Abstract
While the prevalence of patent foramena ovale (PFOs) in the general population is around 25%, it is approximately doubled among cryptogenic stroke (CS) patients. This has generally been attributed to paradoxical embolism and many physicians recommend PFO closure to prevent recurrence. However, the benefit of PFO closure in patients with stroke has not been demonstrated. Further, the epidemiology of stroke recurrence in patients with CS with PFO versus without PFO, and in those with large right-to-left shunts versus small right-to-left shunts, has yielded results that appear difficult to reconcile with the hypothesis that paradoxical embolism is an important cause of stroke recurrence. The purpose of this review is to critically examine the epidemiological evidence that PFO is a potentially modifiable risk factor for stroke recurrence in patients with cryptogenic stroke. The evidence suggests that many patients with CS and PFO have strokes that are PFO-attributable, but that many have strokes that are unrelated to their PFO.. We introduce the concept of “PFO-propensity”, defined as the patient-specific probability of finding a PFO in a patient with cryptogenic stroke based on their age and other risk factors. We show that this value is directly related to the probability that a CS is PFO-attributable. Because there is substantial heterogeneity both in PFO-propensity and in recurrence risk among patients with PFO and cryptogenic stroke, stratification for closure by these joint probabilities will likely prove crucial for appropriate patient selection.
Keywords: Patent foramen ovale, Risk factors for stroke, Secondary stroke prevention, Cryptogenic Stroke
Introduction
Even though it was first proposed as a cause of stroke in 1877, paradoxical embolism through a patent foramen ovale remains controversial1. To some it is a common cause of cryptogenic stroke while to others it is a medical curiosity. Some authors express doubts regarding the importance of paradoxical embolism, even when a PFO is discovered in patients with no other apparent stroke etiology2. Others advocate the prophylactic closure of nearly any discovered PFO3, even though incidental PFOs can be found in approximately 25% of autopsies4. The debate has been intensified in equal measure by the absence of definitive randomized data regarding the effectiveness of PFO closure for stroke and the confusing and paradoxical nature of the epidemiological evidence that PFO is a risk factor for stroke recurrence. The purpose of this review is to critically examine that evidence.
Is PFO a Risk Factor for Stroke Recurrence?
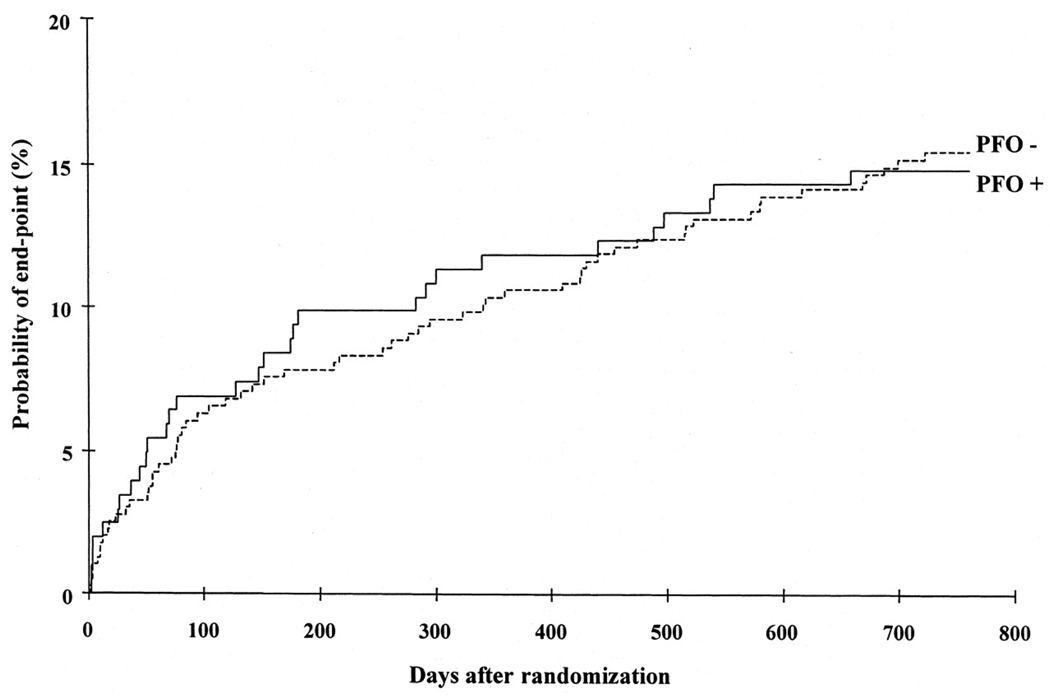
The PFO in Cryptogenic Stroke Study (PICSS) was one of the largest studies to examine the role of PFO in stroke5. Figure 1 shows stroke recurrence rates in patients enrolled in PICCS with and without PFO. The figure, which demonstrates near-identical stroke recurrence in patients with and without PFO, is often invoked by those who caution against the routine closure of PFO in patients with stroke, the apparent logic being that if PFO is not associated with an increased risk of stroke recurrence, then PFO closure is unlikely to reduce this risk. Further, when PFO patients in this trial were subgrouped into those with small versus large PFOs, there was a strong trend to higher recurrence rates in those with smaller PFOs; the 2 year event rates in patients with no, small, and large PFO was 15.4%, 18.5%, and 9.5%, respectively. This again appears to speak against the importance of paradoxical embolism as a risk factor for stroke and stroke recurrence.
Figure 1. Relative Risk of Recurrence in Cryptogenic Stroke with and without PFO.
The cumulative risk of recurrent stroke or death stratified by baseline PFO status in the PFO in Cryptogenic Stroke Study. This figure, from Homma et al5, is based on data from patients with both cryptogenic strokes and strokes of known cause.
However, this straight-forward interpretation of the results of PICSS is likely to be misleading for several reasons. First, despite its name, PICSS enrolled all patients with stroke for whom transesophageal echocardiography (TEE) was performed, including both CS patients and patients with a stroke of determined cause. Indeed, only 250 of the 630 patients enrolled in PICSS had a cryptogenic stroke. Thus, most patients in PICSS, had PFO-unrelated strokes, whether a PFO was discovered at TEE or not. Indeed, when understood in this light, the trend to lower recurrence in patients with large versus small PFO becomes understandable: the group of patients with smaller PFOs has a stroke recurrence rate similar to those without a PFO because they have stroke mechanisms that are similar; the group of patients with larger PFOs is presumably enriched for patients with PFO-related cryptogenic strokes, which may have a lower recurrence risk than other stroke subtypes. Thus, even though patients with large PFOs have the same or even a lower recurrence rate than those without a PFO does not imply that large PFOs do not increase recurrence risk in the patients who actually have them.
Indeed, similar results are seen among patients with cryptogenic stroke. A recent meta-analysis by Almelkhafi et al6, examined four studies that compared recurrence rates in patients with cryptogenic stroke, with versus without PFO. They determined that the presence of a PFO does not increase the risk of recurrence either of stroke or TIA (pooled relative risk [RR] with versus without PFO 1.1 [95% CI 0.8–1.5]) or of stroke alone (pooled RR 0.8 [95% CI 0.5–1.3]). However, just as in PICSS (which included all stroke patients), these similarities in recurrence risks among those with CS does not imply that PFO is not a risk factor for stroke recurrence in patients who have them. The result simply indicates that if a PFO is pathogenic, the strokes that it causes are roughly as likely to recur as cryptogenic strokes caused by other occult mechanism (e.g. occult paroxysmal atrial fibrillation or subthreshold aortic atheroembolic disease).
Indeed, one of the most consistent findings is that CS patients with PFO have substantially lower prevalence of conventional stroke risk factors than CS patients without PFO. Conventional stroke risk factors in the three largest studies examining stroke recurrence in CS are shown in Table 1, for patients with and without PFO7–9. These large and consistent differences have several important implications: 1) PFO is pathogenically important in the index cryptogenic stroke, since otherwise patients with and without this anatomic variant would be expected to be similar; 2) PFO is an important risk factor for stroke recurrence, since it single-handedly compensates for the shortfall in other risk factors such that those with and without PFO have similar recurrence rates; 3) even before a TEE is obtained, the presence or absence of conventional stroke risk factors can be used to estimate the likelihood of finding a PFO. It can be seen that, among CS patients, younger patients without hypertension and/or diabetes and/or coronary artery disease are much more likely to have a PFO than patients with these risk factors.
Table 1.
Prevalence of Conventional Risk Factors in Cryptogenic Stroke Patients with and without PFO
| Risk Factor | Serena (2008)7 | Lamy (2002)8 | Weimar (2009)9 | |||
|---|---|---|---|---|---|---|
| pfo + | pfo − | pfo + | pfo − | pfo + | pfo − | |
| n=297 | n=189 | n=267 | n=314 | n=404 | n=722 | |
| Age (mean) | 53.2 | 60.8 | 40.1 | 44.5 | 53.6 | 60.6 |
| DM (%) | 6.4 | 19.0 | 3.0 | 5.1 | 12.7 | 19.2 |
| HTN (%) | 27.7 | 46.0 | 8.6 | 21.3 | 43.2 | 61.5 |
| CAD (%) | 3.7 | 6.9 | - | - | 6.2 | 11.5 |
| Smoker (%) | 33.0 | 30.3 | 43.4 | 51.6 | 26.7 | 34.8 |
Another finding that has been somewhat confusing, is that CS patients with small and large right-to-left shunts have roughly equivalent stroke recurrence rates. This has been found consistently in the 3 largest studies that have examined this7, 9, 10, whether shunting is measured by TEE or transcranial doppler (TCD). The absence of an effect of shunt size might be explained in part by the poor reliability of shunt measurement, which depends on multiple factors including volume status, procedural technique and inter-reader reliability. However, another explanation also deserves attention: patients with smaller PFOs may be more likely to have occult mechanisms other than paradoxical embolism, with recurrence risks that are the same or even higher than paradoxical embolism. The finding of similar recurrence risks should therefore not be interpreted as implying that CS patients with small and large shunts found on TEE or TCD are equally likely to have a future paradoxical embolism.
PFO in Cryptogenic Stroke: Pathogenic or Incidental
Thus, the fact that patients with a CS and a PFO may have stroke mechanisms that are either PFO-related (e.g. paradoxical emboli) or PFO-unrelated and that it is not possible, with certainty, to segregate these two groups, substantially complicates the clinical epidemiology, as it does the clinical care of these patients. Closing a PFO that is totally incidental to a stroke is not likely to decrease stroke recurrence, and will only add procedural and device-related risks. While it is typically not possible in an individual patient to determine with certainty whether a PFO discovered in the setting of a CS is pathogenic (as opposed to incidental), it is possible to determine the fraction of cryptogenic strokes attributable to the presence of a PFO among patients in whom a PFO is discovered.
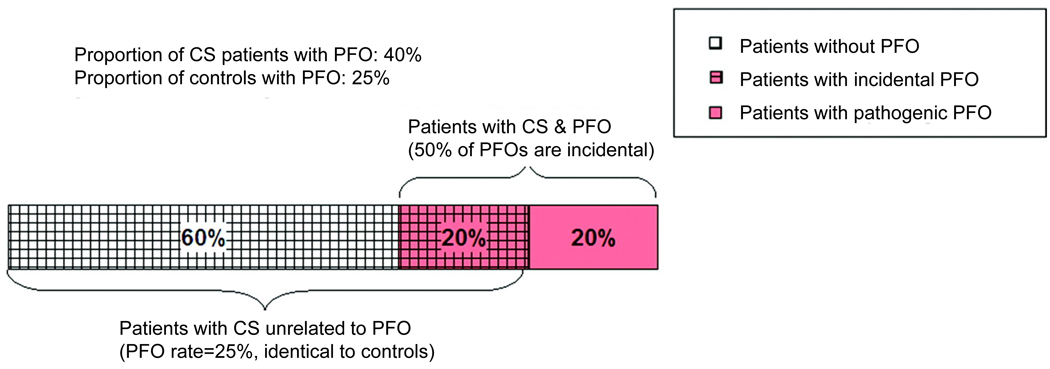
This is shown in Figure 2, using a PFO prevalence of 40% among those with CS and 25% among a control population. Assuming that PFO prevalence among those with CS unrelated to a PFO is 25% (based on the control rate), these figures suggest that approximately 50% of PFOs discovered in the setting of CS would be incidental.
Figure 2. Proportion of Patients with Cryptogenic Stroke and PFO with Incidental PFO.
This figure shows how the proportion of incidental versus pathogenic PFO in patients with CS can be calculated based on the prevalence of PFO in CS patients and in controls. As indicated in Figure 2, when the prevalence of PFO in the CS population is 40% and the prevalence of PFO in the control group is 25%, then 50% of PFOs discovered in CS patients would be incidental. This is based on the assumption that CS patients who have strokes from causes unrelated to PFO will have the same PFO prevalence as the control group (in this case 25%). Adapted from Alsheikh-Ali AA et al11.
This is an application of Bayes’ theorem. Using Bayes theorem to solve for the probability that PFOs are incidental yields the following equation11, 12:
| (Equation 1) |
Thus, as PFO prevalence in CS patients decreases the probability that a discovered PFO will be incidental increases. If the prevalence of PFO in CS patients was equivalent to that in a control population (e.g. 25%) then the probability that a discovered PFO is incidental increases to 100% (i.e. this would be the expected rate if PFO was not a risk factor for cryptogenic stroke).
The equation has another interesting property in that the right hand side is numerically equivalent to the inverse of the odds ratio in case control studies11, 13. This permits the easy conversion of the odds ratio of case control studies associating the prevalence of PFO in CS patients versus controls to the more clinically relevant estimation of the probability that a discovered PFO is incidental.
Figure 3 shows the results of a meta-analysis of case control studies comparing PFO prevalence in patients with CS versus those with stroke of known cause. Only studies without age exclusions were included in this analysis. Panel A shows the results expressed conventionally as an odds ratio and shows that 16 out of 17 studies demonstrated a higher prevalence of PFO in CS patients compared to patients with stroke of known cause, a remarkably strong and consistent result. Following Bayesian transformation (Panel B), these results yield an estimated summary probability of 33% (95% CI: 28%–39%) that a PFO discovered in a patient with a CS is incidental11.
Figure 3. PFO Prevalence in Cryptogenic Stroke vs Stroke of Known Cause.
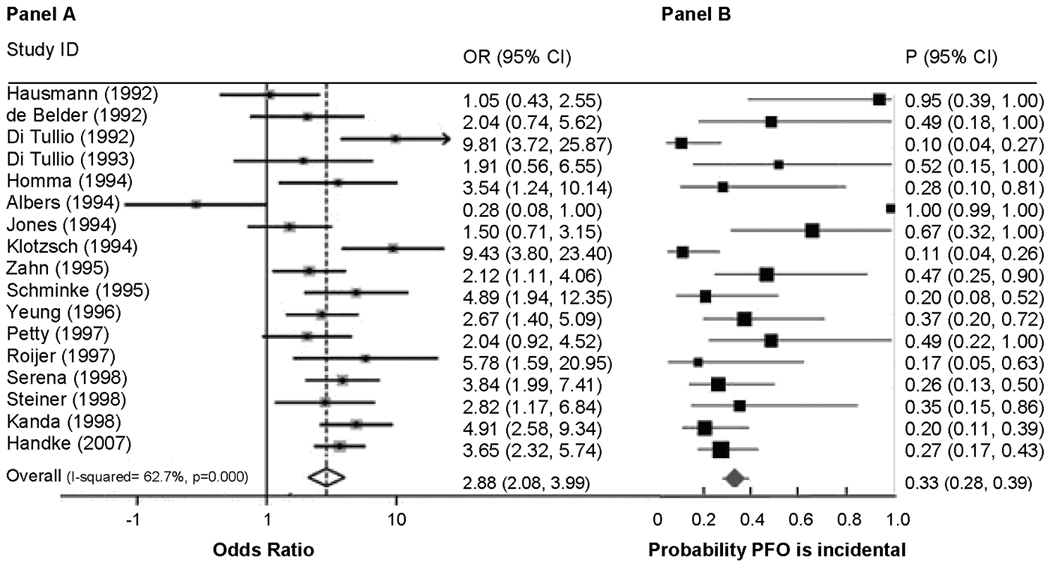
This figure shows forest plots of random-effects meta-analyses of case-control studies examining the prevalence of PFO in cases with CS versus controls with stroke of determined cause. Panel A shows the odds ratio of finding a PFO in patients with cryptogenic stroke versus stroke of known cause. Panel B shows the same data after Bayesian transformation to yield the probability that the PFO is incidental. Adapted from reference Alshiekh-Ali et al11.
However, there is tremendous between-study heterogeneity in this estimate. Between-study differences that might account for this variability include the stringency of the operational definition of CS (i.e. the work-up either required or typically performed to rule out other causes), with more stringent studies yielding lower probabilities of incidental PFO. Bias due to more rigorous TEE examination of CS patients compared to that in patients with known mechanism (i.e. in unblinded studies) would tend diminish the estimate of incidental strokes.
The characteristics of patients enrolled in a study can also affect the PFO-attributable fraction among patients with PFO and CS (Table 2). Among studies enrolling only younger patients, the estimated probability of an incidental PFO is considerably lower; among older patients, considerably higher. The data suggest that when a PFO is found together with an atrial septal aneurysm in the setting of a cryptogenic stroke, this finding is rarely incidental11.
Table 2.
PFO Prevalence in Cryptogenic Stroke vs Stroke of Known Cause
| OR | Probability PFO Incidental (%) | Sensitivity Analysis (%)§ | |
|---|---|---|---|
| Age-inclusive | 2.88 (2.08–3.99) | 33 (28–39) | 48 (39–59) |
| Younger* | 5.07 (3.28–7.84) | 20 (16–25) | 20 (16–25) |
| Older** | 1.95 (1.03–3.67) | 48 (34–66) | 84 (60–100) |
| PFO +ASA | 9.09 (3.25–25) | 11 (4–31) |
Younger is typically defined as < 55 years of age
Older is typically defined as ≥ 55 years of age
Sensitivity Analysis is based on studies using non-stroke controls
Adapted from previously published work by Alsheikh-Ali AA et al11
PFO Propensity
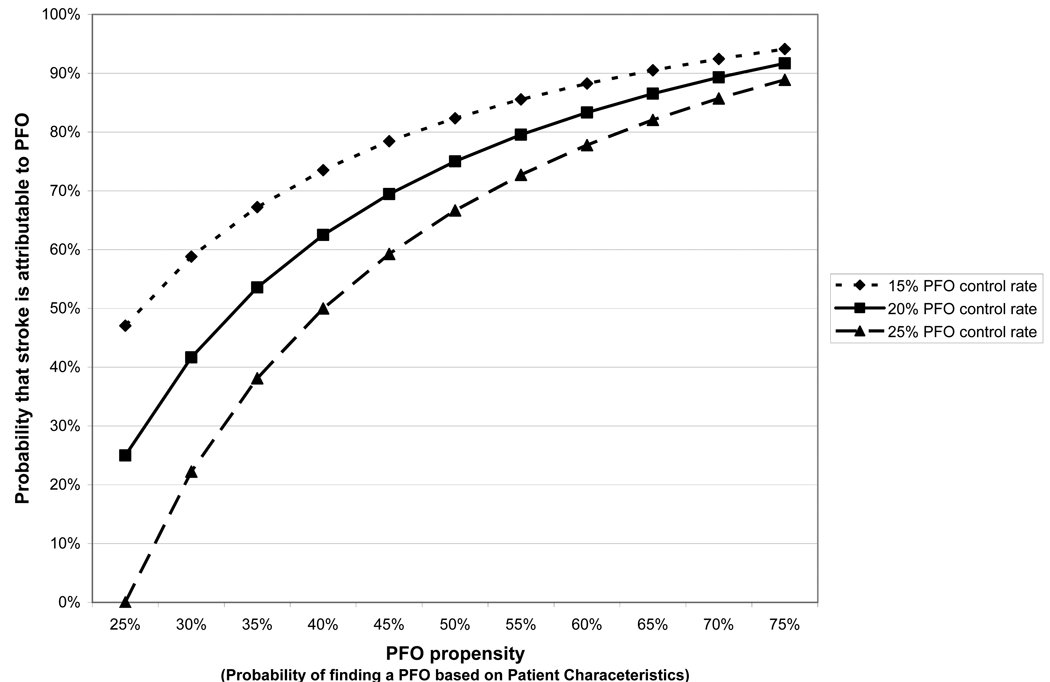
Equation 1 can also be used to estimate, in an individual patient, the likelihood that a discovered PFO is, or is not, incidental based on the prevalence of PFO in CS patients with similar characteristics. We call this individualized prevalence estimate “PFO propensity” (i.e. the probability that a CS patient has a PFO or not based on other characteristics). As seen in Table 1, among CS patients, PFO propensity will be increased by younger age and the absence of conventional stroke risk factors. Figure 4 illustrates the relationship between the PFO attributable fraction (i.e. the fraction of cryptogenic strokes attributable to the PFO in those with both CS and PFO) based on PFO propensity, using Equation 1. Since conventional logistic regression modeling can be used to estimate PFO propensity, these models can be used to stratify patients by the probability that a PFO is pathogenic versus incidental.
Figure 4. PFO Propensity and the Probability that a Stroke is PFO-attributable.
We define PFO propensity as the probability of finding a PFO in a patient, based on patient-specific characteristics (such as age and the presence or absence of hypertension, diabetes and hypercholesterolemia). Through Bayes’ theorem, it is directly (though non-linearly) related to the probability that a cryptogenic stroke is PFO-attributable (in patients with both cryptogenic stroke and PFO).
PFO Closure
We have shown that there is compelling, yet indirect evidence that PFO is an important risk factor for stroke recurrence after an initial cryptogenic stroke. However, the stroke recurrence rate in patients with PFO and CS is relatively low, estimated at around 2% per year4. It is also clear that many of the recurrent strokes, as with the index events, may not be due to paradoxical embolism. Indeed, even among the portion of patients in whom a stroke is PFO-attributable, other mechanisms aside from paradoxical embolism have been proposed, such as in-situ thrombus formation or increased susceptibility to atrial fibrillation14, 15. These mechanisms would presumably not be addressed with mechanical closure.
Thus, the margin of potential benefit for PFO closure is narrow. Even a relatively low rate of procedure- and device-related complications could nullify most or all of the potential benefit. While case series consistently show very low rates of stroke recurrence after device placement and suggest benefit for closure compared to medically treated patients16, for interventions with a low margin for benefit, these observational studies are unreliable, and we must await the completion of randomized clinical trials, which are on-going.
Regardless of the findings of trials, the discussion above suggests that some patients might benefit from PFO closure and others might be harmed, while most will remain stroke free with or without the implanted device. Careful patient selection for closure, and for the trials that study closure, will therefore be crucial. Where risk is determined by multiple patient characteristics, use of risk models may be important for selecting patients who can benefit17. Risk stratification strategies for PFO closure, however, need to include stratification not only for stroke recurrence risk but, more importantly, for the probability that the index event was itself PFO-attributable. Even if many CS patients can benefit from closure, testing the procedure in a population of CS patients with many incidental PFOs may falsely suggest that the procedure is of no benefit.
Acknowledgments and Funding
This manuscript is partially funded by NIH/NINDS grant (R01 NS062153). David Thaler is a consultant to AGA Medical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006 January 17;47(2):440–445. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Tong DC, Becker KJ. Patent foramen ovale and recurrent stroke: closure is the best option: no. Stroke. 2004 March;35(3):804–805. doi: 10.1161/01.STR.0000117964.10781.BA. [DOI] [PubMed] [Google Scholar]
- 3.Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005 April;91(4):444–448. doi: 10.1136/hrt.2004.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005 August 16;112(7):1063–1072. doi: 10.1161/CIRCULATIONAHA.104.524371. [DOI] [PubMed] [Google Scholar]
- 5.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002 June 4;105(22):2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 6.Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology. 2009 July 14;73(2):89–97. doi: 10.1212/WNL.0b013e3181aa2a19. [DOI] [PubMed] [Google Scholar]
- 7.Serena J, Marti-Fabregas J, Santamarina E, Rodriguez JJ, Perez-Ayuso MJ, Masjuan J, Segura T, Gallego J, Davalos A. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke. 2008 December;39(12):3131–3136. doi: 10.1161/STROKEAHA.108.521427. [DOI] [PubMed] [Google Scholar]
- 8.Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, Coste J, Mas JL. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002 March;33(3):706–711. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 9.Weimar C, Holle DN, Benemann J, Schmid E, Schminke U, Haberl RL, Diener HC, Goertler M. Current management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shunt. Cerebrovasc Dis. 2009;28(4):349–356. doi: 10.1159/000229553. [DOI] [PubMed] [Google Scholar]
- 10.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. New England Journal of Medicine. 2001 December 13;345(24):1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 11.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent Foramen Ovale in Cryptogenic Stroke. Incidental or Pathogenic? Stroke. 2009 May 14; doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent DM, Trikalinos TA, Thaler DE. Patent foramen ovale and cryptogenic stroke. N Engl J Med. 2008 April 3;358(14):1519–1520. [PubMed] [Google Scholar]
- 13.Mattle HP, Meier B, Nedeltchev K. Prevention of stroke in patients with patent foramen ovale. Int J Stroke. 2010 April;5(2):92–102. doi: 10.1111/j.1747-4949.2010.00413.x. [DOI] [PubMed] [Google Scholar]
- 14.Berthet K, Lavergne T, Cohen A, Guize L, Bousser MG, Le Heuzey JY, Amarenco P. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke. 2000 February;31(2):398–403. doi: 10.1161/01.str.31.2.398. [DOI] [PubMed] [Google Scholar]
- 15.Mugge A, Daniel WG, Angermann C, Spes C, Khandheria BK, Kronzon I, Freedberg RS, Keren A, Denning K, Engberding R. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995 June 1;91(11):2785–2792. doi: 10.1161/01.cir.91.11.2785. [DOI] [PubMed] [Google Scholar]
- 16.Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Annals of Internal Medicine. 2003 November 4;139(9):753–760. doi: 10.7326/0003-4819-139-9-200311040-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007 September 12;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]