Abstract
Osteoarthritis (OA) is a disabling condition in which multiple initiating events or conditions (heritable and nonheritable) result in eventual loss of articular cartilage. However, the etiology of OA remains poorly understood, and diagnosis of early disease is difficult due to the lack of specific identifiers. Recent literature suggests that a series of inflammatory processes may be involved in initiating and propagating OA. We hypothesized that products of neutrophils and macrophages, namely myeloperoxidase (MPO), a specific enzyme responsible for the production of both highly reactive hypochlorous acid (HOCl) and chlorine gas (Cl2) and chlorinated peptides, may be present in the synovial fluid of patients with OA. We examined the synovial fluid from 30 patients to identify and profile the presence of MPO. We divided the samples into three groups using radiographic and clinical assessment: (1) control, patients with acute knee injury with no history of OA and no radiographic evidence of OA; (2) early OA, patients with a mild OA based on radiographs; and (3) late OA, patients with a longstanding history of OA and with radiographic evidence of complete joint loss. Patients with early OA demonstrated significantly elevated levels of MPO. We also demonstrated the presence of HOCl and Cl2 modified proteins (Cl-peptides) in early OA synovial fluid samples by liquid chromatography and mass spectrometry. Patients in the control and advanced OA groups demonstrated little elevation in MPO levels and Cl-peptides were undetectable. These results indicate that MPO and Cl-peptides may serve as diagnostic markers for the detection of early OA.
Keywords: myeloperoxidase, chlorinated peptides, osteoarthritis, biomarkers
INTRODUCTION
The definitive treatment for end-stage OA is total joint arthroplasty. This procedure, when performed at the appropriate time, will provide pain relief and restore function for the lifetime of the prosthetic joint. Joint arthroplasty, however, has a finite life and particularly in the younger patients is likely to fail, necessitating the need for revision surgery.
OA is a condition that leads to destruction of articular cartilage resulting in complete loss of joint space. Late OA can easily be discerned on radiographs. Early OA, on the other hand, is difficult to diagnose with the routine imaging modalities. It is plausible that diagnosis of this disease at an earlier stage may allow administration of therapeutic and preventative modalities that could halt or retard the progression of the disease. As such, a biomarker with sufficient sensitivity and specificity to identify early OA would be an invaluable tool. From a diagnostic viewpoint, it is also important that OA be distinguished from other arthropathies. Osteoarthritic conditions were previously described as noninflammatory arthropathies, distinguishing them from rheumatoid arthritis, an autoimmune mediated inflammatory disease. Recent evidence, however, has implicated inflammation as contributing to the symptoms and progression of OA.1–9 Signs of acute synovitis and neutrophil infiltration of the synovial tissue are increasingly recognized as common and early features of OA.10 In addition, subclinical inflammation involving macrophage and lymphocyte infiltration of the synovial tissue and synovial fluid,10 elevated serum C-reactive protein,11 and the presence of inflammatory cytokines12, 13 provide evidence that chronic inflammation is associated with primary OA. Furthermore, some studies suggest that interruption of the inflammatory process with pro-inflammatory antagonists may actually have a chondroprotective effect.6, 14
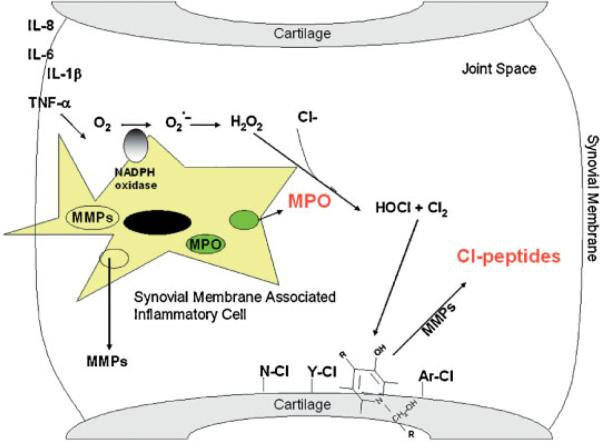
One mechanism by which pro-inflammatory cytokines are thought to signal the destruction of cartilage is through the activation of resident and infiltrating neutrophils and macrophages. When activated, these cells produce reactive oxygen species (ROS) that include superoxide anion , hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) and chlorine gas (Cl2), which have an oxidative modification effect on articular cartilage. Our recent study has demonstrated that the latter products are capable of modifying the pyridinoline crosslinkage of articular cartilage,15 thus initiating an early event in the degradative process (see Scheme 1).
Scheme 1.
Schematic showing the release of MPO from inflammatory cells within the synovial tissue and the chlorination of cartilage matrix proteins. ROS formation is a dynamic process that relies on the initial consumption of oxygen from the microenvironment and its conversion to . The production of by neutrophils and monocytes/macrophages is initiated by the activation assembly of NADPH-oxidase. undergoes spontaneous dismutation to hydrogen peroxide (H2O2) in the presence of hydrogen ions at a rate of 5.0 × 105 M−1s−1 at pH 7.0–7.2. H2O2 is then utilized as a substrate in the myeloperoxidase (MPO)/halide catalyzed reaction producing hypochlorous acid (HOCl), a highly reactive oxygen species, and in the presence of chloride ions at pH 4–5.5, highly reactive chlorine gas (Cl2). The reaction of HOCl and Cl2 with cartilage components, such as tyrosine, tryptophan, lysine, and pyridinoline crosslinks, and subsequent proteolytic cleavage would result in the generation of chlorinated-peptides.
Here, we demonstrate the presence of Cl-peptides in the synovial fluid of patients with early OA and propose that their presence could be of value for the diagnosis and prognosis of arthritic disease. The presence of Cl-proteins indicates the involvement of MPO at an early stage in the disease process and inactivation of this system may provide a target for therapeutic intervention.
MATERIALS AND METHODS
Sample Acquisition
After obtaining institutional review board approval at our institution, synovial fluid was collected at the time of surgery from patients undergoing total knee replacement or knee arthroscopy. Fluid was collected via a 10-cc syringe and samples were kept frozen until time of assay. Sample collection and study methods were reviewed and approved by the IRB committee at Thomas Jefferson University.
Sample Preparation
Synovial fluid samples were centrifuged at 1000 × g for 10 min to pellet any cells or insoluble debris. The top, clear layer was then aspirated and a 100 μL aliquot was placed in a 1.5-mL Eppendorf tube and stored at −20°C for further processing. The remaining synovial fluid was stored at −80°C in a 15-mL centrifuge tube. The centrifuged pellets were stained with Wright's Giemsa and the presence of white blood cells (WBCs) determined. All samples with blood were excluded, as WBCs would be present independent of an active inflammatory process, if the cells were introduced during the aspiration procedure.
Hyaluronidase Digestion
To reduce viscosity, all samples were treated with hyaluronidase (1U/1 μL) (Bovine testes; Calbiochem; La Jolla, CA) prior to characterization. The samples were then incubated with agitation for 12 h at 37°C.
MPO Analysis
To measure the level of MPO in the hyaluronidase-treated samples, we used an enzyme-linked immunosorbent assay (MPO; Oxis International; Portland, OR) and followed the manufacturer's instructions. In brief, antigen was captured in this “sandwich” ELISA by a solid phase monoclonal antibody and detected with a biotin-labeled goat polyclonal anti-MPO. An avidin alkaline phosphatase conjugate was then bound to the biotinylated antibody and p-nitrophenyl phosphate (pNPP) substrate was added. The release of p-nitrophenol was detected spectrophotometrically at 405 nm. The sensitivity threshold detection limit of this assay is 1.5 ng/mL and crossreactivity with eosinophil peroxidase is <2%.
Sample Digestion
Samples were digested prior to high-performance liquid chromatography-mass spectroscopy (HPLC-MS), with CNBr for 6 h at room temperature, in a fume hood. In brief, two to three crystals of CNBr were dissolved in 70% formic acid and 20 μL was added to each sample. The reaction was stopped by adding an equal volume of distilled water. Next, we performed a proteolytic digestion using trypsin (0.05%; 1 mg/mL final concentration, Sigma; Milwaukee, WI) for 24 h at 37°C.
HPLC-MS
All MS experiments were performed at M-Scan Inc., West Chester, PA. To determine the appropriate parameters for detecting chlorine-containing peptides in the synovial fluid from patients with arthritis, the system was initially set up using a Cl-VIP standard. After enzymatic digestion samples were fractionated on a reverse-phase C18 column using a 5% to 70% (v/v) acetonitrile gradient in 0.1% (v/v) trifluoroacetic acid over 40 min. Protein digest products were detected by UV illumination at 214 nm. Fractionated protein was then analyzed by positive ion TOF-MS. The MS peaks were then scanned using the Micromass software search (MassLynx).
RESULTS
Sample Classification and MPO Measurement in Synovial Fluid Samples
Samples were assigned to one of three groups: control (acute injury), early OA, and late OA. These assignments were made by experienced joint surgeons and were based on radiographic, clinical, and arthroscopic findings. Those patients with no arthritic symptoms and no radiographic or arthroscopically visualized changes consistent with OA were assigned to the control group (Table 1). Patients with mild arthritic symptoms who had radiographic findings suggestive of OA were assigned to the early OA group. Patients with longstanding symptoms consistent with OA and radiographic findings suggestive of severe OA were assigned to the late OA group.
Table 1.
Cohort Age, Body Mass Index, and MPO Results of Synovial Fluid Aspirates
| Control (n = 4) | Early OA (n = 11) | Late OA (n = 18) | |
|---|---|---|---|
| Age | 43 ± 9.6 | 61 ± 17.4 | 60 ± 8.1 |
| BMI | 30.5 ± 6.5 | 33.8 ± 8.2 | 33 ± 6.8 |
| MPO (ng/ml) | −(0.04) ± 1.86 | 4.08 ± 1.43a,b | 0.24 ± 1.40 |
MPO levels obtained by ELISA in 33 synovial fluid samples from 30 patients classified as control (n = 4), early OA (n = 11), and late OA (n = 18). There are significant increases in MPO levels of patients in the early OA group. The data is presented as the mean ± SD of a set of experiments repeated at least two times. Statistical significance of the differences between Control and Early OA (a), and control and late OA (b) was determined by a pairwise comparison of correlated groups using a Student's t-test, and statistical significance is defined as p < 0.005. A two-way ANOVA analysis for correlations between age and MPO levels showed that although the development of OA was age related, the amount of MPO was unrelated to the age of the individual.
Using sedimentation of the synovial fluids and Wright's Giemsa stain of the cytospun sediments, we were unable to detect neutrophils or monocytes in the joint fluid of any of the patients in this cohort. This finding is consistent with the production of inflammatory cytokines within and the infiltration of inflammatory cells into the synovial tissue rather than the joint space itself.
We found that synovial fluid in the control group (no OA) and synovial fluid in the late (advanced) OA group had low or undetectable levels of MPO, whereas synovial fluid in the early OA group had significantly increased levels of MPO (Table 1). There was a fourfold increase in MPO levels in patients with early OA (p < 0.005) compared to patient controls and patients with late OA. A two-way ANOVA analysis for correlations between age and MPO levels showed that although the development of OA was age related, the amount of MPO was unrelated to the age of the individual.
HPLC-MS Analysis for Cl-Peptides
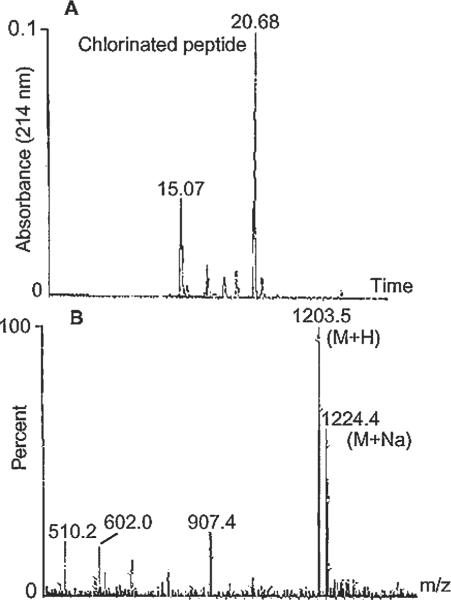
To determine the appropriate parameters for detecting chlorine-containing peptides in the synovial fluid from patients, the system was initially set up using a Cl-VIP standard. It was determined that the signal output from the standard peptide could be further enhanced by digesting Cl-VIP with trypsin prior to use. In brief, a tryptic digest of the Cl-VIP standard was fractionated on a reverse-phase C18 column (Fig. 1A) using a 5% acetonitrile to 70% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid gradient over a period of 40 min. LC fractions containing protein (UV absorbance 214 nm) were further analyzed by positive ion MS. The mass spectrum peaks were then scanned for the presence of chlorinated peptides using the Micromass software search (MassLynx) (Fig. 1B). As expected, a number of digest products were detected by LC fractionation, including the N-terminal, chlorinated peptide, HSDAVF(Cl)TDNY eluting at 20.68 min. The mass spectrum of this peptide demonstrated a strong (M + H) pseudomolecular ion at m/z 1203.5, with an isotope pattern consistent with the presence of Cl.
Figure 1.
Tryptic digest of the Cl-VIP standard (25 μg) fractionated on a reverse-phase C18 column using a 5 to 70% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid gradient over a period of 40 min and analyzed by mass spectrometry for the presence of Cl−. (A) HPLC reverse phase separation profile of a tryptic digest of Cl-VIP peptide monitored at 214nm and (B) positive ion-mass spectrum of the 20.68 min HPLC fraction (5–6 μg) of the N-peptide of Cl-VIP. The mass spectrum of the 20.68 min peak showed a strong (M+H) pseudomolecular ion at m/z 1203.5, with an isotope pattern consistent with the presence of Cl.
HPLC-MS Analysis of Patient Synovial Fluid
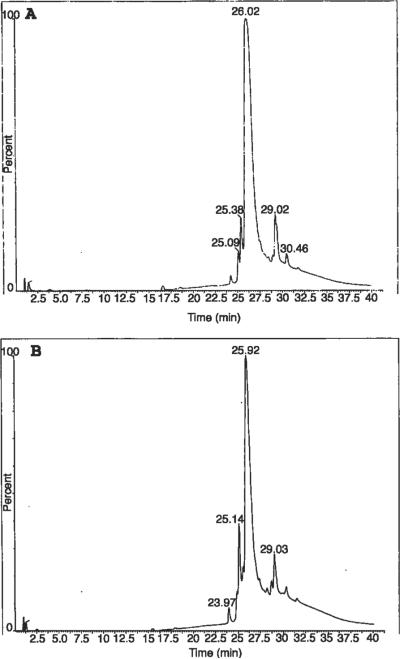
A random selection of nine samples (two controls, three early OA, four late OA) was processed by HPLC-MS to detect Cl-peptides in the synovial fluid. HPLC separation after hyaluronidase treatment alone resulted in elution of a limited number of compounds late in the gradient (~26 m), suggesting the presence of relatively high mass proteins (~66.5 kDa) (Fig. 2A and B). For this reason, we found it necessary to perform a multistep, sequential protein digestion. After enzymatically digesting the samples with hyaluronidase, CNBr was used to cleave methionine groups in peptide backbones facilitating denaturation and increasing susceptibility to subsequent proteolytic digestion with trypsin. This digestion process diminished the intensity of the late eluting, high mass protein peak and gave rise to several smaller protein peaks eluting in the region of lower mass peptides (5–19 m), indicating sufficient protein digestion (Fig. 3A and B).
Figure 2.
HPLC of hyaluronidase treated (1 U/μL, 12 h, 37°C) synovial fluid samples of two patients (A, B). Note the elution of a large, high mass peak ~25–26 m indicating inadequate protein digestion for determining the presence of chlorine.
Figure 3.
HPLC analysis of the samples presented in Figure 2 after additional digestion. The synovial fluid samples were digested with hyaluronidase (1 U/μL, 12 h, 37°C), CNBr (6 h, room temperature) and trypsin (1 mg/mL, 24 h, 37°C) (A, B). Note the diminished intensity of the high mass peak ~25–26 m shown in Figure 2 and the appearance of several lower mass protein peaks eluting in the region of 5–18 m in the CNBr and trypsin-treated samples.
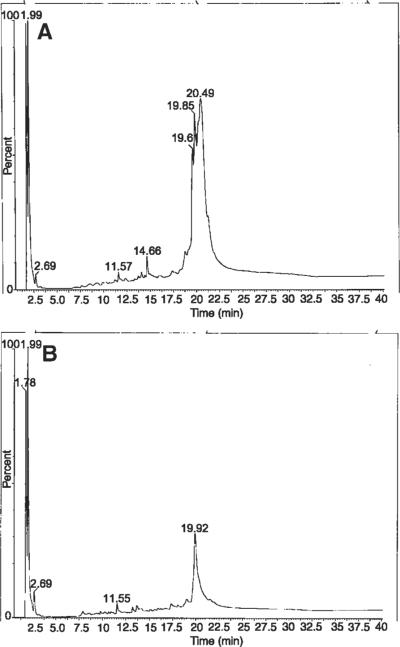
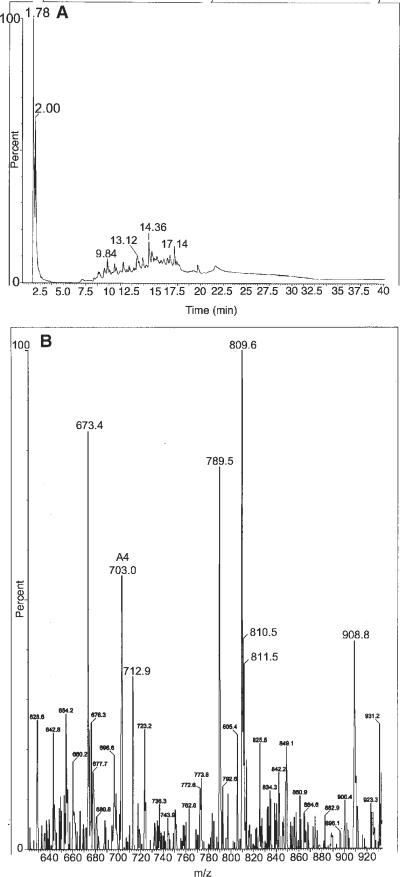
The presence of Cl peptides was detected in the early OA samples tested, but not in the control or the late OA samples (Fig. 4). This analyte appeared at 13.12 m in the chromatography shown in Figure 4A and in the MS appeared as a singularly charged signal, with an isotopic distribution consistent with the presence of Cl at m/z 809.6 (Fig. 4B).
Figure 4.
HPLC-MS results of a synovial fluid sample from the early OA group after thorough protein digestion. Note the HPLC analyte at 13.12 m that correlates with a Cl-peptide (A) and the appearance of a singly charged signal with an isotopic distribution consistent with the presence of Cl at m/z 809.6 (B).
DISCUSSION
This study showed that synovial fluid samples of patients with early OA have elevated levels of MPO. LC-MS showed the presence of Clpeptides in samples that contained MPO. The data presented here provide evidence that the presence of MPO and Cl-peptides in synovial fluid is indicative of an inflammatory involvement in the development of OA. The presence of MPO in synovial fluid indicates that neutrophils and/or macrophages are present within the affected joint, and that an active inflammatory response is present. However, neither cell type was present in the synovial fluid itself. These findings are consistent with reports of mononuclear cell infiltration and overexpression of mediators of inflammation within the synovial membrane (synovitis) of patients with early, but not late OA.2,3,9
The infiltration and activation of neutrophils and macrophages during the early stages of this process are responsible for production of ROS that include , H2O2, HOCl, and Cl2. These products, in turn, are known to have an oxidative modification effect on articular cartilage. The two principle enzymes that neutrophils and macrophages employ to produce ROS are NADPH oxidase16–19 and MPO20–23 (see schematic diagram). NADPH oxidase catalyzes the formation of by reducing oxygen via cytochrome b558. rapidly converts to H2O2 either spontaneously or catalytically through superoxide dismutase. Neither nor H2O2 exhibit significant reactivity with biological compounds; however, MPO converts H2O2 in the presence of Cl− to HOCl and under acidic conditions to Cl2. Of these, HOCl and Cl2 are very reactive and have been shown to oxidize type II collagen (Col-II), one of the major extracellular matrix (ECM) components of articular cartilage, and increase their susceptibility to proteolytic degradation.15 Of the known mammalian peroxidases, MPO is the only enzyme that can oxidize Cl− to Cl+ at physiologic pH and generate chlorinated products that were detected in the synovial fluids of patients with early OA.
Recently, we showed that HOCl and Cl2 oxidizes pyridinoline (PYD) compounds to form chlorinated moieties.15 PYD crosslinks are integral members in the structural composition of articular cartilage that serve to covalently link helical regions of Col-II to one another, type IX collagen (Col-IX) to the surface of Col-II, and Col-IX to other molecules of Col-IX. This high degree of crosslinking functionally stabilizes the collagen fibrillar superstructure, thus making it more resistant to proteolytic degradation. In addition to its importance in the maintenance of the collagen superstructure, PYD also contains amine and phenol groups that are sensitive to HOCl and Cl2 modification. Specifically, HOCl and Cl2 are capable of displacing the phenol group on an aromatic ring of PYD to produce chlorinated 3-chloro-products. They also react with primary amines to generate long-lived N-chloramines, which have a lower oxidizing potential than HOCl and Cl2, but they have longer lifetime (~18 h) and as such may be responsible for distant damage.
In addition to increased MPO, protein targets of HOCl and Cl2 are chlorinated and found in the synovial fluid samples of patients with early OA. Thus, chlorination occurs during a period when cartilage ECM degradation is at its highest level. Under normal circumstances with no cartilage degeneration (control groups) and in advanced stages of OA, where little cartilage remains, MPO activity is minimal and may explain our inability to detect either MPO or chlorinated-peptides.
Current diagnosis of OA is based upon radiographic and clinical data. New tools and criteria for diagnosing and measuring the progression of OA are currently under investigation, including the development of biomarkers. A number of markers have been proposed for the diagnosis of OA, such as, the presence of urine bone hydroxyllysyl pyridinoline, serum cartilage oligomeric matrix protein, glycosaminoglycan, keratan sulphate epitope 5D4, YKL-40, osteocalcin, C-telopeptide of type I collagen, hyaluronan and C-reactive protein, and in synovial fluid, aggrecan or Col-II fragments.24–27 However, none of these markers have sufficient sensitivity or specificity to provide a definitive diagnosis of early OA. It is therefore of importance to characterize additional biomarkers. Knowledge gained from the discovery of biomarkers is expected to also provide critical information needed to develop therapies for the treatment of OA.
We have shown that the presence of Cl-peptides in synovial fluid is unique to subjects with active cartilage degradation. As such, the presence of cartilage specific Cl-peptides in joint fluid should serve as important biomarkers for the early diagnosis of OA, and may be useful in determining disease progression. Availability of such “markers” would aid clinicians in the diagnosis of this condition at an early stage with potential for better delivery of care to preserve the joint. Additionally, the detection of ROS production, particularly the more reactive species points to a mechanism by which articular cartilage surfaces may be modified and may help the physicians to design novel therapeutic approaches, such as administration of antioxidants, that can impact the disease progression. Further clinical studies are needed to evaluate the role of these marker agents in diagnosis of early OA. We are also currently engaged in studies to determine the presence of these biomarkers in the serum and urine.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Orthopaedic Surgery, Thomas Jefferson University, and the Jefferson Health Care system for their support of this work. None of the listed authors has any professional and/or financial affiliations that may be perceived to have biased this presentation. This work was paid for in part by grants awarded to Dr. Marla J. Steinbeck from National Institute of Craniofacial and Dental Research (R29 DE11082) and Dr. Javad Parvizi from The Knee Society and The Aircast Foundation, Inc. (F0405R).
REFERENCES
- 1.Abramson SB. Inflammation in osteoarthritis. J Rheumatol Suppl. 2004;70:70–76. [PubMed] [Google Scholar]
- 2.Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 4.Brooks P. Inflammation as an important feature of osteoarthritis. Bull World Health Organ. 2003;81:689–690. [PMC free article] [PubMed] [Google Scholar]
- 5.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–508. doi: 10.1016/S0140-6736(97)07226-7. see comment. [DOI] [PubMed] [Google Scholar]
- 6.Drynda S, Ringel B, Kekow M, et al. Proteome analysis reveals disease-associated marker proteins to differentiate RA patients from other inflammatory joint diseases with the potential to monitor anti-TNFalpha therapy. Pathol Res Pract. 2004;200:165–171. doi: 10.1016/j.prp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich GE. Osteoarthritis beginning with inflammation. Definitions and correlations. Bull World Health Organ. 2003;81:691–693. 1975. [PMC free article] [PubMed] [Google Scholar]
- 8.Garnero P, Delmas PD. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2003;15:641–646. doi: 10.1097/00002281-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Loeuille D, Chary-Valckenaere I, Champigneulle J, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 10.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. comment. [DOI] [PubMed] [Google Scholar]
- 11.Spector TD, Hart DJ, Nandra D, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Yoshino S, Kato T, et al. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage. 1999;7:401–402. doi: 10.1053/joca.1998.0224. [DOI] [PubMed] [Google Scholar]
- 13.Konttinen YT, Michelsson JE, Tolvanen E, et al. Primary inflammatory reaction in synovial fluid and tissue in rabbit immobilization osteoarthritis. Clin Orthopaed Related Res. 1990;260:280–286. [PubMed] [Google Scholar]
- 14.Caron JP, Fernandes JC, Martel-Pelletier J, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 15.Daumer KM, Khan AU, Steinbeck MJ. Chlorination of pyridinium compounds. Possible role of hypochlorite, N-chloramines, and chlorine in the oxidation of pyridinoline cross-links of articular cartilage collagen type II during acute inflammation. J Biol Chem. 2000;275:34681–34692. doi: 10.1074/jbc.M002003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 17.Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995;2:55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Dewald B, Baggiolini M, Curnutte JT, et al. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979;63:21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause KH, Clark RA. Geneva Biology of Ageing Workshop 2000: phagocytes, inflammation, and ageing. Exp Gerontol. 2001;36:373–381. doi: 10.1016/s0531-5565(00)00221-7. [DOI] [PubMed] [Google Scholar]
- 20.Clark RA, Klebanoff SJ. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979;63:177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen H, Klebanoff SJ. Oxidation of Escherichia coli iron centers by the myeloperoxidase-mediated microbicidal system. J Biol Chem. 1982;257:13731–13735. [PubMed] [Google Scholar]
- 22.Suzuki K, Muso E, Nauseef WM. Contribution of peroxidases in host-defense, diseases and cellular functions. Jpn J Infect Dis. 2004;57 [PubMed] [Google Scholar]
- 23.Nauseef WM, Metcalf JA, Root RK. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983;61:483–492. [PubMed] [Google Scholar]
- 24.Otterness IG, Weiner E, Swindell AC, et al. An analysis of 14 molecular markers for monitoring osteoarthritis. Relationship of the markers to clinical end-points. Osteoarthritis Cartilage. 2001;9:224–231. doi: 10.1053/joca.2000.0379. [DOI] [PubMed] [Google Scholar]
- 25.Moller HJ. Connective tissue markers of rheumatoid arthritis. Scand J Clin Lab Invest. 1998;58:269–278. doi: 10.1080/00365519850186445. [DOI] [PubMed] [Google Scholar]
- 26.Rorvik AM, Grondahl AM. Markers of osteoarthritis: a review of the literature. Vet Surg. 1995;24:255–262. doi: 10.1111/j.1532-950x.1995.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharif M, Kirwan JR, Elson CJ, et al. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]