Abstract
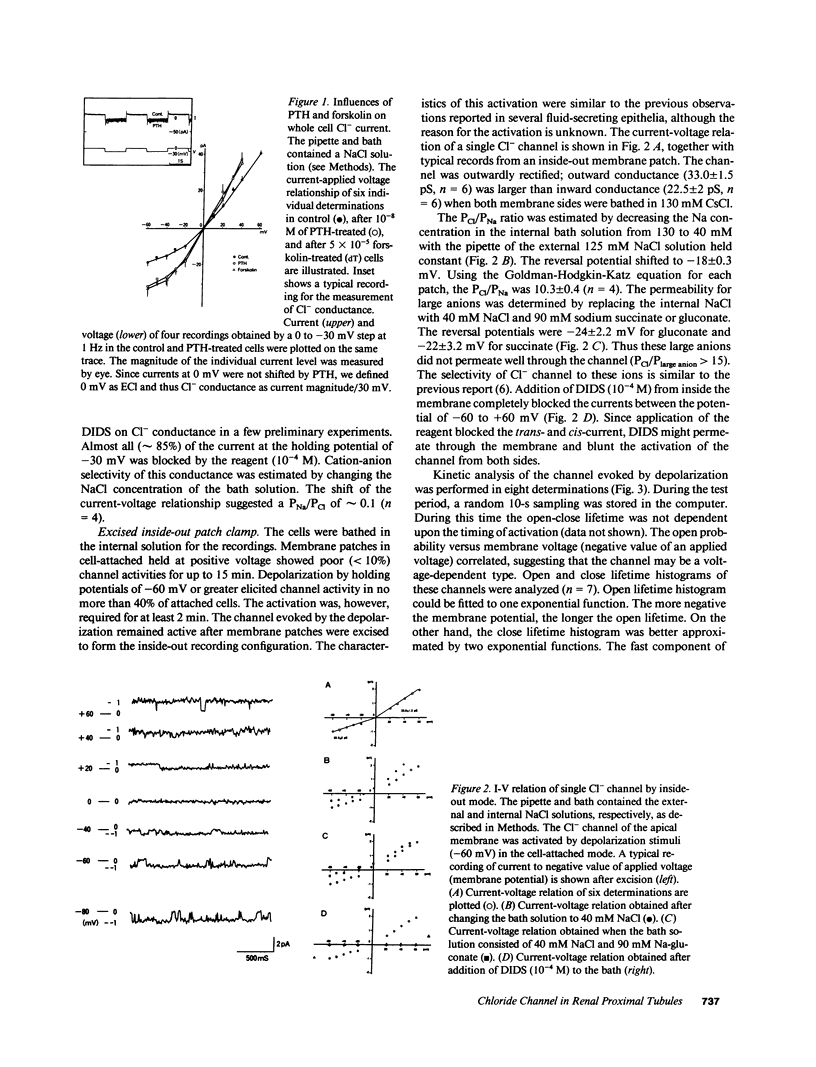
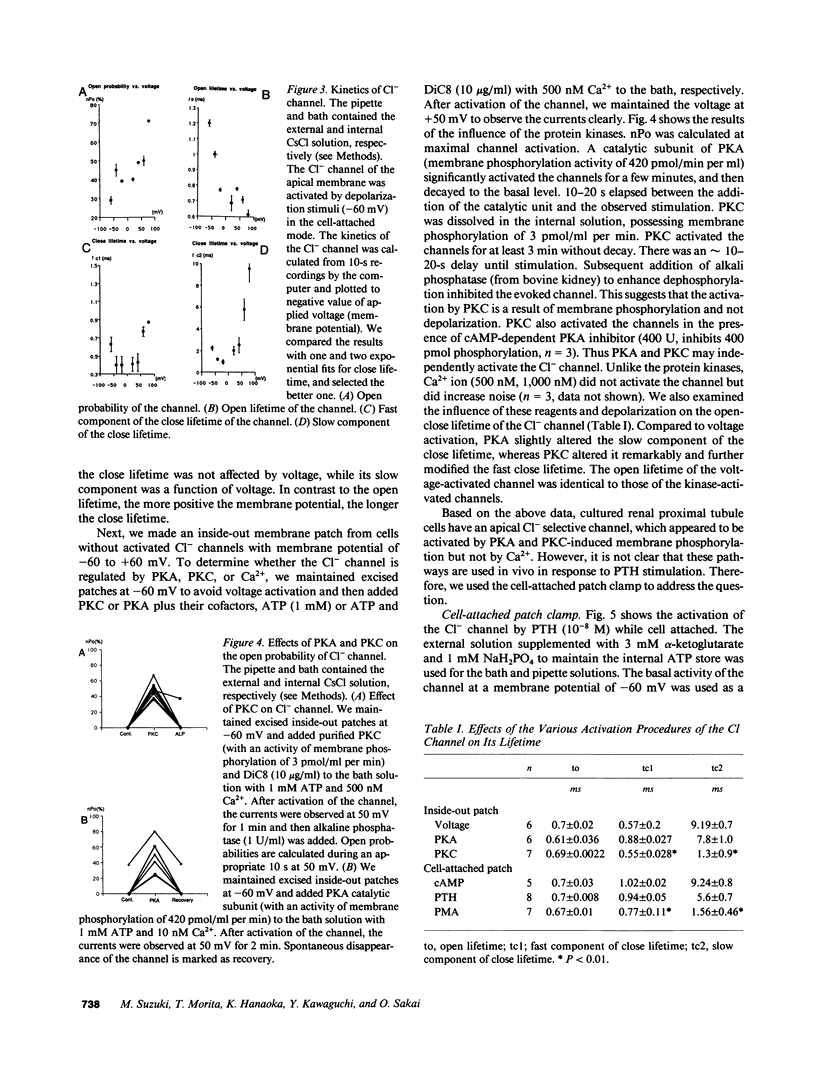
Previous data suggested an active Cl- conductance in the renal proximal convoluted tubule, although single channel conductance and regulation were not found. We have investigated the presence and regulation of the Cl- channel in proximal convoluted tubules by patch clamp analysis. The current-voltage relationship of whole cells with 130 mM NaCl in the pipette was nonlinear. The addition of 1-34 PTH (10(-8) M), forskolin, or cAMP significantly increased whole cell Cl- conductance. We found a single Cl- channel in excised apical membranes possessing conductance of 33 picosiemens (pS) at positive and 22.5 pS at negative potential, which was blocked by 4,4'-diisothiocyanostilbene-2,2'- disulfonic acid (10(-4) M) and was selective to Cl- (Cl/Na = 10). The channel was activated by prolonged membrane depolarization, by a catalytic subunit of protein kinase A (PKA), or by purified kinase C (PKC), but not by Ca2+ (1 microM) inside the membrane. During cell-attached patch clamping, the channel was similarly activated by PTH, phorbol ester, or dibutyryl cAMP in a dose-dependent manner. To investigate second messenger contributions to the PTH-action, the PTH-evoked channels were modified further by the subsequent addition of several blockers of the second messengers. This suggested that PKA and PKC were involved in Cl- channel activation. We therefore conclude that renal proximal convoluted tubule cells possess an apical Cl- channel activated by PTH via the PKA and PKC pathways.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Wasserstein A., Goldfarb S. PTH, calcitonin, cyclic nucleotides and the kidney. Annu Rev Physiol. 1981;43:583–595. doi: 10.1146/annurev.ph.43.030181.003055. [DOI] [PubMed] [Google Scholar]
- Alpern R. J. Apical membrane chloride/base exchange in the rat proximal convoluted tubule. J Clin Invest. 1987 Apr;79(4):1026–1030. doi: 10.1172/JCI112914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Eber S. L., Poelling R. E., Thorne P. K., Forte L. R. A dual mechanism for regulation of kidney phosphate transport by parathyroid hormone. Am J Physiol. 1987 Aug;253(2 Pt 1):E221–E227. doi: 10.1152/ajpendo.1987.253.2.E221. [DOI] [PubMed] [Google Scholar]
- Edelman A., Bouthier M., Anagnostopoulos T. Chloride distribution in the proximal convoluted tubule of Necturus kidney. J Membr Biol. 1981;62(1-2):7–17. doi: 10.1007/BF01870195. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Chloride channels in the luminal membrane of the rectal gland of the dogfish (Squalus acanthias). Properties of the "larger" conductance channel. Pflugers Arch. 1987 Jun;409(1-2):114–121. doi: 10.1007/BF00584757. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Cl- -channels in the apical cell membrane of the rectal gland "induced" by cAMP. Pflugers Arch. 1985 Apr;403(4):446–448. doi: 10.1007/BF00589260. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. A voltage-dependent ionic channel in the basolateral membrane of late proximal tubules of the rabbit kidney. Pflugers Arch. 1986;407 (Suppl 2):S142–S148. doi: 10.1007/BF00584943. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Moskowitz D., Esbrit P., Civitelli R., Westbrook S., Huskey M. Stimulation of inositol trisphosphate and diacylglycerol production in renal tubular cells by parathyroid hormone. J Clin Invest. 1987 Jan;79(1):230–239. doi: 10.1172/JCI112788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Watanabe M., Hidaka H. N-(2-Aminoethyl)-5-isoquinolinesulfonamide, a newly synthesized protein kinase inhibitor, functions as a ligand in affinity chromatography. Purification of Ca2+-activated, phospholipid-dependent and other protein kinases. J Biol Chem. 1985 Mar 10;260(5):2922–2925. [PubMed] [Google Scholar]
- Ishibashi K., Rector F. C., Jr, Berry C. A. Chloride transport across the basolateral membrane of rabbit proximal convoluted tubules. Am J Physiol. 1990 Jun;258(6 Pt 2):F1569–F1578. doi: 10.1152/ajprenal.1990.258.6.F1569. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen M., Vereecke J., Carmeliet E., Van den Berghe H., Cassiman J. J. Outward-rectifying chloride channels in cultured adult and fetal human nasal epithelial cells. J Membr Biol. 1990 Aug;117(2):123–130. doi: 10.1007/BF01868679. [DOI] [PubMed] [Google Scholar]
- Krapf R., Berry C. A., Verkman A. S. Estimation of intracellular chloride activity in isolated perfused rabbit proximal convoluted tubules using a fluorescent indicator. Biophys J. 1988 Jun;53(6):955–962. doi: 10.1016/S0006-3495(88)83176-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Paulais M., Teulon J. cAMP-activated chloride channel in the basolateral membrane of the thick ascending limb of the mouse kidney. J Membr Biol. 1990 Feb;113(3):253–260. doi: 10.1007/BF01870076. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Protein kinase C from rat renal mesangial cells: its role in homologous desensitization of angiotensin II-induced polyphosphoinositide hydrolysis. Biochim Biophys Acta. 1988 May 13;969(3):263–270. doi: 10.1016/0167-4889(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Ishikawa T., Matsushima S., Naka M., Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987 Jun 5;262(16):7796–7801. [PubMed] [Google Scholar]
- Sariban-Sohraby S., Burg M. B., Turner R. J. Apical sodium uptake in toad kidney epithelial cell line A6. Am J Physiol. 1983 Sep;245(3):C167–C171. doi: 10.1152/ajpcell.1983.245.3.C167. [DOI] [PubMed] [Google Scholar]
- Schmid A., Burckhardt G., Gögelein H. Single chloride channels in endosomal vesicle preparations from rat kidney cortex. J Membr Biol. 1989 Nov;111(3):265–275. doi: 10.1007/BF01871011. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Light D. B., Fejes-Toth G., Naray-Fejes-Toth A., Stanton B. A. A GTP-binding protein activates chloride channels in a renal epithelium. J Biol Chem. 1990 May 15;265(14):7725–7728. [PubMed] [Google Scholar]
- Suzuki M., Capparelli A., Jo O. D., Kawaguchi Y., Ogura Y., Miyahara T., Yanagawa N. Phosphate transport in the in vitro cultured rabbit proximal convoluted and straight tubules. Kidney Int. 1988 Aug;34(2):268–272. doi: 10.1038/ki.1988.175. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kawahara K., Ogawa A., Morita T., Kawaguchi Y., Kurihara S., Sakai O. [Ca2+]i rises via G protein during regulatory volume decrease in rabbit proximal tubule cells. Am J Physiol. 1990 Mar;258(3 Pt 2):F690–F696. doi: 10.1152/ajprenal.1990.258.3.F690. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Van Driel R., Jastorff B., Baraniak J., Stec W. J., De Wit R. J. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984 Aug 25;259(16):10020–10024. [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Chloride uptake by brush border membrane vesicles isolated from rabbit renal cortex. Coupling to proton gradients and K+ diffusion potentials. J Clin Invest. 1981 Jan;67(1):103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]



