Abstract
Common components of whole-cell internal recording solutions were tested both in vitro and in patch-clamp experiments for their effects on the activity of cAMP-dependent protein kinase. Potassium fluoride (KF), 440 mM trimethylamine chloride and exclusion of bovine serum albumin (BSA) decreased the activity of the enzyme, while ethylene glycol-bis (β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA) and the potassium salts of aspartate, gluconate, methylsulfate and monobasic phosphate increased its activity. Addition of KF to the internal solution produced a hyperpolarizing shift in the V1/2 of Ih channel activation, consistent with the KF-induced reduction of protein kinase A activity. Therefore, consideration of the composition of internal solutions is warranted when studying channel physiology by patch-clamp techniques.
Keywords: Ion channel, Second messenger, Phosphorylation, Whole-cell voltage-clamp
Second messenger systems often regulate neuronal excitability by modulating the properties of voltage-gated ion channels. This neuromodulation usually involves phosphorylation of specific residues in the ion channel protein. While phosphorylation can be mediated by several protein kinases, the cAMP-dependent protein kinase (PKA) is frequently responsible for channel protein phosphorylation and subsequent regulation of neuronal function. For example, PKA phosphorylation of voltage-gated ion channels such as neuronal Na+ channel α subunits [3,11], the delayed rectifier-type K+ channel [2,6,15], the minK–KvLQT channel complex [1] and the L-type Ca2+ channels [5,18] have been extensively characterized. The hyperpolarization-activated (Ih) channel is also modulated by PKA [4,17] and a consensus PKA phosphorylation site is present in some of the gene products encoding this family of channels [14]. The PKA-dependent modulation of ion channels is not limited to voltage-gated channels; ligand-gated channels are also modulated. For example, PKA-dependent phosphorylation is required to maintain the function of ligand-gated AMPA/kainate channels in hippocampal neurons [7,13,21].
Due to the important role of second messenger-mediated protein phosphorylation in the regulation of nerve cell activity, many studies are performed to test how ion channel phosphorylation affects neuronal properties. Most experiments studying ion channel and neuronal properties are performed using standard whole-cell voltage-clamp recording techniques [9]; however, different results have been observed depending on the components of the internal solutions [8,16]. To date, no studies have been done to independently assess how the function of endogenous cellular protein kinases, in particular PKA, are altered by components of an internal recording solution. Therefore, the purpose of this study was to examine the effects of several common components of internal whole-cell recording solutions on the in vitro activity of PKA. In addition, we compared the voltage dependence of activation of the Ih channel in whole-cell recordings using two internal solutions that differed in their major anionic component. Our results show that the activity of PKA can be significantly altered by the composition of the whole-cell electrode recording solution. Thus, careful attention to the composition of the internal solution is required when studying ion channel physiology and modulation by kinases to ensure that kinase activities are not significantly affected.
The activity of the catalytic subunit of mouse PKA (provided by Dr. Susan Taylor, UCSD) was measured by monitoring incorporation of radioactive phosphate into the synthetic PKA substrate, kemptide (LeuArgArgAla-SerLeuGly) [12], using a P81 phosphocellulose filter paper assay [19]. Phosphorylation reactions (50 µl total volume) were performed in 0.2 ml conical bottom polypropylene thin-wall PCR tubes (8-strip format). Reaction mixtures consisted of one of the test compounds, 50 mM 4-morpho-linpropanesulfonic acid (MOPS), 1 mM dithiothreitol (DDT), 5 mM magnesium acetate, 1 mg ml−1 bovine serum albumin (BSA), 0.16 µg PKA, and 540 µM kemptide, pH 7.0. The compounds tested were: (in mM) 125 potassium fluoride (KF), 125 K-aspartate (KAsp), 125 K-gluconate (KGluc), 125 K-methylsulfate (KMeSO4, ICN Biomedicals, Irvine, CA), 62.5 K-phosphate monobasic (KH2PO4, Mallinckrodt AR; Paris, KY), 125 cesium chloride (CsCl, Aldrich Chemical, Milwaukee, WI), 62.5 trimethylamine n-oxide (TMA oxide), 440 trimethylamine chloride (TMA-Cl), 10 HEPES, 1 glutathione, 5 tetraethyl-ammonium (TEA), and 2 ethylene glycol-bis (β-aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA) + 0.5 calcium chloride (CaCl2). The differences in osmolality or ionic strength of the reaction mixture resulting from the addition of these different compounds were not corrected for. In addition, for one set of experiments, the BSA was omitted from the reaction mixture (indicated as no BSA). All chemicals were obtained from Sigma (St. Louis, MO) unless stated otherwise.
The reaction mixtures were prepared fresh and kept on ice before use. Five minutes before starting the reactions, tubes containing the reaction mixtures were transferred to a 30°C water bath. Reactions were started by the addition of 20 µl of [γ-32P] ATP (250 µM final concentration, specific activity ranged from 1280 to 374 c.p.m. pmol−1, NEN Life Science Products, Boston, MA) using an adjustable 8-channel multipipettor. Samples of each reaction (10 µl) were removed at 2, 5, 10 and 20 min with an 8-channel pipettor and spotted on an 8 × 12 cm sheet of P81 phosphocellulose paper (Whatman) mounted in a 96-well Miniblot I microsample filtration manifold (Schleicher and Schuell; Keene, NH). Immediately after spotting the reaction samples on the P81 paper, 200 µl of 75 mM phosphoric acid was added to each well of the filtration apparatus and allowed to soak through the P81 paper. After the last reaction samples were taken (at 20 min), the sheet of P81 paper was removed from the filtration apparatus and placed in 500 ml of 75 mM phosphoric acid. The sheet was washed in 75 mM phosphoric acid a total of four times (15 min each wash) with gentle stirring. After all the washes were completed, the sheet was briefly soaked in 95% ethanol and dried under a stream of warm air. The sheet was then placed in a FlexiFilter tray and carrier system (Packard Instrument, Meriden, CT), and 10 µl of MicroScint-O (Packard Instrument) scintillation cocktail was added to each well. The top of the FlexiFilter tray was covered with a clear adhesive plastic film and counted in a TopCount scintillation counter (Packard Instrument) configured for counting 96-well microtiter plates.
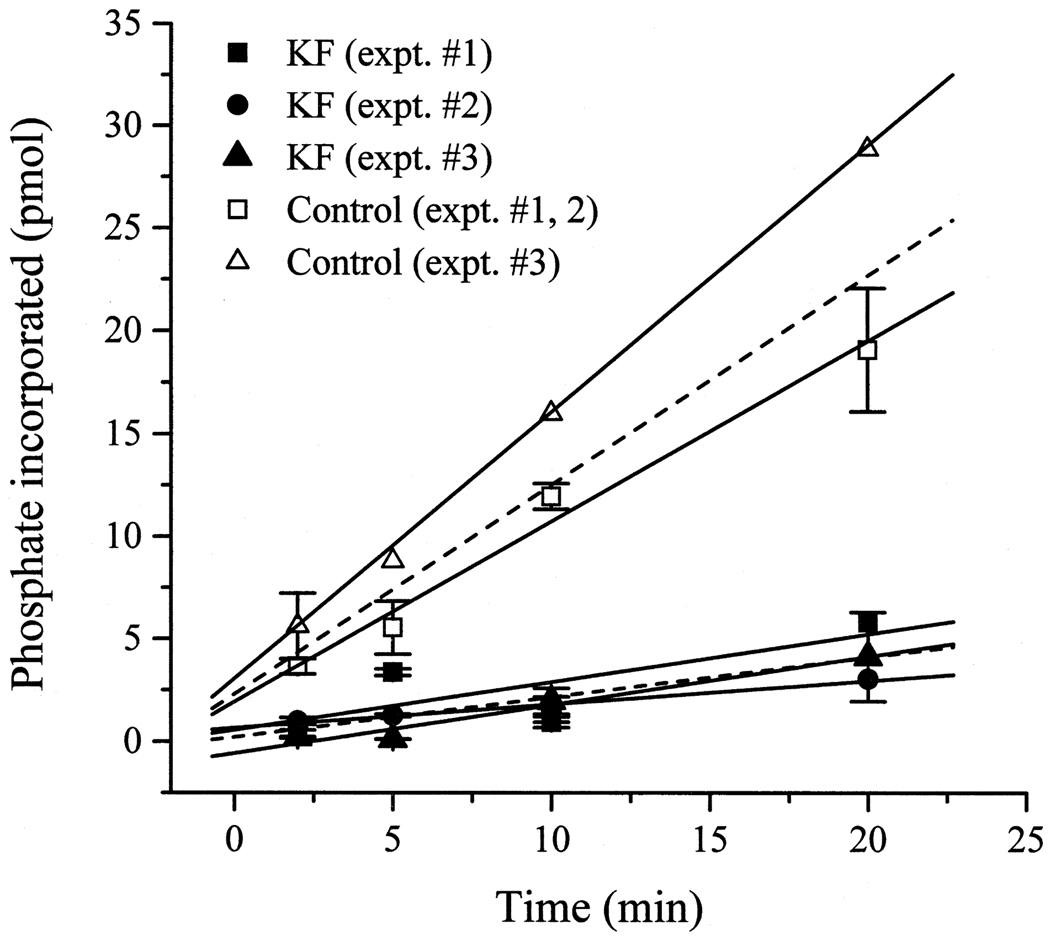
For each of the test compounds, three experiments were performed. Each experiment consisted of a control (reaction mixture without the test compound), the experimental reaction mixture and the corresponding blanks (reaction mixture without the enzyme). At every time point assayed (2, 5, 10 and 20 min), two samples were taken. Back-ground counts (blanks) were subtracted from the control and experimental counts. For each separate experiment, the control and experimental counts obtained for each time point were converted to picomoles of phosphate incorporated into the kemptide (according to the specific activity of the [γ-32P] ATP on the day of the experiment) and averaged across the two samples. An example of the time course of phosphate incorporation under control conditions and in the presence of KF is presented in Fig. 1. Linear regression analysis was performed to obtain the rate of incorporation under control and experimental conditions for each of the three experiments (solid lines, Fig. 1). The experimental rates were expressed as percent of their corresponding control rates. For each test compound, the results were averaged across the three experiments, and are reported as mean ± S.E.M. (n = 3; Fig. 2A–C). Statistical comparisons were made using Student’s t-test and p values < 0.05.
Fig. 1.
Phosphate incorporation into kemptide over time under control and experimental (125 mM KF) conditions. The activity of the catalytic subunit of mouse PKA was measured under control conditions and in the presence of 125 mM KF in three separate experiments (expt. #1, 2 and 3). For each experiment, two samples were assayed at each time point. Counts obtained for each sample were converted to picomoles of phosphate incorporated and averaged across the two samples. Experiments 1 and 2 were run simultaneously; therefore, one control (open square) was run for both experiments. Error bars indicate S.E.M. For each experiment, results were fit by linear regression analysis (solid lines) to obtain the rate of incorporation under control conditions and in the presence of KF. Results obtained from the three experiments were averaged to obtain a mean rate of incorporation (dashed lines). The incorporation of phosphate into kemptide increased linearly over 20 min with a mean rate of incorporation of 6.38 ± 0.49 pmol min−1 µg−1 PKA and 1.19 ± 0.38 pmol min−1 µg−1 PKA under control conditions and in the presence of KF, respectively.
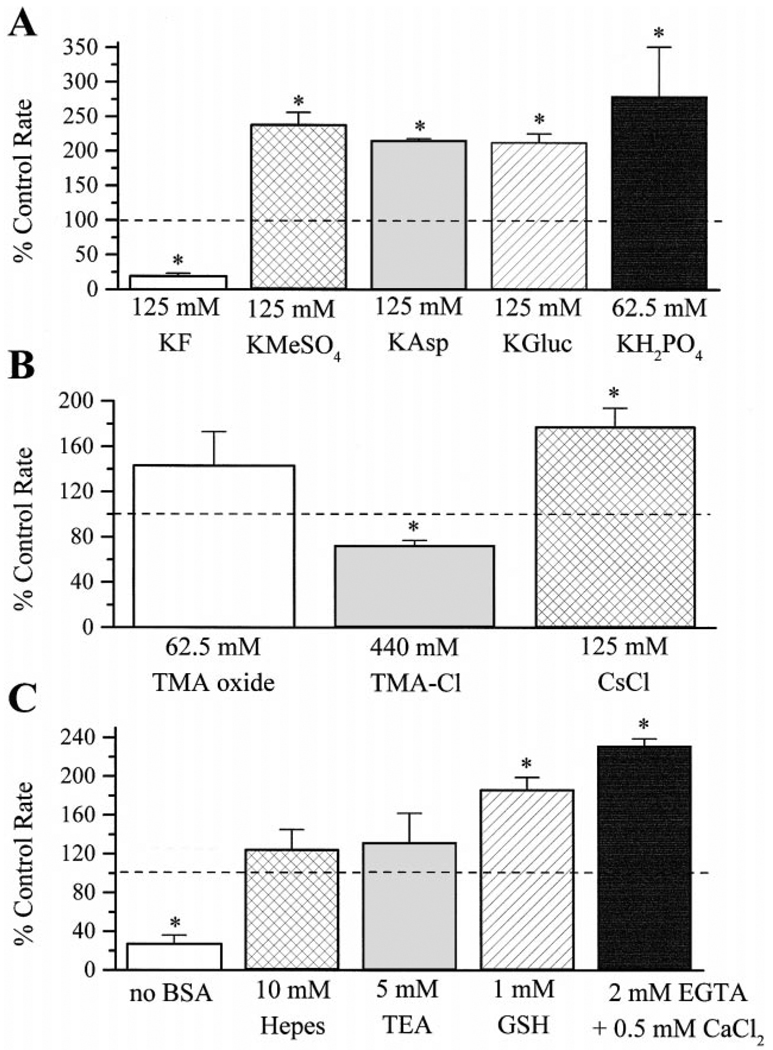
Fig. 2.
Effects of commonly used components of patch-clamp internal recording solutions on the activity of cAMP-dependent protein kinase. The effects of: (A) 125 mM KF, 125 mM KMeSO4, 125 mM KAsp, 125 mM KGluc and 62.5 mM KH2PO4; (B) 62.5 mM TMA oxide, 440 mM TMA-Cl and 125 mM CsCl; and (C) 10 mM HEPES, 5 mM TEA, 1 mM glutathione (GSH), 2 mM EGTA + 0.5 mM CaCl2 and omission of BSA from the reaction mixture on the rate of phosphate incorporation into kemptide were investigated. The rate of phosphorylation was expressed as percent of the corresponding control rate and averaged across the three replicates obtained for each experimental condition. Results shown represent mean ± S.E.M. Asterisks indicate significance at p < 0.05. Dashed lines indicate 100% of control level.
Standard patch-clamp whole-cell recording techniques [9] were performed to study the effects of two different internal recording solutions, on the hyperpolarization-activated Ih channel in cultured rat olfactory receptor neurons (ORNs). Cell culture and voltage-clamp experiments were performed and analyzed as described previously [20]. The recording solutions tested were (in mM): (A) KF internal solution: 125 KF, 11 EGTA; and (B): KH2PO4 internal solution: 62.5 KH2PO4, 62.5 TMA oxide, 62.7 KOH, 0.5 CaCl2, 2 EGTA. Both solutions also contained (in mM) 15 KCl, 10 HEPES, 5 MgCl2, 2 NaATP, 1 glutathione, 5 TEA, pH 7.2, 305–310 mosM.
The activity of PKA was measured in the presence of different components commonly used in patch-clamp recording internal solutions. The test compounds were added to the PKA reaction mixture over a 20 min time course and phosphorylation rates were obtained as described above. Addition of 125 mM KF to the reaction mixture produced an 80 ± 4% decrease in the rate of phosphate incorporation into kemptide (Fig. 2A). In contrast to the inhibition observed with KF, addition of other anions to the reaction mixture produced the opposite effect: 125 mM KMeSO4, 125 mM KAsp, 125 mM KGluc and 62.5 mM KH2PO4 produced a significant 139 ± 17%, 116 ± 2%, 113 ± 12% and 180 ± 71% increase over their corresponding control rate of phosphorylation, respectively (Fig. 2A).
The effect of cation components of patch-clamp internal recording solutions on the activity of PKA was also tested. Experiments were performed as described above and results are shown in Fig. 2B. Addition of 62.5 mM TMA oxide to the reaction mixture produced no significant effect on the rate of phosphate incorporation into kemptide, while addition of 440 mM TMA-Cl produced a 27 ± 4% reduction in the rate of phosphorylation. The effect of 125 mM CsCl was also tested. CsCl produced a 78 ± 16% increase in the rate of phosphate incorporation into kemptide.
Calcium chelating agents, antioxidants and a variety of buffers are also routinely included in patch-clamp recording solutions. The effects of typical examples of these reagents were tested on the activity of PKA, and results are shown in Fig. 2C. The effect of 2 mM EGTA, in combination with 0.5 mM CaCl2, was tested as an example of a Ca2+ chelating agent. When EGTA was tested in combination with CaCl2, a 132 ± 7% increase in the rate of phosphate incorporation was observed. The buffer and antioxidant tested were 10 mM HEPES and 1 mM glutathione, respectively. HEPES produced no significant change in the rate of phosphorylation when compared to its corresponding control, while glutathione produced an 87 ± 12% increase in the rate of phosphate incorporation. In addition, the effect of 5 mM TEA, a K+ channel blocker frequently used in internal recording solutions, was tested. TEA produced no significant change in the rate of phosphate incorporation when compared to its control. In a separate set of experiments, the effect of omitting BSA from the reaction mixture was tested on the activity of PKA. Addition of BSA (or another carrier protein) to the reaction mixture was necessary to protect the enzyme from denaturation and maintain its activity in vitro. When BSA was not included in the reaction mixture, a 73 ± 9% decrease in the rate of phosphorylation was observed.
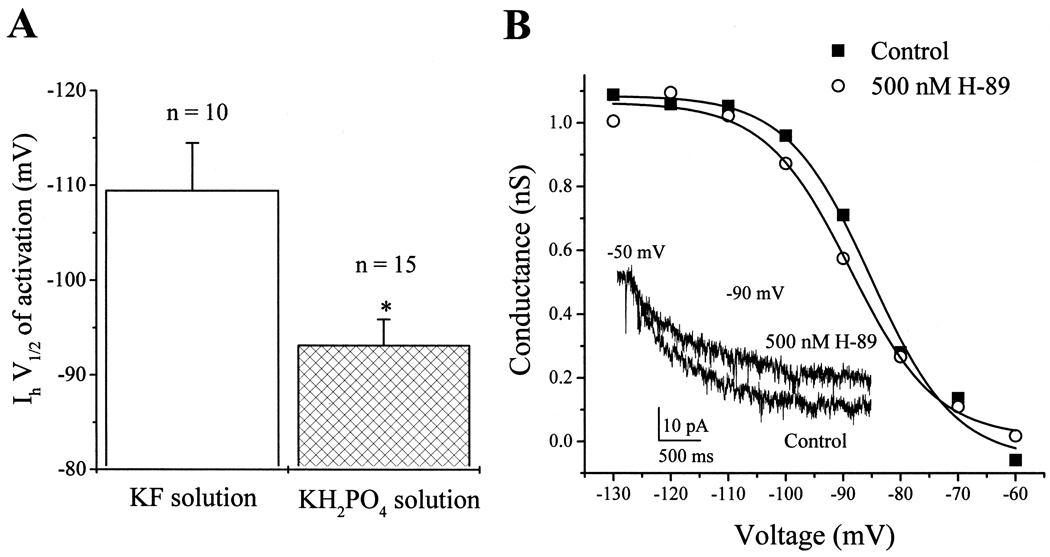
To examine if the changes in PKA activity observed in the in vitro assays can result in changes of ion channel properties during patch-clamp experiments, we studied the effects of two different internal solutions on the hyperpolarization-activated Ih channel in cultured rat ORNs. Ih is modulated by PKA-dependent phosphorylation: inhibition of protein kinase activity results in a hyperpolarizing shift in the voltage dependence of Ih activation (V1/2) in Purkinje cells [4]. Since KF and KH2PO4 produced profound decreases and increases in the in vitro activity of PKA respectively, we compared the V1/2 of Ih activation in rat ORNs when KF and KH2PO4 internal solutions were used during whole-cell voltage-clamp experiments. The average V1/2 for Ih recorded with KF and KH2PO4 internal solution was −109 ± 5 mV and −93 ± 3 mV (mean ± S.E.M.), respectively (Fig. 3A). The 16 mV hyperpolarizing shift in the voltage dependence of Ih activation observed with the KF internal solution is consistent with an inhibition of protein kinase activity. To directly evaluate if inhibition of PKA in rat ORNs results in a hyperpolarizing shift in the V1/2 of Ih activation, we tested the effect of H-89, a PKA inhibitor, on Ih. External application of 500 nM H-89 to a cell produced a 4 mV hyperpolarizing shift in the V1/2 of Ih activation (Fig. 3B) which resulted in a 17% reduction of Ih peak current amplitude at −90 mV (Fig. 3B inset). Similar effects were observed in three other cells. Collectively, the results presented in Fig. 3 show that the negative shift in the V1/2 of Ih activation observed with the KF internal solution was due to an inhibition of PKA activity. These results also demonstrate that the properties of ion channels can be altered by components of internal recording solutions during patch-clamp experiments. A similar dependence of ion channel properties on the components of internal solutions has been described previously for glycine-activated Cl− channels. In this case, addition of KF to the internal solution blocked the PKA-mediated run-up of glycine currents, while substitution of KF for Cs-methanesulphonate preserved the run-up of the currents [8,16]. Although, many recent studies investigating the physiology and modulation of ion channels are performed using perforated-patch recording techniques, the type of pore-former should be considered if KF is included in the internal solution. For example, nystatin perforated-patch recordings [10] will not prevent the KF-induced reduction in PKA activity, since KF can permeate the nystatin pores.
Fig. 3.
Voltage dependence of Ih channel activation in rat ORNs is altered by PKA inhibition. (A) The average V1/2 of Ih activation was obtained for two groups of cells in which Ih was studied by standard patch-clamp recordings using either a KF (n = 10 cells) or a KH2PO4 internal solution (n = 15 cells). The presence of KF in the internal solution produced a 16 mV hyperpolarizing shift in the V1/2 of Ih activation, consistent with an inhibition of PKA activity. Results shown represent mean ± S.E.M. Asterisks indicate significance at p < 0.05. (B) Conductance–voltage relationships for a cell recorded under control conditions (solid squares, V1/2 = −85 mV) and when the cell was perfused with 500 nM H-89 (PKA inhibitor, open circles, V1/2 = −89 mV). H-89 produced a 4 mV hyperpolarizing shift in the V1/2 and a decrease in the peak current activated at −90 mV (inset).
While our assay does not provide direct insights on the mechanisms involved in the effects reported here, nonspecific effects that could aid in the stability of the enzyme can be postulated. Such mechanisms may include antioxidant effects (glutathione), chelation of particular metal ions (EGTA) or minimization of PKA denaturation (BSA). Even though the differences in osmolality and ionic strength between the different reactions mixtures were not corrected for, the wide range of osmolar and ionic concentrations (1 mM to 125 mM) producing different effects in the activity of the enzyme argue against a simple effect due to varying osmolality or ionic concentration. The differences in osmolality and/or ionic strength appear to have an effect only when very high osmolar and/or ionic concentrations were used (440 mM TMA-Cl). The results from these experiments provide important information that should be taken in consideration when using standard patch-clamp whole-cell recording techniques to study the effects of PKA, and probably other enzymes, on the function and physiology of ion channels.
Acknowledgements
The authors wish to thank Dr. Susan Taylor (University of California, San Diego) for providing the recombinant catalytic subunit of PKA used in these experiments. This work was supported by a Ford Foundation Predoctoral Fellowship to G. Vargas, a NIH NIDCD R01 #DC002994-02 to M.T. Lucero and a University of Utah Research Foundation Funding Incentive Seed Grant to D.K. Blumenthal.
References
- 1.Blumenthal EM, Kaczmarek LK. Modulation by cAMP of a slowly activating potassium channel expressed in Xenopus oocytes. J. Neurosci. 1992;12:290–296. doi: 10.1523/JNEUROSCI.12-01-00290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosma MM, Allen ML, Martin TM, Tempel BL. PKA-dependent regulation of mKv1.1, a mouse shaker-like potassium channel gene, when stably expressed in CHO cells. J. Neurosci. 1993;13:5242–5250. doi: 10.1523/JNEUROSCI.13-12-05242.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel α subunit. J. Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang F, Cohen IS, DiFrancesco D, Rosen MR, Tromba C. Effects of protein kinase inhibitors on canine purkinje fibre pacemaker depolarization and the pacemaker current, if. J. Physiol. (Lond) 1991;440:367–384. doi: 10.1113/jphysiol.1991.sp018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 6.Giles W, Nakajima T, Ono K, Shibata EF. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J. Physiol.(Lond) 1989;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Huang LYM. Cross-modulation of glycine-activated Cl− channels by protein kinase C and cAMP-dependent protein kinase in the rat. J. Physiol.(Lond) 1998;506:331–339. doi: 10.1111/j.1469-7793.1998.331bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell free patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 10.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, West JW, Lai Y, Scheuer T, Catterall WA. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- 12.Pearson RB, Kemp BE. Protein kinase phosphorylation site sequence and consensus specific motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 13.Rosenmund C, Carr DW, Bergeson SE, Gajanan N, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 14.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- 15.Soliven B, Nelson DJ. Beta-adrenergic modulation of K+ current in human T lymphocytes. J. Membr. Biol. 1990;117:263–274. doi: 10.1007/BF01868456. [DOI] [PubMed] [Google Scholar]
- 16.Song Y, Huang LYM. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990;348:242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- 17.Tokimasa T, Akasu T. Cyclic AMP regulates an inward rectifying sodium–potassium current in dissociated bull-frog sympathetic neurones. J. Physiol. 1990;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trautwein W, Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G-proteins. Annu. Rev. Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- 19.Underwood CJ, Blumenthal DK. Adaptation of the protein kinase filter paper assay to a 96-well microtiter format. Anal. Biochem. 1999;267:235–238. doi: 10.1006/abio.1998.2985. [DOI] [PubMed] [Google Scholar]
- 20.Vargas G, Lucero MT. Dopamine modulates the inwardly-rectifying hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J. Neurophysiol. 1999;81:149–158. doi: 10.1152/jn.1999.81.1.149. [DOI] [PubMed] [Google Scholar]
- 21.Wang L-Y, Salter MW, MacDonald JF. Regulation of kainate receptor by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]