To the Editor: Triatoma infestans bugs, the main vector of Chagas disease, historically occupied a large area from northeastern Brazil to Chubut Province in Patagonia, Argentina (1). Large-scale insecticide spraying during the 1980s and 1990s reduced its geographic range and abundance and interrupted transmission of Trypanosoma cruzi, mainly in Uruguay, Chile, and Brazil (2). However, T. infestans and transmission of T. cruzi persist in the Gran Chaco, a large ecoregion in Argentina, Bolivia, and Paraguay (3).
Chubut Province has historically been an area with no risk for vector-mediated transmission of T. cruzi; only its extreme northern region was categorized as having a low transmission risk (4,5). However, increased immigration from disease-endemic rural areas in Argentina and Bolivia into Chubut has raised concerns about accidental introduction of T. infestans in travelers’ luggage (1) and establishment of a transmission cycle.
In January 2007, we conducted a province-wide survey of 21 villages in Chubut Province previously infested with T. infestans bugs by using 0.2% tetramethrin as a dislodgant agent (1 person-hour/house); no T. infestans bugs were detected (Appendix Figure). Only T. patagonica bugs were found in 11% of peridomestic structures, and none were infected with T. cruzi. In June 2007, a T. infestans–like bug was found in a primary healthcare center in Comodoro Rivadavia (45°51′S, 67°28′W), a city in southern Chubut Province (Appendix Figure). Healthcare center staff reported visits by immigrants from Bolivia a few days before this finding and suspected them to be the source. The bug was identified morphologically as a T. infestans female and it laid 6 eggs. PCR amplification of kinetoplast DNA showed that it was not infected by T. cruzi.
DNA sequence analysis is useful for investigating evolutionary history and population structure within Triatominae (6). T. infestans bugs from Bolivia and Argentina showed genetic differences for nuclear (7) and mitochondrial markers (6), including mitochondrial cytochrome oxidase I (mtCOI) (8). We used our mtCOI haplotype database, which includes published (8) and new domestic, peridomestic, and sylvatic T. infestans from 65 locations in 13 provinces in Argentina (n = 346) and 3 departments in Bolivia (n = 144), to analyze the mtCOI sequence of the bug found in southern Patagonia and determine if it could be assigned to a known haplotype from Bolivia or Argentina. We investigated phylogenetic relationships with other haplotypes by using neighbor-joining and Bayesian approaches.
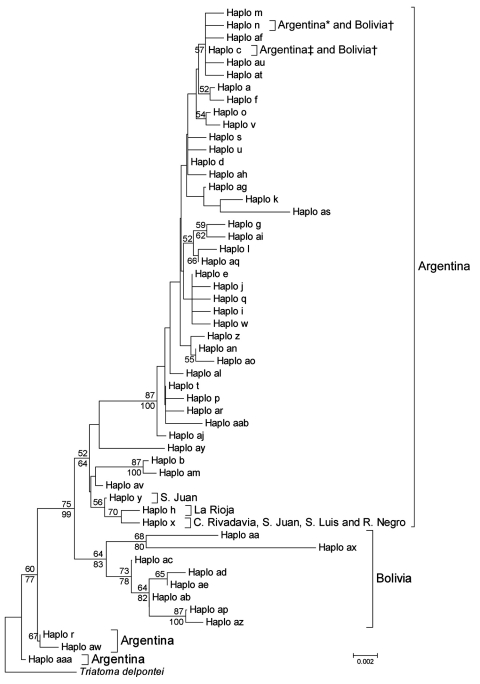
Our mtCOI database included 53 haplotypes: 42 were found in Argentina, 9 in Bolivia, and 2 in both countries (Figure). The bug from southern Patagonia had haplotype x, which has been found in only 3 western or southern provinces in Argentina (San Juan, San Luis, and Rio Negro) (8; Appendix Figure).
Figure.
Phylogenetic relationships between mitochondrial cytochrome oxidase I gene haplotypes of Triatoma infestans from Argentina and Bolivia. The neighbor-joining tree was constructed by using MEGA 4.1 (www.megasoftware.net) and bootstrap values (based on 1,000 replications) >50% are shown above the branches. A Bayesian maximum clade credibility tree was similar, and clade posterior probabilities >50% are shown below the branches of the neighbor-joining tree. MRBAYES 3.1 (http://mrbayes.csit.fsu.edu) default priors were assumed and run for 4 million generations. Convergence of the Markov chain Monte Carlo analysis was investigated with the SD of split frequencies and diagnostics implemented in AWTY (http://ceb.csit.fsu.edu/awty). The model of evolution (Hasegawa-Kishino-Yano + invariable sites+ Γ [HKY + I + Γ]) was chosen with Mrmodeltest 2.3 (www.abc.se/~nylander). Because MEGA 4.1 does not support HKY; the more inclusive Tamura-Nei method (www.megasoftware.net/WebHelp/part_iv___evolutionary_analysis/computing_evolutionary_distances/distance_models/nucleotide_substitution_models/hc_tamura_nei_distance.htm) was used for the neighbor-joining analysis. Haplotypes al, an, ao, ap, aq, at, au, ax, az, aaa, and aab are reported. DNA sequences are available in GenBank (accession nos. EF451005-EF451041, FJ439768, FJ811845–8, and GQ 478993–GQ 479005). *Two provinces in Argentina; †Tarija, Bolivia; ‡10 provinces in Argentina. Scale bar indicates nucleotide substitutions per site.
Results of phylogenetic analyses were congruent (Figure). The neighbor-joining tree showed that haplotype x formed a cluster with haplotype h (Argentina) and haplotypes from Bolivia clustered in 3 other groups: 1) two groups with bootstrap values >70% (one with haplotypes at, n, c, and 33 haplotypes from Argentina, and the other with haplotypes ab, ac, ad, ae, ap, and az); and 2) one group with a bootstrap value of 68% (haplotypes ax and aa). The Bayesian tree showed that haplotypes from Bolivia were arranged in 2 well-supported clades (posterior probabilities >83%) and that haplotype x was not included within any of them. Thus, haplotype x of the bug from southern Patagonia was found only in Argentina and was not closely related to haplotypes from Bolivia.
We investigated the geographic origin of non-native putative attendees of the healthcare center in San Cayetano. These persons were immigrants from Bolivia and from northern (Salta and Jujuy), western (Mendoza, San Juan, and San Luis), and southern Argentina (Rio Negro), i.e., from the 3 putative sources of the bug. These immigrants typically pay extended visits to their home towns at least once a year and transport luggage in which the bug could have traveled. In 2006, San Juan had the highest levels of domestic and peridomestic infestation with T. infestans (35% and 21%, respectively), including urban infestation (9). Mendoza (not in our database) had considerable domestic and peridomestic infestations (both 7%), and San Luis (0.5% and 5.3%, respectively) and Rio Negro (both <0.1%) had low infestations in 2001 (4) and thereafter (C. Spillmann, unpub. data). Bolivia, Salta, and Jujuy are excluded as potential sources of the bug because haplotypes closely related to haplotype x were not found in these places. Active dispersal from a local source can be ruled out because there is no precedent for T. infestans in Comodoro Rivadavia, and the mean temperature in June (8°C) is below the known threshold for flight initiation (23°C) (10).
Our results show that molecular phylogenetics can identify passive transport of insects into areas where a disease is not endemic and rule out putative sources supported only by circumstantial evidence. Our findings reinforce the need for sustained and coordinated vector surveillance and control at a regional level (3).
Supplementary Material
Location of Comodoro Rivadavia (Chubut) and mitochondrial cytochrome oxidase I haplotype frequencies among Triatoma infestans bugs in provinces in Argentina and departments in Bolivia. Colors and patterns in circles indicate frequencies of each haplotype in an area. The haplotype of the bug from southern Patagonia (x) is indicated in red. Shared haplotypes between Argentina and Bolivia are indicated in blue (haplotype c) and green (haplotype n). Yellow areas indicate provinces surveyed. Gray shading indicates areas of Chubut Province where T. infestans bugs were not found in 2007. S. C., Santa Cruz; Sgo., Santiago.
Acknowledgments
We thank Mirko Rojas-Cortez for providing samples from Bolivia, Ricardo Váez for assisting in bug searches, Fernando Garelli and Juan Gurevitz for carefully reading the manuscripts and suggestions, and Marcela Rodriguero for helpful discussions on an earlier version of the manuscript.
This study was supported by research grant no. R01 TW05836 from the National Institutes of Health Fogarty International Center and the National Institute of Environmental Health Sciences to U.K. and R.E.G., and by grants from the Universidad de Buenos Aires and Agencia Nacional de Promoción Científica y Técnica (Argentina) to R.E.G. R.V.P., M.V.C., and R.E.G. are scientific investigators of Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina).
Footnotes
Suggested citation for this article: Piccinali RV, Canale DM, Sandoval AE, Cardinal MV, Jensen O, Kitron U, et al. Triatoma infestans Bugs in southern Patagonia, Argentina [letter]. Emerg Infect Dis [serial on the Internet]. 2010 May [date cited]. http://www.cdc.gov/EID/content/16/5/887.htm
References
- 1.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera: Reduviidae) and their significance as vectors of Chagas’ disease. Bull Am Mus Nat Hist. 1979;163:127–520. [Google Scholar]
- 2.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–8. 10.1016/j.pt.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 3.Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci U S A. 2007;104:16194–9. 10.1073/pnas.0700863104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization. XI Reunión de la Comisión Intergubernamental para la Eliminación de Triatoma infestans y la Interrupción de la Tripanosomiasis Americana por Transfusión, Paraguay. Iniciativa de salud del cono Sur (INCOSUR). 2002. March [cited 2009 Oct 13]. http://www.paho.org/spanish/HCP/HCT/DCH/xi-incosur.htm
- 5.Segura EL, Cura EN, Estani SA, Andrade J, Lansetti JC, de Rissio AM, et al. Long-term effects of a nationwide control program on the seropositivity for Trypanosoma cruzi infection in young men from Argentina. Am J Trop Med Hyg. 2000;62:353–62. [DOI] [PubMed] [Google Scholar]
- 6.Abad-Franch F, Monteiro FA. Molecular research and the control of Chagas disease vectors. An Acad Bras Cienc. 2005;77:437–54. 10.1590/S0001-37652005000300007 [DOI] [PubMed] [Google Scholar]
- 7.Bargues MD, Klisiowicz DR, Panzera F, Noireau F, Marcilla A, Perez R, et al. Origin and phylogeography of the Chagas disease main vector Triatoma infestans based on nuclear rDNA sequences and genome size. Infect Genet Evol. 2006;6:46–62. 10.1016/j.meegid.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 8.Piccinali RV, Marcet PL, Noireau F, Kitron U, Gürtler RE, Dotson EM. Molecular population genetics and phylogeography of the Chagas disease vector Triatoma infestans in South America. J Med Entomol. 2009;46:796–809. 10.1603/033.046.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrizo Páez R, Pickenhayn J, Carrizo Páez M. Chagas urbano en San Juan. Diagnóstico, revisión y propuesta para un sistema integrado de ataque. Rev Argent Cardiol. 2008;76:480–7. [Google Scholar]
- 10.Gurevitz JM, Ceballos LA, Kitron U, Gürtler RE. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J Med Entomol. 2006;43:143–50. 10.1603/0022-2585(2006)043[0143:FIOTIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Location of Comodoro Rivadavia (Chubut) and mitochondrial cytochrome oxidase I haplotype frequencies among Triatoma infestans bugs in provinces in Argentina and departments in Bolivia. Colors and patterns in circles indicate frequencies of each haplotype in an area. The haplotype of the bug from southern Patagonia (x) is indicated in red. Shared haplotypes between Argentina and Bolivia are indicated in blue (haplotype c) and green (haplotype n). Yellow areas indicate provinces surveyed. Gray shading indicates areas of Chubut Province where T. infestans bugs were not found in 2007. S. C., Santa Cruz; Sgo., Santiago.