Abstract
Background
Drug hypersensitivity syndrome (DHS) is a severe, multisystem adverse drug reaction that may occur following the use of numerous medications, including anticonvulsants, sulfonamides, and minocycline hydrochloride. Long-term autoimmune sequelae of DHS have been reported, including hypothyroidism.
Observations
A 15-year-old female adolescent developed DHS 4 weeks after starting minocycline therapy for acne vulgaris. Seven weeks later she developed autoimmune hyperthyroidism (Graves disease), and 7 months after discontinuing minocycline therapy she developed autoimmune type 1 diabetes mellitus. In addition, she developed elevated titers of several markers of systemic autoimmune disease, including antinuclear, anti-Sjögren syndrome A, and anti-Smith antibodies.
Conclusions
Minocycline-associated DHS may be associated with multiple autoimmune sequelae, including thyroid disease, type 1 diabetes mellitus, and elevated markers of systemic autoimmunity. Long-term follow-up is needed in patients with DHS to determine the natural history of DHS-associated sequelae.
Drug hypersensitivity syndrome (DHS), also known as drug reaction with eosinophilia and systemic symptoms (DRESS), is a severe, multiorgan system adverse drug reaction characterized by cutaneous eruption, fever, lymphadenopathy, eosinophilia, hepatitis, and less frequent involvement of the kidneys, lungs, and heart. Autoimmune thyroiditis is a known long-term sequela of DHS; however, other autoimmune manifestations are uncommon. We report a case of minocycline hydrochloride–associated DHS with subsequent development of autoimmune hyperthyroidism, type I diabetes mellitus, and additional serologic findings suggestive of evolving systemic autoimmunity.
REPORT OF A CASE
A 15-year-old female adolescent with no significant medical history developed fever and a diffuse erythematous skin eruption 4 weeks after initiating treatment with minocycline hydrochloride, 100 mg daily, for acne vulgaris. Minocycline therapy was discontinued, and the patient was treated with oral antihistamines for 1 week, followed by 4 days of prednisone therapy at escalating doses from 10 mg once daily to 40 mg twice daily. Despite corticosteroid treatment, she developed progressive erythroderma associated with facial swelling, pruritus, pharyngitis, and diffuse lymphadenopathy, leading to hospital admission (Figure). At the time of admission, she had an elevated white blood cell count (22 000 μL; reference range, 4800–10 800 μL [to convert to × 109/L, multiply by 0.001]) with eosinophilia (14%; reference range, 0%–6%) and reactive lymphocytosis (14%; reference range, 0%–6%). During her hospitalization, she developed elevated transaminase levels (maximum alanine aminotransferase level, 340 U/L [reference range, 10–44 U/L], and maximum aspartate aminotransferase level, 256 U/L [reference range, 0–34 U/L] [to convert both types of transaminase values to microkatals per liter, multiply by 0.0167]), hypoxia, and pyuria, consistent with DHS. A chest radiograph showed no abnormalities. Findings from viral studies were negative for cytomegalovirus, Epstein-Barr virus, toxoplasma, and herpes simplex viruses 1 and 2. The patient did not have any family history of autoimmune disease or drug hypersensitivity.
Figure.
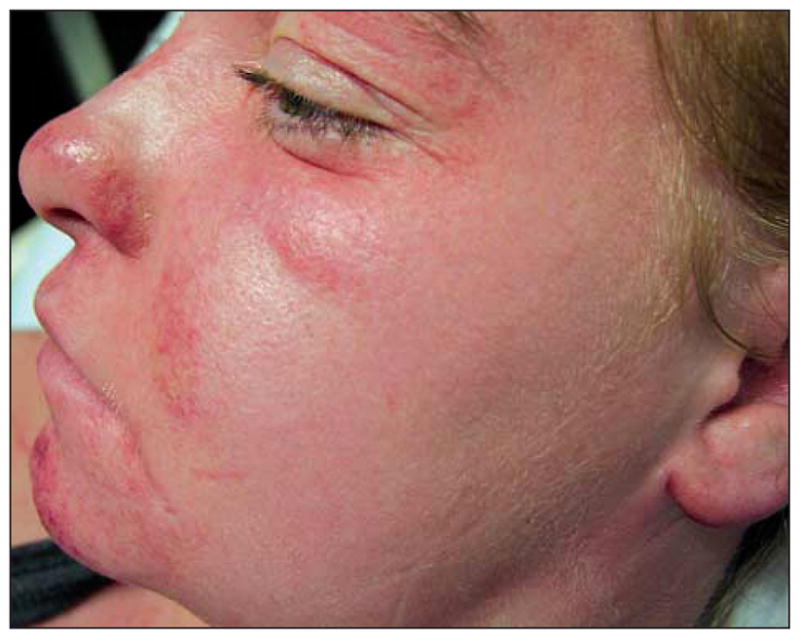
Facial erythema and edema associated with pharyngitis and diffuse lymphadenopathy consistent with drug hypersensitivity syndrome.
The patient remained hospitalized for 9 days and was treated with corticosteroids, with gradual improvement in her cutaneous symptoms, hepatitis, eosinophilia, and leukocytosis. She experienced 2 recurrences of cutaneous symptoms during attempts at corticosteroid tapering and eventually discontinued prednisone therapy 4 months after developing her first DHS symptoms. Although laboratory evaluations performed at the time of hospitalization for DHS revealed no evidence of thyroid or other autoimmune disease, over the months that followed, multiple autoimmune complications were detected.
During the hospitalization, thyroid function test results were normal and antithyroid antibodies were negative. Repeated testing 6 weeks after discharge identified low thyrotropin (TSH) and high free thyroxine (FT4) levels and markedly elevated antithyroglobulin and antithyroid peroxidase antibody titers. The patient manifested no symptoms of hyperthyroidism at that time. Markers of Graves disease (thyroid-stimulating immunoglobulin [TSI] and TSH receptor antibody) were negative, and a diagnosis of autoimmune thyroiditis in the thyrotoxic phase was made. With the exception of a 2-week period of euthyroidism, the patient remained hyperthyroid for the next several months (total triiodothyronine [T3] level, 210–320 ng/dL; reference range, 60–181 ng/dL [to convert to nanomoles per liter, multiply by 0.0154]). Five months after discontinuing minocycline therapy, she developed palpitations, irritability, and difficulty sleeping. Studies at that time identified a further increase in T3 (452 ng/dL) and FT4 (4.9 ng/dL; reference range, 1.1–1.8 ng/dL levels [to convert to picomoles per liter, multiply by 12.871]). Her TSI level also increased to 175% (reference range, <129%) and TSH receptor antibody titer increased to 65% (reference range, <10%). A revised thyroid diagnosis of Graves disease was confirmed by an increased 24-hour uptake of radioactive iodine (75%; reference range, <32%), and she was treated with radioactive iodine thyroid ablation.
Seven months after discontinuing minocycline therapy, the patient developed polydipsia and polyuria. Her fasting blood glucose level was 286 mg/dL (to convert to millimoles per liter, multiply by 0.0555), and glycosuria and ketonuria were present. Her hemoglobin A1c level was elevated at 8.1% (reference range, <5.9%), and elevated glutamic acid decarboxylase (GAD) level and IA2 antibody titer were detected. New-onset type 1 diabetes mellitus was diagnosed, and she began multiple daily injections of insulin. HLA antigen typing identified the following DRB1-DQA1-DQB1 haplotypes: (1) 0401-0303-03 and (2) 1302-0102-0604.
Serum markers of systemic autoimmune disease (anti-Ro, anti-La, anti–double-stranded DNA [dsDNA], anti-Smith, anti-Sm/RNP, antimitochondrial, anti–F-actin, and antihistone antibodies; cardiolipin IgG/M; antinuclear antibody [ANA]; antineutrophil cytoplasmic antibody; C1q binding; and complement factors C3 and C4) were initially negative during the patient’s hospitalization, 10 days after the discontinuation of minocycline therapy. However, repeated serologic testing performed 7 months after minocycline therapy’s discontinuation identified elevated ANA, anti-Smith and anti–Sjögren syndrome-A (SS-A/Ro) antibody titers. Screening for other organ-specific autoantibodies, including those associated with pernicious anemia, celiac disease, and Addison disease, was negative. Twelve months after drug exposure, the patient had not developed signs or symptoms of systemic autoimmune disease, and the anti-Smith antibody was no longer detectable.
COMMENT
Drug hypersensitivity syndrome is a rare and potentially fatal drug reaction. Although most commonly associated with anticonvulsant medications, DHS has been reported with a variety of drugs, including sulfonamides, allopurinol, antiretroviral agents (eg, nevirapine, abacavir), and minocycline.1 The etiology of DHS is unclear but has been linked to reactivation of human herpes virus 6 in several reports.2–5 In certain populations, susceptibility to drug hypersensitivity has also been linked to specific HLA haplotypes, including HLA-B*1502 and HLA-B*5801.6–8
Although minocycline is thought to have anti-inflammatory properties, the drug has been linked to numerous autoimmune phenomena, including drug-induced lupus erythematosus, autoimmune hepatitis, serum sickness–like reactions, and vasculitis.9,10 The mechanisms underlying these reactions are not well understood; proposed hypotheses include decreased production of free radicals,11 inhibition of phospholipase A2,12 and altered expression of tumor necrosis factor and interferon-γ.13
Our patient developed Graves disease, type 1 diabetes mellitus, and positive antinuclear and anti-Smith antibodies over a period of several months following minocycline exposure, suggesting a long-term immune system alteration following DHS rather than a short-term acute effect of minocycline. The delayed onset of autoimmune symptoms is unusual for minocycline-associated lupuslike reactions, which are typically observed with an elevated ANA level and arthralgia symptoms that resolve following a drug’s discontinuation.9 In contrast, our patient did not exhibit arthralgia symptoms at the time of her initial hospitalization and her ANA profile at that time was negative.
Hypothyroidism is a well-recognized but uncommon sequela of DHS (Table). In a series of 202 patients who developed hypersensitivity to anticonvulsants or sulfonamides, 5 developed hypothyroidism 4 to 8 weeks following the drug hypersensitivity reaction.20 On the basis of in vitro thyroid cell toxicity studies, the authors postulated that thyroid peroxidase metabolized the drug into reactive intermediates that damage the thyroid, followed by a secondary autoimmune process.20 Only 1 case of hyperthyroidism has been reported with minocycline use in a patient who developed minocycline-induced lupus, rather than DHS.19 Our patient developed hyperthyroidism with elevated anti–thyroid peroxidase and antithyroglobulin antibodies 7 weeks after the development of DHS, with the subsequent appearance of elevated levels of TSI and TSH receptor antibodies 8 months after the development of DHS. To our knowledge, this pattern of thyroid disease has not been previously reported with DHS and is atypical for autoimmune thyroid disease in the general population.
Table.
Reported Cases of Thyroid Disease and Diabetes Following Drug Hypersensitivity Syndrome (DHS)
| Case | Endocrinopathy | Time From DHSa | Drug Therapyb | Antibodies | Comments |
|---|---|---|---|---|---|
| 114 | Fulminant diabetes | 2 wk | Carbamazepine | Islet cell/GAD negative | |
| 215 | Fulminant diabetes | 0 d | Mexiletine | GAD negative | Preexisting type 2 diabetes mellitus, concurrent pancreatitis |
| 316 | Fulminant diabetes | 7 d | Allopurinol | Not reported | Concurrent pancreatitis |
| 417 | Fulminant diabetes | 2.5 mo | Penicillin, diclofenac, ibuprofen | Islet cell/GAD negative | |
| 518 | Type 1 diabetes mellitus | 4 mo | Methimazole | GAD positive | |
| 6 | Type 1 diabetes mellitus | 7 mo | Minocycline | GAD positive, IA2 positive | Our patient |
| 719 | Hyperthyroidism | 0 d | Minocycline | TPO, TG, TSI negative | Lupuslike syndrome, not DRESS |
| 820 | Hypothyroidism | 4 wk | Phenytoin | TPO positive | |
| 920 | Hypothyroidism | 4 wk | Salazopyrin | TPO positive | |
| 1020 | Hypothyroidism | 8 wk | Phenobarbital | TPO positive | |
| 1120 | Hypothyroidism | 8 wk | Phenobarbital, carbamazepine | unknown | |
| 1220 | Hypothyroidism | Unknown | Phenytoin, phenobarbital, carbamazepine | TPO negative | |
| 13 | Hyperthyroidism | 7 wk | Minocycline | TPO, TG, TSI, TSHR positive | Our patient |
Abbreviations: DRESS, drug reaction with eosinophilia and systemic symptoms; GAD, glutamic acid decarboxylase; TG, thyroglobulin; TPO, thyroid peroxidase; TSHR, thyrotropin-receptor; TSI, thyroid-stimulating immunoglobulin.
Time between presentation of DHS and presentation of endocrinopathy.
Minocycline administered as minocycline hydrochloride.
Rapid loss of insulin secretion (fulminant diabetes) has been described following DHS (Table).14–18 In 3 reported cases findings for anti–islet cell or glutamic acid decarboxylase (GAD) antibodies were negative, indicating that the β-cell failure was not of autoimmune origin. In one report, the presence of antibodies was not described, but the onset of diabetes was associated with severe pancreatitis, which is also suggestive of a nonautoimmune cause.16 One case has been reported of GAD-positive type 1 diabetes mellitus associated with DHS, following exposure to methimazole.18
To our knowledge, this is the first reported case of type 1 diabetes mellitus following minocycline-induced DHS. Since GAD and IA2 antibodies were not obtained in this patient early in the course of her DHS, we cannot establish the exact chronology of the elevated antibody titers against pancreatic islet antigens. Because our patient’s HLA type does not confer a markedly increased genetic risk for type 1 diabetes mellitus,21,22 and given the development of multiple other markers of autoimmunity after exposure to minocycline, we believe that her pancreatic disease is most likely an unusual autoimmune sequela of minocycline-induced DHS.
Although the patient did not fulfill American College of Rheumatology criteria for systemic lupus erythematosus, high titers of ANA and anti-Smith antibodies in the presence of autoimmune thyroid and pancreatic disease raised concern for the future development of additional autoimmune diseases. In a recent report of patients with autoimmune thyroid disease, 74.5% had an elevated anti-dsDNA titer, and 90.1% had an elevated anti–single-stranded DNA titer.23 The prevalence of systemic autoimmune disease (including systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, mixed connective tissue disease, Sjögren syndrome, and polymyositis/dermatomyositis) has been reported to be as high as 51% in patients with Hashimoto thyroiditis and 16% of those with Graves disease.24 An increased prevalence of positive ANA status has also been reported in patients with acne, and higher ANA titers have been associated with minocycline exposure.25 To our knowledge, the presence of anti-Smith antibodies, which are highly specific for systemic lupus erythematosus, have not been reported in the setting of minocycline-induced lupus.26 The significance of the transient elevation of anti-Smith antibody in our patient is not clear but may indicate components of autoimmunity that may finally be diminishing.
In conclusion, long-term autoimmune sequelae may develop following DHS, including hypothyroidism and hyperthyroidism and diabetes. Long-term monitoring of autoimmune markers in patients with minocycline-associated DHS will help establish the natural history of this disorder.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Dr Cowen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Brown and Cowen. Acquisition of data: Mercurio, Wang, and Looney. Analysis and interpretation of data: Brown, Rother, Artman, Mercurio, Wang, Looney, and Cowen. Drafting of the manuscript: Brown, Wang, and Cowen. Critical revision of the manuscript for important intellectual content: Rother, Artman, Wang, and Looney. Administrative, technical, and material support: Wang.
Additional Contributions: This research was supported in part by the Intramural Research Programs of the National Institutes of Health, Center for Cancer Research, National Cancer Institute, and National Institute of Diabetes, Digestive, and Kidney Diseases.
References
- 1.Peyriere H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155(2):422–428. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- 2.Kano Y, Inaoka M, Sakuma K, Shiohara T. Virus reactivation and intravenous immunoglobulin (IVIG) therapy of drug-induced hypersensitivity syndrome. Toxicology. 2005;209(2):165–167. doi: 10.1016/j.tox.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Kano Y, Inaoka M, Shiohara T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. 2004;140(2):183–188. doi: 10.1001/archderm.140.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55(1):1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 5.Wong GA, Shear NH. Is a drug alone sufficient to cause the drug hypersensitivity syndrome? Arch Dermatol. 2004;140(2):226–230. doi: 10.1001/archderm.140.2.226. [DOI] [PubMed] [Google Scholar]
- 6.Chung WH, Hung SI, Chen YT. Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2007;7(4):317–323. doi: 10.1097/ACI.0b013e3282370c5f. [DOI] [PubMed] [Google Scholar]
- 7.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 8.Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichenfield AH. Minocycline and autoimmunity. Curr Opin Pediatr. 1999;11(5):447–456. doi: 10.1097/00008480-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol. 2007;157(3):540–546. doi: 10.1111/j.1365-2133.2007.08056.x. [DOI] [PubMed] [Google Scholar]
- 11.Miyachi Y, Yoshioka A, Imamura S, Niwa Y. Effect of antibiotics on the generation of reactive oxygen species. J Invest Dermatol. 1986;86(4):449–453. doi: 10.1111/1523-1747.ep12285793. [DOI] [PubMed] [Google Scholar]
- 12.Pruzanski W, Greenwald RA, Street IP, Laliberte F, Stefanski E, Vadas P. Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline. Biochem Pharmacol. 1992;44(6):1165–1170. doi: 10.1016/0006-2952(92)90381-r. [DOI] [PubMed] [Google Scholar]
- 13.Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, et al. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40(4):934–940. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekine N, Motokura T, Oki T, et al. Rapid loss of insulin secretion in a patient with fulminant type 1 diabetes mellitus and carbamazepine hypersensitivity syndrome. JAMA. 2001;285(9):1153–1154. doi: 10.1001/jama.285.9.1153. [DOI] [PubMed] [Google Scholar]
- 15.Seino Y, Yamauchi M, Hirai C, et al. A case of fulminant type 1 diabetes associated with mexiletine hypersensitivity syndrome. Diabet Med. 2004;21(10):1156–1157. doi: 10.1111/j.1464-5491.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- 16.Sommers LM, Schoene RB. Allopurinol hypersensitivity syndrome associated with pancreatic exocrine abnormalities and new-onset diabetes mellitus. Arch Intern Med. 2002;162(10):1190–1192. doi: 10.1001/archinte.162.10.1190. [DOI] [PubMed] [Google Scholar]
- 17.Chiou CC, Chung WH, Hung SI, Yang LC, Hong HS. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J Am Acad Dermatol. 2006;54(2 suppl):S14–S17. doi: 10.1016/j.jaad.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki N, Miura Y, Oiso Y. A case of type 1 diabetes followed by methimazole-induced hypersensitivity syndrome. Diabetes Care. 2006;29(5):1179–1180. doi: 10.2337/diacare.2951179. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin RW, Calikoglu AS. Hyperthyroidism and lupus-like syndrome in an adolescent treated with minocycline for acne vulgaris. Pediatr Dermatol. 2007;24(3):246–249. doi: 10.1111/j.1525-1470.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Eggo MC, Uetrecht JP, et al. Drug-induced hypothyroidism: the thyroid as a target organ in hypersensitivity reactions to anticonvulsants and sulfonamides. Clin Pharmacol Ther. 1992;51(1):56–67. doi: 10.1038/clpt.1992.8. [DOI] [PubMed] [Google Scholar]
- 21.Undlien DE, Friede T, Rammensee HG, et al. HLA-encoded genetic predisposition in IDDM: DR4 subtypes may be associated with different degrees of protection. Diabetes. 1997;46(1):143–149. doi: 10.2337/diab.46.1.143. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Kobayashi T, Nakanishi K, et al. Association of HLA-DQ genotype in autoantibody-negative and rapid-onset type 1 diabetes. Diabetes Care. 2002;25(12):2302–2307. doi: 10.2337/diacare.25.12.2302. [DOI] [PubMed] [Google Scholar]
- 23.Pedro AB, Romaldini JH, Americo C, Takei K. Association of circulating antibodies against double-stranded and single-stranded DNA with thyroid autoantibodies in Graves’ disease and Hashimoto’s thyroiditis patients. Exp Clin Endocrinol Diabetes. 2006;114(1):35–38. doi: 10.1055/s-2005-873005. [DOI] [PubMed] [Google Scholar]
- 24.Biró E, Szekanecz Z, Czirjak L, et al. Association of systemic and thyroid auto-immune diseases. Clin Rheumatol. 2006;25(2):240–245. doi: 10.1007/s10067-005-1165-y. [DOI] [PubMed] [Google Scholar]
- 25.Marzo-Ortega H, Baxter K, Strauss RM, et al. Is minocycline therapy in acne associated with antineutrophil cytoplasmic antibody positivity? a cross-sectional study. Br J Dermatol. 2007;156(5):1005–1009. doi: 10.1111/j.1365-2133.2007.07828.x. [DOI] [PubMed] [Google Scholar]
- 26.Benito-Garcia E, Schur PH, Lahita R. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests. Arthritis Rheum. 2004;51(6):1030–1044. doi: 10.1002/art.20836. [DOI] [PubMed] [Google Scholar]


