Abstract
BACKGROUND AND OBJECTIVES:
To provide a contemporary estimate of the economic burden of atherothrombosis in Canada, annual cardiovascular-related hospitalizations, medication use and associated costs across the entire spectrum of atherothrombotic disease were examined.
METHODS:
The REduction of Atherothrombosis for Continued Health (REACH) registry enrolled 1964 Canadian outpatients with coronary artery disease, cerebrovascular disease or peripheral arterial disease (PAD), or three or more cardiovascular risk factors. Baseline data on cardiovascular risk factors and associated medication use, and one-year follow-up data on cardiovascular events, hospitalizations, procedures and medication use were collected. Annual hospitalization and medication costs (Canadian dollars) were derived and compared among patients according to the presence of established atherothrombotic disease at baseline, specific arterial beds affected and the number of affected arterial beds.
RESULTS:
Average annualized medication costs were $1,683, $1,523 and $1,776 for patients with zero, one, and two or three symptomatic arterial beds, respectively. Average annual hospitalization costs increased significantly with the number of beds affected ($380, $1,403 and $3,465, respectively; P<0.0001 for overall linear trend). Mean hospitalization costs for patients with any coronary artery disease, any cerebrovascular disease and any PAD were $1,743, $1,823 and $4,677, respectively. After adjusting for other clinical factors, PAD at baseline was independently associated with a significant increase in hospitalization costs.
CONCLUSION:
Costs associated with vascular-related hospitalizations and interventions for Canadian patients increased with the number of affected arterial beds, and were particularly high for patients with PAD and/or polyvascular disease. These contemporary data provide insight into the economic burden associated with atherothrombotic disease in Canada, and highlight the need for increased preventive strategies to lessen the burden for patients and society.
Keywords: Cerebrovascular disease, Coronary disease, Costs, Hospitalization, Peripheral vascular disease
Abstract
HISTORIQUE ET OBJECTIFS :
Pour fournir une évaluation contemporaine du fardeau économique de l’athérothrombose au Canada, les chercheurs ont examiné les hospitalisations annuelles liées aux troubles cardiovasculaires, l’utilisation de médicaments et les coûts connexes dans tout le spectre des maladies athérothrombotiques.
MÉTHODOLOGIE :
Mille neuf cent soixante-quatre patients externes canadiens ayant une coronaropathie, une maladie cérébrovasculaire ou une maladie artérielle périphérique (MAP) ou au moins trois facteurs de risque cardiovasculaires ont participé au registre REACH pour réduire l’athérothrombose afin de profiter d’une santé constante. Les chercheurs ont colligé les données de départ sur les facteurs de risque cardiovasculaires et l’utilisation connexe de médicaments ainsi que les données de suivi d’un an sur les événements cardiovasculaires, les hospitalisations, les interventions et l’utilisation de médicaments. Ils ont dérivé les coûts annuels d’hospitalisation et des médicaments (en dollars canadiens) et les ont comparés entre patients selon la présence d’une maladie athérothrombotique diagnostiquée au départ, les lits artériels touchés exacts et le nombre de lits artériels touchés.
RÉSULTATS :
Le coût moyen annualisé des médicaments s’élevait à 1 683 $, 1 523 $ et 1 776 $ pour les patients ayant zéro, un et deux ou trois lits artériels symptomatiques, respectivement. Le coût d’hospitalisation annuel moyen a augmentait considérablement, selon le nombre de lits touchés (380 $, 1 403 $ et 3 465 $, respectivement; P<0,0001 pour la tendance linéaire globale). Le coût moyen d’hospitalisation des patients ayant une coronaropathie, une maladie cérébrovasculaire ou une MAP s’élevait à 1 743 $, 1 823 $ et 4 677 $, respectivement. Après rajustement compte tenu d’autres facteurs cliniques, la MAP au départ s’associait de manière indépendante à une augmentation considérable des coûts d’hospitalisation.
CONCLUSION :
Les coûts associés aux hospitalisations et aux interventions découlant de problèmes vasculaires chez les patients canadiens augmentaient selon le nombre de lits artériels touchés et se révélaient particulièrement élevés chez les patients ayant une MAP ou une maladie polyvasculaire. Ces données contemporaines donnent un aperçu du fardeau économique associé à la maladie athérothrombotique au Canada et font ressortir la nécessité d’accroître les stratégies de prévention en vue de réduire le fardeau pour les patients et la société.
Despite downward trends in the total number of deaths and hospitalizations associated with cardiovascular disease, atherothrombotic events remain the leading cause of death and disability throughout most industrialized countries, including Canada (1,2). Atherothrombosis is a systemic disorder affecting arterial beds throughout the body, potentially resulting in manifest coronary artery disease (CAD), cerebrovascular disease (CVD), peripheral arterial disease (PAD) or a combination of these conditions (polyvascular or diffuse vascular disease). Cardiovascular disease accounted for 31% of all deaths in 2005 in Canada, which was somewhat lower than rates from other developed countries (eg, in 2005, cardiovascular disease explained 35% of deaths in the United States and 36% of deaths in the United Kingdom) (2–4). Although continuing national efforts in Canada (5–7) are being directed toward secondary prevention and optimization of treatment strategies, it is expected that due to the aging population and other factors, including the increase in unhealthy dietary patterns, physical inactivity, obesity and diabetes mellitus, both the clinical and economic burden of atherothrombosis will present enormous challenges in the future (1,8,9). In Canada, costs associated with cardiovascular disease, including physician services, hospitalizations, lost wages and decreased productivity, were recently estimated to be more than $22.2 billion per year (10).
The REduction of Atherothrombosis for Continued Health (REACH) registry is a real-world, international, prospective registry aimed at evaluating the long-term risk of atherothrombosis across the entire spectrum of subjects at risk (11). The uniqueness of this registry is derived from its global scope and the fact that it enrolled patients at risk due to established atherothrombotic disease (coronary, cerebrovascular or peripheral) as well as patients without established symptomatic disease, but with three or more risk factors for atherothrombotic events. Baseline data from both the global REACH population and the Canadian REACH patients showed that risk factor profiles of patients with established vascular disease were similar regardless of the vascular bed affected (12,13). In addition, for the global and American REACH patient populations, there was a relatively high rate of polyvascular disease among patients with established vascular disease at baseline (13,14). The aims of the current study were to examine the one-year rates of cardiovascular-related hospitalizations and to provide estimated annual costs associated with these hospitalizations as well as cardiovascular medication use among the Canadian participants enrolled in REACH, stratified according to specific affected arterial beds and the number of arterial beds affected. An improved understanding of the economic burden associated with different risk groups would aid in the optimal targeting of future national prevention efforts aimed at decreasing the burden of cardiovascular disease for both patients and society.
METHODS
Participants and study design
The study characteristics of the overall REACH registry patient population have been described previously (11–13). In brief, 1976 patients were enrolled between January 2004 and October 2004 from 154 outpatient office practices in the Maritimes (Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick), Quebec, Ontario, the Prairies (Alberta, Manitoba and Saskatchewan) and British Columbia; these included family and general practitioners’ offices, as well as specialists’ offices in areas such as cardiology, neurology, angiology, vascular disease and endocrinology. To reflect the Canadian health care system and the emphasis on the primary care character of the study, an a priori target of 75% general practitioners or family physicians was established, with participating physicians required to enrol up to 15 consecutive patients. Compared with the 2005 Canadian population, geographical regions within Canada were represented within 1.5% of their respective populations, with the exception of British Columbia (slightly under-represented with 9.6% of initiated sites versus 13.1% of the population) and the Prairies (slightly over-represented with 19.1% of initiated sites versus 16.8% of the population) (12). Patients were eligible for inclusion if they were 45 years of age or older with at least one of the following four criteria:
Any combination of three or more atherothrombotic risk factors (men 65 years of age or older, women 70 years of age or older, diabetes mellitus, diabetic nephropathy, hypertension, hypercholesterolemia, current smoking, asymptomatic ankle-brachial index of less than 0.90, asymptomatic carotid stenosis of 70% or greater, and presence of one or more carotid plaques);
Documented CAD (stable angina, unstable angina, myocardial infarction, angioplasty/stenting and coronary artery bypass grafting);
Documented CVD (transient ischemic attack or stroke); or
Documented symptomatic PAD (a history of current intermittent claudication with either an ankle-brachial index of less than 0.90, peripheral angioplasty/stenting or amputation) (11).
Data collection
Health care resource use:
Medication use was coded as chronic therapy (yes or no) at baseline and one year, and included antiplatelet agents, oral anticoagulants, nonsteroidal anti-inflammatory drugs, lipid-lowering drugs, cardiovascular agents (calcium channel blockers, beta-blockers, nitrates and other antianginal agents), diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and other antihypertensive drugs, claudication medications and antidiabetic agents. Clinical follow-up at 12 months included the documentation of cardiovascular events, hospitalizations for vascular-related reasons and vascular procedures.
Medication costs:
Patient-level reported medication use for each medication class was assigned to the most commonly prescribed drug and dose based on the expert opinion of two cardiologists. Costs associated with medication use were obtained from the Ontario Drug Benefit Formulary/Comparative Drug Index (15) for all except two medications. For the two drugs not listed in the Ontario Drug Benefit Formulary/Comparative Drug Index, the Alberta Health and Wellness Drug Benefit List (16) was used to derive the cost for rosiglitazone, and the Newfoundland and Labrador Interchangeable Drug Products Formulary (17) was used to derive the cost for glimepiride. All cost estimates were expressed in 2006 Canadian dollars, and all provincial-based costs for medications were adjusted to obtain a value that was representative of national averages using provincial and national health care information provided by the Canadian Institute for Health Information.
Hospitalization costs:
The primary data source for the 2006 hospitalization cost schedules was the Health Funding and Costing Branch of Alberta Health and Wellness (18). Because case costing for hospital care is not completed uniformly among provinces, each reported hospitalization was assigned to one or more Canadian case mix groups (CMGs), based on the primary reason for hospitalization and/or procedures. The system of CMGs was designed to improve the comparability of Canadian health care data. CMGs categorize patients into statistically and clinically homogeneous groups; the most responsible diagnosis is the driver of CMG assignment. All cost estimates were expressed in 2006 Canadian dollars. The reference costs for hospital cases were provided in 2005 Canadian dollars and reflected average inpatient case costs for Alberta. These costs were inflated and converted to 2006 Canadian dollar values that were more reflective of average national case costs for Canada using ratios calculated based on provincial and national health care information provided by the Canadian Institute for Health Information (19). Details regarding unit costs for medications and hospitalizations are presented in the Appendix.
Follow-up events:
Twelve-month clinical follow-up visits were scheduled at patients’ enrolling centres at which physicians documented cardiovascular events, hospitalizations for vascular-related reasons and vascular procedures according to predefined data elements and definitions. Site visits and data audits for all enrolled patients at 6% (randomly selected) of the Canadian sites were conducted between baseline and 12 months (12).
Statistical analysis
One-year follow-up data relating to medication use, clinical events, procedures and associated hospitalizations were available for 1964 of 1976 enrolled patients (99.4%). Neither resource use nor costs were imputed for patients whose follow-up data were completely missing. Missing information related to the use of individual medications at baseline was multiply imputed using baseline characteristics as predictors; when missing at 12 months, individual medication use data were multiply imputed using 12-month follow-up events and baseline medication use as predictors. For the calculation of costs associated with medication use for surviving patients with the complete 12-month follow-up, baseline medication use was assumed from baseline through six months, and 12-month medication use was assumed throughout the seven- to 12-month follow-up period. Patients who were reported to have died during follow-up were included in all analyses, with all costs following their death assumed to be zero.
ANOVA was used to test the relationship between both the number of affected arterial beds (zero, one, or two or three) and the presence or absence of symptomatic disease in each specific arterial bed (ie, any CAD, any CVD and any PAD – note that these are not mutually exclusive groups), and hospitalization costs. Potential demographic and clinical predictors of costs were examined in multivariable regression models, from which mean predicted hospitalization and total costs for various risk groups, adjusted for age and sex, were obtained. Ninety-one per cent of REACH patients enrolled in Canada reported no hospitalizations during the one-year follow-up period and, therefore, had no hospitalization costs. To accommodate this empirical cost distribution, a two-part model was used to separate the analysis into two parts: the prediction of any (ie, one or more) hospitalization event, and the prediction of hospitalization costs in patients with at least one hospitalization. This approach allows the impact of covariates (number of affected arterial beds, age, sex, educational level [completed versus not completed high school], diabetes mellitus, smoking status [previous and current] and history of heart failure) on the probability of any hospitalization to be independent of the impact on hospitalization costs for patients with one or more hospitalization events. For this analysis, logistic regression was used in the first part and a generalized linear model (20), assuming a gamma error distribution and log-link function, was used in the second part. Covariate-adjusted predicted mean hospitalization costs and associated 95% CIs for different risk groups were computed via simulation, based on the two-part model results. For the simulation, the coefficients from the first and second parts of the two-part model were randomly drawn from their respective multivariate normal distributions, from which patient level predicted probabilities of any hospitalization (part 1), and predicted hospitalization costs (part 2) were computed by entering corresponding regression coefficients into the model. By using these patient-level predictions, the average probability and cost for each subgroup were obtained, and the product of these two average predicted values was used as the final predicted cost for the group. Using bootstrapping techniques, this procedure was repeated 1000 times to obtain a distribution of predicted hospitalization costs for each risk group, from which the mean was calculated and the 97.5th and 2.5th percentiles were derived, providing associated upper and lower 95% confidence limits (21).
All analyses were conducted using SAS version 9.1.3 (SAS Institute Inc, USA) and tests for significance were two-tailed with alpha levels of 0.05.
RESULTS
The Canadian risk profile
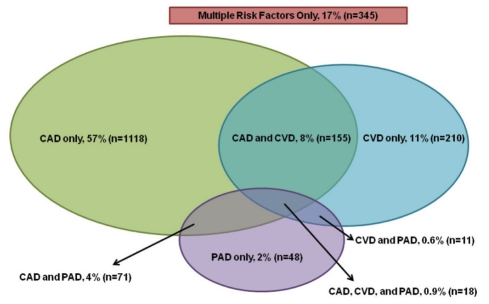
A total of 1998 patients were approached for enrollment by 154 physicians across Canada; of these, 20 did not meet inclusion criteria and two withdrew their consent, leaving 1976 REACH patients enrolled for Canada. The distribution of the 1976 Canadian REACH patients across vascular disease risk groups is presented in Figure 1. The majority (n=1118, 57%) of these patients had CAD only (no symptomatic disease in other arterial beds). The group of patients enrolled on the basis of CVD only consisted of 210 patients (11%), whereas patients with PAD only accounted for 2% (n=48) of the enrolled population. Among the 148 patients enrolled with a history of symptomatic PAD, 63 patients (43%) had a history of peripheral revascularization and 16 patients (11%) had a history of lower limb amputation at baseline. Patients with polyvascular disease (two or more arterial sites affected) accounted for 14% (n=255; of which, 237 patients had three arterial sites affected and 18 patients had two sites affected). A total of 345 patients (17%) were enrolled with no established arterial disease, but with three or more cardiovascular risk factors.
Figure 1).
REduction of Atherothrombosis for Continued Health (REACH) registry: Canadian risk profile (n=1976). CAD Coronary artery disease; CVD Cerebrovascular disease; PAD Peripheral arterial disease
Baseline clinical and demographic characteristics and medication use, both overall and for six different risk groups (risk factors only, one arterial bed affected, two or three arterial beds affected, any CAD, any CVD and any PAD; note that the latter three categories are not mutually exclusive) are presented in Table 1. Due to the limited number of patients with three arterial sites affected, patients with two or three arterial sites affected were combined into one category. The overall mean age was 68 years and 69% were men. Baseline rates of comorbidities and risk factors were high in all patient groups; hypercholesterolemia ranged from 73% to 87%, hypertension ranged from 72% to 88%, and diabetes mellitus was present in 85% of the multiple risk factor patients, and between 34% and 48% of patients with symptomatic disease. A total of 46% of all subjects were former smokers, whereas 15% were current smokers. Active smoking was highest (30%) in subjects with PAD. Obesity was present in more than 50% of the population. The proportion of patients receiving antiplatelet therapy was high in patients with symptomatic disease (range for acetylsalicylic acid between 68% and 84%, and for other antiplatelet medication between 19% and 31%). A total of 82% of patients were prescribed statins and approximately one-half of the patients were on beta-blockers.
TABLE 1.
Baseline patient characteristics of REduction of Atherothrombosis for Continued Health (REACH) patients from Canada, overall and stratified according to affected arterial beds (n=1976)
| Overall (n=1976) | Multiple risk factors (n=345) | Any 1 site (n=1376) | Any 2 or 3 sites (n=255) | Any CAD (n=1362) | Any CVD (n=394) | Any PAD (n=148) | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, years, mean ± SD | 68.4±9.9 | 67.8±9.9 | 68.1±9.8 | 71.1±9.6 | 68.3±9.8 | 71.1±9.8 | 69.3±9.6 |
| Men | 69.0 | 56.8 | 71.6 | 71.0 | 73.4 | 65.5 | 68.9 |
| Socioeconomic factors | |||||||
| High school not completed | 69.3 | 71.4 | 67.7 | 75.1 | 68.3 | 71.0 | 81.3 |
| Nonactive working status | 74.3 | 76.2 | 72.3 | 82.4 | 73.1 | 82.2 | 79.1 |
| Medical history | |||||||
| Hypercholesterolemia | 84.4 | 86.4 | 84.4 | 81.6 | 86.9 | 72.8 | 81.8 |
| Hypertension | 76.6 | 88.4 | 71.9 | 85.5 | 73.2 | 82.5 | 81.8 |
| Diabetes mellitus* | 44.1 | 84.6 | 33.7 | 45.1 | 36.5 | 35.4 | 48.0 |
| Smoking status | |||||||
| Former | 45.7 | 31.6 | 48.8 | 47.8 | 49.4 | 44.9 | 50.7 |
| Current | 15.4 | 20.9 | 13.2 | 19.6 | 13.7 | 13.5 | 30.4 |
| Obesity† | 51.7 | 68.5 | 48.2 | 47.9 | 48.1 | 48.5 | 46.9 |
| Overweight (BMI 25 kg/m2 to <30 kg/m2) | 41.8 | 39.5 | 42.0 | 43.8 | 42.6 | 41.3 | 44.2 |
| Class I (BMI 30 kg/m2 to <35 kg/m2) | 22.9 | 25.1 | 22.6 | 21.9 | 23.1 | 22.0 | 15.0 |
| Class II (BMI 35 kg/m2 to <40 kg/m2) | 6.9 | 9.4 | 6.9 | 3.6 | 6.6 | 3.9 | 6.1 |
| Class III (BMI ≥40 kg/m2) | 3.4 | 6.5 | 2.9 | 2.4 | 3.0 | 2.1 | 2.0 |
| Baseline medication use | |||||||
| Antiplatelet agents | |||||||
| Acetylsalicylic acid | 79.0 | 66.4 | 83.4 | 72.2 | 83.6 | 68.4 | 81.0 |
| Other | 17.0 | 1.7 | 19.0 | 27.1 | 18.8 | 30.9 | 19.0 |
| Oral anticoagulants | 10.9 | 6.0 | 10.1 | 21.6 | 12.1 | 19.8 | 10.9 |
| Nonsteroidal anti-inflammatory drugs | 10.2 | 13.2 | 8.5 | 15.5 | 9.8 | 13.1 | 9.2 |
| Lipid-lowering agents | |||||||
| Statins | 81.5 | 77.7 | 82.2 | 83.1 | 85.3 | 72.8 | 81.1 |
| Other | 8.0 | 10.1 | 8.1 | 4.7 | 8.1 | 4.1 | 6.8 |
| Cardiovascular agents | |||||||
| Calcium channel blockers | 34.6 | 34.8 | 32.6 | 44.9 | 34.6 | 36.5 | 47.9 |
| Beta-blockers | 51.4 | 21.3 | 57.5 | 59.0 | 65.2 | 37.2 | 45.9 |
| Nitrates/other antianginal drugs | 22.8 | 1.4 | 25.0 | 40.1 | 32.3 | 19.9 | 28.0 |
| Diuretics | 39.9 | 51.3 | 34.8 | 52.2 | 36.8 | 45.9 | 49.0 |
| Angiotensin-converting enzyme inhibitors | 57.0 | 57.7 | 56.8 | 57.3 | 58.1 | 52.7 | 57.1 |
| Angiotensin II receptor blockers | 23.5 | 33.4 | 20.6 | 25.7 | 21.5 | 23.3 | 22.9 |
| Other antihypertensive agents | 5.1 | 6.4 | 4.1 | 8.7 | 5.3 | 4.6 | 6.9 |
| Claudication medications‡ | 1.4 | 0.3 | 1.2 | 3.9 | 1.5 | 1.6 | 8.5 |
| Antidiabetic agents | |||||||
| Insulin | 8.8 | 14.0 | 6.8 | 12.2 | 8.3 | 7.2 | 14.3 |
| Biguadines | 25.7 | 55.9 | 18.7 | 23.0 | 20.1 | 17.6 | 23.0 |
| Sulfonylureas | 19.5 | 43.2 | 13.9 | 17.6 | 14.6 | 14.5 | 16.9 |
| Thiazolidinediones | 9.0 | 22.9 | 5.9 | 7.5 | 6.0 | 6.6 | 6.8 |
| Other | 2.8 | 6.2 | 1.7 | 3.9 | 2.1 | 2.1 | 5.5 |
| Any antidiabetic agent | 39.3 | 80.6 | 29.0 | 39.2 | 31.8 | 29.4 | 39.9 |
| Average medications per patient, n§ | 5.1 | 5.2 | 4.9 | 5.8 | 5.2 | 4.8 | 5.4 |
Data presented as % unless otherwise indicated.
Type 1 or 2 diabetes mellitus currently treated with hypoglycemic agents, or history of diabetes;
Men with a waist circumference of 102 cm or larger, and women with a waist circumference of 88 cm or larger;
Claudication medications included cilostazol, pentoxifylline, buflomedil, naftidrofuryl and Ginkgo biloba;
Refers to the overall number of medications. BMI Body mass index; CAD Coronary artery disease; CVD Cerebrovascular disease; PAD Peripheral arterial disease
Medication costs
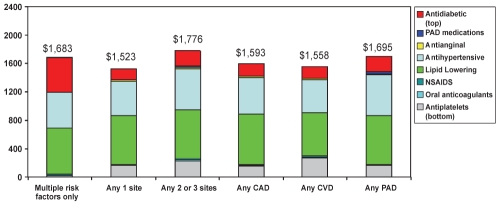
Average annualized medication costs were $1,683, $1,523 and $1,776 for patients with zero, one and more than one symptomatic arterial bed, respectively, and were approximately $1,600 for patients with affected arterial beds (Figure 2). Relatively high costs associated with medication use for patients with risk factors only were due to costs associated with antidiabetic medication use, because diabetes was one of the risk factors for which patients with no established arterial disease could meet the enrollment criteria. Together, lipid-lowering and antihypertensive medications accounted for more than one-half of the medication costs examined.
Figure 2).
Average annual medication costs according to medication class. CAD Coronary artery disease; CVD Cerebrovascular disease; NSAIDS Nonsteroidal anti-inflammatory drugs; PAD Peripheral arterial disease
Cardiovascular events, hospitalization rates and associated costs
One-year cardiovascular event and hospitalization rates for the overall population and according to different risk groups are presented in Table 2. The overall incidence of the combined end point of cardiovascular death/myocardial infarction/stroke/cardiovascular hospitalization varied considerably according to risk group; this rate was particularly high for patients with PAD (18.5%) and among patients with two or more affected arterial beds (symptomatic polyvascular disease, 17.9%), whereas patients with multiple risk factors only had the lowest event rate (3.2%).
TABLE 2.
Percentage of patients experiencing at least one cardiovascular event at one year, and one-year hospitalization rates per 1000 patients, overall and stratified according to affected arterial beds (n=1964)
| Overall (n=1964) | Multiple risk factors (n=342) | Any 1 site (n=1370) | Any 2 or 3 sites (n=252) | Any CAD (n=1356) | Any CVD (n=390) | Any PAD (n=146) | |
|---|---|---|---|---|---|---|---|
| Cardiovascular events, % | |||||||
| Cardiovascular death | 1.1 | 0.3 | 1.0 | 2.8 | 1.3 | 1.8 | 2.7 |
| Cardiovascular death/MI/stroke | 2.6 | 2.0 | 2.1 | 6.3 | 2.7 | 4.6 | 4.8 |
| Cardiovascular death/MI/stroke/cardiovascular hospitalization | 8.0 | 3.2 | 7.4 | 17.9 | 9.1 | 11.5 | 18.5 |
| Hospitalizations involving no vascular interventions, event rates per 1000 patients | |||||||
| Fatal MI | 2.5 | 0.0 | 1.5 | 11.9 | 3.7 | 5.1 | 6.8 |
| Fatal stroke | 2.0 | 0.0 | 2.2 | 4.0 | 2.2 | 5.1 | 0.0 |
| Other cardiovascular death | 6.6 | 2.9 | 6.6 | 11.9 | 6.6 | 7.7 | 20.5 |
| Nonfatal stroke | 11.2 | 17.5 | 4.4 | 39.7 | 8.1 | 25.6 | 34.2 |
| Nonfatal MI | 7.6 | 2.9 | 8.0 | 11.9 | 9.6 | 7.7 | 6.8 |
| PAD – new diagnosis | 5.1 | 2.9 | 3.6 | 15.9 | 6.6 | 5.1 | 13.7 |
| Worsening of claudication | 2.0 | 0.0 | 1.5 | 7.9 | 1.5 | 0.0 | 27.4 |
| Unstable angina | 28.5 | 2.9 | 34.3 | 31.7 | 39.8 | 12.8 | 34.2 |
| Transient ischemic attack | 7.1 | 2.9 | 6.6 | 15.9 | 6.6 | 15.4 | 13.7 |
| Other ischemic event | 9.2 | 2.9 | 4.4 | 43.7 | 11.8 | 5.1 | 75.3 |
| Chronic heart failure | 11.7 | 2.9 | 9.5 | 35.7 | 14.0 | 20.5 | 27.4 |
| Bleeding with transfusion | 1.0 | 0.0 | 0.7 | 4.0 | 1.5 | 2.6 | 0.0 |
| Hospitalizations involving vascular interventions, event rates per 1000 patients | |||||||
| Coronary artery bypass grafting | 13.7 | 0.0 | 14.6 | 27.8 | 19.2 | 15.4 | 20.5 |
| Percutaneous coronary intervention/stenting | 20.9 | 0.0 | 26.3 | 19.8 | 29.5 | 15.4 | 6.8 |
| Carotid surgery | 4.6 | 5.8 | 2.9 | 11.9 | 2.9 | 10.3 | 13.7 |
| Carotid angioplasty/stenting | 0.5 | 0.0 | 0.7 | 0.0 | 0.0 | 2.6 | 0.0 |
| Lower limb amputation | 5.1 | 0.0 | 4.4 | 15.9 | 4.4 | 5.1 | 41.1 |
| Peripheral angioplasty/stenting | 3.1 | 2.9 | 2.9 | 4.0 | 1.5 | 2.6 | 27.4 |
| Peripheral bypass graft | 9.2 | 0.0 | 8.8 | 23.8 | 10.3 | 10.3 | 61.6 |
CAD Coronary artery disease; CVD Cerebrovascular disease; MI Myocardial infarction; PAD Peripheral arterial disease
Hospitalizations were classified into those involving vascular interventions and those not involving vascular interventions for the purpose of costing, because vascular interventions tend to drive CMG assignment and costs. It is important to note that some of the hospitalizations involving revascularization (eg, percutaneous coronary intervention/stenting) also involved a reported clinical event (eg, unstable angina or myocardial infarction). Hospitalization rates corresponding to the characteristic of the hospitalization that determined the CMG assignment are presented in Table 2. For hospitalizations involving no vascular interventions, rates were highest among patients with any PAD and patients with diffuse vascular disease for the majority of indications for hospitalization (fatal myocardial infarction, other cardiovascular death, nonfatal stroke, new PAD diagnosis, worsening of claudication, other ischemic events and chronic heart failure). Consistent with the clinical diagnoses that defined baseline eligibility, hospitalizations associated with unstable angina were specifically high in patients diagnosed with CAD, while hospitalizations for transient ischemic attacks were high in patients with any CVD. For six of seven categories of hospitalization involving vascular interventions, patients with PAD and patients with symptomatic polyvascular disease had the highest event rates; not only for peripheral procedures (carotid surgery, carotid angioplasty/stenting, lower limb amputations, peripheral angioplasty/stenting and peripheral bypass graft) but also for coronary artery bypass grafting. Hospitalizations involving percutaneous coronary intervention were most common in patients with CAD.
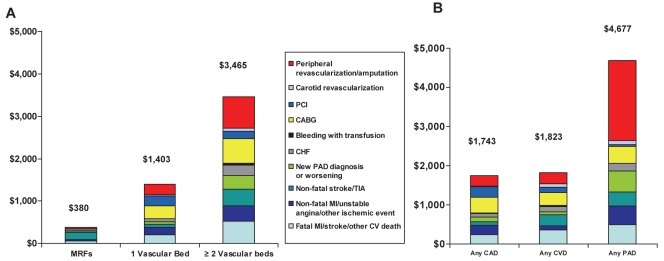
Similar patterns to those described above for hospitalization rates were seen for average one-year hospitalization costs (Figure 3). Total average annual hospitalization costs increased as the number of affected arterial beds increased ($380, $1,403 and $3,465 for zero, one, and two or three affected arterial beds, respectively [P<0.0001 for test of overall linear trend and all pairwise comparisons]). Mean hospitalization costs for patients with any CAD, any CVD and any PAD were $1,743, $1,823 and $4,677, respectively (P<0.0001 for test of overall linear trend and pairwise comparisons). A history of symptomatic PAD at baseline was associated with a significant increase in one-year hospitalization costs (P<0.0001) in unadjusted analyses (Figure 3, right). Coronary revascularizations (percutaneous coronary interventions and coronary artery bypass grafting) were responsible for the majority of costs in patients with established CAD (38.1%, $664) or CVD (24.9%, $454) at baseline, whereas peripheral revascularizations or amputations accounted for most of the costs in patients with PAD (43.6%, $2,040).
Figure 3).
Average one-year costs (Canadian dollars) associated with hospitalizations for vascular reasons per patient stratified according to the number of arterial beds affected (A) and according to the presence or absence of disease in specific arterial beds (B). CABG Coronary artery bypass grafting; CAD Coronary artery disease; CHF Chronic heart failure; CV Cardiovascular; CVD Cerebrovascular disease; MI Myocardial infarction; MRF Multiple risk factors; PAD Peripheral arterial disease; PCI Percutaneous coronary intervention; TIA Transient ischemic attack
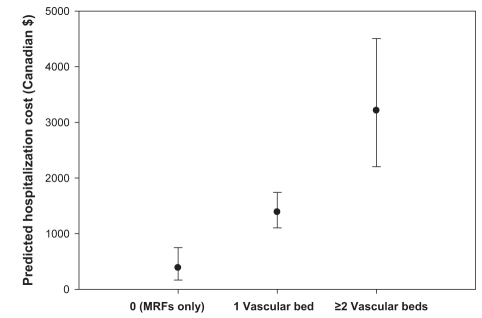
Covariate-adjusted estimates of mean vascular-related hospitalization costs and associated 95% CIs, according to the number of affected arterial beds, are presented in Figure 4. Consistent with the unadjusted results, hospitalization costs increased significantly with the number of symptomatic arterial beds.
Figure 4).
Mean hospitalization costs per patient (with 95% CIs) adjusted for covariates according to the number of affected arterial beds. MRFs Multiple risk factors
DISCUSSION
Results from the present study, based on data collected from the full spectrum of stable outpatients at risk for atherothrombotic events, provide insight into the clinical and economic burden associated with atherothrombosis in Canada. While average annual costs associated with cardiovascular medication use were consistently high for all risk groups examined, considerable variation was observed with respect to cardiovascular event rates, hospitalizations and associated costs across risk groups defined according to the specific arterial beds affected and the extent of arterial disease. Event rates and hospitalization costs increased significantly with an increase in the number of affected arterial beds, and patients with PAD had significantly higher event rates and hospitalization costs than patients with CAD or CVD. The overall economic burden associated with hospitalization costs was substantial, with average annual costs exceeding $3,400 per patient with polyvascular disease, and costs for patients with symptomatic PAD exceeding $4,600 per patient.
These results provide unique insight into the clinical and economic burden associated with atherothrombosis in Canada. No previous study has examined the clinical and economic burden according to the extent of disease (22,23), and recent information on event rates and costs associated with PAD in Canada is lacking. Results presented here provide contemporary estimates of the clinical and economic burden in this important but underdiagnosed and undertreated risk group (24,25).
Despite recent declines in death rates and hospital admissions, there has been rapid growth in the use of costly medical technology and pharmaceutical treatments for patients with cardiovascular disease (23,26,27). With the aging population, the continued adoption of unhealthy lifestyles, and the concomitant increased risk of diabetes and obesity, the health care demands associated with the increased number of patients at risk for atherothrombosis will continue to increase (23,28). In REACH, the average annual costs for vascular-related hospitalizations per patient for patients with established vascular disease ranged from $1,743 for patients with CAD to $4,677 for patients with PAD. To put these costs in perspective, total spending per person on health care in 2006 was $4,171 in Canada (28), indicating that costs associated with in-hospital care for patients with vascular disease are high and account for a large proportion of national health care expenditures. By comparison, the costs associated with hospitalizations related to chronic obstructive pulmonary disease in Canada (for 2000) were estimated at $1,049 (29).
An estimated 1.29 million Canadians were reported to have heart disease in 2005 (27), and it is estimated that approximately 300,000 Canadians are living with the consequences of a stroke, while approximately 800,000 Canadians are affected by PAD (25,27,30–32). Based on these prevalence rates and our estimates, average annual costs for vascular-related hospitalizations would account for more than $2.2 billion for patients with CAD, more than $0.5 billion for patients with CVD, and more than $3.7 billion for patients with PAD. The high costs for patients with PAD are due to the high rate of peripheral revascularization procedures as well as the high costs associated with cardiovascular events and coronary revascularization procedures. The observed high clinical and economic burden associated with polyvascular disease for REACH patients from Canada is consistent with global estimates and estimates calculated for other developed countries such as the United States (14,33).
Despite several awareness and prevention campaigns targeting different cardiovascular risk groups that have been successfully organized in Canada, PAD has not been a priority of these programs (5–7,25). PAD remains an under-recognized and undertreated disease (24,34,35) and is associated with high rates of cardiovascular events relative to other risk groups such as patients with CAD (34). A contributor to these higher event rates is the fact that cardiovascular risk factors are not as effectively controlled for patients with PAD compared with patients with CAD or CVD (24,34,35). Previous results from the global REACH registry revealed that more adequate risk factor control at baseline was associated with subsequently lower one-year cardiovascular event rates (35). While the use of antihypertensive, antiplatelet and lipid-lowering therapies has been shown to be greater for Canadian patients enrolled in REACH, compared with the global REACH population as well as REACH patients enrolled from the United States (12), increased efforts with respect to risk factor control, especially for patients with PAD and/or polyvascular disease, seem warranted.
Limitations
Our findings should be considered in light of several potential limitations. First, regarding hospitalization costs, the REACH registry did not collect information related to hospitalizations, other than those that were vascular-related, and the REACH registry did not collect information relating to outpatient treatment or long-term care such as nursing home and rehabilitation stays following stroke or amputation. These missing cost components may affect some risk groups more than others; thus, the relative economic burden of the different risk groups may differ somewhat from our estimates. Likewise, not included in the present study are the indirect costs associated with lost productivity on the part of the patient, family members and other informal caregivers. Although the REACH registry collected data relating to employment status and number of sick leave days taken by the patient due to vascular events or hospitalizations reported at follow-up, only one-quarter of REACH enrollees from Canada were working at baseline; thus, the available data were too limited to derive estimates of costs associated with lost productivity (14). In addition, the registry did not collect information relating to informal caregiver time – another component of indirect costs – nor did it collect data on nonvascular hospitalizations. Therefore, our cost estimates are likely an underestimation of the total economic burden associated with atherothrombotic disease. The limited number of patients from Canada did not allow us to compare costs between subgroups defined according to the specific arterial beds (and combinations thereof) affected; in particular, the relatively limited number of patients enrolled with established PAD restricts potential generalizability of those results to the overall Canadian population, and the certainty with which conclusions relating to that risk group can be drawn; however, the high event rates and associated costs for REACH patients with PAD from Canada have also been reported for REACH patients from the United States (14).
The findings of the present study collectively illustrate the high economic burden associated with the control of risk factors, and the treatment of major cardiovascular events and other vascular-related hospitalizations in a relatively stable population of outpatients from Canada at risk for atherothrombosis. Patients with polyvascular disease and patients with PAD seem to be at particularly high risk for atherothrombotic events and the concomitant high costs associated with treatment for those events, including expensive revascularization procedures. Public awareness programs, outcomes monitoring and improved prevention efforts in undertreated risk groups, such as patients with PAD, may be important in the effort to forestall the anticipated increased burden on the health care system as the population ages (27).
APPENDIX
Details about unit cost calculations for hospitalizations and medications, and for the Canadian REduction of Atherothrombosis for Continued Health (REACH) registry cost analyses
| Diagnosis | Procedure | Average case cost, $ | Average LOS (days) |
|---|---|---|---|
| Hospitalizations with procedures | |||
| Any | Amputation affecting lower limbs: | ||
| Including toe amputations | 20.528 | 20.1 | |
| Excluding toe amputations | 21.838 | 21.3 | |
| Any | CABG | 20.829 | 9.6 |
| Any | CABG and coronary angioplasty/stenting | 32.823 | 17.8 |
| Any | Carotid angioplasty/stenting | 7.044 | 3.3 |
| Any | Carotid surgery | 7.044 | 3.3 |
| Any | Other interventions for PAD (angioplasty/stenting) | 7.802 | 4.8 |
| Any | Peripheral bypass graft | 15.062 | 8.8 |
| Nonfatal myocardial infarction | Coronary angioplasty/stenting | 11.823 | 5.3 |
| Unstable angina | Coronary angioplasty/stenting | 11.823 | 5.3 |
| Other ischemic arterial event | Coronary angioplasty/stenting | 8.898 | 2.6 |
| Congestive heart failure | Coronary angioplasty/stenting | 11.823 | 5.3 |
| No clinical outcomes leading to hospitalization reported | Coronary angioplasty/stenting | 8.018 | 1.8 |
| Hospitalizations without procedures | |||
| Fatal myocardial infarction | None | 16.057 | 12.0 |
| Fatal stroke | None | 26.653 | 23.7 |
| Other cardiovascular death | None | 18.837 | 18.0 |
| Stroke – ischemic | None | 8.621 | 8.9 |
| Stroke – hemorrhagic | None | 8.621 | 8.9 |
| Stroke – unknown origin | None | 8.621 | 8.9 |
| Nonfatal myocardial infarction | None | 5.577 | 4.9 |
| Peripheral artery disease – new diagnosis | None | 6.116 | 6.3 |
| Worsening of claudication related to PAD | None | 7.109 | 7.8 |
| Unstable angina | None | 3.298 | 3.5 |
| Transient ischemic attack | None | 3.998 | 3.7 |
| Other ischemic arterial event | None | 4.360 | 4.1 |
| Congestive heart failure | None | 6.863 | 8.8 |
| Episode of bleeding with transfusion | None | 11.130 | 10.4 |
| Medication | Typical medication | Dose, mg | Doses per day |
Cost, $ |
||
|---|---|---|---|---|---|---|
| Per dose | Per day | Per month | ||||
| Antiplatelet drugs | ||||||
| Acetylsalicylic acid | Acetylsalicylic acid | 325 | 1 | 0.01 | 0.01 | 0.381 |
| Other | Clopidogrel | 75 | 1 | 2.41 | 2.41 | 73.192 |
| Oral anticoagulants | Warfarin | 5 | 1 | 0.19 | 0.19 | 5.772 |
| NSAIDs | Ibuprofen | 400 | 3 | 0.11 | 0.34 | 10.261 |
| Lipid-lowering agents | ||||||
| Statins | Atorvastatin | 40 | 1 | 2.16 | 2.16 | 65.568 |
| Other | Gemfibrozil | 600 | 2 | 0.77 | 1.54 | 46.770 |
| Cardiovascular agents | ||||||
| Calcium channel blockers | Amlodipine | 5 | 1 | 1.28 | 1.28 | 39.036 |
| Beta-blockers | Atenolol | 50 | 1 | 0.46 | 0.46 | 13.864 |
| Nitrates/other antiangina agents | Isordil | 40 | 3 | Not a benefit | 0.000 | |
| Isosorbide dinitrate | 40 | 3 | 0.06 | 0.19 | 5.855 | |
| Diuretics | Furosemide | 40 | 1 | 0.06 | 0.06 | 1.718 |
| ACE inhibitors | Lisinopril | 10 | 1 | 0.68 | 0.68 | 20.565 |
| Angiotensin II antagonists | Losartan | 50 | 1 | 1.10 | 1.10 | 33.546 |
| Other antihypertensives | Hydralazine | 25 | 3 | 0.16 | 0.49 | 15.041 |
| Claudication medications | Cilostazol | 100 | 2 | Not available | 1.14 | 34.675 |
| Antidiabetic agents | ||||||
| Insulin | Regular insulin | 0.5–1 unit/kg | 1.20 | 36.534 | ||
| Biguadines | Metformin | 500 | 2 | 0.16 | 0.32 | 9.594 |
| Sulfonylureas | Glimeperide | 2 | 1 | 0.71 | 0.71 | 21.493 |
| Thiazolinediones | Rosiglitazone | 8 | 1 | 2.87 | 2.87 | 87.221 |
| Other | Glyburide | 5 | 1 | 0.11 | 0.11 | 3.353 |
Costs are presented in 2006 Canadian dollars. ACE Angiotensin-converting enzyme; CABG Coronary artery bypass grafting; LOS Length of stay; NSAIDs Nonsteroidal anti-inflammatory drugs; PAD Peripheral arterial disease
Footnotes
FUNDING SOURCES: The REACH registry was sponsored by a grant from Bristol-Myers Squibb (USA), sanofi-aventis (France) and the Waksman Foundation (Japan). The REACH registry is endorsed by the World Heart Federation. A complete list of REACH investigators is accessible online at www.reachregistry.org. The REACH registry enforces a no ghost-writing policy. This manuscript was written and edited by the authors, who take full responsibility for its content. The first draft was written by Dr Smolderen. Dr Smolderen was supported by an award from the American Heart Association Pharmaceutical Roundtable, and David and Stevie Spina.
ROLE OF THE FUNDING SOURCE: All manuscripts in the REACH registry are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding sponsors have the opportunity to review manuscript submissions but do not have authority to change any aspect of a manuscript.
CONFLICT OF INTEREST DISCLOSURES: Dr Smolderen has no disclosures. Dr Bell has received research grants and/or speakers honoraria from the following organizations: Pfizer (Canada), sanofi-aventis (Canada), AstraZeneca (Canada), Bristol-Myers Squibb (Canada), Novartis (Canada), Bayer (Canada) and Boehringer Ingelheim (Canada). Ms Lei has no disclosures. Dr Cohen has received research grants from sanofi-aventis (Canada), Novartis (Canada) and AstraZeneca (Canada); speaker’s honoraria from sanofi-aventis (Canada), Bristol-Myers Squibb (Canada) and AstraZeneca (Canada); and consulting fees from sanofi-aventis (Canada) and AstraZeneca (Canada). Dr Steg has received research grant support from Servier (France; 2009 to 2014); is a consultant or advisory board member for Astellas (Netherlands), AstraZeneca (Sweden), Bayer (France), Boehringer Ingelheim (France), BMS (France), Daiichi-Sankyo-Lilly (France), GlaxoSmithKline (France), Medtronic (Netherlands), Otsuka (USA), Roche (Switzerland), sanofi-aventis (France), Servier (France) and The Medicines Company (France); and is a stockholder for Aterovax (France). Dr Bhatt has received institutional research funding from AstraZeneca (Sweden), Bristol-Myers Squibb (USA), Eisai (Japan), sanofi-aventis (France) and The Medicines Company (USA). Dr Mahoney has received grant support from sanofi-aventis (France), sanofi-aventis (Canada), Bristol-Myers Squibb (USA), Daiichi-Sankyo (USA) and Eli Lilly (USA), and has received honoraria from sanofi-aventis (France), Bristol-Myers Squibb (USA) and Bristol-Myers Squibb (Canada).
REFERENCES
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Statistics Canada. Mortality Summary List of Causes. Ottawa: Statistics Canada; 2005. 2009. [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Allender S, Scarborough P, Peto V, et al. European cardiovascular disease statistics 2008. Brussels: European Heart Network; 2008. [Google Scholar]
- 5.Campbell NR, Tu K, Brant R, Duong-Hua M, McAlister FA. The impact of the Canadian Hypertension Education Program on antihypertensive prescribing trends. Hypertension. 2006;47:22–8. doi: 10.1161/01.HYP.0000196269.98463.fd. [DOI] [PubMed] [Google Scholar]
- 6.Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: A randomized trial. CMAJ. 2005;173:40–5. doi: 10.1503/cmaj.1041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich MW. Multidisciplinary heart failure clinics: Are they effective in Canada? CMAJ. 2005;173:53–4. doi: 10.1503/cmaj.050705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT. The Canadian obesity epidemic, 1985–1998. CMAJ. 2002;166:1039–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Katzmarzyk PT, Gledhill N, Shephard RJ. The economic burden of physical inactivity in Canada. CMAJ. 2000;163:1435–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Canadian Heart Health Strategy and Action Plan Steering Committee. Building a Heart Healthy Canada. Canadian Heart Health Strategy and Action Plan. 2009. [DOI] [PMC free article] [PubMed]
- 11.Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: An international, prospective, observational investigation in subjects at risk for atherothrombotic events – study design. Am Heart J. 2006;151:786.e1–10. doi: 10.1016/j.ahj.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Bell A, Hill MD, Herman RJ, Girard M, Cohen E. Management of atherothrombotic risk factors in high-risk Canadian outpatients. Can J Cardiol. 2009;25:345–51. doi: 10.1016/s0828-282x(09)70088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney EM, Wang K, Cohen DJ, et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. doi: 10.1161/CIRCOUTCOMES.108.775247. [DOI] [PubMed] [Google Scholar]
- 15.Ontario Ministry of Health and Long-Term Care. Ontario Drug Benefit Formulary/Comparative Drug Index. Toronto: Ontario Ministry of Health and Long-Term Care. 2009.
- 16.Alberta Health and Wellness. Alberta Health and Wellness Drug Benefit List. Edmonton: Alberta Health and Wellness; 2009. [Google Scholar]
- 17.Health and Community Services. Newfoundland and Labrador Interchangeable Drug Products Formulary. St John’s: Newfoundland & Labrador Department of Health and Community Services. 2009.
- 18.Health MIS and Costing Unit. Health Costing in Alberta, 2006 Annual Report. Edmonton: Health Authority Funding and Financial Accountability Costing Branch. Alberta Health and Wellness; 2006. [Google Scholar]
- 19.Statistics Canada. Latest release from the Consumer Price Index. Ottawa: Statistics Canada; Dec 16, 2006. 2006. [Google Scholar]
- 20.McCullagh P, Nelder JA. Generalized Linear Models. 2nd edn. London: Chapman and Hall; 1989. [Google Scholar]
- 21.Mullahy J. Much ado about two: Reconsidering retransformation and the two-part model in health econometrics. J Health Econ. 1998;17:247–81. doi: 10.1016/s0167-6296(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 22.Caro JJ, Migliaccio-Walle K, Ishak KJ, Proskorovsky I, O’Brien JA. The time course of subsequent hospitalizations and associated costs in survivors of an ischemic stroke in Canada. BMC Health Serv Res. 2006;6:99. doi: 10.1186/1472-6963-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu JV, Nardi L, Fang J, Liu J, Khalid L, Johansen H. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180:E118–25. doi: 10.1503/cmaj.081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 25.Lovell M, Harris K, Forbes T, et al. Peripheral arterial disease: Lack of awareness in Canada. Can J Cardiol. 2009;25:39–45. doi: 10.1016/s0828-282x(09)70021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Chiu M, Manuel DG, et al. Trends in risk factors for cardiovascular disease in Canada: Temporal, socio-demographic and geographic factors. CMAJ. 2009;181:E55–66. doi: 10.1503/cmaj.081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Expenditure Trends, 1975 to 2008. Ottawa: Canadian Institute for Health Information; 2008. [Google Scholar]
- 29.Chapman KR, Bourbeau J, Rance L. The burden of COPD in Canada: Results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S23–31. doi: 10.1016/s0954-6111(03)80022-7. [DOI] [PubMed] [Google Scholar]
- 30.Heart and Stroke Foundation of Canada. Tipping the Scales of Progress: Heart Disease and Stroke in Canada. Ottawa: Heart and Stroke Foundation of Canada; 2006. [Google Scholar]
- 31.Abramson BL, Huckell V, Anand S, et al. Canadian Cardiovascular Society Consensus Conference: Peripheral arterial disease – executive summary. Can J Cardiol. 2005;21:997–1006. [PubMed] [Google Scholar]
- 32.Field TS, Green TL, Roy K, Pedersen J, Hill MD. Trends in hospital admission for stroke in Calgary. Can J Neurol Sci. 2004;31:387–93. doi: 10.1017/s0317167100003504. [DOI] [PubMed] [Google Scholar]
- 33.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 34.Welten GM, Schouten O, Hoeks SE, et al. Long-term prognosis of patients with peripheral arterial disease: A comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–96. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub PP, Abola MT, Baumgartner I, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) registry. Atherosclerosis. 2009;204:e86–92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]