Abstract
Biocompatible, degradable, polymer scaffolds combined with cells or biological signals are being investigated as alternatives to traditional options for tissue reconstruction and transplantation. These approaches are already in clinical use as engineered tissues that enhance wound healing and skin regeneration. The continued enhancement of these material strategies is highly dependent on the ability to promote rapid and stable neovascularization (new blood vessel formation) within the scaffold. While neovascularization therapies have shown some promise for the treatment of ischemic tissues, vascularization of polymer scaffolds in tissue engineering strategies provide a unique challenge due to the volume and complexity of the tissues targeted. In this article we examine recent advances in research focused on promoting neovascularization in polymer scaffolds for tissue engineering applications. These approaches include the use of growth factors, cells, and novel surgical approaches to both enhance and control the nature of the vascular networks formed. The continued development of these approaches may lead to new tissue engineering strategies for the generation of skin and other tissues or organs.
Introduction
Wounds resulting from trauma, burn, or tumor resection remain some of the more challenging issues in tissue reconstruction. The reconstruction of these defects depends on the size of the defect, the donor site availability and health status of the patient. In a healthy adult, a wound less than 5 centimeters in its largest dimension can be healed by secondary intention. Healing can be enhanced through a variety of therapies, such as vacuum assisted closure (VAC) and/or hyperbaric oxygen (HBO) treatment. If the defect is larger than 5 cm, then local or free flap transfer may be required for coverage of the defect. In a purely soft tissue defect with healthy soft tissue or muscle covering the wound bed, the wound can be easily treated with a skin graft. However, skin grafts have particular difficulty surviving when there is exposed bone and tendon. In addition, chronic wounds resulting from aging, diabetes and peripheral artery occlusive disease are often refractory to conventional treatment.
New biologically active polymers that initiate or enhance wound healing are currently available potential alternative approaches for the treatment of large volume, or poorly healing, wounds.1 In polymer design, engineers and scientists must consider the optimization of biomaterial properties in order to modulate important biological responses, including epithelialization, infection, and scar formation. In addition, the success of these strategies is highly dependent on whether the materials are able to promote rapid and stable neovascularization (new blood vessel formation) within the scaffold, typically prior to complete material degradation. There are a variety of approaches that have been explored for promoting neovascularization. Here we will describe some of the more common approaches and where they currently stand in regards to application for engineering skin, tissues, and organs.
Growth Factors
Scaffold Delivery of Growth Factors
The most common approach for controlling neovascularization in biomaterial scaffolds is based on the incorporation of naturally occurring growth factors into the scaffolds. A complex temporal and spatial expression of multiple growth factors occurs in tissues regulating neovascularization in response to tissue ischemia, inflammation, or disease. The direct injection of growth factors into tissues has shown promise clinically for the treatment of ischemic diseases.2 For many tissue engineering applications, including skin, the scaffolds often need to permit extensive neovascularization prior to the bulk degradation of the material. This presents issues not encountered when promoting vascularization in ischemic tissues. The specific growth factors to use, their delivery kinetics, and spatial presentation must be optimized with material degradation in order to coordinate both vascularization and tissue regeneration.
Isoforms of vascular endothelial growth factor (VEGF) have received significant attention as therapeutic agents and shown promise in clinical studies for the treatment of peripheral limb and myocardial ischemia. In these applications, the protein or DNA are typically delivered via multiple bolus injections into the diseased tissue. This can result in abnormally high levels that may stimulate vessels with abnormal structure, high permeability, and poor long-term stability.3,4 Addition of VEGF proteins into polymer scaffolds has been shown to enhance vascularization. The VEGF is typically incorporated in a form in which it diffuses from the scaffolds, or is released as the polymer degrades, stimulating local vessels to sprout towards the implanted material. The proteins can also be covalently incorporated into polymer scaffolds. When attached to poly (ethylene glycol) (PEG) or fibrin-based materials, VEGF release is delayed, prolonging its biological function and reducing risk of ectopic effects.5,6 The attached VEGF had been shown to have improved activity and promote more extensive neovascularization relative to freely diffusing VEGF.6
A number of members of the fibroblast growth factor (FGF) family of proteins have been also been investigated for their ability to stimulate vessel formation within polymer scaffolds. The most popular proteins of this family are FGF-1 (or acidic FGF) and FGF-2 (basic FGF), which are potent endothelial cell (EC) mitogens and are known to stimulate neovascularization. We have recently shown that the controlled delivery of FGF-1 from alginate microbeads results in a more persistent neovascularization response relative to bolus delivery.7–9 The incorporation of these beads into collagen scaffolds results in both an increase in initial vessel invasion into the collagen and a longer persistence of the vascular network fomed.10 In addition, by delivering FGF-1 from alginate the dose required to achieve vascularization is lower than what is required when the protein is suspended in the scaffold.9 While the sustained delivery from alginate increases the lifetime of the vessels, measures of vessel maturity did not increase.
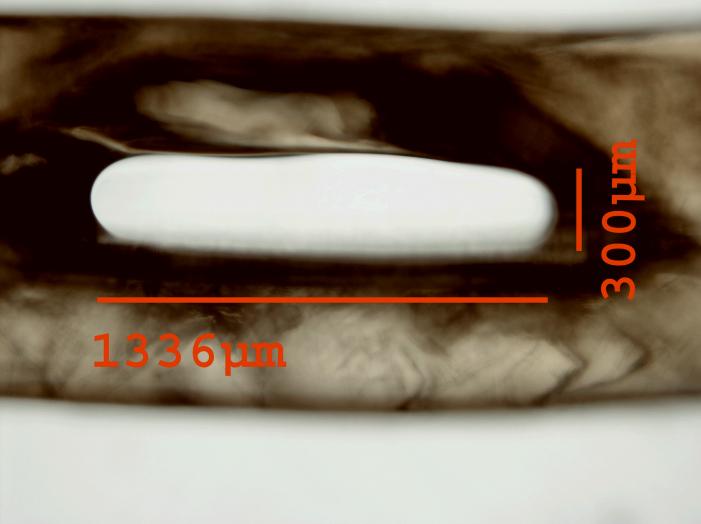
The sequential delivery of a protein that stimulates vessel sprouting and invasion followed by a factor involved in vessel stabilization can increase the stability, or maturity, of the vessels formed. While vessels stimulated by VEGF alone may regress, this regression can be inhibited by the delivery of angiopoietin-1 (Ang-1), a vessel-stabilizing factor.11 The combination of PDGF-BB with FGF-2 increased arteriogenesis, or collateralization, in models of tissue ischemia.12 Multiple growth factor delivery has also been used to enhance scaffolds neovascularization where VEGF followed by platelet derived growth factor-BB (PDGF-BB) lead to larger vessels and greater mural cell interactions.13 This research shows that controlled delivery of multiple growth factors may enhance neovascularization over a single factor. However, the delivery methods still need to be optimized. We have recently developed a technique for generating multilayer alginate microbeads that could be used for dual growth factor delivery with distinct release kinetics from the different alginate layers (Figure 1).14
Figure 1.

A mutlilayer alginate microbead in which each layer contains fluorescently labeled albumin. The composition of each layer can be controlled allowing for distinct release kinetics of proteins encapsulated in each of the layers.
Growth Factor Gradients
While the identity of growth factor and/or growth factor combinations is important in stimulating neovascularization, the spatial presentation of these factors plays a major role in this process. In studies in the post-natal retina, Gerhardt et al. showed that the migration of ECs on the tips of sprouting vessels was dependent on the gradient of VEGF, while proliferation was a function of its concentration.15 The approaches described in the previous section generate gradients due to the diffusion of the growth factor from the polymer scaffold and into the surrounding tissue. It may also be beneficial to generate scaffolds with controlled gradients of growth factors to accelerate and guide vessel invasion.
Barkefors et al. investigated EC migration in response to soluble gradients of VEGF and FGF-2 using a novel microfluidic chemotaxis chamber.16 An advantage of the MCC as compared to more traditional chamber assays is that it allows for predictable gradients of various shapes and imaging of migratory cell paths using time-lapse microscopy. Gradients of VEGF resulted in a more potent chemotactic effect for ECs as compared to similar gradients of FGF-2. Further analysis of cell migration in different regions of the gradient indicated a maximal chemotactic response in exponential gradient as compared to linear gradient regions suggesting that the shape of the gradient dictates migratory response. These results suggest the complicated nature of the EC response to gradients of immobilized factors. The response depends on the specific growth factor and the shape of its concentration profile.
Growth factors and ECM molecules simultaneously regulate the migration of cells. Liu et al. created surface density gradients of both VEGF and fibronectin on gold substrates with varying gradient slopes generated by self-assembled monolayers using an electrochemical approach.17 To our knowledge, this was the first study to create surface density gradients of VEGF. ECs seeded on surface density gradients of fibronectin, VEGF, or both proteins exhibited increased cell displacement on gradient surfaces towards higher surface protein density as compared to surfaces with a uniform protein density. A two-fold increase in cell migration was observed on VEGF gradient as compared to fibronectin gradient surfaces and a further two-fold increase in migratory response was observed on surfaces with combined gradients of both factors as compared to those with immobilized VEGF or fibronectin gradients alone.
While the above-mentioned studies have provided significant insight of EC haptotactic response on 2D surfaces, the creation of gradients within three-dimensional polymeric scaffolds more accurately mimics the physiological environment. Hydrogels of PEG formed via photopolymerization have the potential to be used to create immobilized gradients of growth factors and cell adhesion sites. The advantage of this approach to engineer polymer scaffolds for tissue engineering applications is that both the physical properties and the incorporation of proteins and peptides can be controlled by polymerization conditions (e.g., polymerization time, PEG macromer molecular weight, precursor concentration). Immobilized gradients of FGF-2 were incorporated into PEG-based hydrogels formed using a gradient maker.18 In this case cells preferentially migrated up the gradient, but it is not clear whether the magnitude of the gradient influenced migratory behavior. In addition, while the hydrogel is 3D the cells only migrated on the surface resulting in a 2D cell migratory response, and only smooth muscle cell migration was examined. Creation of 3D gradients within PEG hydrogels would be a better benefit for applications in regenerative medicine.
Microfluidic devices provide the versatility for multi-parameter manipulation as well as the ability to image 3D cellular migration within scaffolds in response to local changes in their microenvironment.19–21 Studies have utilized these platforms to investigate the effects of gradients of growth factors.19 ECs were cultured in a channel in direct contact with collagen scaffolds localized in these microfluidic devices. Mass transport behavior is the exploited to create gradients within the collagen by injection of VEGF in channels surrounding the collagen scaffolds. ECs in contact with the VEGF gradient rapidly migrated into the scaffold (up the gradient), while cells in contact with control channels in the absence of VEGF gradient exhibited significantly less migration.22
There have been significant efforts to enhance EC migration and neovascularization by incorporation of growth factors within polymer scaffolds. The biological response to these materials depends on a number of factors, including which growth factors are used and their temporal and spatial presentation. In addition, the optimal approach is likely to depend on the specific tissue to be regenerated as the microcirculation varies within every tissue and organ.23 The ability to generate tissue-specific vascular structures has received little attention.
Cells
Endothelial Cells
Growth factor delivery alone may not stimulate vessel invasion rapidly enough to completely vascularize scaffolds used for the treatment of large volume wounds.23 In these conditions, the central region of the scaffolds can become necrotic or not regenerate sufficiently. The addition of ECs either individually or organized into vascular structures prior to implantation may accelerate scaffold vascularization. If the cells can form network structures and inosculate with the host circulation then, in theory, large volumes of tissues can rapidly establish a patent blood supply in a manner similar to transplanted organs and free tissue transfer.
Endothelial cells mixed with Matrigel™, a solution of basement membrane proteins, and seeded onto highly porous PLLA scaffolds assemble into microvessels within 5 days after implantation.24 These capillary-like structures inosculated with the host circulation after 14 days and recruited host derived support cells by 21 days. It is not clear if this approach would work for large scaffolds, as even the transplanted ECs are sensitive to hypoxic conditions within the scaffolds. However, the same research group showed that transfecting the cells to express an anti-apoptotic protein could enhance the survival of the transplanted ECs.25
The use of adult ECs is hindered by cell availability and altered function of cells due to co-morbidities and age in the potential patient population.26,27 Endothelial progenitor cells or embryonic stem cells offer an alternative source of cells for therapeutic applications.28,29 Human embryonic stem cells can be induced to form ECs that assemble into networks in vitro and enhance patent vascular formation in PLLA/PLGA scaffolds when implanted in vivo.28 The addition of embryonic fibroblasts to the scaffolds improves network formation and stabilization of these vessels.29
Multicellular Structures
The assembly of ECs into network structures is often enhanced due to the presence of other cells due to either the production of soluble factors30 or stabilizing cell-EC interactions31. The co-implantation of ECs and mesenchymal stem cells (MSCs) in fibronectin–type I collagen gel resulted in both a greater number and longer lasting vessels than ECs alone.31 The MSCs appeared to largely differentiate into mural cells that stabilized the vessels. The ECs still assembled into vascular networks without MSCs but little perfusion was observed. The importance of co-implantation with other cells has also been shown with fibrin gels prevascularized with fibroblasts and ECs.32 In this case, the cells were induced to form networks prior to implantation. The vascularized gels had an increase in the number of perfused vessels and decreased time required for perfusion relative to controls.
Patterning Vascular Structures
The techniques described above rely on the ability of the ECs to self assemble into networks, resulting in largely a single type of vessel with limited control over spatial distribution. As an alternative to designing scaffolds with the appropriate signals to stimulate vascularization upon implantation in vivo, vascularized tissue constructs may be fabricated in vitro. In an effort to engineer polymer scaffolds that mimic the complexity and microarchitecture of biological tissues and result in the formation of functional vascular structures, numerous micropatterning (μpatterning) techniques have been exploited such as microcontact printing (μcP), micromolding, photolithography, micromachining, and laser guided writing.
Early efforts focused on EC patterning on rigid substrates such as gold,33 silicon and Pyrex34,35. Using μcP gold substrates with adhesive patterns of fibronectin of various geometries were created.33 EC cell spreading and shape was found to be dependent on the size and geometry of the adhesive patterns. ECs cultured on single islands of fibronectin greater than 1500 μm2 entered the growth phase, whereas restriction of the cells to areas less than 500 μm2 resulted in cell apoptosis. These results suggested that local changes in cell growth and apoptosis, which are critical to the formation of tissue structures, may be generated by local changes in EC-ECM interactions. An extension of this study investigated whether defined patterned geometries resulted in cell differentiation.34 ECs cultured on 10 μm wide lines of fibronectin resulted in extensive cell-cell contacts and formed capillary tubular structures with a central lumen, whereas cells adhered to 30 μm wide lines of fibronectin resulted in EC monolayers. Thus, alterations in the geometry of cell spreading influences EC genetic programs which may be switched between growth, apoptosis, and differentiation.
Micromachining techniques have also been used to create vascular structures. Kaihara et al. formed vascular capillary networks with diameters as small as 10 μm that were etched into silicon and Pyrex surfaces that served as templates.35 ECs and hepatocytes cultured on these two-dimensional branched structures lifted off as cell monolayers and were then implanted. Upon implantation, the fabricated structures resulted in the formation of vascularized hepatic tissue. While these techniques have contributed significantly towards the creation of vascularized structures on rigid substrates their application is limited to patterning in two-dimensions (2D) with extension to 3D resulting from replication of the 2D patterns in 3D.36
In an effort to extend 2D patterning technology to 3D application, natural as well as synthetic polymer systems have been utilized as substrates. A three-dimensional printing process (3DP™) was used to create a vascularized “mini-liver” in vitro.37 Using this approach 200 μm channels were created within biodegradable copolymer scaffolds of PLLA and poly(lactic-co-glycolic acid). After five weeks in static culture, ECs attached and filled the channels. Mixed populations of hepatocytes and ECs seeded into these scaffolds demonstrated substantial reorganization and the formation of structures that appeared similar to sinusoids when cells bridged across channels. This scaffold system also demonstrated features of vessels and parenchymal space. Although this patterning technique was successful in creating hepatic sinusoids, it would be difficult to apply towards the creation of channels with smaller feature sizes (5–10 μm) similar to the dimensions of capillary networks. Another study focused on combining melt micromolding with thermal fusion bonding to create stacked, aligned, and interconnected multilayered microfluidic networks analogous to those of the microvasculature using PLGA biodegradable scaffolds.38 Channel widths ranged from 30–3000 μm with height dimensions of 35 μm. When these microfluidic networks were perfused they showed no signs of leaks or occlusions.
Patterns of vascular networks with channel widths varied from 3 mm (at the inlet and outlet) to capillary dimensions on the order of 45 μm with fixed 30 μm depth have been created within biodegradable cell adhesive elastomers of poly(glycerol sebacate).39 Vascular structures were formed by etching the patterns onto silicon wafers. These scaffolds contained inlet and outlet ports enabling perfusion. When these devices were perfused at physiological flow rates they resulted in complete endothelialization after 14 days and remained stable for 4 weeks in culture illustrating the potential of this technique for inducing vascularization in engineered tissues.
Chrobak et al. used micromolding to generate functional perfused microvascular cylindrical channels within collagen gels that demonstrated strong barrier properties after 5 days in culture and resisted leukocyte adhesion.40 Confluent EC monolayers resulted in the formation of channel diameters between 75–150 μm after maturation, with EC tube length spanning the entire collagen gel (5–7 mm). Cell induced defects of these channels were minimized by optimizing the concentration of collagen as well as the temperature required for gel formation.
Other approaches to create microvascular patterns within scaffolds with high resolution have focused on laser-induced patterning. Laser-guided direct writing (LGDW) is a patterning technique used to confine multiple cell types in a laser beam and deposit them on various surfaces including biological gels with μm accuracy allowing for direct cell patterning within the engineered tissue scaffolds.41 This approach was used to pattern ECs on Matrigel in 2D as well as in 3D multilayers. ECs elongated and formed tube-like structures along the patterns; however, stabilization and maturation of these structures into capillaries was not observed. In a later study, Chen et al. utilized biological laser printing (BioLP™) to directly deposit patterns of ECs onto Matrigel.42 Initial unconnected EC patterns resulted in interconnected patterns of different geometries suggesting that growth was achieved without surface modification and was solely a result of cell-cell interactions and cell differentiation.
Another patterning strategy that can be used to vascularize polymer scaffolds is the combination of photopolymerization of synthetic hydrogel precursors with non-contact photolithography. We used interfacial photopolymerization (IP) to produce microvascular patterns within multilayered PEG-DA hydrogels with feature sizes between 50–70 μm using vascular photomasks (Figure 2, A and B).43 IP was induced by the addition of the PEG-DA precursor solution onto a surface that was covalently immobilized with a photoinitiator (Eosin Y) followed by exposure to visible light (γ = 514 nm). This resulted in the formation of a hydrogel that grew from the surface outward with thickness controlled solely as a function of polymerization conditions without the need of spacers or molds. The addition of a PEG aminoacrylate macromer to the hydrogel precursor resulted in the formation of a hydrogel containing pendant amines onto which the eosin was covalently attached enabling the formation of covalently attached multilayers (Figure 2, C and D). This approach could be exploited to allow formation of 3D multilayered structures with distinct pattern formation in each layer.
Figure 2.
PEG hydrogel patterns formed by interfacial photopolymerization and non-contact photolithography. Vascular hydrogel patterns (A) and (B). Multilayer hydrogels with distinct pattern formation in each layer (1, 2, 3, indicate first, second and third hydrogel layers, respectively). (C) Top view (D) side view. Adapted and reprinted with permission from Papavasiliou G. et al. [43].
Building on these results, we constructed microchannels within PEG hydrogels using two photopolymerizable macromers, PEG-DA and PEG-PLLA diacrylate (PEG-PLLA-DA) (Figure 3).44 Hydrogel patterns of PEG-PLLA-DA could be formed within PEG hydrogels. Multiple layers could be created with distinct PEG-PLLA patterns. The PEG-PLLA serves as a sacrificial polymer that can be selectively degraded away after patterning due to its greater susceptibility to hydrolysis. After completion of the multilayer process, the hydrogel was incubated in a high pH environment resulting in complete degradation of the PEG-PLLA-DA hydrogel patterns. Using this approach multilayered interconnected channels were fabricated through hydrolytic degradation of PEG-PLLA-DA hydrogel patterned regions within distinct layers. The channels can be functionalized with cell adhesion sequences to support EC growth to form capillary like channels.44
Figure 3.
Microchannel generated in poly(ethylene glycol) hydrogels generated by selective degradation of patterned microstructures.
The studies described above have provided significant insight and developments into how cell interactions with polymer scaffolds can be designed to improve, or accelerate, vessel assembly. Patterning technologies are far from clinical application, but have been shown to allow precise control over the generation of vascular structures and microchannels. Regardless, whether the cells are allowed to form networks on their own or are directly patterned into distinct regions, the source of the cells that would be used for future clinical applications is not clear. Studies are needed that address the capabilities of these specific cells to form networks and inosculate with host vasculature.
Surgical Approaches
Whether using growth factors, cells, or combinations of the two to promote neovascularization in polymer scaffolds, the approaches can be very successful at generating microvascular networks in small volume scaffolds. However, clinical application for the treatment of large defects requires a more complex vessel hierarchy within larger scaffold volumes. We have approached this issue by exploiting prefabrication approaches developed in the field of reconstructive microsurgery.9,45–50 In these approaches the scaffolds are implanted in a “donor” tissue location that would promote greater neovascularization than the defect location. After a period of prefabrication time, the scaffolds with an extensive vascular network would then be transferred to the recipient site.
In one embodiment scaffolds are implanted around a vascular pedicle composing a sizable artery and vein. The pedicle allows de novo vascularization of the scaffolds that could then be transferred to the recipient defect with or without microsurgical techniques. Using a rodent vascular pedicle model, we have shown that with the same FGF-1 delivery strategy from alginate beads there is greater overall vascularization when the beads are implanted around a vascular pedicle model9 than other microcirculatory beads7,8. In addition, these newly vascularized scaffolds along with the pedicle may be transferred together using microsurgical anastomoses to the recipient vessels. It may be difficult to translate the pedicle model to clinical application, but others have shown that larger volumes of vascularized tissues can be created by implantation of materials around microsurgically created vessel loops.51–56
We have also shown that the implantation of a cell-matrix-growth factor mixture into a highly vascularized donor location in the body can be used to guide fabrication of large volumes of vascularized tissues with complex 3D shape.47 The application of prefabricated or premainated flap has been widely applied in clinical cases.57–59 This approach was successfully applied clinically where the prefabricated tissue construct with neo-vascularization was easily transferred to the recipient location.48 The use of established surgical approaches to enhance vascularization in large volume scaffolds has received little attention for applications in tissue engineering. When combined with a novel growth factor and/or cell strategy surgical techniques may help optimize the volumes of scaffolds vascularized.
Future Needs
The ability to stimulate neovascularization within polymer scaffolds is critical to the success of tissue engineering strategies for the treatment of large wounds and engineering new tissues and organs. While this issue has received significant attention in recent years, there remains a significant number of issues to overcome, including:
Generation of new vessels in large, complex volumes of tissues. Research up to this point has focused on small polymer scaffolds, which do not approach the clinical volumes needed.
While numerous efforts have focused on strategies to incorporate growth factors or cells within synthetic polymer, studies to date have not quantified how their 3D spatial presentation influences vessel assembly. These studies would significantly contribute to our understanding on how polymer scaffolds can be better designed to induce vascularization
Addressing fundamental alterations in neovascularization in targeted patient populations. While not addressed in this article, many in the population targeted for these therapeutic approaches, including the elderly27, people with diabetes60, and other co-morbidities23 have a reduced capacity for vessel assembly. These issues need to be considered directly in the development of new therapeutic approaches.
The continued development of novel approaches to these issues will likely lead to new therapeutic approaches to applications for engineering skin and other tissues.
Acknowledgments
The authors would like to acknowledge the contributions of Omaditya Khanna and Dr. Monica Moya to Figure 1 and Yu-Chieh Chiu to Figure 3. This work was supported by funds from the National Science Foundation (0852048, 0731201, and 0854430), the Veterans Administration, the National Institutes of Health (R21HL094916 and RO1 DK 080897), Chang Gung Memorial Hospital (CRPG371861) and the Taiwan National Science Council (NSC96-2314-B-182A-075-MY2).
This work was supported by funds from the National Science Foundation (0852048, 0731201, and 0854430), the Veterans Administration, the National Institutes of Health (R21HL094916 and RO1 DK 080897), Chang Gung Memorial Hospital (CRPG371861) and the Taiwan National Science Council (NSC96-2314-B-182A-075-MY2)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–80. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 2.Nikol S, Baumgartner I, Van Belle E, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16(5):972–8. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak HF, Detmar M, Claffey KP, et al. Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol. 1995;107(1–3):233–5. doi: 10.1159/000236988. [DOI] [PubMed] [Google Scholar]
- 5.Seliktar D, Zisch AH, Lutolf MP, et al. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68(4):704–16. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 6.Ehrbar M, Metters A, Zammaretti P, et al. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release. 2005;101(1–3):93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Moya ML, Garfinkel MR, Liu X, et al. Fibroblast Growth Factor-1 (FGF-1) Loaded Microbeads Enhance Local Capillary Neovascularization. J Surg Res. 2009 doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moya ML, Lucas S, Francis-Sedlak M, et al. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78(2):142–7. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Moya ML, Cheng MH, Huang JJ, et al. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31(10):2816–26. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am J Surg. 2006;192(5):604–9. doi: 10.1016/j.amjsurg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Peirce SM, Price RJ, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am J Physiol Heart Circ Physiol. 2004;286(3):H918–25. doi: 10.1152/ajpheart.00833.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cao R, Brakenhielm E, Pawliuk R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9(5):604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 13.Richardson TP, Peters MC, Ennett AB, et al. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 14.Khanna O, Moya ML, Opara EC, et al. Synthesis of multilayered alginate microcapsules for the sustained-release of fibroblast growth factor-1. J Biomed Mater Res. doi: 10.1002/jbm.a.32883. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkefors I, Le Jan S, Jakobsson L, et al. Endothelial cell migration in stable gradients of Vascular Endothelial Growth Factor A and Fibroblast Growth Factor 2 - Effects on chemotaxis and chemokinesis. J Biol Chem. 2008;283(20):13095–13912. doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Ratner BD, Sage EH, et al. Endothelial cell migration on surface-density gradients of fibronectin, VEGF, or both proteins. Langmuir. 2007;23(22):11168–11173. doi: 10.1021/la701435x. [DOI] [PubMed] [Google Scholar]
- 18.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Chung S, Sudo R, Mack PJ, et al. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9(2):269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 20.Chung S, Sudo R, Vickerman V, et al. Microfluidic platforms for studies of angiogenesis, cell migration, and cell-cell interactions. Ann Biomed Eng. 2010;38(3):1164–1177. doi: 10.1007/s10439-010-9899-3. [DOI] [PubMed] [Google Scholar]
- 21.Chung S, Sudo R, Zervantonakis IK, et al. Surface-treatment-induced three-dimensional capillary morphogenesis in a microfluidic platform. Adv Mater. 2009;21(47):4863–4867. doi: 10.1002/adma.200901727. [DOI] [PubMed] [Google Scholar]
- 22.Vickerman V, Blundo J, Chung S, et al. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8(9):1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brey EM, Uriel S, Greisler HP, et al. Therapeutic neovascularization: contributions from bioengineering. Tissue Eng. 2005;11(3–4):567–84. doi: 10.1089/ten.2005.11.567. [DOI] [PubMed] [Google Scholar]
- 24.Nor JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81(4):453–63. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 25.Nor JE, Christensen J, Liu J, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61(5):2183–8. [PubMed] [Google Scholar]
- 26.Brey EM, Greisler HP. Telomerase expression in somatic cells. Lancet. 2005;365(9477):2068–9. doi: 10.1016/S0140-6736(05)66715-3. [DOI] [PubMed] [Google Scholar]
- 27.Poh M, Boyer M, Solan A, et al. Blood vessels engineered from human cells. Lancet. 2005;365(9477):2122–4. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 28.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(7):4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 30.Ghajar CM, Blevins KS, Hughes CC, et al. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12(10):2875–88. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 31.Koike N, Fukumura D, Gralla O, et al. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428(6979):138–9. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Aledia AS, Ghajar CM, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15(6):1363–71. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CS, Mrksich M, Huang S, et al. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998;14(3):356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 34.Dike LE, Chen CS, Mrksich M, et al. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35(8):441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 35.Kaihara S, Borenstein J, Koka R, et al. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6(2):105–117. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 36.Borenstein JT, Weinberg EJ, Orrick BK, et al. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13(8):1837–44. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 37.Griffith LG, Wu B, Cima MJ, et al. In vitro organogenesis of liver tissue. Ann NY Acad Sci. 1997;831:382–397. doi: 10.1111/j.1749-6632.1997.tb52212.x. [DOI] [PubMed] [Google Scholar]
- 38.King KR, Wang CCJ, Kaazempur-Mofrad MR, et al. Biodegradable microfluidics. Adv Mater. 2004;16(2):2007–2012. [Google Scholar]
- 39.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, et al. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11(1–2):302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 40.Chrobak KM, Potter DR, J T. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71(3):185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Nahmias Y, Schwartz RE, Verfaillie CM, et al. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–136. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Barron JA, BR R. Cell patterning without chemical surface modification: Cell-cell interactions between printed bovine aortic endothelial cells (BAEC) on a homogeneous cell-adherent hydrogel. Appl Surf Sci. 2006;252(24):8641–8645. [Google Scholar]
- 43.Papavasiliou G, Songprawat P, Perez-Luna VH, et al. Three-dimensional patterning of poly(ethylene glycol) hydrogels through surface-initiated photopolymerization. Tissue Eng Part C Methods. 2008;14(2):129–140. doi: 10.1089/ten.tec.2007.0355. [DOI] [PubMed] [Google Scholar]
- 44.Chiu YC, Larson JC, Perez-Luna VH, et al. Formation of microchannels in poly(ethylene glycol) hydrogels by selective degradation of patterned microstructures. Chem Mater. 2009;21(8):1667–1682. [Google Scholar]
- 45.Uriel S, Huang JJ, Moya ML, et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29(27):3712–9. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Cheng MH, Brey EM, Allori A, et al. Ovine model for engineering bone segments. Tissue Eng. 2005;11(1–2):214–25. doi: 10.1089/ten.2005.11.214. [DOI] [PubMed] [Google Scholar]
- 47.Cheng MH, Brey EM, Allori AC, et al. Periosteum-guided prefabrication of vascularized bone of clinical shape and volume. Plast Reconstr Surg. 2009;124(3):787–95. doi: 10.1097/PRS.0b013e3181b17a91. [DOI] [PubMed] [Google Scholar]
- 48.Cheng MH, Brey EM, Ulusal BG, et al. Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast Reconstr Surg. 2006;118(1):1e–4e. doi: 10.1097/01.prs.0000221120.11128.1a. [DOI] [PubMed] [Google Scholar]
- 49.Cheng MH, Uriel S, Moya ML, et al. Dermis-derived hydrogels support adipogenesis in vivo. J Biomed Mater Res. 2009 doi: 10.1002/jbm.a.32410. In Press. [DOI] [PubMed] [Google Scholar]
- 50.Brey EM, Cheng MH, Allori A, et al. Comparison of guided bone formation from periosteum and muscle fascia. Plast Reconstr Surg. 2007;119(4):1216–22. doi: 10.1097/01.prs.0000254361.74614.bb. [DOI] [PubMed] [Google Scholar]
- 51.Hofer SO, Knight KM, Cooper-White JJ, et al. Increasing the volume of vascularized tissue formation in engineered constructs: an experimental study in rats. Plast Reconstr Surg. 2003;111(3):1186–92. doi: 10.1097/01.PRS.0000046034.02158.EB. discussion 1193–4. [DOI] [PubMed] [Google Scholar]
- 52.Mian R, Morrison WA, Hurley JV, et al. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6(6):595–603. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- 53.Mian RA, Knight KR, Penington AJ, et al. Stimulating effect of an arteriovenous shunt on the in vivo growth of isografted fibroblasts: a preliminary report. Tissue Eng. 2001;7(1):73–80. doi: 10.1089/107632700300003305. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Sung KC, Tsutsumi A, et al. Tissue engineering skin flaps: which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast Reconstr Surg. 2003;112(6):1636–44. doi: 10.1097/01.PRS.0000086140.49022.AB. [DOI] [PubMed] [Google Scholar]
- 55.Staudenmaier R, Hoang TN, Kleinsasser N, et al. Flap prefabrication and prelamination with tissue-engineered cartilage. J Reconstr Microsurg. 2004;20(7):555–64. doi: 10.1055/s-2004-836127. [DOI] [PubMed] [Google Scholar]
- 56.Demirtas Y, Engin MS, Aslan O, et al. The Effect of “Minimally Invasive Transfer of Angiosomes” on Vascularization of Prefabricated/Prelaminated Tissues. Ann Plast Surg. doi: 10.1097/SAP.0b013e31819b6c6e. [DOI] [PubMed] [Google Scholar]
- 57.Guo L, Pribaz JJ. Clinical flap prefabrication. Plast Reconstr Surg. 2009;124(6 Suppl):e340–50. doi: 10.1097/PRS.0b013e3181bcf094. [DOI] [PubMed] [Google Scholar]
- 58.Mathy JA, Pribaz JJ. Prefabrication and prelamination applications in current aesthetic facial reconstruction. Clin Plast Surg. 2009;36(3):493–505. doi: 10.1016/j.cps.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Pribaz JJ, Fine NA. Prefabricated and prelaminated flaps for head and neck reconstruction. Clin Plast Surg. 2001;28(2):261–72. vii. [PubMed] [Google Scholar]
- 60.Francis-Sedlak ME, Moya ML, Huang JJ, et al. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. doi: 10.1016/j.mvr.2009.12.005. In Press. [DOI] [PubMed] [Google Scholar]