Abstract
Efforts to develop an effective vaccine against ricin are focused on the engineering of attenuated and stable recombinant forms of the toxin’s enzymatic A subunit (RTA). While several candidate antigens are in development, vaccine design and efficacy studies are being undertaken in the absence of a fundamental understanding of those regions of RTA that are critical in eliciting protective immunity. In this present study, we produced and characterized a collection of monoclonal antibodies (MAbs) directed against five distinct immunodominant regions on RTA, and used these MAbs to identify several key neutralizing epitopes on the toxin. Protective MAbs were directed against α-helices located in RTA folding domains 1 and 2, whereas non-neutralizing antibodies recognized random coils and loops that were primarily confined to folding domain 3. These data offer insights into the immunodominant and structural determinants on RTA that give rise to protective immunity, and for the first time provide an immunological rationale for ricin vaccine design.
1. Introduction
Ricin toxin is a natural product of the castor bean plant, Ricinis communis, which is cultivated on an industrial scale around the world for the production of castor oil. The toxin constitutes up to 5% of the total protein of the castor bean and can be extracted from the mash produced as a by-product of castor oil production, through several simple enrichment steps. Ricin, in semi-purified or purified form, is extremely toxic [1–3]. Although few cases of ricin intoxication in humans have been reported [4], animal studies confirm that the toxin can be fatal by injection, inhalation or ingestion. Ricin has a history of being used both as a biological weapon and biothreat agent [5], a fact that is disconcerting considering no available vaccines or antidotes are currently available to prevent or counteract the effects of the toxin. For these reasons, the Centers for Disease Control and Prevention (CDC) and the Department of Health and Human Services (HHS) have classified ricin as a Category B select agent, and the National Institutes of Health consider the development of countermeasures against ricin toxin an integral part of their biodefense research program.
Ricin is a member of the family of type II ribosome-inactivating proteins, a family that includes abrin and shiga toxins [6, 7]. Ricin toxin consists of a 32 kDA enzymatic A subunit (RTA) joined by a disulfide bond to a 34-kDA lectin B subunit (RTB). RTA is an RNA N-glycosidase whose target is a conserved adenine residue in the so-called sarcin/ricin loop (SRL) of eukaryotic 28S ribosomal RNA [8]. RTB recognizes, with low affinity, α(1-3)-linked galactose and N-acetylgalactosamine residues on the surface of almost every cell type, and it mediates toxin internalization via both clathrin-dependent and clathrin-independent mechanisms [9–11]. Once internalized, the toxin exploits multiple endocytic pathways, and traffics in a retrograde fashion from early endosomes to the trans-Golgi network, eventually reaching the endoplasmic reticulum (ER) [12, 13]. In the ER, RTA and RTB dissociate, and the A-subunit is retro-translocated across the ER membrane to the cytoplasm [14, 15].
Although a number of candidate ricin vaccines have been explored over the past several decades, current efforts are focused on the development of recombinant, attenuated derivatives of RTA. The most advanced vaccine in terms of clinical development is RiVax, a recombinant RTA subunit carrying two site-directed point mutations: one mutation in a catalytic tyrosine residue (Y80A) and the other in a valine (V76M) residue postulated to promote vascular leak syndrome [16–19]. This recombinant derivative of RTA is attenuated several thousand fold relative to the native protein, but retains immunogenicity. RiVax is currently in Phase I clinical trials (E. Vitetta and J. Smallshaw, UT Southwestern, personal communication). The second RTA derivative being pursued as a vaccine was developed by the U.S. Army and is called RTA 1-33/44-198 [20–22]. This variant carries a deletion of an N-terminal proximal exposed loop region (T34-P43) as well as a truncation of the C-terminus (A199- F267). Carra and colleagues have demonstrated that these deletions result in a thermostable protein that is both non-toxic and immunogenic in a mouse model [20].
While considerable effort has been invested in the engineering of attenuated derivatives of RTA, these studies are being conducted with only a limited understanding of the regions of the toxin that are important in eliciting protective antibodies. This is potentially problematic because immunization with RTA is known to elicit a mixture of neutralizing, non-neutralizing, and toxin-enhancing antibodies [23, 24]. Indeed, we have speculated that there may be discrete regions or domains on RTA that are primarily responsible for stimulating antibodies capable of neutralizing the toxin [25]. It is therefore surprising that, to date, that only a handful of B cell epitopes on RTA have been identified. Lebeda and Olson described a 26-amino acid loop-helix-loop motif (Y91-T116) on RTA that was postulated to serve as an important target of neutralizing monoclonal antibodies (MAbs) [26]. In support of their hypothesis, we recently demonstrated that residues N97-F108 are the target of the protective MAb R70 [25, 27]. In the same study, we also established that antibodies against amino acids T161-M175 are capable of conferring protection against both systemic and mucosal ricin challenge, at least in mice.
With the long-term goal of both improving and evaluating the humoral response to candidate ricin vaccines, the immediate objective of this present study was to better define the neutralizing and non-neutralizing B-cell epitopes on RTA. Since the two best characterized protective B-cell epitopes on RTA are linear determinants, we sought to identify the immunodominant linear regions on RTA so as to gain insight into the regions that are critical for eliciting both neutralizing and non-neutralizing antibodies. In this study we have produced and characterized a collection of MAbs directed against five of the six (I–VI) distinct immunodominant linear regions on RTA. We used both cell-based cytotoxicity assays, as well as animal challenge studies, to determine which of these MAbs was capable of neutralizing ricin in vitro and in vivo. We found that neutralizing MAbs were primarily directed at α-helices located in RTA folding domains 1 and 2, whereas non-neutralizing antibodies recognized random coils and loops that were primarily confined to folding domain 3. These data provide insight into immunodominant and structural determinants on RTA that give rise to protective immunity, and for the first time provide an immunological rationale for ricin vaccine design.
2. Materials and Methods
2.1 Chemicals, biological reagents and cell lines
Ricin, RTA, and RTB were purchased from Vector Laboratories (Burlingame, CA). Ricin toxoid (RT) was produced by treatment of holotoxin with paraformaldehyde (4% v/v), as described previously [28]. Ricin and RT were dialyzed against PBS at 4°C in 10,000 MW cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL), prior to use in cytotoxicity studies. Paraformaldehyde (16%) was purchased from Electron Microscopy Sciences (Fort Washington, PA). RiVax™ vaccine, from Soligenix, Inc (Princeton, NJ), was prepared by adsorption of recombinant RTA (Y80A; V76M) to AlOH (Alhydrogel® in 10 mM histidine, pH 6.0, 144 mM NaCl, so that the total vaccine contained 0.85 mg aluminum equivalents/ml and 200 μg RTA protein/ml. GlutaMax™, fetal calf serum and goat serum were purchased from Gibco-Invitrogen (Carlsbad, CA). A ClonaCell HY™ kit for hybridoma production was purchased from STEMCELL Technologies (Vancouver, BC, Canada). Unless noted specifically, all other chemicals were obtained from the Sigma-Aldrich Company (St. Louis, MO). Vero cells, irradiated MRC-5 human lung fibroblast cells, and the murine myeloma cell line P3X63.Ag8.653 were purchased from the American Type Culture Collection (Manassas, VA). Cell culture media were prepared by the Wadsworth Center Media Services facility. Monoclonal antibody SyH7 was affinity-purified on a protein G column by the Wadsworth Center protein expression core. Cell lines and hybridomas were maintained in a humidified incubator at 37° C with 5% CO2. Pooled affinity-purified rabbit anti-RiVax polyclonal antibody (PAb) was provided by Drs. Ellen S. Vitetta and Joan Smallshaw (University of Texas Southwestern Medical Center, Dallas, TX).
2.2 Mouse strains, animal care and immunizations
Female BALB/c mice approximately 10–12 weeks of age were purchased from Taconic Labs (Hudson, NY). Animals were housed under conventional, specific pathogen-free conditions and were treated in compliance with the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC) guidelines.
For serum profiling by RTA peptide array, and antibody competition analysis by BIAcore, female BALB/c mice were immunized by either the subcutaneous (sc) or intraperitoneal (i.p.) route with RiVax (10 μg per animal) or with RT (50 μg per animal) three times at 10 day intervals. Ten days after the third immunization, blood was collected from the tail vein. For hybridoma production, female BALB/c mice were primed parenterally (i.p.) with either RT (50 μg) or RiVax (10 μg) on day 0, and then boosted by the same route with RT (50 μg) or RiVax (10 μg) on days 10 and 20.
2.3 B-cell hybridoma production
Four days after the second boost with either RT (50 μg) or RiVax (10 μg), mice were euthanized, and total splenocytes were fused with the myeloma cell line P3X63.Ag8.653, using polyethylene glycol (PEG). The resulting hybridomas were either seeded into wells of 96-well cell culture-treated microtiter plates containing a layer of irradiated MRC-5 feeder cells and cloned by limiting dilution [28, 29], or else seeded in methylcellulose and cloned as per the instructions in the ClonaCell -HY™ hybridoma cloning manual (STEMCELL Technologies, Vancover, BC, Canada). Hybridomas secreting antibodies of interest were expanded and cultured in either a 1:1 mixture of NCTC (Sigma Co.) and RPMI medium containing 10% fetal calf serum, oxaloacetate, pyruvate, and insulin (OPI), 8 mM GlutaMax™, and penicillin-streptomycin, or else in medium A (STEMCELL Technologies) before being transitioned to CD Hybridoma, a serum-free, protein-free, antibiotic-free medium (Gibco-Invitrogen).
2.4 ELISAs and RTA peptide arrays
ELISAs and peptide arrays were performed as previously described [25]. Briefly, Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific) were coated overnight with ricin, RTA, RTB, BSA (0.1 μg/well) or individual peptides in PBS (pH 7.4) before being treated with primary mouse or rabbit sera, hybridoma supernatants, or purified MAbs. Horseradish peroxidase (HRP)-labeled goat anti-mouse or rabbit IgG-specific polyclonal antibodies (SouthernBiotech) were used as secondary reagents. The ELISA plates were developed using the colorimetric detection substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Labs, Gaithersburg, MD) and were analyzed with a SpectroMax 250 spectrophotometer, with Softmax Pro 5.2 software (Molecular Devices, Sunnyvale, CA). The RTA peptide array used in this study consisted of 44 12-mers, each overlapping its neighbors by 6 amino acids, collectively spanning the RTA sequence (Table 1). The peptides were synthesized, unbound, in 96 individual tubes, in 96-well plate format, and were provided at 2.5 μmol scale (1.8 mg per peptide, on average) at >75% purity (New England Peptide, Gardner, MA). The peptides were solubilized in DMSO, and aliquots were stored at −20°C.
TABLE 1.
Sequences of RTA Peptides
 |
2.5 Vero cell cytotoxicity assays
Vero cell cytotoxicity assays were performed as previously described [25]. Briefly, Vero cells were trypsinized, adjusted to approximately 0.5–1.0 × 105 cells per ml, and seeded (100 μl/well) onto white 96-well plates (Corning), and allowed to adhere overnight. Vero cells were then treated with ricin (10 ng/ml), ricin:MAb mixtures, or medium alone (negative control) for 2 h at 37°C. The cells were washed to remove non-internalized toxin or toxin:MAb mixtures, and were then incubated for 40 h. Cell viability was assessed using CellTiter-GLO reagent (Promega). All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only.
2.6 Passive protection studies
Individual MAbs were diluted into endotoxin-free PBS and then administered in a final volume of 0.4 ml to female BALB/c mice by i.p. injection at a concentration of 40 ug/animal. Twenty-four hours later, the mice were injected by the same route with ricin (50 μg/kg) that had been diluted into PBS to a final volume of 0.4 ml. Survival was monitored over a 3-day period. In addition, hypoglycemia was used as a surrogate marker of intoxication. Blood (<5 μl) was collected from the tail vein of the animals at 18–24 h intervals. Blood glucose levels were measured with an Aviva ACCU-CHEK handheld blood glucose meter (Roche, Indianapolis, IN). Mice were euthanized when they became overtly moribund and/or blood glucose levels fell below 25 mg/dl. For statistical purposes, readings at or below the meter’s limit of detection of ~12 mg/dl were set to that value.
2.7 Antibody affinity measurements and competition analysis
Affinity of antibodies for ricin toxin was determined by surface Plasmon resonance (SPR) using a Biacore 3000 (GE Healthcare) with ricin attached to a CM5 chip surface. HEPES-buffered saline, pH 7.4, with EDTA and P20 added (HBS-EP, pH 7.4) was used as the running buffer at a flow-rate of 30 μl/ml. Serial dilutions of each antibody were made in HBS-EP, pH 7.4, from an initial 600 nM down to 18.75 nM. Each concentration series run also contained a buffer-alone injection. The concentration series was run with a 3–4 min injection time, followed by 10 min of dissociation time. The chip surface was regenerated with two 30 sec injections, at a flow-rate of 50 μl/ml, of 10 mM glycine, pH 1.5, followed by a 2 min equilibration time. All kinetic experiments were performed at 25°C. Kinetic constants for the antibody/ricin interactions were obtained with the BIA evaluation software.
Antibody and mouse anti-RiVax serum competitive binding assays by Biacore were performed with HBS-EP, pH 7.4 as the running buffer at a flow-rate of 10μl/min. The first MAb or murine anti-RiVax serum was injected until saturation was achieved, (i.e., when no significant additional rise in resonance units (RU) was observed after antibody injection.) The second, competing MAb was then injected using a 2-min injection time. The amount of second MAb bound to the chip, in RU, was calculated as the RU value at 15s after the injection minus the RU value at 15s preceding the start of the injection. The chip surface was regenerated by short pulses with 10 mM glycine, pH 1.5, until the RU values had returned to baseline.
2.8 In vitro translation assays
RTA (1 ng) was incubated with individual MAbs (250 to 500 ng) for 10 min at room temperature, and the mixture was then added to a cell-free reticulocyte lysate mixture containing ribosomes, amino acids, and ATP (Retic Lysate IVT; Ambion, Austin, TX). The cocktail was incubated at 25°C for 20 min before the addition of uncapped in vitro-transcribed template RNA (1 μg) encoding firefly luciferase (Luc) or Xenopus elongation factor 1 (XeF-1), each obtained from Promega (Madison, WI). The xef-1 template was used as a negative-control mRNA, since the translated XeF-1 protein is incapable of cleaving the luciferase substrate. The samples (25 μl) were incubated in microcentrifuge tubes for 3 h in a 30°C water bath and were then transferred to white 96-well plates (Corning, Lowell, MA) before the addition of Bright-Glo luciferin substrate at a 1:1 ratio of sample (Promega). Luminescence was measured with a SpectraMax L luminometer interfaced with SoftMax Pro software (version 5.2; Molecular Devices). All experiments were performed in triplicate.
2.9 Statistical analysis and software
Statistical analysis was carried out with Excel 2003 (Microsoft, Redmond WA) and GraphPad Prism 5 (GraphPad software). Paired and Unpaired t-tests with Welch’s correction were used where indicated. The significance level threshold was set at α = 0.05. The open-source molecular visualization software PyMOL(DeLano Scientific LLC, Palo Alto, CA) was used for epitope modeling.
3. Results
3.1 Neutralizing antibodies constitute only a minority of the total antibodies elicited against RTA
To gain a rough estimate of the relative proportions of neutralizing and non-neutralizing antibodies elicited in response to RTA, we generated and screened a library of B cell hybridomas derived from the spleens of RT-immunized BALB/c mice. Hybridoma supernatants were screened for reactivity with ricin, RTA and RTB, and were simultaneously assessed for neutralizing activity in a Vero cell cytotoxicity assay. In a representative fusion (Supplementary Fig. 1), ~50% (497/1000) of the hybridomas secreted MAbs that reacted with ricin holotoxin. Among these, 84% were directed against RTA, 13% against RTB, and the remaining 3% reacted equally well with either subunit. Interestingly, among the RTA-specific hybridomas, only 6% (24/418) were capable of neutralizing ricin; the remaining 94% had no discernable effect on preventing toxin-induced cell death. Furthermore, the majority of these neutralizing RTA-specific MAbs bound to conformation-dependent (or discontinuous) epitopes (~88%), given that they failed to react with a 12-mer peptide library spanning RTA. Analysis of hybridomas derived from RiVax-immunized mice confirmed that only a fraction (~6%) of the total RTA-specific MAbs possessed toxin neutralizing activity (data not shown). These data suggest that neutralizing Abs constitute only a small fraction of the polyclonal response to RTA.
3.2 Serum antibodies from RiVax-immunized mice and rabbits react with six distinct linear regions on RTA
To identify the immunodominant linear B-cell epitopes on RTA, we subjected immune sera from BALB/c mice (n=13) that had been immunized with RiVax or RT to pepscan analysis. Immune sera were examined for reactivity with a peptide array consisting of 44 overlapping 12-mers spanning the length of the RTA primary sequence (Table 1). Peptides of 12 amino acid in length were used in this study since in general mouse B-cell epitopes are 8–20 amino acids in size. The antisera from these animals reacted with peptides spanning six distinct regions (I–VI) on RTA, that were designated as follows: region I (30–60), region II (78–108), region III (120–150), region IV (170–190), region V (204–240), and region VI (258–264) (Fig. 1A, C). To examine whether this immune reactivity profile is similar between species, we subjected a pool of affinity-purified rabbit anti-RiVax polyclonal antibodies to RTA peptide array analysis. These rabbit antibodies reacted with peptides spanning almost exactly the same six regions of RTA that were recognized by the mouse immune sera (Fig. 1B), suggesting that the immunodominant linear epitopes recognized by serum antibodies following RiVax immunization between mice and rabbits are conserved. PyMOL was used to model the locations of these six immunodominant linear B cell epitopes on the tertiary structure of RTA (Fig. 2A).
Fig. 1. Anti-RiVax antibodies from mice and rabbits react with linear peptides encompassing six distinct regions of RTA.
Serum samples from RiVax immunized (A) BALB/c mice (n=13) and (B)affinity purified anti-RiVax polyclonal antibody (PAb) from New Zealand white rabbits (20 animals) were assessed for reactivity with 44 overlapping 12-mer RTA peptides spanning the primary amino acid sequence of RTA (X-axis). The peptides are color coded is the same scheme as shown in Table 1. For comparative purposes, the average reactivity of the sera or PAb with RTA (yellow bar) is shown on the far right. Serum or PAb reactivity (Y-axis) is expressed as the average optical density (OD) measured at 450nm, as determined by ELISA. In the case of the mouse peptide arrays, the serum from each of the 13 mice was run on an individual peptide array and the average OD for each peptide for all 13 mice was determined. Reactivity was defined as activity in an ELISA in which signal detected against a specific peptide was greater than 3 standard deviations above background signal. (C) A linear depiction of the RTA sequence indicating the proposed six immunodominant regions (I–VI), as described in the text.
Fig. 2. Modeling of immunodominant regions and epitopes on RTA.
The ricin holotoxin crystal structure (PDB code 2AAI) and the open-source molecular visualization software PyMOL were used to model the location of the immunodominant linear regions on RTA, and map the specific epitopes of the anti-RTA MAbs raised as part of the present study. (A) PyMOL surface representations of RTB (black) and RTA (light grey). The six immunodominant regions (I–VI) of RTA are color coded according to the colors used in Figure 1C. The active site (AS) is highlighted in purple. (B) PyMOL surface representation of RTA (light grey) in which the 12-mer linear epitopes recognized by MAbs FGA12, PB10, GD12, SyH7, SB1, BD7 are depicted. Each epitope is colored according to the scheme used in Figure 1A. (C) PyMOL cartoon diagram of RTA (light grey) with the epitopes indicated in Panel B. It should be noted that the secondary structures of the epitopes recognized by MAbs in regions I, V, and VI are mainly comprised of loops and random coils, while the targets of MAbs in regions II and IV are primarily α-helices.
In general, the RiVax sera demonstrated hyper-reactivity with only one peptide, or in some cases two peptides, within each of the six regions. For example, within region I, immunoreactivity was directed almost entirely to peptide E2 (residues D37-R48). In regions II III, V, and VI reactivity was primarily against peptides E12 (N97-F108), F5 (E127-E138), G9 (Q223-R234), and H3 (C238-F267), respectively. The immune reactivity peak in region IV did not localize to one specific peptide, but spanned peptides F11, F12, G1, G2, and G3. Interestingly, each of these six immunodominant regions was predicted by the Kolaskar and Tongaonkar antigenicity method [30], as well as ABCpred [31], to contain linear B cell epitopes, especially the hyper-reactive peptides E2 (region I), E12 (region II), F5 (region III), G9 (region V), and H3 (region VI). Although the above results suggest that the MAbs bind to linear B-cell epitopes, one cannot exclude the possibility that peptides may assume some degree of secondary structure that may or may not resemble the native “epitope”, and so the MAbs may be binding to a conformational epitope within a continuous stretch of residues.
3.3 Identification of MAbs against the immunodominant regions on RTA
We wished to determine which of the six immunodominant regions on RTA are targets of neutralizing antibodies. To accomplish this, we produced a collection of B-cell hybridomas from the spleens of BALB/c mice that had been immunized with RT or RiVax. Hybridoma supernatants were screened by ELISA for reactivity with RTA, and then subsequently for reactivity with RTA-peptides encompassing the six immunodominant regions depicted in Figure 1. Hybridomas whose supernatants reacted with one or more peptides of interest were cloned by limiting dilution or by single cell cloning in 2% methylcellulose (see Materials and Methods). We screened ~3000 hybridomas and identified a single MAb each against regions I, II, V and VI, and two MAbs against region IV (Fig. 3 and Table 2). We were unsuccessful in identifying a MAb against region III. The hybridomas of interest were expanded and supernatants were collected for MAb characterization. The following parameters were determined for each of the six MAbs identified: (i) epitope specificity, as determined by RTA peptide array (Fig. 3); (ii) subunit specificity, as determined by ELISA using plates coated with ricin holotoxin, RTA, or RTB (Supplementary Fig. 2); (iii) IgG subclass (Table 2); and (iv) affinity for ricin holotoxin, as determined by SPR (Table 2). All MAbs had moderate (KD= 1×10−6 – 1×10−7 M) to high (KD>1 ×10−7 M) affinities for ricin (Table 2). The region II specific MAb, PB10, an IgG2b, bound to the same peptide as the well-characterized neutralizing MAb R70, although PB10 recognizes ricin with slightly lower affinity [27].
Fig. 3. Epitope mapping of immunodominant linear B-cell epitopes on RTA.
Anti-RTA MAbs (10 μg/ml) were applied to RTA peptide arrays for confirmation of their specificity for key reactive epitopes on RTA. (A) FGA12 reacted very specifically with peptide E2 in region I. (B) PB10 reacted exclusively with peptide E12 in region II. (C) GD12 reacted with peptide F11 in region IV (previously described in reference [25]). (D) SyH7 bound specifically to peptide G3, also in region IV. (E) SB1 reacted equally with peptides G9 and G10 in region V. (F) BD7 bound almost exclusively to peptide H3 in region VI. Each peptide array was performed at least three independent times with similar results. The data shown are from one representative experiment. The reactivities (Y-axis) of the MAbs refer to the values (optical densities at 450 nm [OD450]) minus the background obtained by the peptide array ELISA.
Table 2.
Physical characteristics of MAbs specific for linear epitopes on RTA
| MAb | Isotype | Epitope | Range (aa) | Neutralizing | KD[M]b |
|---|---|---|---|---|---|
| FGA12 | IgG1 | DVRHEIPVLPNR | 37 – 48 | − | NA |
| PB10 | IgG2b | NQEDAEAITHLF | 97 – 108 | + | 4.01 × 10−8 |
| R70 | IgG1 | NQEDAEAITHLF | 97 – 108 | + | 3.2 × 10−9 |
| GD12a | IgG1 | TLARSFIICIQM | 163 – 174 | + | 2.9 × 10−9 |
| SyH7 | IgG1 | EMRTRIRYNRRS | 187 – 198 | + | 2 × 10−8 |
| SB1 | IgG2a | QGAFASPIQLQRRNGSKF | 223 – 240 | − | 1.07 × 10−8 |
| BD7 | IgG1 | CAPPPSSQF | 259 – 267 | − | 1.48 × 10−7 |
Previously described in reference (24).
Determined with a ricin coated CM5 chip. NA, not available.
3.4 In vitro and in vivo neutralizing activity of MAbs directed against immunodominant regions of RTA
We used a Vero cell cytotoxicity assay to assess the capacity of each MAb to neutralize ricin in vitro. Ricin (10 ng/ml) was incubated with each MAb at a range of concentrations (0.1–3.0 μg/ml) for 1 h, and then applied in triplicate to Vero cells grown in 96-well microtiter plates. The viability of the Vero cells was determined 48 h later. MAbs FGA12, SB1, and BD7, directed against regions I, V and VI, respectively, did not protect the Vero cells from the cytotoxic effects of ricin at any of the concentrations tested (Fig. 4Aa). On the other hand, the region II-specific MAb, PB10, as well as the region IV-specific MAbs, SyH7 and GD12, neutralized ricin in a dose-dependent manner (Fig. 4A). GD12, SyH7, and PB10 achieved estimated 50% inhibitory concentrations (IC50) of 0.25 μg/ml, 0.17 μg/ml and 0.07 μg/ml, respectively.
Fig. 4. In vitro and in vivo neutralizing activities of MAbs directed against immunodominant regions of RTA.
(A) MAbs were each assessed for their capacity to neutralize ricin in a Vero cell cytotoxicity assay. Ricin (10 ng/ml) was incubated for 1 h with each MAb at the indicated concentrations, and then the mixture was applied in triplicate to Vero cells grown in 96-well microtiter plates. Cell viability was assessed 48 h later. Each data point represents the average of at least three replicate wells. The error bars represent the standard deviation. The visibility of the y-axis values for FGA12, SB1 and BD7 were improved by “nudging” the y-axis values of FGA12 by 2 data units and BD7 by -1 data unit using the nudge option in Graphpad Prism 5. PB10 (region II), SyH7 and GD12 (region IV) protected Vero cells from ricin cytotoxicity, but FGA12 (region I), SB1 (region V) and BD7 (region VI) did not protect Vero cells from the cytotoxic effects of ricin in vitro. (B) BALB/c mice were passively immunized with MAbs SyH7, PB10 or SB1 (40 μg/animal) and then challenged 24 h later with 5 LD50’s of ricin. Mice passively immunized with SyH7 (dashed gray line) or PB10 (sky blue line) survived, whereas mice treated with SB1 (brown line) succumbed 24 h post ricin challenge. The visibility of the values for the SyH7 treated and toxin only treated mice were improved by “nudging” the y-axis values for SyH7 mice by -2 data units and x-axis values for the toxin only treated mice by 0.5 data units, using the “nudge” option in Graphpad Prism 5. (C) Blood glucose levels in mice following ricin challenge. Blood glucose levels in the groups of mice described in Panel B were determined at 24 h intervals following ricin challenge. By paired t-test, blood glucose levels of mice from groups treated with MAbs SyH7 (P=0.2307) or PB10 (P=0.8624) 72 hour post challenge were not significantly from pre-challenge levels or control (no toxin treatment) mice.
We next evaluated the ability of PB10, SyH7, and SB1 to protect mice from a lethal dose of ricin. MAb GD12 has previously been demonstrated to be protective [25], and therefore was not included in this study. MAbs were passively administered to groups of BALB/c mice (5 mice per group) by i.p. injection. Twenty-four hours later, the animals were challenged with 5 LD50’s of ricin given i.p., and then monitored over a 3 day period for morbidity as well as hypoglycemia, a quantitative indicator of ricin intoxication. Mice passively administered PB10 or SyH7 survived toxin challenge, and demonstrated normal blood glucose levels at 72 h (Fig. 4B–C). In contrast, mice treated with SB1 succumbed to ricin intoxication within 24 h of toxin challenge (Fig. 4B–C). These data demonstrate that the physical association of a MAb with RTA is itself insufficient to neutralize ricin. Rather, they would imply epitope specificity is the primary determinant of protection against ricin intoxication.
RTA consists of three folding domains: domain 1 spans residues 1-117, domain 2 spans residues 118-210 and domain spans 3 residues 211-276 (Fig. 5A). Interestingly, the neutralizing MAbs GD12, SyH7 and PB10 recognize regions of RTA that are α-helical in structure (Fig. 5C) situated within folding domains 1 and 2 (Fig. 5A,B). In contrast, the non-neutralizing MAbs recognize random coils and loops (Fig. 5C), primarily localized within folding domain 3 of RTA (Fig. 5A,B). The exception to this rule is FGA12, a non-neutralizing MAb that binds an epitope that, while spatially very close to folding domain 3, is in fact in folding domain 1 (Fig. 5A,B). Nonetheless, these data imply that neutralizing and non-neutralizing MAbs localize to distinct structural and functional domains of RTA.
Fig. 5. Modeling of neutralizing and non-neutralizing epitopes on RTA.
(A) PyMOL surface representations of ricin depicting the folding domains of RTA. RTA folding domains are coded as follows: domain 1 (green), domain 2 (brown) and domain 3 (orange). For reference, RTB is shown in black. (B–C) Regions of RTA recognized by neutralizing (NAb; red) and non-neutralizing antibodies (non-NAbs; blue). The active site on RTA is depicted in purple. For reference, RTB is shown in black. Note that the neutralizing epitopes are α-helical in structure and are located in folding domains 1 and 2, while the non-neutralizing epitopes are random coils and loops, primarily situated in domain 3 of RTA.
3.5 Competition between RTA-specific neutralizing and non-neutralizing MAbs
The observation that neutralizing and non-neutralizing MAbs recognize epitopes that are in close proximity to one another on the surface of RTA raised the possibility that non-neutralizing MAbs interfere with neutralizing MAbs to associate with ricin. Interfering antibodies have been described in the case of hepatitis C viruses as well as other pathogens [32, 33]. To examine this possibility in the case of ricin, we performed competitive in vitro neutralizing assays using the non-neutralizing MAb SB1 and the neutralizing MAb SyH7; these two MAbs bind to epitopes immediately adjacent to one another on the surface of RTA (Fig. 6A). SyH7 (0.125 μg/ml) was mixed with a 10-fold molar excess of SB1 (1.25 μg/ml), and the combination was incubated with ricin for 1 h before being applied to Vero cells. Cell viability was measured 48 h later. When tested individually, SyH7 (0.125 μg/ml) provided ~ 65% protection from ricin toxicity, whereas SB1 (1.25 μg/ml) had no discernable neutralizing activity (Fig. 6B). The addition of 10-fold molar excess SB1 to SyH7 had little effect (<8%) on SyH7’s capacity to neutralize ricin. These data indicate that, although the epitopes for these two MAbs are in extremely close proximity, the non-neutralizing MAb, even in 10-fold molar excess, did not interfere with the ability of the neutralizing MAb to inactivate ricin.
Fig. 6. Evaluation of interference of neutralizing MAbs by non-neutralizing MAbs.

(A) PyMOL surface representation of RTA depicting the epitopes (circled) recognized by SyH7 (neutralizing) and SB1 (non-neutralizing). (B) Neutralizing activity in a Vero cell cytotoxicity assay of SyH7 (0.125 μg/ml), SB1(1.25 μg/ml) or a mixture of both. Each data point represents the average from at least three replicate wells. The error bars represent the standard deviation.
We used a competitive antibody binding assay in a Biacore instrument to examine whether SyH7 was preventing SB1 from associating with RTA, or whether the two MAbs were capable of simultaneously occupying the toxin subunit. A CM5 chip coated with ricin was subjected successively to SyH7 and SB1 (or vice versa) and the respective reactivities were determined (see Materials and Methods). We found that neither MAb affected the ability of the other to associate with ricin (Table 3), suggesting that the two MAbs are capable of co-occupying RTA. In contrast, when the chip was first treated to polyclonal ricin-specific antiserum, the binding of SyH7 (or PB10) to ricin was completely abrogated (Supplementary Fig. 2).
Table 3.
Non-neutralizing MAbs do not interfere with the binding of neutralizing MAbs to ricin.
 |
The same competitive Biacore analysis strategy was used to examine the abilities of the other anti-RTA MAbs, in addition to an anti-RTB MAb, 24B11, to co-occupy ricin. The first MAb (Table 3) was injected onto the ricin coated chip until no increase in resonance units (RU) was observed, indicating that the chip capacity had reached saturation. The second MAb antibody was injected onto the ricin coated chip over a period of 120 seconds and its ability to bind to ricin was determined by an increase in RU. The “percent inhibition of binding” was calculated relative to the RU obtained for each MAb when they were used to probe ricin-coated chips in the absence of a competitive (or primary) MAb. As shown in Table 3, checkerboard analysis of five MAbs from this study revealed: (i) the neutralizing MAbs PB10 and GD12 were each capable of interfering with one another’s ability to bind ricin; (ii) PB10 and GD12 also interfered with the abilities of the non-neutralizing MAbs SB1 and BD7 to associate with ricin, even though the epitopes recognized by the PB10 and GD12 were relatively far from those recognized by SB1 and BD7; (iii) the domain 3 MAbs, SB1 and BD7, inhibited one another from binding to ricin; and finally, (iv) the RTB-specific MAb 24B11 interfered with SB1 and BD7, possibly because SB1 and BD7 recognize epitopes near the RTA-RTB interface. However, 24B11 also affected to some degree GD12 from binding to ricin, despite the fact that these two MAbs are predicted to bind opposite sides of the holotoxin.
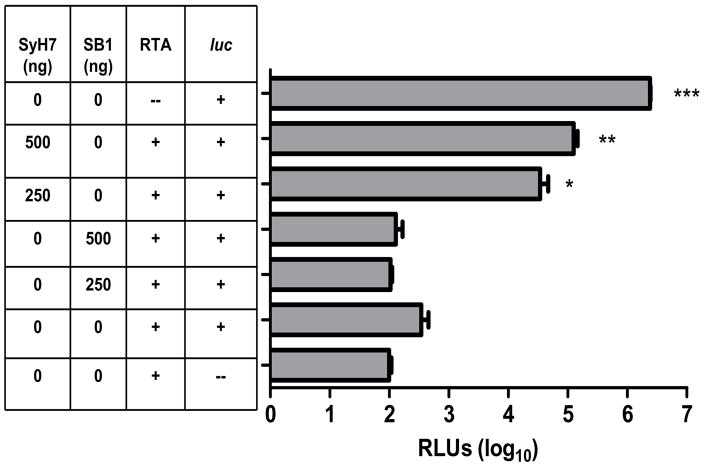
3.6 SyH7 but not SB1 interferes with the enzymatic action of RTA
We and others have postulated that anti-RTA MAbs neutralize ricin by interfering with the toxin’s capacity to depurinate ribosomal RNA, and consequently arrest protein synthesis [24]. Indeed, we recently demonstrated that neutralizing MAbs GD12 and R70 partially interfere with the enzymatic action of RTA [25]. To examine whether this is the case for SyH7, we incubated SyH7 or SB1 with RTA (1 ng) for 10 min at room temperature and then added it to a rabbit reticulocyte lysate mixture containing a source of methionine, other amino acids, ATP, ribosomes and luciferase (luc) messenger RNA as a template. Luminescence was used as an indicator of protein synthesis. In control reactions in which luc mRNA was combined with the cell-free in vitro translation mixture in the absence of ricin and MAbs, robust luciferase activity was detected (Fig. 7). The addition of RTA to this mixture reduced luc dependent luciferase activity by >3 logs, demonstrating the effect of the toxin on ribosome function. The addition of SyH7 reduced (but did not eliminate) RTA’s enzymatic activity in a dose-dependent manner (P < 0.009) (Fig. 7). In contrast, the non-neutralizing mAb, SB1, had no detectable effect on the enzymatic activity of RTA (Fig. 7). These data are consistent with a model in which neutralizing MAbs interfere with RTA’s enzymatic activity, whereas non-neutralizing mAbs do not.
Fig. 7. SyH7 but not SB1 interferes with the enzymatic activity of RTA.
RTA and either SyH7 or SB1 (at the indicated concentrations) were incubated with a cell-free in vitro translation mixture before the addition of luciferase mRNA. Luciferase activity, reported in relative luciferase units (RLUs), serves as an indicator of protein translation. Each experiment was performed in triplicate; error bars represent standard deviation. The asterisks indicate that the values represented by the horizontal bars are statistically different that the RTA + luc mRNA control (second from the bottom); *p <= 0.05, **p <= 0.001, ***p <= 0.0001, no *p > 0.05.
4. Discussion
There are currently two candidate ricin vaccines under development. The first is a recombinant derivative of RTA that contains a point mutation in the toxin’s active site, as well as a mutation in a region of RTA attributed to eliciting vascular leak syndrome [16]. The second is a recombinant form of RTA known as 1-33/44-198 that carries a deletion of an exposed 10 amino acid hydrophobic loop (T34-P43) within folding domain 1, as well as a truncation of all of folding domain 3 (A199 - F267) [20–22]. In both cases, however, RTA has been engineered in the absence of a fundamental understanding of the regions of the toxin that are critical in eliciting protective immunity. In an effort to address this concern, we have embarked upon a systematic identification of the neutralizing and non-neutralizing B-cell epitopes on RTA. In the present study we have (i) identified six immunodominant linear B cell epitopes on RTA; (ii) produced MAbs against five of the six immunodomainant regions, and finally; (iii) tested these MAbs for the ability to neutralize ricin in vitro and in vivo. The results of this study suggest that there are “hot spots” on RTA responsible for eliciting neutralizing antibodies. Specifically, we found that neutralizing MAbs were primarily directed against α-helices situated within RTA folding domains 1 and 2, whereas non-neutralizing MAbs targeted random loops and coils largely localized to domain 3. The results of this study would suggest that there are distinct regions or domains of RTA that are disproportionally responsible for eliciting protective immunity, and that further delineation of these regions will have important implications for refinement of a candidate RTA subunit vaccine.
A systematic analysis of a collection of B-cell hybridomas suggested that only a minority (~6%) of the RTA-specific antibodies induced in mice following immunization with RT or RiVax possessed ricin neutralizing activity. The remainder (>90%) of the antibodies bound ricin holotoxin as determined by ELISA, but demonstrated no detectable neutralizing activity. While this methodology provides only a limited (and possibly biased) snapshot of the immune response to RTA, results of other studies of RTA-specific MAbs are consistent with our conclusion here that only a fraction of the total RTA-specific antibodies within a polyclonal response elicited following immunization are responsible for protective immunity to ricin [23, 24]. Such a phenomenon is not unprecedented; it is well established that HIV [34, 35] and hepatitis C virus (HCV) [33] induce high anti-viral antibody titers that lack detectable neutralizing activity. Indeed, our data may explain the often observed discrepancy between ricin-specific antibody titers and ricin neutralizing activity in serum samples from rabbits [18], mice and macaques (J. O’Hara, R. Brey, and N. Mantis, unpublished data), and even humans [36]. For example, in a recently completed pilot Phase I clinical trial of RiVax, two individuals with virtually identical anti-RTA serum IgG levels (4.73±0.02 μg/ml and. 4.36±0.16 μg/ml) had toxin neutralizing titers that differed by >10-fold (1.4±0 and 0.13±0.02) [36]. Furthermore, our preliminary data on serum profiling of individual mouse anti-RiVax serum using RTA peptide arrays suggests that ricin neutralizing activity can be correlated with reactivity of specific B-cell epitopes on RTA (data not shown). Based on our studies in mice, we speculate that RiVax immunization in humans elicits a mixture of ricin neutralizing and non-neutralizing antibodies, and that the ratio of the two populations of antibodies varies from individual to individual.
Using immune serum profiling by peptide array (“pepscan”), we identified six distinct immunodominant linear B cell epitopes (I–VI) on RTA, and produced MAbs directed against five of the six regions. MAbs directed against regions II and IV, but not against regions I, V, and VI, neutralized ricin in vitro. Only MAbs against regions II and IV protected mice against a lethal toxin challenge. When visualized using PyMOL, the neutralizing and non-neutralizing epitopes on RTA clustered into distinct folding domains (not to be confused with immunodominant domains) and could be further distinguished on the basis of their secondary structure. RTA is composed of three distinct folding domains: folding domain 1 spans residues 1-117, domain 2 spans residues 118-210, and domain 3 spans residues 211-267. The epitopes recognized by non-neutralizing MAbs were random loops and coils primarily found in folding domain 3, while the epitopes bound by neutralizing MAbs were α-helices confined to folding domains 1 and 2. FGA12 is the only non-neutralizing MAb that we have identified to date that recognizes an epitope within folding domain 1. Interestingly, the FGA12 epitope lies within the exact exposed hydrophobic loop that was deleted by McHugh and colleagues in an effort to stabilize the recombinant derivative of RTA [21, 22]. While our data suggest an intriguing correlation between epitope structure and neutralizing activity, additional MAbs against each of these regions are needed to fully validate this conclusion.
It should be noted that the two best-characterized protective epitopes on RTA are located in folding domains 1 and 2. The first, defined by residues N97-F108, was originally described by Lemley and colleagues as being the target of the MAb R70 [25, 27]. We have subsequently identified two additional MAbs against precisely the same epitope: 23D7, a monoclonal IgA [28], and PB10 (this study). It is becoming increasingly clear that this epitope represents one of the dominant targets of neutralizing antibodies induced following immunization with RT or RTA, at least in mice. In fact, we estimate that >60% of the neutralizing MAbs elicited against linear epitopes on RTA react with peptide N97-F108 (J. O’Hara and N. Mantis, unpublished data). The epitope is solvent-exposed and contains charged residues that have been hypothesized to participate in RTA-ribosome binding and/or enzymatic activity [26]. Lebeda and Olson demonstrated that this region of RTA is conserved in four additional plant-derived ribosome inactivating proteins [26]. The second best characterized epitope (T163 -M174) located in folding domain 2, was originally identified by Castelletti and colleagues [37] as being a primary target of serum antibodies from humans who had been treated with ricin immunotoxin. MAb GD12 was identified as being able to bind to a peptide spanning T16-M174 and was shown to be capable of protecting mice against both i.p. and intragastric ricin challenge [25].
We have now identified a second epitope on folding domain 2 that may be important in eliciting protective immunity to ricin. The epitope is recognized by SyH7, and spans residues E187-S198. This stretch of amino acids is rich in arginine residues, and forms a positively charged patch likely responsible for the initial contacts between RTA and rRNA [38, 39]. SyH7 partially inhibited RTA enzymatic activity in an in vitro translation assay, suggesting that this MAb interferes with the ribosomal binding or enzymatic activity of RTA. Interestingly, the epitope recognized by SyH7 overlaps with another neutralizing epitope identified by Colombatti [23]. Moreover, this same epitope lies within a 30-mer peptide identified by Castelletti as being recognized by human sera from individuals treated with an RTA-immunotoxin [37]. Therefore, supporting the idea that residues E187-S198 are an important target of protective antibodies in rodents and humans.
Our studies suggest that antibody-mediated ricin neutralization activity is determined primarily by epitope specificity, rather than by antibody affinity or isotype. For example, MAbs SyH7 and SB1 had virtually identical affinities (KD) for ricin, yet disparate neutralizing activities. SyH7 was a potent inhibitor of ricin toxicity in vitro and in vivo, whereas SB1 failed to demonstrate even meager neutralizing activity. Antibody isotype is also not the sole determinant of protection against ricin, because both neutralizing (GD12, R70) and non-neutralizing MAbs (FGA12, BD7) were found to be IgG1’s. We therefore speculate that epitope specificity is the primary determinant of an antibodies ability to neutralize ricin. If our hypothesis is correct, then it would be largely in accordance with what is known about the mechanisms of antibody-mediated neutralization of other toxins, such as botulinum [40, 41], tetanus [42], and anthrax [43, 44].
The preponderance of non-neutralizing MAbs elicited following RTA immunization led us to examine whether these MAbs interfere with the ability of neutralizing antibodies to recognize and/or inactivate ricin. Interfering antibodies are generally defined as functionally non-neutralizing antibodies that sterically hinder the binding of neutralizing antibodies to their respective epitopes. Interfering Abs have been demonstrated in the sera of individuals chronically infected with HCV, for example. Zhang and colleagues [33] were able to demonstrate that the functional removal of interfering antibodies through affinity depletion and peptide inhibition, revealed a previously unrecognized population of neutralizing antibodies in the sera of chronically HCV-infected patients. In the case of ricin, we tested the ability of the non-neutralizing MAb SB1, when provided in 10 fold molar excess, to interfere with the functional capability of neutralizing MAb SyH7. We found that SB1 did not interfere with the neutralizing capacity of SyH7, even though the two MAbs recognize epitopes in extremely close proximity to one another. Competitive binding assays by SPR confirmed that SyH7 and SB1 can co-occupy ricin. Indeed, there was no evidence, when checkerboard analysis was performed, that either of the two non-neutralizing MAbs, SB1 and BD7, interfered with the three neutralizing antibodies tested, namely PB10, SyH7, and GD12. On the other hand, we found that two neutralizing MAbs, PB10 and GD12, were each able to block the binding of SB1 and BD7 to ricin. Because SB1 and BD7 did not competitively inhibit the binding of PB10 or GD12, it is tempting to speculate that PB10 and/or GD12 may induce conformational changes in RTA that influence the conformation of distant epitopes.
It is striking that the regions deleted in the ricin vaccine currently being developed by the U.S. Army, notably residues 34-43 and 198-267, correspond exactly to the regions on RTA that we have identified as being targets of non-neutralizing antibodies. Those regions were deleted by Carra and colleagues in an effort to improve overall stability and antigenicity of the RTA subunit vaccine [20]. We hypothesize that residues 34-43 and 198-267, while clearly immunogenic, give rise to antibodies that do not contribute to protective immunity. If this is in fact the case, we would predict that the so-called RTA 1-33/44-198 vaccine would induce a proportionally greater number of neutralizing antibodies as compared to native RTA or RTA carrying point mutations in the active site. This remains to be tested. Nonetheless, in summary, our analysis in this study of a collection of murine MAbs directed against linear B cell epitopes on RTA provides insight of the overall B cell epitopes likely to exist on ricin. Future efforts aimed at identifying conformation-dependent epitopes on RTA, as well as serum profiling of antibodies from RiVax immunized humans, will provide an even greater (and much needed) understanding of the B cell epitopes that exist on RTA.
Supplementary Material
Hybridoma supernatants from RT-immunized mice were screened by ELISA for reactivity with ricin holotoxin, RTA and RTB. The RTA-specific MAbs were then tested for the ability to neutralize ricin in a Vero cell cytotoxicity assay. An RTA peptide array was used to determine if the MAbs bound to linear epitopes on RTA. The numbers in parenthesis represent the percentage of cells within the respective group. These data reflect the results of two fusions in which 524 ricin positive hybridomas were further screened.
Individual MAbs (FGA12, PB10, SyH7, SB1, BD7) at the indicated concentrations were examined for reactivity with ricin (circles), RTA (squares), RTB (triangle), or BSA (inverted, filled triangle), as determined by ELISA. Microtiter plates were coated with 0.1 μg/well of each of the target antigens. Each data point represents the average value of three replicate wells. The roman numerals in parenthesis to the right of each MAb corresponds to the immunodominant region on RTA recognized by each respective MAb.
Using SPR as a method to detect antibody binding to ricin holotoxin, in a BIAcore unit, serum from individual mice immunized with RT was injected onto a CM5 chip coated with ricin holotoxin until the chip was saturated with antibody. SyH7 (A) or PB10 (B) neutralizing MAbs were then injected onto the chip and the amount of antibody bound was measured by an increase in RU. The RU’s in the boxed legend indicate the RU values for the respective MAb when it is injected onto the ricin coated chip in the absence of the mouse serum. In each case, there was very little increase in RU indicating SyH7 or PB10 NAbs were largely unable to bind to ricin in the presence of the mouse anti-toxoid sera. This suggests that antibodies directed against folding domains 1 and 2 are elicited in the polyclonal response to ricin toxoid.
Acknowledgments
We would like to thank Dr. Karen Chave and Marcella Powell of the Protein Expression Core at the Wadsworth Center for monoclonal antibody purification. We would also like to acknowledge Drs. Ellen Vitetta and Joan Smallshaw at UT, Southwestern Medical Center (Dallas, TX) for kindly providing affinity-purified rabbit anti-RiVax polyclonal antibody. This work was supported by grants AI070624 (to RJB) and AI081053 (to NJM) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005 Nov 9;294(18):2342–51. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 2.Brey RN, Mantis NJ. Vaccines for Ricin – a Type II Ribosome Inactivating Protein. In: Barrett ADT, Stanberry LR, editors. Vaccines for Biodefense and Neglected Diseases. New York: Elsevier Inc; 2009. [Google Scholar]
- 3.Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005 Jun 17;57(9):1424–39. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Coopman V, De Leeuw M, Cordonnier J, Jacobs W. Suicidal death after injection of a castor bean extract (Ricinus communis L.) Forensic science international. 2009 Aug 10;189(1–3):e13–20. doi: 10.1016/j.forsciint.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Schier JG, Patel MM, Belson MG, Patel A, Schwartz M, Fitzpatrick N, et al. Public health investigation after the discovery of ricin in a South Carolina postal facility. American journal of public health. 2007 Apr;97(Suppl 1):S152–7. doi: 10.2105/AJPH.2006.099903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004 Sep 15;44(4):361–70. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004 Sep 15;44(4):371–83. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–12. [PubMed] [Google Scholar]
- 9.Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. JBiolChem. 1979;254(19):9795–9. [PubMed] [Google Scholar]
- 10.Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326(6113):624–6. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- 11.Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978 Sep 26;536(1):18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 12.Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3783–8. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, et al. Pathways followed by ricin and Shiga toxin into cells. HistochemCell Biol. 2002;117(2):131–41. doi: 10.1007/s00418-001-0346-2. [DOI] [PubMed] [Google Scholar]
- 14.Argent RH, Parrott AM, Day PJ, Roberts LM, Stockley PG, Lord JM, et al. Ribosome-mediated folding of partially unfolded ricin A-chain. J Biol Chem. 2000 Mar 31;275(13):9263–9. doi: 10.1074/jbc.275.13.9263. [DOI] [PubMed] [Google Scholar]
- 15.Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002 Sep 10;20(27–28):3422–7. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 17.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003 Apr;21(4):387–91. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 18.Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005 Sep 15;23(39):4775–84. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007 Oct 16;25(42):7459–69. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, et al. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine. 2007 May 22;25(21):4149–58. doi: 10.1016/j.vaccine.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.McHugh CA, Tammariello RF, Millard CB, Carra JH. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci. 2004 Oct;13(10):2736–43. doi: 10.1110/ps.04897904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein Eng Des Sel. 2004 Apr;17(4):391–7. doi: 10.1093/protein/gzh043. [DOI] [PubMed] [Google Scholar]
- 23.Colombatti M, Pezzini A, Colombatti A. Monoclonal antibodies against ricin: effects on toxin function. Hybridoma. 1986;5(1):9–19. doi: 10.1089/hyb.1986.5.9. [DOI] [PubMed] [Google Scholar]
- 24.Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol. 2004 May 15;172(10):6221–8. doi: 10.4049/jimmunol.172.10.6221. [DOI] [PubMed] [Google Scholar]
- 25.Neal LM, O’Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010 Jan;78(1):552–61. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebeda FJ, Olson MA. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int J Biol Macromol. 1999 Jan;24(1):19–26. doi: 10.1016/s0141-8130(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 27.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–21. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 28.Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006 Jun;74(6):3455–62. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 30.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990 Dec 10;276(1–2):172–4. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 31.Saha S, Raghava GP. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006 Oct 1;65(1):40–8. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- 32.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002 Dec 12;420(6916):678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 33.Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, et al. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A. 2009 May 5;106(18):7537–41. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis KL, Gray ES, Moore PL, Decker JM, Salomon A, Montefiori DC, et al. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology. 2009 May 10;387(2):414–26. doi: 10.1016/j.virol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, et al. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS pathogens. 2009 Sep;5(9):e1000598. doi: 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2268–73. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, et al. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin’s lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol. 2004 May;136(2):365–72. doi: 10.1111/j.1365-2249.2004.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katzin BJ, Collins EJ, Robertus JD. Structure of ricin A-chain at 2.5 A. Proteins. 1991;10(3):251–9. doi: 10.1002/prot.340100309. [DOI] [PubMed] [Google Scholar]
- 39.Li XP, Chiou JC, Remacha M, Ballesta JP, Tumer NE. A two-step binding model proposed for the electrostatic interactions of ricin a chain with ribosomes. Biochemistry. 2009 May 12;48(18):3853–63. doi: 10.1021/bi802371h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravichandran E, Al-Saleem FH, Ancharski DM, Elias MD, Singh AK, Shamim M, et al. Trivalent vaccine against botulinum toxin serotypes A, B, and E that can be administered by the mucosal route. Infect Immun. 2007 Jun;75(6):3043–54. doi: 10.1128/IAI.01893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi T, Joshi SG, Al-Saleem F, Ancharski D, Singh A, Nasser Z, et al. Localization of the sites and characterization of the mechanisms by which anti-light chain antibodies neutralize the actions of the botulinum holotoxin. Vaccine. 2009 Apr 28;27(19):2616–24. doi: 10.1016/j.vaccine.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volk WA, Bizzini B, Snyder RM, Bernhard E, Wagner RR. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect Immun. 1984 Sep;45(3):604–9. doi: 10.1128/iai.45.3.604-609.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly-Cirino CD, Mantis NJ. Neutralizing Monoclonal Antibodies Directed against Defined Linear Epitopes on Domain 4 of Anthrax Protective Antigen. Infect Immun. 2009 Aug 24; doi: 10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reason D, Liberato J, Sun J, Keitel W, Zhou J. Frequency and domain specificity of toxin-neutralizing paratopes in the human antibody response to anthrax vaccine adsorbed. Infect Immun. 2009 May;77(5):2030–5. doi: 10.1128/IAI.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hybridoma supernatants from RT-immunized mice were screened by ELISA for reactivity with ricin holotoxin, RTA and RTB. The RTA-specific MAbs were then tested for the ability to neutralize ricin in a Vero cell cytotoxicity assay. An RTA peptide array was used to determine if the MAbs bound to linear epitopes on RTA. The numbers in parenthesis represent the percentage of cells within the respective group. These data reflect the results of two fusions in which 524 ricin positive hybridomas were further screened.
Individual MAbs (FGA12, PB10, SyH7, SB1, BD7) at the indicated concentrations were examined for reactivity with ricin (circles), RTA (squares), RTB (triangle), or BSA (inverted, filled triangle), as determined by ELISA. Microtiter plates were coated with 0.1 μg/well of each of the target antigens. Each data point represents the average value of three replicate wells. The roman numerals in parenthesis to the right of each MAb corresponds to the immunodominant region on RTA recognized by each respective MAb.
Using SPR as a method to detect antibody binding to ricin holotoxin, in a BIAcore unit, serum from individual mice immunized with RT was injected onto a CM5 chip coated with ricin holotoxin until the chip was saturated with antibody. SyH7 (A) or PB10 (B) neutralizing MAbs were then injected onto the chip and the amount of antibody bound was measured by an increase in RU. The RU’s in the boxed legend indicate the RU values for the respective MAb when it is injected onto the ricin coated chip in the absence of the mouse serum. In each case, there was very little increase in RU indicating SyH7 or PB10 NAbs were largely unable to bind to ricin in the presence of the mouse anti-toxoid sera. This suggests that antibodies directed against folding domains 1 and 2 are elicited in the polyclonal response to ricin toxoid.