Abstract
The Drosophila seminal fluid protein (SFP) sex peptide (SP) elicits numerous post-mating responses, including increased egg laying and decreased sexual receptivity, in the mated female. Unlike other SFPs, which are detectable in mated females for only a few hours post mating, SP is maintained—and its effects are sustained—for several days. The persistence of SP in the mated female's reproductive tract is thought to be a consequence of its binding to, and gradual release from, sperm in storage, which maintains SP's ability to act within the female reproductive tract. Recent studies have shown that several other SFPs, acting in a network, are needed for SP's localization to sperm and are necessary for the efficient release of sperm from storage. This result suggested an additional new role for SP modulating the release of sperm from storage. We tested for this possibility by examining sperm storage parameters in mated females that did not receive SP. We found that while sperm accumulation into storage was unaffected, sperm depletion from storage sites was significantly decreased (or impaired) in the absence of SP. Mates of males expressing a modified SP that is unable to be released from sperm showed a similar phenotype, indicating that release of sperm-bound SP is a necessary component of normal sperm depletion. Additionally, SP null males were more successful in a sperm competitive environment when they were first to mate, which is likely a consequence of higher retention of their sperm due to defective sperm release. Our findings illustrate a direct role for SP in the release of sperm from storage.
IN Drosophila, males affect the fertility, reproductive physiology, and behavior of females through the transfer of sperm, seminal fluid proteins (SFPs), and cuticular hydrocarbons during mating (reviewed in Chapman and Davies 2004; Dickson 2008; Wolfner 2009). Sperm and SFPs influence the mated female's egg production and her sexual receptivity to subsequent males in two distinct phases: within the first 24 hr of mating (the “short-term response,” or STR), where increased egg production and reduced sexual receptivity depend on the receipt of SFPs (Kalb et al. 1993; Xue and Noll 2000; Bretman et al. 2010), and beyond 24 hr, when the maintenance of these processes requires both sperm and SFPs (the “long-term response,” or LTR; Manning 1962, 1967; Kalb et al. 1993; Chapman et al. 2003; Liu and Kubli 2003; Peng et al. 2005a). In particular, the sex peptide (SP; Acp70A), a 36-amino-acid SFP, plays a central role in the LTR (reviewed in Kubli 2003; Ravi Ram and Wolfner 2007a; Chapman 2008), increasing egg production (Soller et al. 1999) and decreasing female receptivity to remating (Chen et al. 1988; Chapman et al. 2003; Liu and Kubli 2003); SP also affects other behaviors and hormonal physiology (Moshitzky et al. 1996; Peng et al. 2005b; Wigby and Chapman 2005; Carvalho et al. 2006; Mueller et al. 2007; Isaac et al. 2010).
SP's mechanism of action is well characterized. Upon transfer to the female, a fraction of the transferred SP enters the circulatory system (Pilpel et al. 2008). The SP that remains within the female's reproductive tract localizes—with the help of several other SFPs (CG1652, CG1656, CG9997, and CG17575)—to the sperm storage organs, binding to sperm and/or to the lumen of the sperm storage organs (Peng et al. 2005a; Ravi Ram and Wolfner 2009). SP's activity in egg laying and receptivity is mediated through the sex peptide receptor (SPR), a G-protein-coupled receptor that is broadly expressed in the female reproductive tract and nervous system (Yapici et al. 2008). In particular, SPR mediates SP action through a specific set of neurons—those expressing both fruitless and pickpocket—that innervate the female reproductive tract (Hasemeyer et al. 2009; Yang et al. 2009).
Mated females' STR to SP does not require sperm in storage, but the persistence of SP's effects does (Chapman et al. 2003; Liu and Kubli 2003). The binding of SP to sperm, and its subsequent cleavage to release SP's active C-terminal portion, is proposed to be the basis for the LTR within mated females (Peng et al. 2005a). Although the role of stored sperm in the function of SP has been documented, the reverse—whether SP influences sperm storage—has not been tested. A role for SP in sperm storage was suggested by the observation of sperm retained in storage up to 20 days post mating in SP null mates (Liu and Kubli 2003), a time when singly mated females are normally no longer fertile. Also suggesting the possibility of SP's role in sperm storage is an observation about several SFPs that are needed for SP's localization to sperm (Ravi Ram and Wolfner 2009). Removal of these gene products individually from the male seminal fluid (via RNA interference) resulted in the retention of sperm in storage in their mates (Ravi Ram and Wolfner 2007b). This phenotype gave these males an advantage in a sperm competitive environment, presumably the result of the higher number of sperm retained in storage (when compared to control mates) at the time of remating (Avila and Wolfner 2009).
While these results suggested indirectly that SP might play a role in female sperm storage, the possibility of such a role has never been examined. Sperm storage in Drosophila is influenced by post-mating events involved in the entry of sperm into storage, maintenance of sperm in storage, and the release/depletion of sperm from storage. A role for SP in sperm storage could be detected in early events involved in sperm entry into storage or in later events involved in sperm release. One early condition implicated in the entry of sperm into storage is the conformational state of the uterus. Upon mating, the female reproductive tract undergoes a series of morphological changes implicated in the entry of sperm into storage (Adams and Wolfner 2007; Avila and Wolfner 2009). These changes are mediated by Acp36DE, an SFP required for efficient accumulation of sperm into storage (Neubaum and Wolfner 1999; Bloch Qazi and Wolfner 2003; Avila and Wolfner 2009). Upon entry into storage, sperm are maintained in the storage organs before they are released for utilization; both of these mechanisms are influenced by SFPs (Ravi Ram and Wolfner 2007a; Wong et al. 2008). Here, we determined the role of SP in sperm dynamics by examining uterine conformation, total sperm storage, sperm release, and sperm competition in the presence or absence of SP in the male ejaculate. We also examined sperm storage when males transferred a mutant, noncleavable form of SP that is unable to be released from sperm. We found that, while the absence of SP does not affect uterine conformational change, entry of sperm into storage, or total sperm stored, SP is required for the normal release of sperm in the mated female. This study identifies a new role for SP, in addition to its functions related to egg production and overt behaviors.
MATERIALS AND METHODS
Flies:
The SP null mutant line (SP0/TM3, Sb ry), the SP deficiency line (Δ130/TM3, Sb ry), and the SP-TGQQ line (y w/Y; SP-TGQQ/ SP-TGQQ; Δ130/TM3, Sb Ser) were kind gifts of Eric Kubli (University of Zurich, Zurich, Switzerland). SP null and control males were obtained by crossing the SP null mutant line to the SP deficiency line, generating SP0/Δ130 (experimental) and Δ130/TM3, Sb ry (control) males. SP cleavage mutant males (SP-TGQQ) were obtained by crossing the SP null mutant line to the SP-TGQQ line, generating w/Y; SP-TGQQ/+; SP0/Δ130 (experimental) males and w/Y; SP-TGQQ/+; Δ130/TM3, Sb ry (control) males. Matings were carried out by crossing 3- to 5-day-old unmated mutant or control males to 3- to 5-day-old virgin females of the Canton-S strain of Drosophila melanogaster. All flies were maintained on a standard yeast–glucose medium at room temperature (22° ± 1°) and a 12:12 light:dark cycle.
Uterine conformational assay:
Uterine conformation of mated females was determined as in Avila and Wolfner (2009). Briefly, an unmated male and a virgin female were placed together into an empty glass vial containing moistened Whatman filter paper. Their time of mating initiation was recorded, and females were frozen at −20° at 35 min after the start of mating (ASM). Reproductive tracts from mated females were dissected in 0.7% NaCl and visualized using an Olympus SZ61 dissection microscope. Uteri were staged as in Adams and Wolfner (2007). Stage distribution of mutant vs. control mates was analyzed using a Wilcoxon test (rank sums) in JMP software (JMP IN, 5.1.2).
Sperm counts:
To examine the direct effects of SP presence and release/cleavage from sperm on storage dynamics, we counted the number of sperm stored in female sperm storage organs (the paired spermathecae and the seminal receptacle) at various times ASM. Briefly, an unmated male (SP-null mutant SP0/Δ130 or control Δ130/TM3, Sb ry, and cleavage mutant w/Y; SP-TGQQ/+; SP0/Δ130 or control w/Y; SP-TGQQ/+; Δ130/TM3, Sb ry) and virgin female were placed together in a vial containing standard medium until mating occurred. Males were removed shortly after mating to ensure that females mated only once. Females remained alone in their vials for 2 hr, 4 days, or 10 days ASM (as in Ravi Ram and Wolfner 2007b) at which time they were frozen until processed for sperm counts. To visualize sperm, female reproductive tracts were removed, processed, and stained with orcein using previously described methods (Neubaum and Wolfner 1999; Mueller et al. 2008; Bloch Qazi and Hogdal 2010). Sperm in the female storage organs were counted using a transillumination microscope with ×1000 magnification. We avoided treatment biases in counting by coding samples prior to their quantification. A repeatability of >93% for each experiment, based on duplicate counts of a subset of samples examined, indicated high counting precision. Differences between females mated to SP mutant or control males were analyzed using t-tests with SPSS software (SPSS 15.0) where data did not violate the assumptions of parametric statistical tests. For those cases that did not fit into the assumptions of parametric statistical tests, we analyzed the data using the Wilcoxon test in JMP software (JMP IN, 5.1).
Sperm competition assays:
Defensive sperm competition assays (in which an experimental male is the first to mate) were done as in Clark et al. (1995) using a cn bw (white-eyed) strain of D. melanogaster. In brief, 3- to 5-day-old virgin cn bw females were mated to either SP null males or their sibling control males. Each female was remated on the third day to a single cn bw male, placed alone into a fresh food vial, and allowed to lay eggs for 10 days. Paternity of the progeny was determined using eye color. JMP software was used for statistical analysis (JMP IN, 5.1.2).
RESULTS AND DISCUSSION
SP is not required for sperm entry into, or accumulation within, storage:
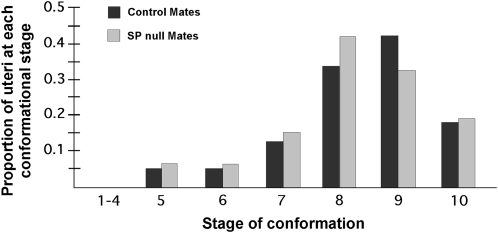
To test for a role for SP in sperm storage events, we first asked whether SP is necessary for entry of sperm into storage. To address this, we examined the effect of SP on two measures of sperm entry into storage: (1) the conformational state of the uterus and (2) the number of sperm stored at early time points post mating. Conformational changes of the uterus initiated during mating are associated with sperm entry into storage (Adams and Wolfner 2007; Avila and Wolfner 2009). We therefore compared the uterine conformational stage distribution in reproductive tracts of females mated to SP null or control males. We observed that progression and completion of uterine changes in SP null mates were comparable—in extent and rate—to those of their controls (Wilcoxon: Z = −0.05, P > 0.86; Figure 1).
Figure 1.—
Progression of different conformational changes in the uteri of females mated to SP null or control males. Mates of SP null and control males had a similar uterine stage distribution at 35 min ASM. SP has no significant effects on the distribution of uterine conformation stages (Ncont = 24, NΔSP = 22, P > 0.86).
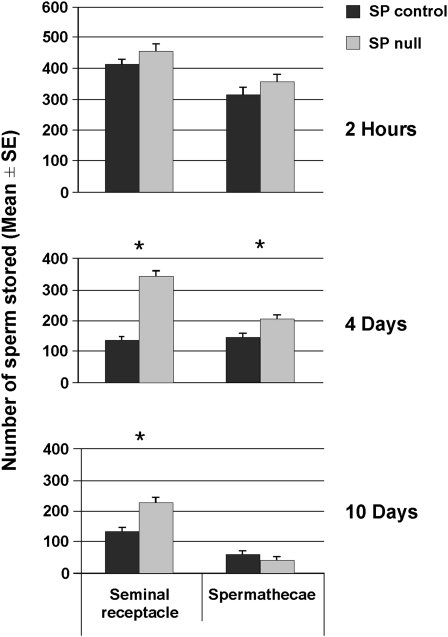
To determine if the number of sperm stored at early time points post mating is influenced by SP, we counted sperm in storage at 2 hr ASM. By this time, eggs have just begun to be ovulated and sperm storage is at maximal levels. Mutants that affect the entry of sperm into storage (e.g., Acp36DE1; Neubaum and Wolfner 1999; Bloch Qazi and Wolfner 2003) show phenotypes at this time point. We counted sperm at 2 hr ASM in SP null and control mates. Mates of SP null and control males did not differ significantly in the number of sperm stored in the seminal receptacle or spermathecae at 2 hr ASM (seminal receptacle: t-test, t = −1.41, d.f. = 29, P = 0.17; spermathecae: t = −1.32, d.f. = 43, P = 0.20; Figure 2), suggesting that lack of SP did not affect the transfer of sperm or their entry into either the seminal receptacle or the spermathecae. Taken together, these results suggest that SP is not required for the initial events necessary for the storage of sperm. This is consistent with observations from previous studies showing that depletion of SFPs needed for SP's localization/retention in mated females affects neither uterine conformation nor sperm entry into storage (Ravi Ram and Wolfner 2007b, 2009; Avila and Wolfner 2009).
Figure 2.—
Females mated to SP null males retain sperm long term. Mean (+1 SE) number of sperm stored in the seminal receptacles (SR) and spermathecae (Spp) of females mated to SP null (shaded bars) and control (solid bars) males at 2 hr, 4 days, and 10 days ASM. Asterisks indicate a statistically significant difference between female groups. We did not detect a significant difference in the number of sperm stored by mates of SP null males compared to their controls in the seminal receptacles or spermathecae at 2 hr ASM (SR: t-test, t = −1.41, Ncont = 18, NΔSP = 13, P = 0.17; Spp: t = −1.32, Ncont = 23, NΔSP = 22, P = 0.20), but we observed a significant difference in the number of sperm stored in seminal receptacles and spermathecae at 4 days ASM (SR: t = −12.39, Ncont = 47, NΔSP = 47, P < 0.0005; Spp: t = −3.63, Ncont = 37, NΔSP = 36, P = 0.001) and in the SR, but not in the Spp at 10 days ASM (SR: t = −4.26, Ncont = 29, NΔSP = 34, P <0.0005; Spp: t = 1.11, Ncont = 29, NΔSP = 34, P = 0. 274).
SP affects the release of stored sperm in mated females:
The long-term persistence of post-mating changes in D. melanogaster requires at least four SFPs: CG9997, CG1652/CG1656, and CG17575 along with SP (Peng et al. 2005a; Ravi Ram and Wolfner 2007b). Mates of CG9997, CG1652/CG1656, or CG17575 knockdown males are also defective in the release of sperm from storage at 4 days and/or 10 days ASM, respectively (Ravi Ram and Wolfner 2007b). Further, SP requires these four SFPs for its localization to sperm and/or the sperm storage organs (Ravi Ram and Wolfner 2009). These findings suggested a link between SP and sperm release, but the potential for such a link had yet to be explored directly. To test if sperm are stored and released normally in females mated to SP null males at levels comparable to their controls, we analyzed sperm storage in females mated to SP null males and their controls at 4 and 10 days ASM.
By 4 days ASM, mates of SP null males retained 153% more sperm in their seminal receptacles (t-test: t = −12.39, d.f. = 92, P < 0.0005; Figure 2) and 41% more sperm in their spermathecae (t = −3.625, d.f. = 71, P = 0.001; Figure 2) than did mates of control males. These differences were maintained as long as 10 days ASM, although low numbers of stored sperm at this later time point appear to decrease the magnitude of the SP effect. Females that did not receive SP from their mates retained 72% more sperm in their seminal receptacles at 10 days ASM than females mated to control males (t = −4.26, d.f. = 61, P < 0.0005; Figure 2). There was no difference between the female groups in numbers of sperm stored in the spermathecae (t = 1.11, d.f. = 61, P = 0.274; Figure 2) at 10 days ASM. The differences in sperm release efficiency between the seminal receptacle and spermathecae may be due, in part, to chemical differences within the lumens of these organs. The two types of organ differ in the secretable proteins that they produce (Allen and Spradling 2008; Prokupek et al. 2008, 2010). Thus the sperm stored within them are likely to be exposed to different environments. Indeed, there is some suggestive evidence, in addition to what we report here, that sperm release may be regulated differently between the organ types: the inability of SP to bind sperm has major effects on sperm release in the seminal receptacle but not in spermathecae (Ravi Ram and Wolfner 2007b, 2009).
Our results demonstrate that a lack of SP impairs the depletion of sperm from the seminal receptacle and, to a lesser extent, the spermathecae in mated females. This potentially explains a previous report of high sperm numbers in the storage organs of females mated to SP null males at 20 days post mating (Liu and Kubli 2003). Our results also suggest that the defect in sperm release from the seminal receptacle observed in mates of CG9997, CG1652, CG1656, or CG17575 knockdown males is likely the result of SP's failure to localize to the sperm and/or sperm storage organs in those females.
Release of sperm-bound SP is required for long-term sperm release:
Because the release of sperm-bound SP is crucial for the LTR (Peng et al. 2005a), we analyzed sperm release in females who received a form of SP unable to be cleaved from stored sperm. To do so, we used transgenic males, referred to as SP-TGQQ males, that express (and transfer to females) only a modified SP that is mutated at a trypsin cleavage site near the N terminus of SP (R7-Q7, K8-Q8) (Peng et al. 2005a). SP expressed from this allele is able to bind sperm but is unable to be released once bound. As we observed the requirement of SP for release of sperm stored to be more robust in the seminal receptacle, we focused on sperm stored in this organ in these experiments.
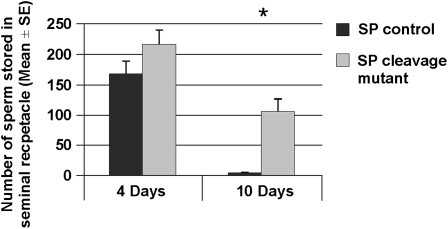
The total number of sperm stored in the seminal receptacle of females mated to SP-TGQQ males or their controls at 4 and 10 days ASM was recorded. We did not observe any significant difference between control and SP-TGQQ mates in the number of sperm stored in their seminal receptacles at 4 days ASM (t = −1.54, d.f. = 34, P = 0.065; Figure 3). However, at 10 days ASM, females mated to SP-TGQQ males had significantly more sperm in their seminal receptacles compared to controls (Wilcoxon: Z = −2.68, P < 0.003; Figure 3). The differences in total stored sperm that we observed between SP null and SP-TGQQ mates are likely due to our use of males of differing genetic backgrounds. As such, genetic background effects might influence sperm release from storage or ovulation—both of which can impact SR storage in ways that will be apparent by 10 days post mating. Additionally, subtle differences in media, culture density, and timing of matings may affect female fertility, producing variation in total sperm stored.
Figure 3.—
Females mated to males whose SP is unable to be cleaved from sperm retain sperm longer in the seminal receptacles. The number of sperm was counted in the seminal receptacles of females mated to males expressing a modified SP [see text and Peng et al. (2005a,b)] or their controls at 4 days and 10 days ASM. Asterisk indicates a statistically significant difference between groups. Total sperm stored by mates of SP-TGQQ mutant males was comparable to controls at 4 days ASM (SR: t = −1.54, Ncont = 17, NSP-TGQQ = 19, P = 0.065), but we observed a significant difference in the number of sperm stored in the seminal receptacle at 10 days ASM (SR: Wilcoxon: Z = −2.68, Ncont = 10, NSP-TGQQ = 10, P < 0.003).
These results show that sperm release from storage is defective in females when SP cannot be cleaved from sperm, but this defect appears later than in females who do not receive any SP. We expect that free, non-sperm-bound SP remaining in the female after mating may account for the differences that we observed between SP null and SP-TGQQ mates at 4 days ASM; free SP transferred by SP-TGQQ males may allow initial sperm release from storage at normal rates. However, sperm release from storage in the long term, i.e., >4 days ASM, requires the presence and release of sperm-bound SP. These results also suggest that the observed decline in the rate of sperm depletion from female storage sites in response to infrequent exposure to fresh oviposition/food quality sites (Bloch Qazi and Hogdal 2010) could potentially be caused by decreases in available sex peptide.
SP affects defensive sperm competition:
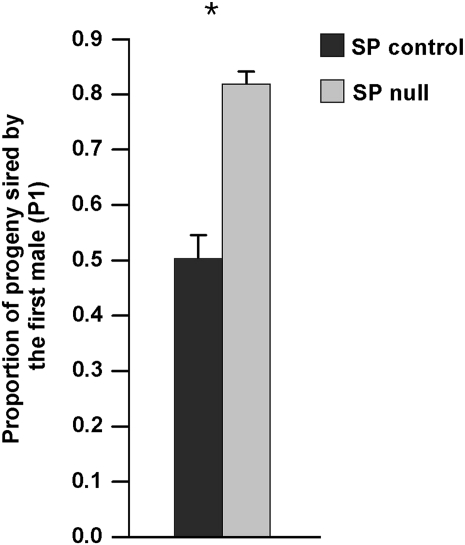
The role of seminal proteins in sperm storage/release has been shown to impact sperm competition (Neubaum and Wolfner 1999; Chapman et al. 2000; Wong et al. 2008; Avila and Wolfner 2009). For example, males lacking SFPs required for normal sperm release (Ravi Ram and Wolfner 2007b) were better competitors, compared to controls, in defensive sperm competition assays (Avila and Wolfner 2009). Given our finding that SP null mates are defective in efficient sperm release, we hypothesized that lack of SP from the male ejaculate would have a similar effect in defensive sperm competition experiments to that of knockdown of SFPs needed for SP's localization to sperm: i.e., SP null males should sire more progeny in a defensive sperm competition experiment. Consistent with this hypothesis, mates of SP null males sired >31% more progeny than did control males when each was the first of two mating males (Wilcoxon: Z = −5.2, P < 0.0001; Figure 4). Since sperm release is compromised in the absence of SP—resulting in the retention of sperm from SP null males in storage—this result suggests that the second mating male provided the full complement of SFPs (including SP), thereby facilitating the release and subsequent utilization of stored sperm from SP null males, a phenomenon termed “copulation complementation” (Xue and Noll 2000).
Figure 4.—
First-mating SP null males sire a higher proportion of progeny than do controls (Wilcoxon: Z = −5.2, Ncont = 28, NΔSP = 31, P < 0.0001). Asterisk indicates a statistically significant difference between groups.
The results reported here are consistent with a recent report that shows that SP null males sire more progeny than second-mating males when remating occurred at 6 and 24 hr (Fricke et al. 2009). Our results are also in agreement with studies that show an association of P1—the proportion of progeny sired by the first-mating male—with natural alleles of SP (Fiumera et al. 2007), as well with a recent study showing a genetic male by female interaction between extracted chromosome lines that carry wild-derived variant alleles of SP and SPR (C. Y. Chow, M. F. Wolfner and A. G. Clark, unpublished results). Sperm release efficiency, resulting from the sperm release defect observed in this study, potentially has consequences in terms of progeny production in females who do not remate.
Conclusion:
SP, implicated in diverse physiological and behavioral functions, is a major player in Drosophila reproductive processes. The results presented here show an additional function for SP, providing the first direct link between SP and sperm storage events. We show that SP is required for efficient sperm release/utilization: females who fail to receive SP retain more sperm in their sperm storage organs. Receipt of free SP is not sufficient to alleviate this defect as mates of SP-TGQQ males also retain sperm at 10 days ASM, suggesting that a constant SP signal to the female is needed for proper sperm utilization. SP has a substantially larger effect on sperm usage in the seminal receptacle, as we did not detect a significant difference in total sperm stored in the spermathecae in the presence vs. absence of SP at 10 days ASM. The sperm retention that we observed has a pronounced effect on sperm in a competitive environment, since SP null males sired significantly more progeny than their sibling controls. Thus, the findings in this study indicate that a continuous SP signal to the female is needed not only to decrease female receptivity and increase egg production, as has been previously shown, but also for proper sperm utilization. It is interesting to speculate on the selective advantage for SP's sperm-release function, given that SP binding to sperm maintains SP's presence in mated females. Perhaps since SP also increases egg production, its regulation of sperm release may aid in coordinating gamete availability.
Acknowledgments
We thank the members of the Wolfner lab for helpful discussion in the preparation of this manuscript, and anonymous reviewers for helpful suggestions. Special thanks go to Eric Kubli, University of Zurich, Zurich, Switzerland, for providing the sex peptide stocks. This research was supported by National Institutes of Health grant R01-HD038921 to M.F.W., including an American Recovery and Reinvestment Act (ARRA) supplement to support M.C.B.Q.
References
- Adams, E. M., and M. F. Wolfner, 2007. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J. Insect Physiol. 53 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. K., and A. C. Spradling, 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 135 311–321. [DOI] [PubMed] [Google Scholar]
- Avila, F. W., and M. F. Wolfner, 2009. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA 106 15796–15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi, M. C., and L. Hogdal, 2010. Hold on: females modulate sperm depletion from storage sites in the fly Drosophila melanogaster. J. Insect Physiol. 56 1332–1340. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi, M. C., and M. F. Wolfner, 2003. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J. Exp. Biol. 206 3521–3528. [DOI] [PubMed] [Google Scholar]
- Bretman, A., M. K. Lawniczak, J. Boone and T. Chapman, 2010. A mating plug protein reduces early female remating in Drosophila melanogaster. J. Insect Physiol. 56 107–113. [DOI] [PubMed] [Google Scholar]
- Carvalho, G. B., P. Kapahi, D. J. Anderson and S. Benzer, 2006. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol. 16 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, T., 2008. The soup in my fly: evolution, form and function of seminal fluid proteins. PLoS Biol. 6 e179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, T., and S. J. Davies, 2004. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides 25 1477–1490. [DOI] [PubMed] [Google Scholar]
- Chapman, T., D. M. Neubaum, M. F. Wolfner and L. Partridge, 2000. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc. Biol. Sci. USA 267 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, T., J. Bangham, G. Vinti, B. Seifried, O. Lung et al., 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 100 9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. S., E. Stummzollinger, T. Aigaki, J. Balmer, M. Bienz et al., 1988. A male accessory gland peptide that regulates reproductive behavior of female Drosophila melanogaster. Cell 54 291–298. [DOI] [PubMed] [Google Scholar]
- Clark, A. G., M. Aguade, T. Prout, L. G. Harshman and C. H. Langley, 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, B. J., 2008. Wired for sex: the neurobiology of Drosophila mating decisions. Science 322 904–909. [DOI] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2007. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics 176 1245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke, C., S. Wigby, R. Hobbs and T. Chapman, 2009. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J. Evol. Biol. 22 275–286. [DOI] [PubMed] [Google Scholar]
- Hasemeyer, M., N. Yapici, U. Heberlein and B. J. Dickson, 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61 511–518. [DOI] [PubMed] [Google Scholar]
- Isaac, R. E., C. Li, A. E. Leedale and A. D. Shirras, 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 277 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb, J. M., A. J. DiBenedetto and M. F. Wolfner, 1993. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc. Natl. Acad. Sci. USA 90 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli, E., 2003. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 60 1689–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., and E. Kubli, 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100 9929–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, A., 1962. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature 194 252–253. [Google Scholar]
- Manning, A., 1967. The control of sexual receptivity in female Drosophila. Anim. Behav. 15 239–250. [DOI] [PubMed] [Google Scholar]
- Moshitzky, P., I. Fleischmann, N. Chaimov, P. Saudan, S. Klauser et al., 1996. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 32 363–374. [DOI] [PubMed] [Google Scholar]
- Mueller, J. L., J. L. Page and M. F. Wolfner, 2007. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics 175 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J. L., J. R. Linklater, K. Ravi Ram, T. Chapman and M. F. Wolfner, 2008. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics 178 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum, D. M., and M. F. Wolfner, 1999. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., S. Chen, S. Busser, H. Liu, T. Honegger et al., 2005. a Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15 207–213. [DOI] [PubMed] [Google Scholar]
- Peng, J., P. Zipperlen and E. Kubli, 2005. b Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 15 1690–1694. [DOI] [PubMed] [Google Scholar]
- Pilpel, N., I. Nezer, S. W. Applebaum and Y. Heifetz, 2008. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem. Mol. Biol. 38 320–330. [DOI] [PubMed] [Google Scholar]
- Prokupek, A., F. Hoffmann, S. I. Eyun, E. Moriyama, M. Zhou et al., 2008. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution 62 2936–2947. [DOI] [PubMed] [Google Scholar]
- Prokupek, A. M., S. I. Eyun, L. Ko, E. N. Moriyama and L. G. Harshman, 2010. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. J. Evol. Biol. 23 1386–1398. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. a Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47 427–445. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. b Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 3 e238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram, K. and M. F. Wolfner, 2009. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Natl. Acad. Sci. USA 106 15384–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller, M., M. Bownes and E. Kubli, 1999. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208 337–351. [DOI] [PubMed] [Google Scholar]
- Wigby, S., and T. Chapman, 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15 316–321. [DOI] [PubMed] [Google Scholar]
- Wolfner, M. F., 2009. Battle and ballet: molecular interactions between the sexes in Drosophila. J. Hered. 100 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, A., S. N. Albright, J. D. Giebel, K. R. Ram, S. Ji et al., 2008. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics 180 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, L., and M. Noll, 2000. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc. Natl. Acad. Sci. USA 97 3272–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. H., S. Rumpf, Y. Xiang, M. D. Gordon, W. Song et al., 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici, N., Y. J. Kim, C. Ribeiro and B. J. Dickson, 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451 33–37. [DOI] [PubMed] [Google Scholar]