination is generated by means other than RecBCD-mediated digestion of duplex DNA ends.
RecG limits recombination during normal growth:
A central tenet of the model in Figure 1 is that recombinogenic 3′ flaps are generated more frequently in cells lacking RecG because of the increased incidence of unscheduled fork collisions. Accordingly, and on the basis that a ruv mutant would have no means to resolve Holliday junctions efficiently by junction cleavage (Zhang et al. 2010), a Δruv ΔrecG cell should be more sensitive to a reduction in 3′ exonuclease activity than a ruv single mutant. We used a ruv+ recG+ derivative of pRC7 to test this possibility and examined the effect of eliminating any two of ExoI, ExoVII, and SbcCD. Removing all three would be uninformative as we have shown this to compromise the viability of both Δruv and ΔrecG strains. With all three nucleases present, ΔruvABC ΔrecG cells show no major reduction in viability in that they form good colonies without the covering plasmid (Figure 7A, i and ii). If recombinogenic 3′ flaps do arise more frequently in the absence of RecG, then it seems that these can be removed efficiently by exonuclease digestion.
Figure 7.—
RecG limits the requirement for RuvABC to maintain rapid growth of ExoI− ExoVII− SbcCD− cells. (A and B) Synthetic lethality assays showing the effect of 3′ ssDNA exonucleases on the viability of a strain lacking RuvABC and RecG.
ExoI proved sufficient to maintain robust viability (Figure 7A, iii). ExoVII also keeps the cells alive, but the slightly smaller size of the plasmid-free colonies observed with the relevant construct suggests that it might be a little less effective (Figure 7A, iv). SbcCD proved rather ineffective, with the cells forming small and rather sickly colonies without the covering plasmid (Figure 7B, i). Robust growth is maintained in this case if RecG is present or RecA eliminated (Figure 7B, ii and iii).
Taken together, these data indicate that potentially recombinogenic substrates do arise more frequently in the absence of RecG but can be eliminated efficiently via the action of 3′ ssDNA exonucleases, particularly by ExoI. However, the inability of ExoI− ExoVII− SbcCD− cells lacking either RuvABC or RecA to establish good colonies on LB agar indicates that recombinogenic substrates are generated under conditions promoting rapid growth even with RecG present and that the cells must engage in and complete recombination if they are to stay alive. If these recombinogenic substrates are 3′ flaps, or dsDNA branches arising from subsequent PriA-mediated replication, RecG is clearly unable to convert all 3′ flaps to 5′ flaps, or if it can, then RecJ cannot eliminate all the 5′ flaps formed. The fact that eliminating RecA enables SbcCD to maintain robust viability in the absence of RecG is significant (Figure 7B, iii). It is consistent with the formation of dsDNA branches that provoke RecBCD-mediated recombination (Figure 1C, iii and iv). Without RecA to load on the 3′ ssDNA exposed after an encounter with χ, RecBCD recombinase activity is aborted and its dsDNA exonuclease activity (ExoV) becomes rampant (Dabert et al. 1992; Kuzminov and Stahl 1997). SbcCD removes the ssDNA, enabling the DNA to be targeted and further digested by another RecBCD molecule (Zahradka et al. 2009). This combination of nuclease activities facilitates complete removal of the DNA branch, thus possibly explaining the restoration of robust viability.
PriA activates recombination in cells lacking RecG:
Previous studies revealed that most, if not all, aspects of the recG mutant phenotype are suppressed by mutations reducing or eliminating the helicase activity of PriA (Al-Deib et al. 1996; Jaktaji and Lloyd 2003; Rudolph et al. 2009a; Zhang et al. 2010). We exploited priA300, which encodes helicase-defective PriAK230R, to investigate whether the same holds true for the inviability caused in ExoI− ExoVII− SbcCD− cells. Synthetic lethality constructs revealed that priA300 restores robust viability (Figure 8A, i–iv; Table 3). The priA300 allele also improves the ability of ExoI− ExoVII− SbcCD− cells lacking RuvABC or RecA to form colonies on LB agar, although viability is still compromised in each case, as evident from the more robust growth of cells retaining the covering plasmid (Figure 8A, v and vi; Table 3). However, priA300 does not confer viability in the absence of both RecG and RecA (Figure 8, vii). It also does not suppress the sensitivity of ExoI− ExoVII− SbcCD− cells to mitomycin C and 2-AP (Figure 8B). These observations, together with the fact that priA300 is not otherwise a suppressor of ruv or recA (Jaktaji and Lloyd 2003), demonstrate that it is PriA, and in particular some activity that depends on its ability to unwind DNA, that is responsible for the recombination provoked in cells lacking ExoI, ExoVII, and SbcCD and for making these cells dependent on RecG for their viability. However, it is also clear from the weak growth of cells lacking RuvABC or RecA (Figure 8A, v and vi) and the inviability of those lacking both RecG and RecA that eliminating PriA helicase activity does not prevent recombination altogether.
Figure 8.—
Effect of priA300 on ExoI− ExoVII− SbcCD− cells depleted of RecA, RecG, RuvABC, or UvrD. (A) Synthetic lethality assays. (B) Spot dilution assays showing sensitivity to mitomycin C and 2-AP.
We also examined priA300 cells lacking ExoI, ExoVII, SbcCD, and UvrD and found that they, too, form robust colonies on LB agar (Figure 8A, viii; Table 3). Their vigorous growth contrasts sharply with the feeble colonies formed by the equivalent priA+ cells (Figure 2D, vi), which we have demonstrated to be due in large measure to some consequence of abortive mismatch repair as it can be alleviated by eliminating MutS (Figure 3E, i). Therefore, we suspect that the abortive mismatch repair in these cells creates substrates that PriA can exploit via its helicase activity to load DnaB and thus initiate replication in a manner that reduces viability. We were unable to investigate the effect of priA300 on ExoI− ExoVII− SbcCD− cells lacking Rep, as we were unable to make the relevant construct. Previous studies established that priA300 rep double-mutant cells have reduced viability (Sandler 2000; Mahdi et al. 2006).
RNase HI-deficient cells require ssDNA exonucleases to stay alive:
The data presented above fit with the idea that the inviability of ExoI− ExoVII− SbcCD− cells lacking RecG is a consequence of SDR initiated via PriA helicase activity. However, we were conscious that RecG is needed to facilitate recovery of recombinants in crosses with ruv strains, a fact previously interpreted as evidence that RecG and RuvABC provide alternative ways for processing recombination intermediates (Lloyd 1991). To investigate whether it is SDR that is responsible rather than some recombination defect, we analyzed exonuclease-deficient constructs lacking RNase HI (ΔrnhA). This enzyme degrades RNA from RNA:DNA duplexes, including from R-loops, and its absence is known to trigger a high level of cSDR, high enough in fact to sustain viability in the absence of origin firing (Kogoma 1997). There is no evidence that it has any direct role in recombination.
We observed that removing RNase HI mimics the effect of removing RecG in that ΔrnhA cells are inviable if ExoI, ExoVII, and SbcCD are eliminated. The synthetic lethality constructs that were exploited yielded no plasmid-free colonies on either LB agar or minimal salts agar (Figure 9, i–iii; Table 1; data not shown). This is consistent with SDR being the trigger for the inviability of exonuclease-depleted ΔrecG cells. However, removing RNase HI differs in that priA300 does little to restore viability. The plasmid-free cells form tiny colonies on minimal agar but fail to grow on LB agar (Figure 9A, iv and v; Table 3). The cells are also inviable on LB agar if ExoI, ExoVII, and RecJ are missing but grow well on minimal agar (Figure 9B, i and ii). As with cells lacking RecG, the presence of either ExoI or ExoVII is sufficient to maintain viability, even if both SbcCD and RecJ are missing (Figure 9B, iii and iv; Table 1; data not shown). The two differences may be explained by the incomplete suppression of SDR by priA300 and the presence of RecG, which could convert some 3′ flaps to 5′ flaps. Without RecJ and ExoVII, PriA might then initiate pathological re-replication by targeting these flaps (Figure 1F). The PriAK230R protein would retain the ability to do so as the template for DnaB loading is already single stranded, eliminating the need for helicase activity.
Figure 9.—
RNase HI promotes survival of cells depleted of ssDNA exonucleases, RuvABC, or RecG. (A and B) Synthetic lethality assays. (C) Sensitivity to UV light.
Removing RNase HI also mimics the effect of removing RecG in that it increases the sensitivity of ruv mutant cells to killing by UV light. However, the effect is not as severe (Figure 9C). On the other hand, even with a full complement of ssDNA exonucleases available, removing RNase HI makes ΔrecG cells inviable, as reported (Hong et al. 1995), whereas it only reduces the viability of ruv cells (Figure 9B, v and vi). The differences observed may be explained by the possibility that RNase HI may also help to process Okazaki fragments during replication (Ogawa and Okazaki 1984; Kornberg and Baker 1992). Without it, gaps may accumulate in the lagging strand, compounding any difficulties arising from the increase in SDR but also compromising viability even when SDR is reduced.
DISCUSSION
Recent studies of how the integrity of the genome and cell viability are maintained during the course of DNA replication in bacteria have focused on describing what happens to replication forks as they encounter blocking lesions in or on the DNA template or on dissecting the molecular mechanisms that enable cells to overcome such blocks and complete replication (Heller and Marians 2006b; Mahdi et al. 2006; Michel et al. 2007; Rudolph et al. 2007; Guy et al. 2009; Boubakri et al. 2010; Gabbai and Marians 2010). By comparison, much less attention has been paid to what happens when one fork runs into another, an event that usually happens only once at the termination of replication during the bacterial cell cycle, but which occurs many times along each chromosome during S-phase in eukaryotes. In this article, we have presented evidence consistent with the idea that the termination of DNA synthesis does not always run smoothly, at least in E. coli, and that, consequently, replication fork encounters need to be limited.
The data presented demonstrate that ΔrecG cells need a 3′ ssDNA exonuclease to stay alive and that this requirement can be satisfied by any one of ExoI, ExoVII, or SbcCD or eliminated by inactivating the helicase activity of PriA. The data are consistent with a model in which unscheduled initiation of replication via PriA-mediated replisome assembly at sites remote from oriC (SDR) compromises the highly evolved replichore arrangement that orchestrates duplication and transmission of the E. coli chromosome and that normally limits fork collisions to a single event during each cycle of cell growth and division. The data fit with the idea that a major role of RecG is to limit such replication (Figure 1). Taken together, the data suggest that the ability of PriA to secure chromosome duplication in times of replicative stress comes at a price (Rudolph et al. 2009a,b).
Previous studies demonstrated that mutations reducing or eliminating PriA helicase activity suppress the sensitivity of recG mutants to mitomycin C (Al-Deib et al. 1996; Gregg et al. 2002; Jaktaji and Lloyd 2003). They also eliminate most of the delay in division observed following irradiation of ΔrecG cells with UV light (Rudolph et al. 2009a). However, it is significant that ΔrecG cells are quite healthy, with a doubling time close to that of wild type. This implies that PriA becomes toxic to ΔrecG cells only when these cells have suffered damage to their DNA. Thus, given that ΔrecG cells lacking ExoI, ExoVII, and SbcCD are inviable, it would seem that DNA must somehow become “damaged” in these cells even without application of external genotoxic agents, i.e., as a result of events that occur during normal growth.
We considered the possibility that this “damage” might reflect abortive repair of DNA base-pair mismatches generated during chromosome replication (Iyer et al. 2006). Both ExoI and ExoVII are implicated in nascent strand removal following initiation of mismatch repair by the MutHLS proteins (Burdett et al. 2001; Viswanathan et al. 2001). Inactivating these two enzymes, and any possible substitute, might therefore leave recombinogenic nicks or gaps in the nascent strands, which might then account for the fact that homologous recombination and means to resolve Holliday junctions are essential for the survival of cells lacking ExoI, ExoVII, and SbcCD (Figures 5A and 6A). Indeed, we discovered that cells lacking ExoI, ExoVII, and SbcCD are sensitive to 2-AP, one of the hallmarks of a DNA mismatch repair defect downstream of the initiation step (Glickman and Radman 1980). These cells also need UvrD to grow well on LB agar, a DNA helicase required for displacement of the nascent stand containing the mismatched base (Iyer et al. 2006). Eliminating MutS restores resistance and reduces the need for UvrD, establishing that the cells do indeed suffer from abortive mismatch repair. However, eliminating MutS does not remove the requirement for RecG and RuvABC, indicating that the cells must have at least one additional defect.
A reduced ability to resolve conflicts between DNA replication and transcription is indicated by the fact that, in addition to UvrD protein, ExoI− ExoVII− SbcCD− cells need Rep to grow on LB agar, but not on minimal salts agar. Previous studies revealed that rep uvrD double mutants have the same growth constraint and that this can be alleviated by mutations shown to destabilize RNAP transcription complexes (Guy et al. 2009). We suggest that, in the absence of ExoI, ExoVII, and SbcCD to remodel a fork stalled at a ternary transcription complex, UvrD might have greater difficulty compensating for the absence of Rep and vice versa. Consistent with this, we discovered that the requirement for UvrD and Rep is alleviated in the presence of an rpoC mutation encoding an R919H substitution in the β′-subunit of RNAP. This mutation and other previously described rpoB alleles also alleviates the sensitivity of ExoI− ExoVII− SbcCD− cells to 2-AP and mitomycin C. However, they do not eliminate the need for RecG or for RecA, indicating that there is yet another defect in these cells.
Studies with strains carrying sulA∷lacZ fusions revealed that the SOS response is constitutively elevated in ExoI− ExoVII− SbcCD− cells and that ExoI, ExoVII, and SbcCD all contribute to keeping its expression at a low level during normal growth in LB broth. Furthermore, SOS expression remains high in the presence of the rpoC[R919H] suppressor mutation, indicating that the SOS-inducing signal is generated at the same high level even when conflicts between DNA replication and transcription might be reduced.
The high level of SOS expression, coupled with the fact that cells lacking ExoI, ExoVII, and SbcCD become inviable if RecA, RecG, or RuvABC is eliminated is significant. It indicates (1) that ssDNA species with free 3′ ends accessible to these nucleases are generated with or without RecG present; (2) that these strands enable RecA to initiate recombination with high efficiency; and (3) that this recombination is essential, providing the only means apart from exonuclease digestion of dealing with the exposed strands. So, how do these recombinogenic 3′ strands arise? And why are they particularly problematic in the absence of RecG? There is clearly a deficiency in mismatch repair and most probably a reduced ability to resolve conflicts between DNA replication and transcription. However, neither defect is sufficient to explain why ExoI− ExoVII− SbcCD− cells need RecA, RecG, and RuvABC to stay alive.
Chromosome breakage would most certainly expose a need for RecA. Breakages occur when forks encounter nicks or gaps in the template strands or when they stall and reverse to establish a Holliday junction structure that could be targeted and cleaved by RuvABC (Kuzminov 1995; Seigneur et al. 1998). RecBCD enzyme would normally be expected to unwind the duplex DNA end exposed in both cases and to degrade both strands via its ExoV activity until it encounters a χ-sequence in the strand ending 3′, whereupon its activity is modified in some manner that is not fully understood but that focuses subsequent degradation on the strand ending 5′, leaving a 3′ tail on which RecBCD then loads RecA to initiate recombination (Smith 1990; Kowalczykowski 2000; Singleton et al. 2004; Amundsen et al. 2007; Dillingham and Kowalczykowski 2008). However, although RecBCD is crucial for the repair of DNA breaks, recBC single mutants are viable, as are recA mutants, which would suggest that chromosome breakage is rather rare under normal growth conditions. Breaks are detectable in recBC cells (Seigneur et al. 1998), and viability is reduced (Capaldo-Kimball and Barbour 1971), but some of the breaks may be pathological in origin, being a consequence of RecBCD inactivation. ExoV is thought to be particularly instrumental in eliminating the Holliday junction structure formed at reversed forks, thus limiting fork breakage via RuvABC (Seigneur et al. 1998). It is therefore significant that cells lacking ExoI, ExoVII, and SbcCD do not require RecBCD to stay alive. Furthermore, the viability of recB recG and recB ruv cells is no different from that of recBC single mutants (Lloyd et al. 1987; Lloyd and Buckman 1991). Taken together, these observations indicate that increased chromosome breakage is not the primary reason for the failure of cells lacking ExoI, ExoVII, and SbcCD to form colonies when RecG, RuvABC, or RecA is inactivated and that something other than the processing of duplex DNA ends is responsible for the initial generation of potentially toxic 3′ ssDNA.
Hiasa and Marians (1994) presented evidence indicating that a 3′ flap might be generated when the replisome of one replication fork displaces the 3′ end of the nascent leading strand of the fork coming in the other direction. This led us to propose that 3′ flaps might be generated in this way quite frequently in a ΔrecG strain as a result of the increase in SDR, which leads to additional and unscheduled replication fork encounters outside of the normal termination area (Rudolph et al. 2009a,b, 2010). Flaps of this nature would be expected to be particularly problematic for cells lacking RecG and depleted of 3′ ssDNA exonucleases. As outlined in Figure 10, they could provide templates for RecA loading and strand exchange. The loading of RecA might also provoke recombination. Otherwise, PriA could target the branch point to initiate further replication, which would convert the flap to a dsDNA branch, thus providing an alternative route to recombination via RecBCD enzyme. Such exchanges would generate a network of partially replicated chromosomes with numerous DNA branches (Figure 1C, iv and v), as observed (Rudolph et al. 2009b).
Figure 10.—
Models illustrating how DNA molecules containing single-strand flaps might be processed by RecG translocase or by ssDNA exonucleases or targeted by RecA or PriA to provoke recombination (see discussion for further details).
The suggestion that cells lacking RecG and depleted of 3′ ssDNA exonucleases suffer damage to their DNA as a result of unscheduled replication fork collisions is consistent with the fact that the problem largely disappears when the helicase activity of PriA is eliminated (Figure 8), especially as PriA helicase mutants reduce SDR (Tanaka et al. 2003). Given that ΔrecG and ΔrnhA cells have in common a high level of cSDR initiated at R-loops (Kogoma 1997), it is also consistent with the fact that eliminating RNase HI mimics the effect of eliminating RecG (Figure 9). However, priA300 does little to eliminate the problem in this case. Furthermore, RecJ is also needed to maintain viability. These two observations are exactly what we would predict if the RecG present in these cells were able to convert a 3′ flap to a 5′ flap (Figure 10). PriA might then be able to initiate replisome assembly without need of its helicase activity as there would be no lagging strand to get in the way of DnaB loading, but alternatively, PriC might do so (Heller and Marians 2005, 2006a,b). However, cells lacking RNase HI not only are less effective in eliminating R-loops but also may be defective in Okazaki fragment processing (Ogawa and Okazaki 1984), which, if true, would create at least two different problems for chromosome replication and thus make it difficult to draw definitive conclusions from rnhA derivatives of ExoI− ExoVII− SbcCD− cells.
By reducing fork collisions to a single event per cell cycle and restricting termination to a defined area, the replichore arrangement of the chromosome enables complete replication of the chromosome to be achieved with minimum conflict with transcription and in a way that allows the 8-bp KOPS (FtsK orienting polar sequences) DNA elements to be arranged symmetrically in each chromosome arm to direct efficient FtsK-mediated chromosome segregation during cell division (Bigot et al. 2005; Reyes-Lamothe et al. 2008). With RecG present to unwind R-loops, D-loops, and 3′ ssDNA flaps, and a full complement of exonucleases with the ability to digest any ssDNA flaps and dsDNA branches that may arise, wild-type cells are able to maintain the advantages conferred.
However, we cannot exclude other interpretations of the data presented, especially because RecG, PriA, SbcCD, and ExoVII act on a variety of substrates in vitro. Thus, cells lacking multiple exonucleases may accumulate so much ssDNA that they simply die when DNA macromolecular metabolism is further compromised by the elimination of RecG. We did not test the viability of ΔrecG cells lacking every possible combination of ssDNA exonucleases to eliminate this possibility. Furthermore, the RecBCD enzyme has a potent ssDNA exonuclease activity that might be able to eliminate a 3′ flap. Thus, the lesion responsible for the inviability of exonuclease-depleted ΔrecG cells, rather than being a 3′ ssDNA flap generated during unscheduled fork collisions as we suggest, may be some downstream consequence of replication fork blockage resulting from an inability to process DNA strands by nuclease digestion. RecG itself has been shown to drive replication fork reversal in vitro, a key feature of models for promoting replication restart in both bacteria and eukaryotes that incorporate the need to process the nascent DNA strands (Seigneur et al. 1998; McGlynn and Lloyd 2000; Gari et al. 2008; Sun et al. 2008). However, although RecG might be targeted to replication forks via its interaction with SSB (Lecointe et al. 2007), direct evidence that it promotes replication fork reversal in vivo is distinctly lacking. Furthermore, it exhibits a strong preference in vitro for unwinding forks that mimic a 3′ flap structure of the type depicted in Figure 1 (McGlynn and Lloyd 2001).
Acknowledgments
We thank Carol Buckman and Lynda Harris for excellent technical help and colleagues identified in Table S1 for plasmids and strains. This work was supported by a program grant to R.G.L. from the UK Medical Research Council and by an early career fellowship to C.J.R. from the Leverhulme Trust.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.120691/DC1.
References
- Al-Deib, A. A., A. A. Mahdi and R. G. Lloyd, 1996. Modulation of recombination and DNA repair by the RecG and PriA helicases of Escherichia coli K-12. J. Bacteriol. 178 6782–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen, S. K., and G. R. Smith, 2003. Interchangeable parts of the Escherichia coli recombination machinery. Cell 112 741–744. [DOI] [PubMed] [Google Scholar]
- Amundsen, S. K., A. F. Taylor, M. Reddy and G. R. Smith, 2007. Intersubunit signaling in RecBCD enzyme, a complex protein machine regulated by Chi hot spots. Genes Dev. 21 3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, T., and T. Kogoma, 1994. Roles of ruvA, ruvC and recG gene functions in normal and DNA damage-inducible replication of the Escherichia coli chromosome. Genetics 137 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, T. G., and P. A. de Boer, 2004. Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Mol. Microbiol. 52 1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot, S., O. Saleh, C. Lesterlin, C. Pages, M. El Karoul et al., 2005. KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J. 24 3770–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubakri, H., A. L. de Septenville, E. Viguera and B. Michel, 2010. The helicases DinG, Rep and UvrD cooperate to promote replication across transcription units in vivo. EMBO J. 29 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett, V., C. Baitinger, M. Viswanathan, S. T. Lovett and P. Modrich, 2001. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc. Natl. Acad. Sci. USA 98 6765–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball, F., and S. D. Barbour, 1971. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J. Bacteriol. 106 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centore, R. C., R. Lestini and S. J. Sandler, 2008. XthA (exonuclease III) regulates loading of RecA onto DNA substrates in log phase Escherichia coli cells. Mol. Microbiol. 67 88–101. [DOI] [PubMed] [Google Scholar]
- Chalker, A. F., D. R. F. Leach and R. G. Lloyd, 1988. Escherichia coli sbcC mutants permit stable propagation of DNA replicons containing a long DNA palindrome. Gene 71 201–205. [DOI] [PubMed] [Google Scholar]
- Chase, J. W., and C. C. Richardson, 1974. Exonuclease VII of Escherichia coli: mechanism of action. J. Biol. Chem. 249 4553–4561. [PubMed] [Google Scholar]
- Connelly, J. C., L. A. Kirkham and D. R. Leach, 1998. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc. Natl. Acad. Sci. USA 95 7969–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly, J. C., E. S. de Leau and D. R. Leach, 1999. DNA cleavage and degradation by the SbcCD protein complex from Escherichia coli. Nucleic Acids Res. 27 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, M. M., 2007. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 42 41–63. [DOI] [PubMed] [Google Scholar]
- Dabert, P., S. D. Ehrlich and A. Gruss, 1992. χ sequence protects against RecBCD degradation of DNA in vivo. Proc. Natl. Acad. Sci. USA 89 12073–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko, K. A., and B. L. Wanner, 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermic, D., 2006. Functions of multiple exonucleases are essential for cell viability, DNA repair and homologous recombination in recD mutants of Escherichia coli. Genetics 172 2057–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillingham, M. S., and S. C. Kowalczykowski, 2008. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 72 642–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggin, I. G., R. G. Wake, S. D. Bell and T. M. Hill, 2008. The replication fork trap and termination of chromosome replication. Mol. Microbiol. 70 1323–1333. [DOI] [PubMed] [Google Scholar]
- Eykelenboom, J. K., J. K. Blackwood, E. Okely and D. R. Leach, 2008. SbcCD causes a double-strand break at a DNA palindrome in the Escherichia coli chromosome. Mol. Cell 29 644–651. [DOI] [PubMed] [Google Scholar]
- Fukuoh, A., H. Iwasaki, K. Ishioka and H. Shinagawa, 1997. ATP-dependent resolution of R-loops at the ColE1 replication origin by Escherichia coli RecG protein, a Holliday junction-specific helicase. EMBO J. 16 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbai, C. B., and K. J. Marians, 2010. Recruitment to stalled replication forks of the PriA DNA helicase and replisome-loading activities is essential for survival. DNA Repair (Amst.) 9 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gari, K., C. Decaillet, M. Delannoy, L. Wu and A. Constantinou, 2008. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc. Natl. Acad. Sci. USA 105 16107–16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman, B. W., and M. Radman, 1980. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc. Natl. Acad. Sci. USA 77 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg, A. V., P. McGlynn, R. P. Jaktaji and R. G. Lloyd, 2002. Direct rescue of stalled DNA replication forks via the combined action of PriA and RecG helicase activities. Mol. Cell 9 241–251. [DOI] [PubMed] [Google Scholar]
- Guy, C. P., J. Atkinson, M. K. Gupta, A. A. Mahdi, E. J. Gwynn et al., 2009. Rep provides a second motor at the replisome to promote duplication of protein-bound DNA. Mol. Cell 36 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, R. S., K. J. Ross, M. J. Lombardo and S. M. Rosenberg, 1998. Mismatch repair in Escherichia coli cells lacking single-strand exonucleases ExoI, ExoVII, and RecJ. J. Bacteriol. 180 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, R. C., and K. J. Marians, 2005. The disposition of nascent strands at stalled replication forks dictates the pathway of replisome loading during restart. Mol. Cell 17 733–743. [DOI] [PubMed] [Google Scholar]
- Heller, R. C., and K. J. Marians, 2006. a Replication fork reactivation downstream of a blocked nascent leading strand. Nature 439 557–562. [DOI] [PubMed] [Google Scholar]
- Heller, R. C., and K. J. Marians, 2006. b Replisome assembly and the direct restart of stalled replication forks. Nat. Rev. Mol. Cell Biol. 7 932–943. [DOI] [PubMed] [Google Scholar]
- Hiasa, H., and K. J. Marians, 1994. Tus prevents overreplication of oriC plasmid DNA. J. Biol. Chem. 269 26959–26968. [PubMed] [Google Scholar]
- Hong, X., G. W. Cadell and T. Kogoma, 1995. Escherichia coli RecG and RecA proteins in R-loop formation. EMBO J. 14 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, R. R., A. Pluciennik, V. Burdett and P. L. Modrich, 2006. DNA mismatch repair: functions and mechanisms. Chem. Rev. 106 302–323. [DOI] [PubMed] [Google Scholar]
- Jaktaji, R. P., and R. G. Lloyd, 2003. PriA supports two distinct pathways for replication restart in UV-irradiated Escherichia coli cells. Mol. Microbiol. 47 1091–1100. [DOI] [PubMed] [Google Scholar]
- Kogoma, T., 1997. Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol. Mol. Biol. Rev. 61 212–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, A., and T. A. Baker, 1992. DNA polymerase I of E. coli, pp. 113–164 in DNA Replication, Ed. 2. W. H. Freeman, New York.
- Kowalczykowski, S. C., 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25 156–165. [DOI] [PubMed] [Google Scholar]
- Krabbe, M., J. Zabielski, R. Bernander and K. Nordstrom, 1997. Inactivation of the replication-termination system affects the replication mode and causes unstable maintenance of plasmid R1. Mol. Microbiol. 24 723–735. [DOI] [PubMed] [Google Scholar]
- Kushner, S. R., H. Nagaishi, A. Templin and A. J. Clark, 1971. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc. Natl. Acad. Sci. USA 68 824–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov, A., 1995. Collapse and repair of replication forks in Escherichia coli. Mol. Microbiol. 16 373–384. [DOI] [PubMed] [Google Scholar]
- Kuzminov, A., and F. W. Stahl, 1997. Stability of linear DNA in recA mutant Escherichia coli cells reflects ongoing chromosomal DNA degradation. J. Bacteriol. 179 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe, F., C. Serena, M. Velten, A. Costes, S. McGovern et al., 2007. Anticipating chromosomal replication fork arrest: SSB targets repair DNA helicases to active forks. EMBO J. 26 4239–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman, I. R., and R. Nussbaum, 1964. The deoxyribonucleases of Escherichia coli. V. On the specificity of exonuclease I. J. Biol. Chem. 239 2628–2636. [PubMed] [Google Scholar]
- Lloyd, R. G., 1991. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J. Bacteriol. 173 5414–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, R. G., and C. Buckman, 1985. Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J. Bacteriol. 164 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, R. G., and C. Buckman, 1991. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J. Bacteriol. 173 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, R. G., C. Buckman and F. E. Benson, 1987. Genetic analysis of conjugational recombination in Escherichia coli K-12 strains deficient in RecBCD enzyme. J. Gen. Microbiol. 133 2531–2538. [DOI] [PubMed] [Google Scholar]
- Lombardo, M. J., I. Aponyi, M. P. Ray, M. Sandigursky, W. A. Franklin et al., 2003. xni-deficient Escherichia coli are proficient for recombination and multiple pathways of repair. DNA Repair (Amst.) 2 1175–1183. [DOI] [PubMed] [Google Scholar]
- Lovett, S. T., and R. D. Kolodner, 1989. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 86 2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi, A. A., G. J. Sharples, T. N. Mandal and R. G. Lloyd, 1996. Holliday junction resolvases encoded by homologous rusA genes in Escherichia coli K-12 and phage 82. J. Mol. Biol. 257 561–573. [DOI] [PubMed] [Google Scholar]
- Mahdi, A. A., C. Buckman, L. Harris and R. G. Lloyd, 2006. Rep and PriA helicase activities prevent RecA from provoking unnecessary recombination during replication fork repair. Genes Dev. 20 2135–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians, K. J., 2000. Replication and recombination intersect. Curr. Opin. Genet. Dev. 10 151–156. [DOI] [PubMed] [Google Scholar]
- Markovitz, A., 2005. A new in vivo termination function for DNA polymerase I of Escherichia coli K12. Mol. Microbiol. 55 1867–1882. [DOI] [PubMed] [Google Scholar]
- Matson, S. W., 1986. Escherichia coli helicase II (uvrD gene product) translocates unidirectionally in a 3′ to 5′ direction. J. Biol. Chem. 261 10169–10175. [PubMed] [Google Scholar]
- McGlynn, P., and R. G. Lloyd, 2000. Modulation of RNA polymerase by (p)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell 101 35–45. [DOI] [PubMed] [Google Scholar]
- McGlynn, P., and R. G. Lloyd, 2001. Rescue of stalled replication forks by RecG: simultaneous translocation on the leading and lagging strand templates supports an active DNA unwinding model of fork reversal and Holliday junction formation. Proc. Natl. Acad. Sci. USA 98 8227–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn, P., A. A. Al-Deib, J. Liu, K. J. Marians and R. G. Lloyd, 1997. The DNA replication protein PriA and the recombination protein RecG bind D-loops. J. Mol. Biol. 270 212–221. [DOI] [PubMed] [Google Scholar]
- Messer, W., 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26 355–374. [DOI] [PubMed] [Google Scholar]
- Michel, B., H. Boubakri, Z. Baharoglu, M. LeMasson and R. Lestini, 2007. Recombination proteins and rescue of arrested replication forks. DNA Repair (Amst.) 6 967–980. [DOI] [PubMed] [Google Scholar]
- Miller, J. H., 1972. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Morimatsu, K., and S. C. Kowalczykowski, 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 11 1337–1347. [DOI] [PubMed] [Google Scholar]
- Mulcair, M., P. Schaeffer, A. Oakley, H. Cross, C. Neylon et al., 2006. A molecular mousetrap determines polarity of termination of DNA replication in E. coli. Cell 125 1309–1319. [DOI] [PubMed] [Google Scholar]
- Ogawa, T., and T. Okazaki, 1984. Function of RNase H in DNA replication revealed by RNase H defective mutants of Escherichia coli. Mol. Gen. Genet. 193 231–237. [DOI] [PubMed] [Google Scholar]
- Reyes-Lamothe, R., X. Wang and D. Sherratt, 2008. Escherichia coli and its chromosome. Trends Microbiol. 16 238–245. [DOI] [PubMed] [Google Scholar]
- Rudolph, C. J., P. Dhillon, T. Moore and R. G. Lloyd, 2007. Avoiding and resolving conflicts between DNA replication and transcription. DNA Repair (Amst.) 6 981–993. [DOI] [PubMed] [Google Scholar]
- Rudolph, C. J., A. L. Upton, L. Harris and R. G. Lloyd, 2009. a Pathological replication in cells lacking RecG DNA translocase. Mol. Microbiol. 73 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph, C. J., A. L. Upton and R. G. Lloyd, 2009. b Replication fork collisions cause pathological chromosomal amplification in cells lacking RecG DNA translocase. Mol. Microbiol. 74 940–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph, C. J., A. L. Upton, G. S. Briggs and R. G. Lloyd, 2010. Is RecG a general guardian of the bacterial genome? DNA Repair (Amst.) 9 210–223. [DOI] [PubMed] [Google Scholar]
- Sandler, S. J., 2000. Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics 155 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler, S. J., and K. J. Marians, 2000. Role of PriA in replication fork reactivation in Escherichia coli. J. Bacteriol. 182 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassanfar, M., and J. W. Roberts, 1990. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 212 79–96. [DOI] [PubMed] [Google Scholar]
- Seigneur, M., V. Bidnenko, S. D. Ehrlich and B. Michel, 1998. RuvAB acts at arrested replication forks. Cell 95 419–430. [DOI] [PubMed] [Google Scholar]
- Shereda, R. D., A. G. Kozlov, T. M. Lohman, M. M. Cox and J. L. Keck, 2008. SSB as an organizer/mobilizer of genome maintenance complexes. Crit. Rev. Biochem. Mol. Biol. 43 289–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, M. R., S. Scaife and D. B. Wigley, 2001. Structural analysis of DNA replication fork reversal by RecG. Cell 107 79–89. [DOI] [PubMed] [Google Scholar]
- Singleton, M. R., M. S. Dillingham, M. Gaudier, S. C. Kowalczykowski and D. B. Wigley, 2004. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature 432 187–193. [DOI] [PubMed] [Google Scholar]
- Smith, G. R., 1990. RecBCD enzyme, pp. 78–98 in Nucleic Acids and Molecular Biology, edited by F. Eckstein and D. M. J. Lilley. Springer-Verlag, Berlin.
- Sun, W., S. Nandi, F. Osman, J. S. Ahn, J. Jakovleska et al., 2008. The FANCM ortholog Fml1 promotes recombination at stalled replication forks and limits crossing over during DNA double-strand break repair. Mol. Cell 32 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T., and H. Masai, 2006. Stabilization of a stalled replication fork by concerted actions of two helicases. J. Biol. Chem. 281 3484–3493. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., C. Taniyama, K. Arai and H. Masai, 2003. ATPase/helicase motif mutants of Escherichia coli PriA protein essential for recombination-dependent DNA replication. Genes Cells 8 251–261. [DOI] [PubMed] [Google Scholar]
- Trautinger, B. W., and R. G. Lloyd, 2002. Modulation of DNA repair by mutations flanking the DNA channel through RNA polymerase. EMBO J. 21 6944–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger, B. W., R. P. Jaktaji, E. Rusakova and R. G. Lloyd, 2005. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol. Cell 19 247–258. [DOI] [PubMed] [Google Scholar]
- Umezu, K., N. Chi and R. D. Kolodner, 1993. Biochemical interaction of the Escherichia coli RecF, RecO, and RecR proteins with RecA protein and single-stranded DNA binding protein. Proc. Natl. Acad. Sci. USA 90 3875–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, S. D., A. A. Mahdi and R. G. Lloyd, 1996. The RecG branch migration protein of Escherichia coli dissociates R-loops. J. Mol. Biol. 264 713–721. [DOI] [PubMed] [Google Scholar]
- Viswanathan, M., and S. T. Lovett, 1998. Single-strand DNA-specific exonucleases in Escherichia coli: roles in repair and mutation avoidance. Genetics 149 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan, M., and S. T. Lovett, 1999. Exonuclease X of Escherichia coli. A novel 3′-5′ DNase and DnaQ superfamily member involved in DNA repair. J. Biol. Chem. 274 30094–30100. [DOI] [PubMed] [Google Scholar]
- Viswanathan, M., V. Burdett, C. Baitinger, P. Modrich and S. T. Lovett, 2001. Redundant exonuclease involvement in Escherichia coli methyl-directed mismatch repair. J. Biol. Chem. 276 31053–31058. [DOI] [PubMed] [Google Scholar]
- Yarranton, G. T., and M. L. Gefter, 1979. Enzyme-catalyzed DNA unwinding: studies on Escherichia coli rep protein. Proc. Natl. Acad. Sci. USA 76 1658–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradka, K., M. Buljubasic, M. Petranovic and D. Zahradka, 2009. Roles of ExoI and SbcCD nucleases in “reckless” DNA degradation in recA mutants of Escherichia coli. J. Bacteriol. 191 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., A. A. Mahdi, G. S. Briggs and R. G. Lloyd, 2010. Promoting and avoiding recombination: contrasting activities of the Escherichia coli RuvABC Holliday junction resolvase and RecG DNA translocase. Genetics 185 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]