Abstract
Home characteristic questions are used in epidemiological studies and clinical settings to assess potentially harmful exposures in the home. The objective of this study was to determine whether questionnaire-reported home characteristics can predict directly measured pollutants. Sixty home inspections were conducted on a subsample of the 2006 population-based Toronto Child Health Evaluation Questionnaire. Indoor/outdoor air and settled dust samples were analyzed. Mean Fel d 1 was higher (p < 0.0001) in homes with a cat (450.58 μg/g) versus without (22.28 μg/g). Mean indoor NO2 was higher (p = 0.003) in homes with gas stoves (14.98 ppb) versus without (8.31 ppb). Self-reported musty odours predicted higher glucan levels (10554.37 μg/g versus 6308.58 μg/g, p = 0.0077). Der f 1 was predicted by the home’s age, but not by reports of carpets, and was higher in homes with mean relative humidity > 50% (61.30 μg/g, versus 6.24 μg/g, p = 0.002). Self-reported presence of a cat, a gas stove, musty odours, mice, and the home’s age and indoor relative humidity over 50% predicted measured indoor levels of cat allergens, NO2, fungal glucan, mouse allergens and dust mite allergens, respectively. These results are helpful for understanding the significance of indoor exposures ascertained by self-reporting in large epidemiological studies and also in the clinical setting.
Keywords: allergens, environmental exposure, house dust, indoor air pollution, questionnaire
1. Introduction
Asthma is the most common chronic childhood disease in North America; its prevalence is increasing, and it is a leading cause of emergency department visits, hospitalizations and school absenteeism [1–3]. Because North American children spend a significant proportion of their time indoors at home [4], the role of the home environment in the triggering and exacerbation of childhood asthma has been studied extensively. For example, carpeted floors in homes tend to harbour greater levels of dust mites, which are known to be a risk factor for asthma [5,6]. High dust weight alone has also been associated with increased respiratory symptoms [7]. Dampness and mould exposure in the home [8,9], cat exposure [10], exposure to cockroach allergens [11,12], indoor particulate matter exposure [13], as well as the presence of gas stoves and elevated nitrogen dioxide levels [14,15] are all known to exacerbate childhood asthma. High endotoxin exposures in the home have been associated with increased asthma severity [16,17] and recent research suggests that endotoxins may also be a risk factor for the development of childhood asthma [18–20]. Exposure to mouse allergens in the home has also recently been reported to be associated with asthma morbidity and an increased risk of wheeze in childhood [21–23]. Consequently, environmental modification forms a major part of present asthma management guidelines [24,25].
In the process of gathering indoor air pollutant exposure data, both home inspections and questionnaires can be used. Home inspections assess the direct presence of specific pollutants and may involve dust sampling and air pollution monitoring. Questionnaires, on the other hand, focus on housing characteristics that tend to be associated with pollutant exposures. To ensure that responses serve as adequate predictors of actual exposure, questionnaires must be validated. A review of the literature reveals that questionnaire reports of specific aspects of the home environment, such as a cat in the home, cockroaches, dampness and mould, and the presence of a gas range have been associated with their intended measures of exposure of increased cat allergens, cockroach allergens, fungal concentrations, and nitrogen dioxide, respectively [26–29]. However, there have also been conflicting reports in the literature with regards to whether certain self-reported housing characteristics predict actual measurements. For example, studies conducted in different countries have reported inconsistent results with regards to the ability of the number and type of pets to predict endotoxin levels [30,31]. There have also been differing reports on whether certain housing characteristics, such as humidity, predict dust mite levels [32]. Some of these discrepancies may be due to differences in geographic region or in the population studied (e.g., a low-income population versus the general population). It is therefore important to carry out direct measurements of pollutants in samples of subjects that are representative of study populations. This would assist the interpretation of home environment questions in a particular study setting and population. In Canada, where 79% of children reside in urban settings [33], it is vital to determine what air pollutants are present in homes at what levels, how they compare to other countries and settings, and the validity of self-reporting pertaining to these pollutants assessed via questionnaire.
The purpose of this study is to validate questions that reflect indoor air pollutant exposure in the Toronto Child Health Evaluation Questionnaire (T-CHEQ). Questionnaire responses are compared to data obtained through direct measurements during home inspections. Specifically, this research seeks to determine whether certain characteristics indicated on the questionnaire (presence of a gas stove, cat, any other pets, carpets, air conditioner, cockroaches, mice, damp spots, visible moulds or fungi) predict, respectively, increased levels of NO2, cat allergen, endotoxin, dust mite allergens, cockroach allergens, mouse allergens, and fungi.
2. Methods
2.1. Study Population
The Toronto Child Health Evaluation Questionnaire (T-CHEQ) is a population-based study that examines the relationship between air pollution and childhood asthma [34]. Between January and May of 2006, questionnaires were completed by parents of 5,559 grade 1 and 2 schoolchildren to assess asthma prevalence. A section of the T-CHEQ asked parents to answer questions reflecting indoor air pollutant exposures. Some of this data was used to predict indoor concentrations of air pollutants. During August 1 to November 24, 2006 and July 4 to 18, 2007, home inspections were conducted on a subsample of 60 homes that were randomly selected from the T-CHEQ population. This subsample included only families who did not live in apartments, who did not smoke inside the home, and who owned their homes. Smokers were excluded from the subsample because cigarette smoke has already been well-characterized as a strong predictor of indoor particulate matter [35–37] and its presence may have obscured the associations between other sources and indoor particulate matter. All home owners provided written informed consent to participate and the home inspection response rate was 78% [34].
2.2. Home Inspections and Measurements
All 60 home inspections were carried out by two research assistants. In addition to dust collection and air pollution monitoring, a visual inspection and a home inspection questionnaire (HI questionnaire) were completed for each home. All families were asked not to vacuum for at least four days prior to dust collection.
Dust samples were collected using a Shop-Vac QAM70 vacuum with X-Cell 100 filters and samples were taken from the home’s living space where the child spent most of his/her time when not in the bedroom. An area of 2 m2 for carpeted floors and 4 m2 for hardwood floors was vacuumed for 3 minutes. Collected samples were analyzed at Paracel Laboratories in Ottawa (Ontario, Canada). After being weighed and sieved to exclude particles greater than 300 μm in size, the dust was analyzed for dust mite, cat and cockroach allergen content using immunoassays employing monoclonal antibodies for Der p 1, Der f 1, Fel d 1, and Bla g 1, respectively [38]. Detection limits were 10 ng/g of dust for Der p 1 and Der f 1, 3.12 ng/g for Fel d 1, and 0.04 U/g for Bla g 1. Mouse allergen concentration was determined using an enzyme-linked immunosorbent assay (ELISA) with purified rabbit polyclonal IgG antibodies specific for mouse urinary protein allergen, Mus m 1. The limit of detection for Mus m 1 was 0.001 μg/g of dust. Bacterial endotoxin was measured by washing with pyrogen free water and submitting the water extracts to Limulus Amoebocyte Lysate (LAL) assay using a chromogenic test kit in a kinetic assay on an MR 5,000 microplate reader [39]. The detection limit for endotoxin was 0.625 ng per gram of dust. The fungal component, (1→3)-β-d-glucan, was measured via the “factor G” LAL-based analytical method [40]. Dust samples were also analyzed specifically for the Alternaria alternata fungus, using an ELISA with monoclonal antibodies for Alt a 1. The limit of detection was 62.5 ng/g for glucan and 0.032 μg/g for Alt a 1. There is a lack of consensus as to whether allergen concentrations should be expressed by unit weight of dust or by unit area sampled [41,42]. In order to compare current results to reported values in the literature and to previously reported notional thresholds of sensitization, all results are expressed as both concentrations per gram of dust (μg/g) and per area (μg/m2).
Air pollution monitoring was completed over a 6-day period and all measurements were taken as averages over this time period. Air exchange rates within the home were measured using perfluorocarbon emitters (PFT), which were placed in the four corners of the main floor, and a capillary adsorption tube (CAT) detector, which was located in a central location on the main floor [43]. Sampling of indoor and outdoor particulate matter (PM2.5) and nitrogen dioxide (NO2) concentrations were conducted using an R&P Chempass Multi-pollutant sampler (R&P/Thermo, Waltham, MA, USA) that housed a passive Ogawa badge (Ogawa & Co, FL, USA) [44]. Indoor and outdoor continuous PM2.5 was also measured over the 6 days using a DustTrak (Model 8520, TSI, St. Paul, MN, USA).
2.3. Data Analysis
Basic bivariate statistics were calculated on all variables using Statistical Analysis Software (SAS) v9.1 (SAS Institute Inc, Cary, NC, USA). Measures of allergens were positively-skewed and were transformed by natural logarithm for data analysis; a square root transformation was used for glucan in μg/g. Pollutants were analyzed as continuous variables. Dust mite and cat allergen were also dichotomized according to previously reported notional thresholds of sensitization (Der p 1 or Der f 1 > 2 μg/g, Fel d 1 > 1 μg/g) and of asthma morbidity (Der p 1 or Der f 1 > 10 μg/g, Fel d 1 > 8 μg/g) [42,45–47]. All data analyses were run using predictors chosen a priori based on existing evidence in the literature. Indoor relative humidity (RH) was analyzed as the percentage of time RH was over 50% in the home; this variable was also dichotomized into homes with mean indoor RH above or below 50%. This was done based on current recommendations for dust mite allergen reduction [24] as well as previous work which showed that maintaining a mean daily RH below 50% effectively restricts dust mite growth and allergen production [48]. A linear regression was performed between dust mite levels and relative humidity. To determine whether gas appliances predicted increased indoor NO2 levels while adjusting for outdoor NO2 and air exchange rate, a multiple regression was performed. Student’s t-tests were employed to compare dichotomous T-CHEQ and HI questionnaire responses with continuous pollutant concentrations. Fisher’s exact tests were employed to assess the relationship between dichotomized pollutant measures and questionnaire responses.
3. Results
A summary of respondent and home characteristics as reported in the T-CHEQ and the home inspection (HI) questionnaire is found in Table 1. Of note, cockroaches were reported in only one home; however, Bla g 1 was below the detection limit of 0.04 U/g in all homes except one, where there was an insufficient amount of sampled dust to perform the analysis. Air monitoring measurements are shown in Table 2 and the distribution of allergen levels as measured in dust are shown in Table 3.
Table 1.
Participant and Dwelling Characteristics.
| VARIABLE | T-CHEQ PHASE 3 (n = 60)* | T-CHEQ PHASE 1 (N = 5559)* | ||||
|---|---|---|---|---|---|---|
| Frequency (%) | 95%Confidence Limits | Frequency (%) | 95%Confidence Limits | |||
|
PARTICIPANT CHARACTERISTICS |
||||||
| Sex | ||||||
| Male | 28 (46.7) | (33.7, 59.7) | 2,744 (50.2) | (48.9, 51.5) | ||
| Household Income Adequacy | ||||||
| Lowest | 0 (0) | - | 935 (18.0) | (17.0, 19.1) ‡ | ||
| Lower Middle Income | 4 (6.8) | (0.2, 13.4) | 1,156 (22.3) | (21.2, 23.4) ‡ | ||
| Upper Middle Income | 13 (22.0) | (11.1, 32.9) | 1,188 (22.9) | (21.8, 24.1) | ||
| Highest Income | 42 (71.2) | (59.3, 83.1) | 1,904 (36.7) | (35.4, 38.0) ‡ | ||
| Lifetime Asthma | 11 (18.3) | (8.3, 28.4) | 836 (15.5) | (14.5, 16.4) | ||
|
DWELLING CHARACTERISTICS |
||||||
| Type Of Dwelling | ||||||
| Single Detached House | 50 (83.3) | (73.6, 93.0) | 2,359 (44.6) | (43.3, 46.0) ‡ | ||
| Double (Semi Detached) | 7 (11.7) | (3.3, 20.0) | 746 (14.1) | (13.2, 15.1) | ||
| Row Or Terrace House | 3 (5.0) | (0.0, 10.7) | 282 (5.3) | (4.7, 5.9) | ||
| Duplex/triplex/low rise apt (<=5 stories) | EXCLUDED | 446 (8.4) | (7.7, 9.2) | |||
| High rise (>5 stories) | EXCLUDED | 1,368 (25.9) | (24.7, 27.1) | |||
| Institution, hotel; rooming/lodging | EXCLUDED | 84 (1.6) | (1.3, 1.9) | |||
| house/camp, mobile home, other | ||||||
| Year Dwelling Built | ||||||
| 1990 Or Later | 5 (8.3) | (1.1, 15.5) | NA | NA | ||
| 1969 To 1989 | 9 (15.0) | (5.7, 24.3) | NA | NA | ||
| 1949 To 1969 | 16 (26.7) | (15.1, 38.2) | NA | NA | ||
| Before 1949 | 30 (50.0) | (37.0, 63.0) | NA | NA | ||
| Cooking Fuel Used At Present† | ||||||
| Gas | 19 (31.7) | (19.5, 43.8) | 1,066 (19.6) | (18.5, 20.7) | ||
| Electric | 41 (68.3) | (56.2, 80.5) | 4,374 (80.4) | (79.3, 81.5) | ||
| Cat In Home At Present† | 9 (15.0) | (5.7, 24.3) | 751 (13.5) | (12.6, 14.4) | ||
| Any Pets At Home At Present† | 38 (63.3) | (50.8, 75.9) | 1,495 (29.4) | (28.1, 30.6) ‡ | ||
| Carpets in Child’s Bedroom | 46 (76.7) | (65.4, 87.7) | 2,358 (42.4) | (41.1, 43.7) ‡ | ||
| Air Conditioning | 58 (96.7) | (92.0, 100.0) | 4,074 (73.3) | (72.1, 74.5) ‡ | ||
| Roaches At Present† | 1 (1.7) | (0, 5.0) | 546 (9.8) | (9.0, 10.6) ‡ | ||
| Mice At Present | 10 (16.7) | (7.0, 26.4) | 5,013 (90.2) | (9.0, 10.6) | ||
| Damp Spots In House At Present† | 7 (11.7) | (3.3, 20.0) | 384 (6.9) | (6.2, 7.6) | ||
| Mould In House At Present | 28 (46.7) | (33.7, 59.7) | 336 (6.0) | (5.4, 6.7) | ||
T-CHEQ sample size (excludes missing data). T-CHEQ sample has previously been shown to be representative of the population of grades 1 and 2 school children living in Toronto in 2006 [34].
Income adequacy: a derived variable defined by Statistics Canada as (income, persons in household) Lowest income: <$15,000, 1–2 or <$20,000, 3–4 or <$30,000, 5+; Lower middle income: $15,000 to $29,999, 1–2, or $20,000 to $39,999, 3–4, or $30,000 to $59,999, 5+; Upper middle income: $30,000 to $59,999, 1–2, or $40,000 to $79,999, 3–4, or $60,000+, 1–2, or $80,000+, 3+ ; Highest income: $60,000+, 1 or 2, or $80,000+, 3+.
“Present” refers to 2006 in the case of T-CHEQ Phase 1, and 2007–08 in the case of Phase 3 .
Statiscically Significant difference based on non-overlapping 95% confidence intervals NA: Not Available
Table 2.
Levels if NO2, PM2.5 and air monitoring measurements in homes.
| Mean (SD) | Median | |
|---|---|---|
| Indoor temperature (°C) | 22.5 (2.1) | 22.6 |
| Indoor relative humidity (%) | 51.7 (7.4) | 50.7 |
| Ventilation (air changes/hour) | 0.36 (0.34) | 0.30 |
| Indoor NO2 (ppb) | 10.0 (7.5) | 8.6 |
| Outdoor NO2 (ppb) | 15.6 (4.8) | 14.9 |
| Indoor PM2.5 (μg/m3) | 9.2 (5.6) | 7.8 |
| Outdoor PM2.5 (μg/m3) | 9.6 (3.7) | 9.0 |
Table 3.
Distribution and levels of pollutants in dust samples.
| N | % | Mean (SD) [μg/g] | Median [μg/g] | Mean (SD) [μg/m2] | Median [μg/m2] | |
|---|---|---|---|---|---|---|
| Fel d 1 | 87.62 (268.52) | 1.42 | 31.73 (118.08) | 0.49 | ||
| Insufficient dust to perform analysis | 1 | 1.67 | ||||
| ≤1 μg/g | 26 | 43.33 | ||||
| >1 μg/g to 8 μg/g | 20 | 33.33 | ||||
| >8 μg/g | 13 | 21.67 | ||||
| Der p 1 | 0.60 (1.90) | 0.04 | 0.29 (0.91) | 0.02 | ||
| Insufficient dust to perform analysis | 2 | 3.33 | ||||
| Below detection limit (<0.01 μg/g) | 16 | 26.67 | ||||
| 10 ng/g to ≤2 μg/g | 39 | 65.00 | ||||
| >2 μg/g to 10 μg/g | 2 | 3.33 | ||||
| >10 μg/g | 1 | 1.67 | ||||
| Der f 1 | 44.50 (135.05) | 2.87 | 16.07 (63.79) | 0.77 | ||
| Insufficient dust to perform analysis | 1 | 1.67 | ||||
| ≤2 μg/g | 25 | 41.67 | ||||
| >2 μg/g to 10 μg/g | 14 | 23.33 | ||||
| >10 μg/g | 20 | 33.33 | ||||
| Bla g 1 | NA | NA | NA | NA | ||
| Insufficient dust to perform analysis | 1 | 1.7 | ||||
| Below detection limit (<0.04 U/g) | 59 | 98.3 | ||||
| Mus m 1 | 0.11(0.34) | 0.02 | 0.08 (0.36) | 0.01 | ||
| Insufficient dust to perform analysis | 8 | 13.33 | ||||
| =<0.001 μg/g | 1 | 1.67 | ||||
| Above detection limit (>0.001 μg/g) | 51 | 85.00 | ||||
| Endotoxin | 9.68 (6.67) | 8.93 | ||||
| Above detection limit (>0.000625 μg/g) | 60 | 100.00 | ||||
| (1→3)-β-D-glucan | 8,734.74 (6,985.22) | 6,562.32 | 4,384.81 (6,932.37) | 1,939.75 | ||
| Insufficient dust to perform analysis | 4 | 6.67 | ||||
| Above detection limit (>0.0625 μg/g) | 56 | 93.33 | ||||
| Alt a 1 | NA | NA | NA | NA | ||
| Insufficient dust to perform analysis | 7 | 11.7 | ||||
| Below detection limit (<0.032 μg/g) | 53 | 88.3 |
NA: Not available.
3.1. Indoor NO2
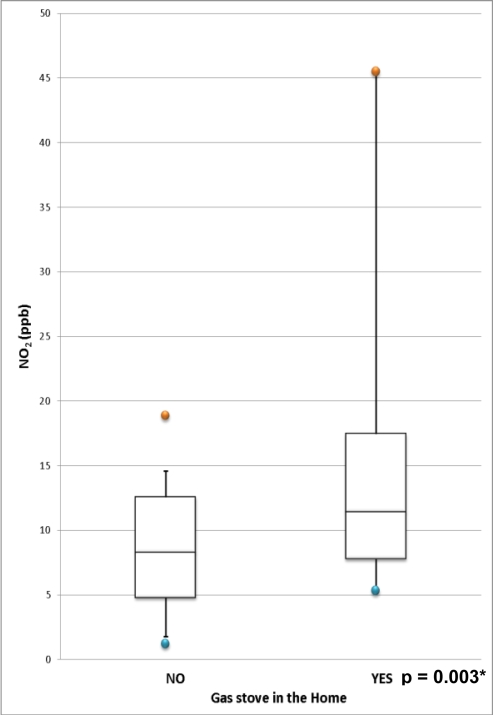
Indoor NO2 concentrations in all homes were below Health Canada’s acceptable long-term exposure range of ≤50 ppb for indoor air [49]. In homes where gas was the main cooking fuel, there were two homes with particularly high NO2 levels (Figure 1).
Figure 1.
Distribution of NO2 (ppb) comparing homes with and without gas stoves.
*Two-sample t-test comparing Ln-transformed population means.
The maximum value (NO2 = 45.5 ppb) was obtained at a home in which the gas stove was not vented. The other outlier (NO2 = 40.2 ppb) occurred in a home where there was a gas stove as well as a gas dryer, both of which were vented. A gas stove was designated as “vented” during the home inspection if the range hood above the stove vented directly to the outside. Mean indoor NO2 was significantly higher (p = 0.003) in homes that reported the main cooking fuel as gas in the T-CHEQ (14.98 ppb) than in homes that reported electricity as the main cooking fuel (8.31 ppb). Report of gas appliances in the HI questionnaire included stove, dryer, fireplace, water heater, and water boiler. In bivariate analyses, only gas stove was a significant predictor of indoor NO2. In a multiple regression that accounted for ventilation and outdoor NO2 levels, gas stove (p = 0.01) remained a significant predictor of indoor NO2 (R2 = 0.35).
3.2. Indoor PM2.5
Indoor PM2.5 concentrations for all homes were below Health Canada’s acceptable long-term exposure range of ≤40 μg/m3 for indoor air [49]. PM2.5 levels could not be obtained for one home due to problems with the sampling equipment. No significant predictors of indoor PM2.5 were found. In particular, HI questionnaire report of vacuuming frequency in the living room and interior wood storage in the home did not predict indoor PM2.5. Measured outdoor PM2.5 and air change rates also did not exhibit a significant relationship with indoor PM2.5.
3.3. Cat Allergens
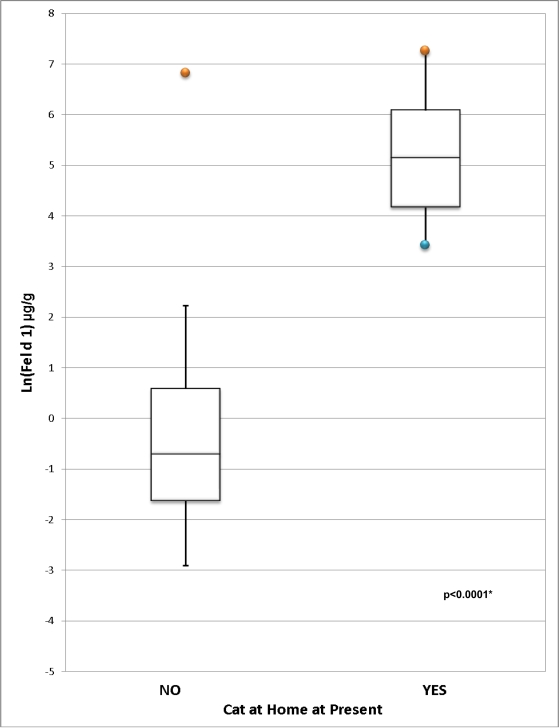
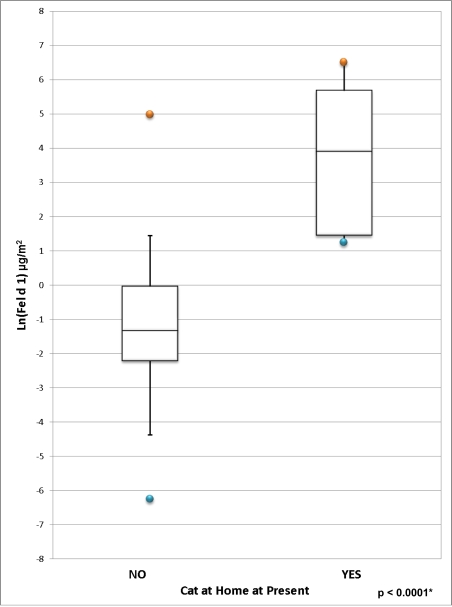
Detectable levels of Fel d 1 were found in all homes except one, where there was not enough dust to perform this analysis. The distribution of Fel d 1 in homes with and without cats is displayed in Figure 2. Mean Fel d 1 levels were significantly higher (p < 0.0001) in homes with cats (187.82 μg/m2, 450.58 μg/g) than in homes without cats (3.63 μg/m2, 22.28 μg/g). All homes with a cat had Fel d 1 levels > 8 μg/g, the asthma symptom threshold, whereas only 7.8% of homes without a cat had Fel d 1 levels > 8 μg/g (p < 0.0001, κ = 0.78). By contrast, all 9 homes with a cat had Fel d 1 > 1 μg/g, the notional sensitization threshold; however, 47.1% of homes without a cat also had Fel d 1 > 1 μg/g (p = 0.003, κ = 0.25).
Figure 2.
(a) Distribution of Ln-transformed Fel d 1 concentration (μg/g) in homes with and without cats. (b) Distribution of Ln-transformed Fel d 1 load (μg/m2) in homes with and without cats.
*Two-sample t-test comparing Ln-transformed population means. (a)
* Two-sample t-test comparing Ln-transformed population means. (b)
3.4. Dust Mite Allergen
Der f 1 was the more prevalent dust mite allergen in the 60 homes sampled. Detectable levels of Der f 1 were found in all homes; one home did not have enough sample available to perform this analysis. By contrast, Der p 1 was below the detection limit of 10 ng/g in 27.6% of homes, and below the notional sensitization threshold of 2 μg/g in 91.6% of homes.
No significant predictors of Der p 1 were found. Results of bivariate analyses of predictors of Der f 1 are displayed in Table 4. Mean Der f 1 levels were significantly higher in homes built before 1990 compared to those built after 1990 (47.61 μg/g versus 10.96 μg/g, p = 0.043; 17.54 μg/m2, versus 0.2 μg/m2, p = 0.012)
Table 4.
Univariate predictors of dust mite allergen (Der f 1) in the living space of the home where the child spent most of his/her time when not in the bedroom.
| N | Der f 1 (μg/g) | Der f 1 (μg/m2) | |||
|---|---|---|---|---|---|
| Mean | p-value | Mean | p-value | ||
| Self-reported variables from the T-CHEQ | |||||
| Any carpet in the child’s bedroom | 0.95 | 0.49 | |||
| Yes | 31 | 45.09 | 21.41 | ||
| No | 28 | 43.86 | 10.16 | ||
| Air conditioning in the home at present | 0.25 | 0.16 | |||
| Yes | 51 | 40.29 | 15.81 | ||
| No | 8 | 71.35 | 17.73 | ||
| Total number of people in the household | 0.63 | 0.52 | |||
| 3 or fewer | 11 | 28.26 | 7.65 | ||
| 4 | 27 | 44.63 | 9.40 | ||
| 5 | 17 | 63.70 | 36.04 | ||
| 6 or more | 4 | 4.36 | 0.23 | ||
| Report from HI questionnaire | |||||
| Year home was built | |||||
| Pre-1990 | 54 | 47.61 | 0.04* | 17.53 | 0.01* |
| 1990 or later | 5 | 10.96 | 0.20 | ||
| Frequency of humidifier use | 0.23 | 0.56 | |||
| Never | 23 | 46.07 | 12.80 | ||
| Yes for less than 60 days | 9 | 26.11 | 19.63 | ||
| Yes for at least 60 days | 26 | 98.60 | 15.91 | ||
| Frequency of central air conditioning use | |||||
| Less than 30 days per year | 20 | 46.36 | 0.06 | 29.51 | 0.01* |
| At least 30 days per year | 29 | 33.82 | 5.20 | ||
| Variables from home inspection measurements | |||||
| Sampling surface | 0.67 | 0.06 | |||
| Carpet | 47 | 50.73 | 19.20 | ||
| Hardwood | 10 | 14.02 | 0.74 | ||
| Calendar season | 0.96 | 0.80 | |||
| Summer | 32 | 51.62 | 20.98 | ||
| Fall | 27 | 32.54 | 7.81 | ||
| Mean indoor relative humidity | 0.002* | 0.007* | |||
| Mean RH ≤ 50% | 18 | 6.24 | 1.68 | ||
| Mean RH > 50% | 14 | 61.30 | 22.39 | ||
| Correlation coefficient (r) | Correlation coefficient (r) | ||||
| Percent of time indoor RH > 50% | 0.39 | 0.002* | 0.36 | 0.005* | |
| Mean indoor temperature | −0.01 | 0.95 | −0.04 | 0.75 | |
Significant predictor of Der f 1
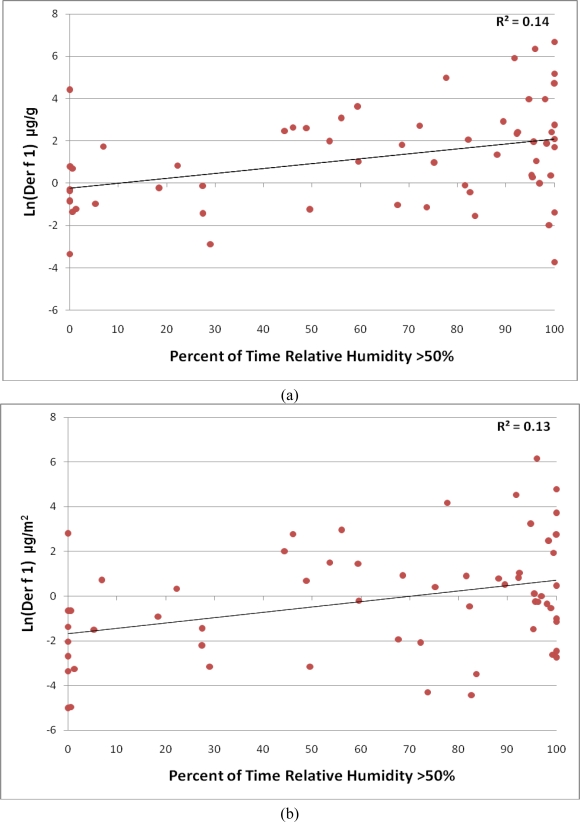
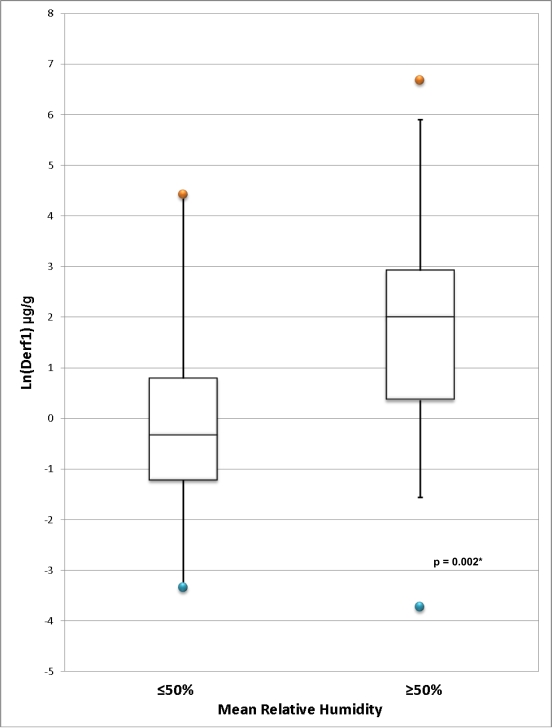
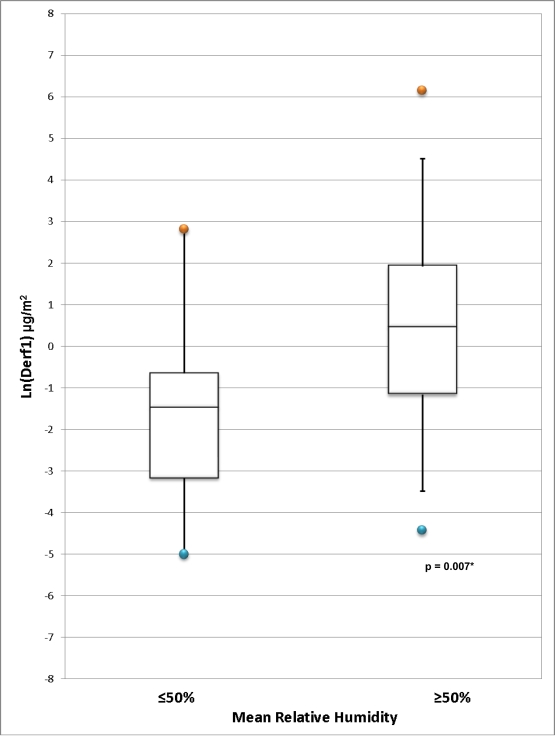
A sampling surface of carpet, compared to hardwood, predicted dust weight (1.13 g versus 0.30 g; p < 0.001), but not Der f 1 levels expressed as μg/g (p > 0.6); carpet sampling surface approached significance as a predictor of Der f 1 levels expressed as μg/m2 (p = 0.06). Homes that reported using central air conditioning for less than 30 days per year had higher mean Der f 1 levels expressed as μg/m2 compared to homes that reported using central air conditioning for at least 30 days per year (29.50 μg/m2 versus 5.20 μg/m2; p = 0.009). A significant, moderate correlation was found between Der f 1 levels in μg/g and the percentage of time in which indoor relative humidity was above 50% (p = 0.002, r = 0.39; Figure 3A). This relationship was also significant for Der f 1 levels in μg/m2 (p = 0.005, r = 0.36; Figure 3B). The distribution of Der f 1 in homes with mean RH above and below 50% is displayed in Figure 4. Der f 1 levels were significantly higher in homes with mean RH over 50% (61.30 μg/g versus 6.24 μg/g, p = 0.002; 22.39 μg/m2 versus 1.68 μg/m2, p = 0.007).
Figure 3.
(a) Ln-transformed Der f 1 (μg/g) concentration by percent of time relative humidity exceeds 50%. (b) Ln-Transformed Der f 1 load (μg/m2) by percent of time relative humidity exceeds 50%.
Figure 4.
(a) Distribution of Ln-transformed Der f 1 concentration (μg/g) in homes comparing mean relative humidity. (b) Distribution of Ln-Transformed Der f 1 load (μg/m2) in homes comparing mean relative humidity.
*Two-sample t-test comparing Ln-transformed population means. (a)
*Two-sample t-test comparing Ln-transformed population means. (b)
3.5. Mouse Allergens
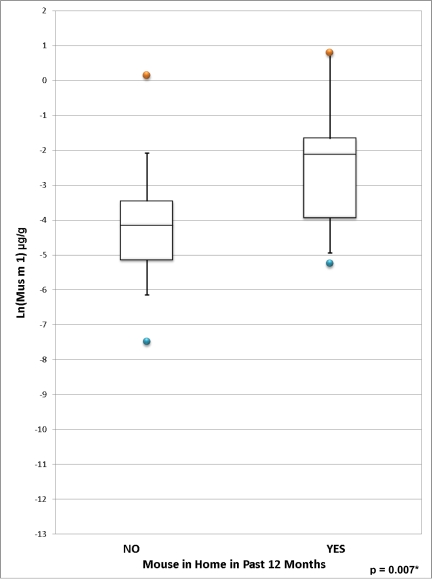
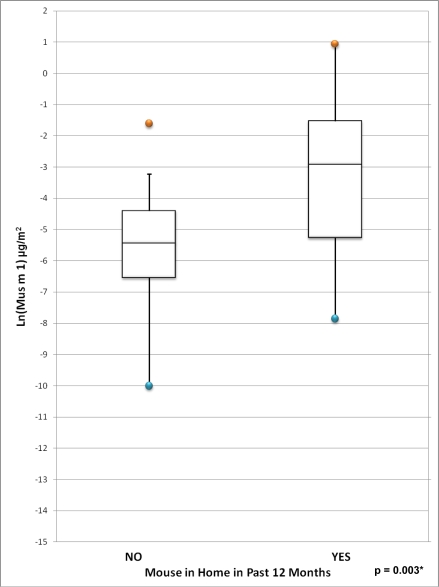
Detectable levels of Mus m 1 were found in all homes except eight, where there was insufficient dust to perform the analysis. Figure 5 displays the distribution of Mus m 1 in homes that did versus did not report mice as pests in the past 12 months. Mean Mus m 1 was significantly higher in homes that reported mice as pests (0.36 μg/g versus 0.06 μg/g, p = 0.007; 0.36 μg/m2 versus 0.02 μg/m2, p = 0.003). Reported age of the home in the HI questionnaire was not predictive of Mus m 1. No significant association was found between the reported presence of a cat in the home and Mus m 1 levels.
Figure 5.
(a) Distribution of Ln-transformed Mus m 1 concentration (μg/g) in homes with and without mice in the past 12 months. (b) Distribution of Ln-transformed Mus m 1 Load (μg/m2) in homes with and without mice in the past 12 months.
*Two-sample t-test comparing Ln-transformed population means. (a)
*Two-sample t-test comparing Ln-transformed population means. (b)
3.6. Endotoxin
Detectable levels of endotoxin were found in all 60 homes. The presence of a cat, a dog, or any pet in the home, as reported in the T-CHEQ, did not predict levels of endotoxin in μg/g or μg/m2.
3.7. Mould
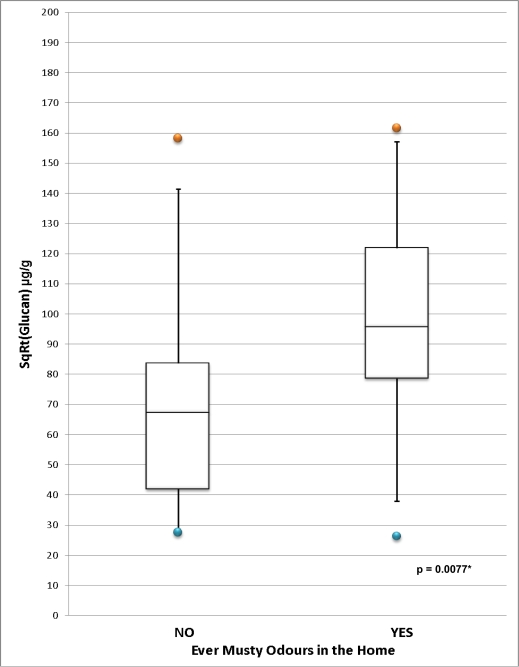
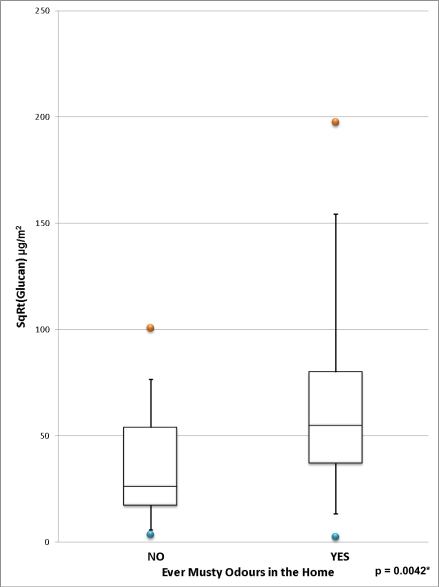
Alt a 1 was below the detection limit of 32 ng/g in all homes, with the exception of 7 houses, where there was insufficient dust to perform the analysis. However, detectable levels of glucan were present in all homes except 4, where there was insufficient dust to perform this analysis. Figure 6 displays the distribution of glucan in homes according to whether a musty odour was reported. Of note, 34 homes reported experiencing musty odours in the home in the HI questionnaire, but in the T-CHEQ, only 1 reported damp spots and 3 reported mould. Report of musty odours in the HI questionnaire was a significant predictor of glucan levels (10,554.37 μg/g versus 6,308.58 μg/g, p = 0.0077; 6,173.62 μg/m2 versus 1,999.73 μg/m2, p = 0.042).
Figure 6.
(a) Distribution of Glucan Concentration (μg/g) in Homes Reporting and Not Reporting Musty Odours. (b) Distribution of Glucan Load (μg/m2) in Homes Reporting and Not Reporting Musty Odours.
*Two-sample t-test comparing square root transformed population means. (a)
*Two-sample t-test comparing square root transformed population means. (b)
4. Discussion
Self-reported age of the home, presence of a gas stove, a cat, mice as pests, and musty odours from questionnaire data predicted objectively measured levels of pollutants in the home that may be associated with adverse health outcomes. No other self-reported home characteristics were predictive of potentially harmful pollutants. This has relevance both for epidemiological studies that assess the effects of indoor air pollutants on adverse health outcomes, as well as for clinical practice, where simple questions may be used to assess harmful exposures at home. Although neither a carpet sampling surface nor the presence of an air conditioner predicted increased levels of dust mite allergen, relative humidity above 50% predicted higher dust mite levels, supporting current guideline recommendations of reducing relative humidity as part of allergen exposure reduction [50,51].
4.1. Strengths and Limitations
Few studies have collected both dust and air quality measurements in the same homes. Our analysis of indoor pollutants also includes Mus m 1 which have not been well-characterized previously, especially in Canada.
One of the main strengths of this study lies in its population-based sampling approach. However, because all sampled homes were owned and not rented, the results may not reflect conditions in types of dwellings such as multi-unit apartments that are more likely to be rented than owned. Restricting the sample to owned dwellings also skewed it to households with higher incomes. In the 2001 Canadian Census, data for the Toronto census metropolitan area restricted to households with at least one child aged 5 to 17 years revealed that 43.5% of this population were in the highest income adequacy group [52]. 37% of the overall T-CHEQ population (n = 5619) was in the highest income category, while 71% of the home inspection subsample (n = 60) was in the highest income category. While this indicates that our results may not be readily generalizable to the wider population, it is interesting to note that we found detectable and even high levels of certain pollutants, such as cat and dust mite allergens in a mostly high-income group of homes. On the other hand, for pollutants such as NO2 and mouse allergen, even when significant associations existed between specific home characteristics and increased pollutants, overall concentrations tended to be low compared to reports from other studies. As a result, in our study population, although some self-reported characteristics via questionnaire were found to be predictive of objective measures of the corresponding pollutant, they were not necessarily indicative of pollutant levels that may lead to adverse health outcomes.
4.2. Indoor NO2
Questionnaire report of a gas stove, in conjunction with air change rates and outdoor NO2 contributions accounted for about one-third of the variance (R2 = 0.35) in indoor NO2 levels. In a previous study in Quebec City, gas heating systems, gas stoves, and air change rates were identified as significant predictors of indoor NO2 concentrations, explaining 48% of the variance in a multiple regression model [53]. Gas furnace was not included as a predictor in our model for indoor NO2 because measurements were conducted in the summer and fall, when furnaces were not in operation.
In our study, the measured indoor NO2 concentrations were relatively low, even in homes with gas appliances (mean NO2 = 12.1 ppb). Similar levels were also observed in the Stockholm BAMSE birth cohort in homes with gas stoves (mean NO2 = 12.0 ppb) [54]. By contrast, recent U.S. studies have reported markedly higher indoor NO2 concentrations in homes with gas stoves and have found increased NO2 levels to be associated with asthma morbidity [55,56]. However, these study populations were primarily composed of inner city residents and lower income groups, and many homes had gas stoves that were not vented [56]. Consequently, in our study, although the presence of a gas stove in the home was predictive of increased NO2, the presence of these gas appliances may not necessarily reflect exposures that would lead to adverse health outcomes.
4.3. Indoor PM2.5
No self-reported home characteristics were found to predict indoor PM2.5 in either the T-CHEQ or the HI questionnaire. Because all home inspections were conducted in the summer or fall, the effect of operating wood-burning fireplaces could not be effectively examined. Outdoor PM2.5 and air change rates did not predict indoor PM2.5 concentrations, although other studies have found both indoor sources such as cooking and cleaning, and outdoor sources to contribute to PM2.5 levels inside the home; air change rates have also been found to modulate the extent to which indoor PM2.5 levels reflect outdoor or indoor sources [57,58]. Human activities in the home, such as walking or vacuuming were not assessed in detail via questionnaire; however, these activities have been shown to contribute to indoor particulate matter via the resuspension of house dust [59]. More recently, increased early life exposure to traffic-related air pollutants have been found to increase the risk of asthma diagnosis [60] and self-report of truck traffic on the street of residence has also been positively associated with increased report of asthma symptoms [61]. Future research would be of interest to determine whether a questionnaire report of traffic in the vicinity of the home is a good predictor of measured concentrations of particulate matter and other traffic-related pollutants both inside and outside the home.
4.4. Cat Allergen
The indication of a cat in the home via questionnaire was found to predict increased cat allergen as a continuous variable expressed in μg/g and μg/m2, as well as cat allergen levels above 1 μg/g and above 8 μg/g. This is consistent with a previous study of homes in the Boston area, in which report of one or more cats currently in the home was found to be the best predictor for Fel d 1 levels ≥ 1 and ≥8 μg/g of dust [26]. The presence of cat allergen above the notional sensitization threshold in 47.1% of homes without cats, and above the asthma symptom threshold in 7.8% of homes without cats, suggest that cat allergen burden within the home may not be solely attributable to a cat currently in the home. The US National Survey of Lead and Allergens in Housing (NSLAH) also found that, for Fel d 1, 55.7% of homes without an indoor cat were above the notional sensitization threshold and 15.7% of homes without an indoor cat were above the asthma symptom threshold [45]. Previous work has shown that clothing and automobiles can be vehicles of pet allergen dispersal [62] and it is therefore likely that the tracking in of cat allergen from outdoors contributes to cat allergen concentrations in the home. Therefore, while report of a cat in the T-CHEQ is a good predictor of high cat allergen, report of no cat in the home is a poor indication of the absence of cat allergen. With respect to health implications, although exposure to cat allergen can exacerbate asthma symptoms in sensitized individuals, and may also lead to a higher risk of developing cat sensitization in children, current evidence remains inconclusive as to whether an association exists between cat exposure and the development of asthma itself [63,64].
4.5. Dust Mite Allergen
Having an older home built prior to 1990 was a significant predictor of increased Der f 1 levels. Older homes were also a significant predictor of dust mite allergen in the NSLAH [65] and in a recent pooled analysis of nine U.S. asthma studies [29]. The T-CHEQ question on flooring pertained only to the child’s bedroom, whereas dust sampling was conducted in the living space where the child spent most of his/her time when not in the bedroom. Neither report of carpets in the child’s bedroom nor a sampling surface of carpet were significant predictors of dust mite allergen concentrations, although previous studies have found them predictive of increased Der p 1 and Der f 1 levels [5,26]. The low levels of Der p 1 that were found in this study are similar to what has been previously reported for Toronto in the Child Asthma Management Program (CAMP), where over 90% of homes had Der p 1 below the notional sensitization threshold and Der f 1 was the predominant dust mite allergen [66]. Although report of an air conditioner in the home was not predictive of dust mite allergen, the percentage of time that measured indoor relative humidity was above 50% was significantly associated with Der f 1 levels. Our results are consistent with previous findings that a mean RH below 50% effectively restricts dust mite growth [48], and our findings support current guideline recommendations to reduce dust mite allergen by maintaining RH below 50% [24,48].
4.6. Cockroach Allergen
Only one home reported the presence of cockroaches and Bla g 1 levels were below the detection limit of 0.04 U/g in all homes. The absence of cockroach allergen may be attributable to the fact that all homes in this study were owned, single-family dwellings; in a study of homes in the Boston area, the odds of recovering detectable levels of cockroach allergen (Bla g 1 or Bla g 2 ≥ 0.025 U/g) have been found to be lower for houses and duplexes compared to apartments [26]. Furthermore, in the CAMP study, cockroach allergen was undetectable (Bla g 1 < 0.4 U/g) in 97.5% of Toronto homes (n = 118). The relatively small sample size of 60 homes in this study may have limited our ability to detect cockroach allergen.
4.7. Mouse Allergen
Report of mice as pests in the past 12 months in the home inspection questionnaire was found to predict increased levels of mouse allergen expressed as both μg/g and μg/m2. This is consistent with the results of a Baltimore inner-city study, where mouse infestation predicted detectable mouse allergen [67]. Reports of rodents were also associated with increased mouse allergen in both the NSLAH and a pooled analysis of nine U.S. asthma studies [29,68]. The presence of detectable mouse allergen in all homes with sufficient sample to perform this analysis suggests that this pollutant is quite prevalent in Toronto homes, despite the large proportion of high-income households in this study population. However, indoor levels of Mus m 1 in this study, even in homes that reported mice as pests, were markedly lower than values reported for US homes [22,68,69]. Only three homes had Mus m 1 concentrations above 0.25 μg/g, of which two homes had Mus m 1 concentrations above 0.5 μg/g—concentrations that have been previously associated with allergic sensitization and allergic symptoms [22]. Because sensitization and asthma symptom thresholds have not been firmly established for this allergen, it is unclear whether the low, widespread levels of mouse allergen in this study, and even the relatively increased levels in homes reporting mice as pests, have significant adverse health effects.
4.8. Endotoxin
Report of a cat, a dog, or any pet in the T-CHEQ did not predict increased levels of endotoxin in the home. Current evidence in the literature is not conclusive with respect to the predictors of endotoxin levels. In a study of 405 German homes, the presence of a cat, the presence of a dog, and poor hygienic conditions in the home were associated with high endotoxin concentrations in settled dust [70]. The Cincinnati Childhood Asthma and Air Pollution study of 532 homes reported that while homes with dogs alone and cats alone exhibited higher endotoxin loads compared to homes without pets, this relationship was only statistically significant for homes with dogs [30]. In the AIRALLERG study of homes of 1,065 German, Dutch and Swedish pre-school children, although there was an association between high endotoxin levels in floor dust and the presence of a cat, a dog, and other pets in the home, not all of these associations were statistically significant for all three countries [31]. The AIRALLERG study results also showed that, in general, the questionnaire variables on housing characteristics accounted for a low proportion of variance in endotoxin concentrations and did not accurately predict endotoxin concentrations in house dust [31].
4.9. Mould
Report of musty odours in the HI questionnaire was a significant predictor of glucan levels in the home. In the T-CHEQ, however, low reporting of dampness or mould in the home, even in the presence of detectable glucan levels, suggests that these questions may not be accurate predictors of actual fungi levels in the home. Questionnaire report of mould, musty odours, or moisture-related problems have been previously associated with levels of fungi in dust in the NSLAH [28] and in a study of 403 homes in Wallaceburg, Ontario [71]. The discrepancy between parental T-CHEQ responses and actual mould in the home may indicate that, by only ascertaining the presence or absence of mould and dampness, the T-CHEQ questions are not sufficiently detailed to capture the true conditions of the home environment. Alternatively, the discrepancy may be due to the inability of residents to detect mould on hidden surfaces or within walls. It may also reflect the potential unwillingness of parents to report the presence of such factors that are known to be detrimental to health, particularly within the context of a questionnaire related to their child’s health. The latter may have been the case in the home with the highest level of glucan, where mould was not reported in the T-CHEQ, but was identified by the resident to research assistants during the home inspection.
Our findings that A alternata allergen was below the detection limit of 0.032 μg/g in all homes contrast with the results of the NSLAH, which found Alternaria antigens in 95% to 99% of dust samples from US homes [28]. It is possible that geographic and climatic differences may explain the low prevalence of A alternata as an indoor fungus in Toronto homes. However, Schmechel et al. recently reported that the polyclonal antibodies used to detect A alternata in the NSLAH cross-reacted broadly with other fungi and were not therefore Alternaria-specific [72]. A species-specific monoclonal antibody was used to detect the Alt a 1 allergen in this study and it is therefore possible that the indoor prevalence of the A alternata fungus is indeed quite low in our study population.
5. Conclusions
Questionnaire reports of a cat, of a gas stove, of mice as pests, of musty odours, and of the home’s age predicted objectively measured concentrations of pollutants in the home at levels that may be associated with adverse health outcomes. No other questions pertaining to home characteristics were predictive of potentially harmful pollutant exposures. This highlights the importance of using valid questions in large epidemiological surveys that assess the effects of indoor air pollutants on adverse health outcomes. These results are also relevant for clinical practice, and suggest that single questions, such as whether there are carpets in the house, may not be sufficient to provide an accurate assessment of harmful exposures at home.
Acknowledgments
The authors would like to thank Ryan Kulka and Hongyu You for training the field staff and carrying out equipment maintenance, Robin Coombs for conducting home inspections, and Keith Van Ryswyk and Branka Jovic for data management. This work was funded by Health Canada and the Program of Energy Research and Development, Natural Resources Canada. Jennifer Loo was supported by an Ontario Thoracic Society Summer Studentship.
References
- 1.Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Rep. 2008;19:45–50. [PubMed] [Google Scholar]
- 2.Millar WJ, Hill GB. Childhood asthma. Health Rep. 1998;10:9–21. [PubMed] [Google Scholar]
- 3.Taylor WR, Newacheck PW. Impact of childhood asthma on health. Pediatrics. 1992;90:657–662. [PubMed] [Google Scholar]
- 4.Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It’s about time: A comparison of Canadian and American time-activity patterns. J. Expos. Anal. Environ. Epidemiol. 2002;12:427–432. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 5.Meijer GG, van der Heide S, Postma DS, de Reus DM, Koeter GH, van Aalderen WM. House dust mite exposure in asthmatic and healthy children: The difference is carpeting. Pediatr. Allergy Immunol. 1995;6:187–191. doi: 10.1111/j.1399-3038.1995.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 6.Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin. Experiment. Allergy. 1989;19:419–424. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 7.Elliott L, Arbes SJ, Harvey ES, Lee RC, Salo PM, Cohn RD, London SJ, Zeldin DC. Dust weight and asthma prevalence in the National Survey of Lead and Allergens in Housing (NSLAH) Environ. Health Perspect. 2007;115:215–220. doi: 10.1289/ehp.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17:284–296. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 9.Rylander R, Lin RH. (1-->3)-beta-D-glucan—Relationship to indoor air-related symptoms, allergy and asthma. Toxicology. 2000;152:47–52. doi: 10.1016/s0300-483x(00)00291-2. [DOI] [PubMed] [Google Scholar]
- 10.Apelberg BJ, Aoki Y, Jaakkola JJ. Systematic review: Exposure to pets and risk of asthma and asthma-like symptoms. J. Allerg. Clin. Immunol. 2001;107:455–460. doi: 10.1067/mai.2001.113240. [DOI] [PubMed] [Google Scholar]
- 11.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J. Allerg. Clin. Immunol. 2001;107:41–47. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 12.Salam MT, Li Y-F, Langholz B, Gilliland FD. Children’s health S. early-life environmental risk factors for asthma: findings from the Children’s Health Study. Environ. Health Perspect. 2004;112:760–765. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, Trenga CA, Larson T, Liu LJS. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ. Health Perspect. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Amer. J. Respir. Crit. Care Med. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Amer. J. Respir. Crit. Care Med. 1998;158:891–895. doi: 10.1164/ajrccm.158.3.9701084. [DOI] [PubMed] [Google Scholar]
- 16.Michel O, Ginanni R, Duchateau J, Vertongen F, Le Bon B, Sergysels R. Domestic endotoxin exposure and clinical severity of asthma. Clin. Experiment. Allergy. 1991;21:441–448. doi: 10.1111/j.1365-2222.1991.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 17.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Amer J. Respir. Crit. Care Med. 1996;154:1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 18.Dales R, Miller D, Ruest K, Guay M, Judek S. Airborne endotoxin is associated with respiratory illness in the first 2 years of life. Environ. Health Perspect. 2006;114:610–614. doi: 10.1289/ehp.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavernier GOG, Fletcher GD, Francis HC, Oldham LA, Fletcher AM, Blacklock G, Stewart L, Gee I, Watson A, Frank TL, Frank P, Pickering CAC, Niven RML. Endotoxin exposure in asthmatic children and matched healthy controls: results of IPEADAM study. Indoor Air. 2005;15:25–32. doi: 10.1111/j.1600-0668.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 20.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Amer. J. Respir. Crit. Care Med. 2005;172:1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, Diette GB. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann. Allergy Asthma Immunol. 2006;97:514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 22.Phipatanakul W, Celedon JC, Hoffman EB, Abdulkerim H, Ryan LM, Gold DR. Mouse allergen exposure, wheeze and atopy in the first seven years of life. Allergy. 2008;63:1512–1518. doi: 10.1111/j.1398-9995.2008.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipatanakul W, Litonjua AA, Platts-Mills TA, Naccara LM, Celedon JC, Abdulkerim H, Hoffman EB, Gold DR. Sensitization to mouse allergen and asthma and asthma morbidity among women in Boston. J. Allerg. Clin. Immunol. 2007;120:954–956. doi: 10.1016/j.jaci.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allerg. Clin. Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Guide to Community Preventive Services; Asthma Control. Community Guide Branch, National Center for Health Marketing (NCHM), Centers for Disease Control and Prevention; Atlanta, GA, USA: 2008. Available online: www.thecommunityguide.org/asthma/index.html. (accessed on 18 March 2010) [Google Scholar]
- 26.Chew GL, Burge HA, Dockery DW, Muilenberg ML, Weiss ST, Gold DR. Limitations of a home characteristics questionnaire as a predictor of indoor allergen levels. Am. J. Respir. Crit. Care Med. 1998;157:1536–1541. doi: 10.1164/ajrccm.157.5.9708011. [DOI] [PubMed] [Google Scholar]
- 27.Lee K, Xue J, Geyh AS, Ozkaynak H, Leaderer BP, Weschler CJ, Spengler JD. Nitrous acid, nitrogen dioxide, and ozone concentrations in residential environments. Environ. Health Perspect. 2002;110:145–150. doi: 10.1289/ehp.02110145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salo PM, Yin M, Arbes SJ, Jr, Cohn RD, Sever M, Muilenberg M, Burge HA, London SJ, Zeldin DC. Dustborne Alternaria alternata antigens in US homes: Results from the National Survey of Lead and Allergens in Housing. J. Allerg. Clin. Immunol. 2005;116:623–629. doi: 10.1016/j.jaci.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson J, Dixon SL, Bresse P, Jacobs D, Adamkiewicz G, Chew GL, Dearborn D, Krieger J, Sandel M, Spanier A. Housing and allergens: A pooled analysis of nine US studies. Environ. Res. 2010;110:189–198. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus ZL, Cho S-H, Khurana Hershey GK, Lockey J, Villareal M, Stanforth S, Lemasters G, Bernstein DI. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J. Allerg. Clin. Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giovannangelo M, Gehring U, Nordling E, Oldenwening M, Terpstra G, Bellander T, Hoek G, Heinrich J, Brunekreef B. Determinants of house dust endotoxin in three European countries—The AIRALLERG study. Indoor Air. 2007;17:70–79. doi: 10.1111/j.1600-0668.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 32.Simpson A, Woodcock A, Custovic A. Housing characteristics and mite allergen levels: To humidity and beyond. Clin. Exp Allergy. 2001;31:803–805. doi: 10.1046/j.1365-2222.2001.01110.x. [DOI] [PubMed] [Google Scholar]
- 33.2006 Census Table—Persons 0—11 Years Old in Private Households in Occupied Private Dwellings by Age Groups, by Selected Characteristics, for Canada, 2006 Census [Custom Data File] Statistics Canada; Ottawa, ON, Canada: 2009. Available on line: http://www.phacaspc.gc.ca/publicat/2009/cphorsphc-respcacsp/cphorsphc-respcacsp06-eng.php#c3-1 (accessed on 22 December 2009) [Google Scholar]
- 34.Dell SD, Foty RG, Gilbert NL, Jerret M, To T, Walter SD, Stieb DM. Asthma and allergic disease prevalence in a diverse sample of Toronto school children: Results from the Toronto Child Health Evaluation Questionnaire (T-CHEQ) Study. Can. Resp. J. 2010;17:1–6. doi: 10.1155/2010/913123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, Williams DAL, Buckley TJ, Eggleston PA, Diette GB. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ. Res. 2008;106:148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Deusen A, Hyland A, Travers MJ, Wang C, Higbee C, King BA, Alford T, Cummings KM. Secondhand smoke and particulate matter exposure in the home. Nicotine Tob. Res. 2009;11:635–641. doi: 10.1093/ntr/ntp018. [DOI] [PubMed] [Google Scholar]
- 37.Wallace L. Indoor particles: A review. J.Air Waste Manage. Assoc. 1996;46:98–126. doi: 10.1080/10473289.1996.10467451. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton RG, Chapman MD, Platts-Mills TAE, Adkinson NF. House dust aeroallergen measurements in clinical practice: A guide to allergen-free home and work environments. Immunol. Allergy Pract. 1992;14:9–25. [Google Scholar]
- 39.Remillard JF, Roslansky PF, Novitsky TJ. Quantification of endotoxin using the LAL kinetic turbidmetric assay in an incubating microplate reader. LAL Update. 1992;10:1–5. [Google Scholar]
- 40.Foto MS. An Analytical Method to Quantify 1,3-b-D-Glucan using the LAL Assay. Carleton University; Ottawa, ON, Canada: 2002. (M.Sc. thesis); [Google Scholar]
- 41.Colloff MJ, Ayres J, Carswell F, Howarth PH, Merrett TG, Mitchell EB, Walshaw MJ, Warner JO, Warner JA, Woodcock AA. The control of allergens of dust mites and domestic pets: A position paper. Clin. Experiment. Allergy. 1992;22:1–28. doi: 10.1111/j.1365-2222.1992.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 42.Platts-Mills TA, Thomas WR, Aalberse RC, Vervloet D, Champman MD. Dust mite allergens and asthma: report of a second international workshop. J. Allerg. Clin. Immunol. 1992;89:1046–1060. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 43.Dietz RN, Cote EA. Air infiltration measurements in a home using a convenient perfluorocarbon tracer technique. Environ. Int. 1982;8:419–433. [Google Scholar]
- 44.Demokritou P, Kavouras IG, Ferguson S, Koutrakis P. Development and laboratory performance evaluation of a personal multipollutant sampler for simultaneous measurements of particulate and gaseous pollutants. Aerosol Sc. Tech. 2001;35:741–752. [Google Scholar]
- 45.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J. Allerg. Clin. Immunol. 2004;114:111–117. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Amer. Rev. Resp. Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 47.Liccardi G, D’Amato G, Russo M, Canonica GW, D’Amato L, De Martino M, Passalacqua G. Focus on cat allergen (Fel d 1): immunological and aerodynamic characteristics, modality of airway sensitization and avoidance strategies. Int. Arch. Allergy Immunol. 2003;132:1–12. doi: 10.1159/000073259. [DOI] [PubMed] [Google Scholar]
- 48.Arlian LG, Neal JS, Vyszenski-Moher DL. Reducing relative humidity to control the house dust mite Dermatophagoides farinae. J. Allerg. Clin. Immunol. 1999;104:852–856. doi: 10.1016/s0091-6749(99)70298-8. [DOI] [PubMed] [Google Scholar]
- 49.Health Canada . Exposure guidelines for residential indoor air quality: Part A. substances with exposure guidelines—Non-carcinogenic effects. Health Canada; Ottawa, ON, Canada: 2008. Available online: http://www.hc-sc.gc.ca/ewh-semt/pubs/air/exposure-exposition/non-carcinoeng.php (accessed on 18 August 2010) [Google Scholar]
- 50.Arlian LG, Neal JS, Morgan MS, Vyszenski-Moher DL, Rapp CM, Alexander AK. Reducing relative humidity is a practical way to control dust mites and their allergens in homes in temperate climates. J. Allerg. Clin. Immunol. 2001;107:99–104. doi: 10.1067/mai.2001.112119. [DOI] [PubMed] [Google Scholar]
- 51.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention 2008 Update. Available online: http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=1561 (accessed on 2 July 2009)
- 52.Special Tabulation Using 2001 Census Microdata File (N = 13368 households), Canada Census. Statistics Canada; Ottawa, ON, Canada: 2001. [Google Scholar]
- 53.Gilbert NL, Gauvin D, Guay M, Heroux M-E, Dupuis G, Legris M, Chan CC, Dietz RN, Levesque B. Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ. Res. 2006;102:1–8. doi: 10.1016/j.envres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Emenius G, Pershagen G, Berglind N, Kwon HJ, Lewne M, Nordvall SL, Wickman M. NO2, as a marker of air pollution, and recurrent wheezing in children: a nested case-control study within the BAMSE birth cohort. Occup. Environ. Med. 2003;60:876–881. doi: 10.1136/oem.60.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, McCormack MC, Williams DL, Breysse PN. Home indoor pollutant exposures among inner-city children with and without asthma. Environ. Health Perspect. 2007;115:1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J. Allerg. Clin. Immunol. 2007;120:618–624. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Abt E, Suh HH, Allen G, Koutrakis P. Characterization of indoor particle sources: A study conducted in the metropolitan Boston area. Environ. Health Perspect. 2000;108:35–44. doi: 10.1289/ehp.0010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cyrys J, Pitz M, Bischof W, Wichmann HE, Heinrich J. Relationship between indoor and outdoor levels of fine particle mass, particle number concentrations and black smoke under different ventilation conditions. J. Expos. Anal. Environ. Epidem. 2004;14:275–283. doi: 10.1038/sj.jea.7500317. [DOI] [PubMed] [Google Scholar]
- 59.Ferro AR, Kopperud RJ, Hildemann LM, Ferro AR, Kopperud RJ, Hildemann LM. Source strengths for indoor human activities that resuspend particulate matter. Environ. Sci. Technol. 2004;38:1759–1764. doi: 10.1021/es0263893. [DOI] [PubMed] [Google Scholar]
- 60.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ. Health Perspect. 2009;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunekreef B, Stewart AW, Anderson HR, Lai CKW, Strachan DP, Pearce N. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: A global relationship in ISAAC phase 3. Environ. Health Perspect. 2009;117:1791–1798. doi: 10.1289/ehp.0800467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neal JS, Arlian LG, Morgan MS, Neal JS, Arlian LG, Morgan MS. Relationship among house-dust mites, Der 1, Fel d 1, and Can f 1 on clothing and automobile seats with respect to densities in houses. Ann. Allergy Asthma Immunol. 2002;88:410–415. doi: 10.1016/S1081-1206(10)62373-3. [DOI] [PubMed] [Google Scholar]
- 63.Chen C-M, Tischer C, Schnappinger M, Heinrich J. The role of cats and dogs in asthma and allergy—A systematic review. Int. J. Hyg. Environ. Health. 2010;213:1–31. doi: 10.1016/j.ijheh.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Kerkhof M, Wijga AH, Brunekreef B, Smit HA, de Jongste JC, Aalberse RC, Hoekstra MO, Gerritsen J, Postma DS. Effects of pets on asthma development up to 8 years of age: The PIAMA study. Allergy. 2009;64:1202–1208. doi: 10.1111/j.1398-9995.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 65.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, Zeldin DC. House dust mite allergen in US beds: Results from the First National Survey of Lead and Allergens in Housing. J. Allerg. Clin. Immunol. 2003;111:408–414. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 66.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J. Allerg. Clin. Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 67.Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, Eggleston PA. Airborne mouse allergen in the homes of inner-city children with asthma. J. Allerg. Clin. Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC, Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J. Allerg. Clin. Immunol. 2004;113:1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 69.Phipatanakul W, Eggleston PA, Wright EC, Wood RA, National Coooperative Inner-City Asthma Study Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J. Allerg. Clin. Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 70.Bischof W, Koch A, Gehring U, Fahlbusch B, Wichmann HE, Heinrich J, Indoor E, Genetics in Asthma Study Group Predictors of high endotoxin concentrations in the settled dust of German homes. Indoor Air. 2002;12:2–9. doi: 10.1034/j.1600-0668.2002.120102.x. [DOI] [PubMed] [Google Scholar]
- 71.Dales R, Miller D, McMullen E. Indoor air quality and health: validity and determinants of reported home dampness and moulds. Int. J. Epidemiol. 1997;26:120–125. doi: 10.1093/ije/26.1.120. [DOI] [PubMed] [Google Scholar]
- 72.Schmechel D, Green BJ, Blachere FM, Janotka E, Beezhold DH. Analytical bias of cross-reactive polyclonal antibodies for environmental immunoassays of Alternaria alternata. J. Allerg. Clin. Immunol. 2008;121:763–768. doi: 10.1016/j.jaci.2007.09.046. [DOI] [PubMed] [Google Scholar]