Abstract
Rationale
Activation of metabotropic glutamate (mGlu) 2/3 receptors may provide a novel strategy for treating schizophrenia. This effect is thought to be mediated through dopamine-independent mechanisms because mGlu2/3-receptor agonists have no considerable affinity for dopamine receptors. These agonists, however, reduce amphetamine-induced hyperlocomotion suggesting that they influence dopamine neurotransmission.
Objective
We evaluated whether the inhibitory effect of mGlu2/3-receptor activation on amphetamine-induced hyper-locomotion correlates with attenuated dopamine release. We also assessed whether mGlu 2/3 receptor activation has inhibitory effects on activity-dependent vesicular release of dopamine in behaving animals.
Methods
Microdialysis was used to measure extracellular levels of dopamine in the dorsal striatum (DStr) and nucleus accumbens (NAc) of freely moving rats. The effect of the mGlu2/3-receptor agonist LY354740 on dopamine release and locomotion elicited by amphetamine, electrical stimulation of the ventral tegmental area, or L-dopa was assessed.
Results
We find that the inhibitory effect of mGlu2/3 activation on amphetamine-induced hyperlocomotion correlates with an attenuated increase in dopamine release in the NAc and DStr. However, when dopamine levels were increased by electrical stimulation of dopamine neurons or by administration of the dopamine precursor L-dopa, activation of mGlu2/3 receptors had no effect on dopamine release or on behavior.
Conclusions
Activation of mGlu2/3 receptors attenuates amphetamine-induced dopamine release through a mechanism that does not affect activity dependent vesicular release, reuptake or synthesis of dopamine.
Keywords: Schizophrenia, Antipsychotic, Microdialysis, Nucleus accumbens, Striatum, Amphetamine
Introduction
The metabotropic glutamate (mGlu) receptors are a relatively novel family of eight receptor subtypes that are categorized into three subgroups based on sequence homology and signal transduction mechanisms. Group II mGlu receptors, consisting of mGlu receptors two and three, are widely expressed throughout the brain (Richards et al. 2005) and have been shown to reduce presynaptic neurotransmitter release through inhibition of adenylyl cyclase (AC) activity (Dohovics et al. 2003; Kilbride et al. 1998). Agonists of mGlu 2/3 receptors recently have received attention as potential novel targets for treatment of schizophrenia (Patil et al. 2007). Pharmacological activation of mGlu 2/3 receptors ameliorates some of the behavioral and cellular characteristics of the NMDA hypofunction model of this disease. These include reducing NMDA antagonist-induced hyperlocomotion and stereotypy in rodents (Moghaddam and Adams 1998;Cartmell et al. 1999; Homayoun et al. 2004; Rorick-Kehn et al. 2007;Schlumbergeret al. 2009b;Uslaner et al. 2009) and impairments in working memory in rodents and humans (Moghaddam and Adams 1998; Krystal et al. 2005). In addition, mGlu 2/3 agonists normalize changes in cortical glutamate efflux (Moghaddam and Adams 1998; Lorrain et al. 2003) and single-unit spiking activity by NMDA antagonism (Homayoun et al. 2004).
Although the potential therapeutic effects of mGlu2/3 agonists are generally considered to be independent of dopaminergic mechanisms, several recent studies have demonstrated that activation of mGlu2/3 receptors attenuates the locomotor stimulating effects of amphetamine (Cartmell et al. 1999, 2000; Fell et al. 2008; Rorick-Kehn et al. 2007; Uslaner et al. 2009; Woolley et al. 2008) and blocks the expression of locomotor sensitization to amphetamine (Kim and Vezina 2002). These data may suggest a dopaminergic involvement in the therapeutic effects of these drugs, and there currently is some controversy over whether mGlu2/3 agonists such as LY354740 and LY379268 have activity as partial agonists at the dopamine D2 receptor (Seeman et al. 2008; but see Fell et al. 2009).
Amphetamine increases extracellular levels of dopamine (and other monoamines) by blocking vesicular transport, as well as reversing the direction of the dopamine transporter (Sulzer et al. 1995; Sulzer et al. 2005). This increase is thought to be responsible for the behavioral activation by amphetamine because locomotor-enhancing effects of amphetamine in rodents are blocked by dopamine-receptor antagonists (Lapin 1995; O'Neill and Shaw 1999). In the case of mGlu2/3 agonist's inhibitory effects on amphetamine, however, the correlation between its behavioral and neurochemical effects, and whether the inhibitory influence generalizes to other hyper-dopamine states, remains largely unknown. The purpose of the current study was to assess whether mGlu 2/3 activation attenuates the effect of amphetamine administration on dopamine release in the NAc and DStr. We also sought to determine whether any effects of mGlu 2/3 activation on dopamine release are specific to impulse flow independent dopamine release mechanisms, such as that caused by amphetamine (Sulzer et al. 2005), or generalize to activity dependent, vesicular dopamine release mechanisms.
Material and methods
Subjects
Adult male Sprague–Dawley rats (n=62, 300–370 g, Harlan Labs, St. Albans, VT) were pair-housed in a temperature and humidity controlled environment on a 12 h light/dark cycle (lights on at 7am). All animals had ad libitum access to food and water in their home cages and were allowed to acclimate to the housing facility for at least 1 week before any surgical procedure. All procedures were in accordance with the National Institute of Health's Guide to the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee prior to the start of the study.
Surgical and microdialysis procedures
Rats were anesthetized with isoflurane and placed on an electric heating pad within a stereotaxic frame. A small incision was made in the skin over the skull, into which lidocaine was perfused. Concentric dialysis probes or bipolar stimulating electrodes were secured in a head cap consisting of dental acrylic and were fastened to the skull using two skull screws. Immediately following surgery, the probes were connected to a liquid swivel balance arm assembly, and the rats were singly housed in a clear polycarbonate shoebox cage with fresh bedding. The cages were situated in a temperature and humidity controlled room on the same light/dark schedule used in the vivarium. Rats were given approximately 24 h to recover from the surgery prior to the collection of microdialysis samples, which is accepted as a sufficient period of time for brain tissue to normalize after probe implantation (Krebs-Kraft et al. 2007). Rats had ad libitum access to food and water during recovery, but not during the experiment. Probes were perfused with a Ringer's solution containing 145 mM of NaCl, 2.7 mM of KCl, 1.0 mM of MgCl2, and 1.2 mM of CaCl2. The flow rate was set at 1.3 μL/min during recovery and at 2.0 μL/min during the collection of dialysis samples. During the experiment, dialysis samples were collected every 20 min and immediately injected into a high-performance liquid chromatography system with electrochemical detection for the analysis of dopamine, as described previously (Adams and Moghaddam 1998).
Dialysis probes had an outer diameter of 330 μm and a 2.0-mm exposed tip. In most animals, dopamine was measured simultaneously from the probes placed in the left or right hemisphere DStr and the contralateral NAc. For experiments involving electrical stimulation of the left or right hemisphere ventral tegmental area (VTA), dopamine was measured from a dialysis probe placed ipsilaterally in the NAc. Coordinates for the dialysis probes and stimulating electrodes were according to the atlas of Paxinos and Watson (Paxinos and Watson 1998) and were as follows in millimeter relative to bregma: DStr (ante-roposterior (AP), +1.6; mediolateral (ML), +/−1.8; dorsoventral (DV), −4.0 to −6.0), NAc (AP, +1.2; ML, +/−1.1; DV, −6.4 to −8.4), VTA (AP, −5.3; ML +/− 0.8; DV, −8.3). Animals were used once and, in cases where technical issues were encountered with one of the probes, data from the other probe and the behavior data were included in the analysis.
Drugs
LY354740 (10.0 mg/kg; a gift from Eli Lilly, Indianapolis, IN, USA) and amphetamine (1.0 mg/kg; Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline and frozen for a maximum of 1 week before use. 3,4-Dihydroxyphenylala-nine methyl ester HCl (L-dopa, 100 mg/kg; Sigma-Aldrich) was dissolved in 0.9% saline prior to each experiment and was used immediately. All injections were given at a volume of 1 ml/kg of body weight.
The dose of LY354740 used in this study was chosen based on previous studies conducted by this laboratory, which demonstrated that 1.0 and 10.0 mg/kg of LY354740 do not significantly alter basal dopamine release in the striatum (Moghaddam and Adams 1998), but are active on both behavioral and electrophysiological measures (Homayoun et al. 2005; Moghaddam and Adams 1998). The dose chosen (10 mg/kg) is the highest dose that reverses the behavioral effects of pro-psychotic drugs without producing considerable locomotor (Cartmell et al. 1999) or neurochemical effects on its own. A relatively low dose of amphetamine (1.0 mg/kg) was used because the behavioral effects of the higher doses are not reduced by 10.0 mg/kg of LY354740 (Cartmell et al. 1999; Schlumberger et al. 2009a).
Electrical stimulation parameters
The VTA was stimulated for 20 min in a noncontinuous burst pattern (1 ms pulses at 100 Hz for 200 ms, interburst interval of 500 ms, amplitude was 60 μA) as described before (Lecourtier et al. 2008).
Locomotor activity
A stainless-steel frame housing with an array of infared beams (Hamilton-Kinder, LLC, Poway, CA) was placed outside the animals’ cage so they could remain in the home-cage environment throughout the experiment. Beam breaks were monitored over the entire course of sample collection by the Kinder Scientific MotorMonitor program. After the experiment, locomotor activity data expressed in terms of fine movements and ambulations were pooled into 20-min bins corresponding to the collection of dialysis samples.
Experimental design
The purpose of experiment one was to test the hypothesis that the attenuated amphetamine-induced hyperlocomotion previously observed after the treatment with mGlu2/3 agonists is correlated with attenuated increases in dopamine release in the DStr and NAc. After a stable baseline of dopamine release (defined for all experiments as less than 20% variation over three consecutive samples from a given brain region) was achieved, rats received an injection of either LY354740 or 0.9% saline, followed 20 min later by amphetamine.
In experiment two, we sought to determine whether mGlu2/3 activation was capable of attenuating increased dopamine release and hyperlocomotion caused by an impulse-flow dependent mechanism. It was reasoned that, if mGlu2/3 activation reduced amphetamine-induced dopa-mine release through an effect on vesicular release or synaptic reuptake mechanisms, it also would affect the increase in extracellular levels of dopamine caused by electrical stimulation of the VTA. Rats received either 0.9% saline or LY354740, followed 20 min later by VTA stimulation using the parameters stated above.
Experiment three was designed to test whether mGlu2/3 activation attenuated dopamine release through an effect on dopamine synthesis. Rats received either an injection of LY354740 or 0.9% saline 20 min prior to an injection of L-dopa.
In each experiment, recovery from surgery and testing occurred in the home-cage environment. All rats were randomly assigned to experimental conditions. No subject was used in more than one experiment.
Histology
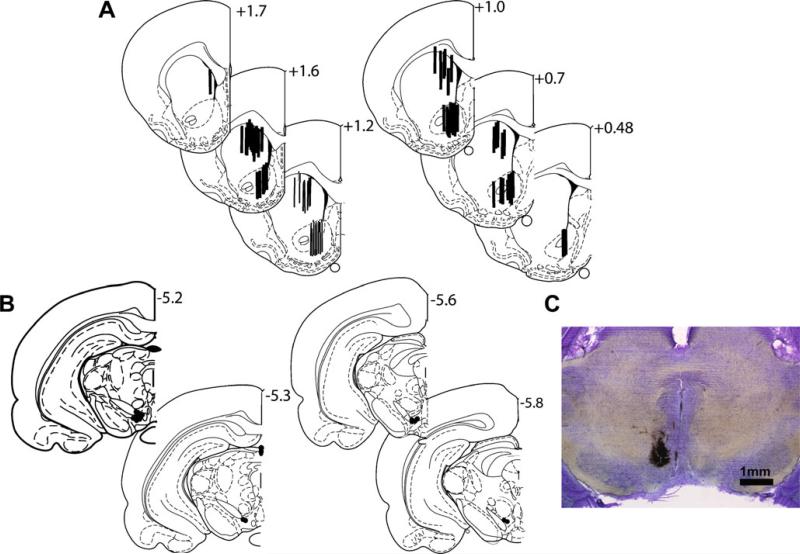
After the completion of each experiment, the rats were anesthetized with chloral hydrate (400 mg/kg) and transcardially perfused with saline followed by a 10% neutral-buffered formaldehyde solution. The brains were removed and stored in formaldehyde for several days prior to being cut in serial sections at 250-μm intervals. In order to verify the microdialysis probe and electrode placements, slices were mounted on slides and stained using cresyl violet. Only data from placements within the brain region of interest were used for further analysis (Fig. 1).
Fig. 1.
Histology. a, b Schematic representations of probe placements in the DStr and NAc, and stimulating electrode placements in the VTA, respectively. c Micrograph of a representative electrode placement in the VTA
Data analysis
Microdialysis data are expressed as the percentage of baseline±sem. Baseline was defined as the average of the three dialysis samples immediately prior to the first injection. For behavioral activation, both ambulation and fine movements were measured. Briefly, ambulations were defined as movements where the animals moved its entire body (i.e., a new beam was broken while a previously broken beam was released). Fine movements were defined as smaller movements where a new beam is broken without releasing a previously broken beam.
Statistical analysis of these dependent measures was conducted using two-factor repeated measures, ANOVAs with the treatment group as the between-subjects factor and time as the within-subjects factor. In situations where no differences were found between treatment groups in terms of dopamine efflux or locomotor activity, data from the experimental groups were combined and reanalyzed using a separate one-factor repeated measures ANOVA examining the time factor only. Neuman–Keuls post hoc tests were used where appropriate for either type of ANOVA. Finally, a further analysis using the Pearson product-moment correlation was conducted in order to examine the relationship between peak dopamine release from the DStr or NAc (defined as the bin with the highest average dopamine concentration for the group) and time-matched locomotor activity data. Statistical significance was set at p<0.05 for all analyzes.
Data from individual subjects meeting Peirce's criteria for outliers (Ross 2003) were omitted from statistical analysis of that dependent measure.
Results
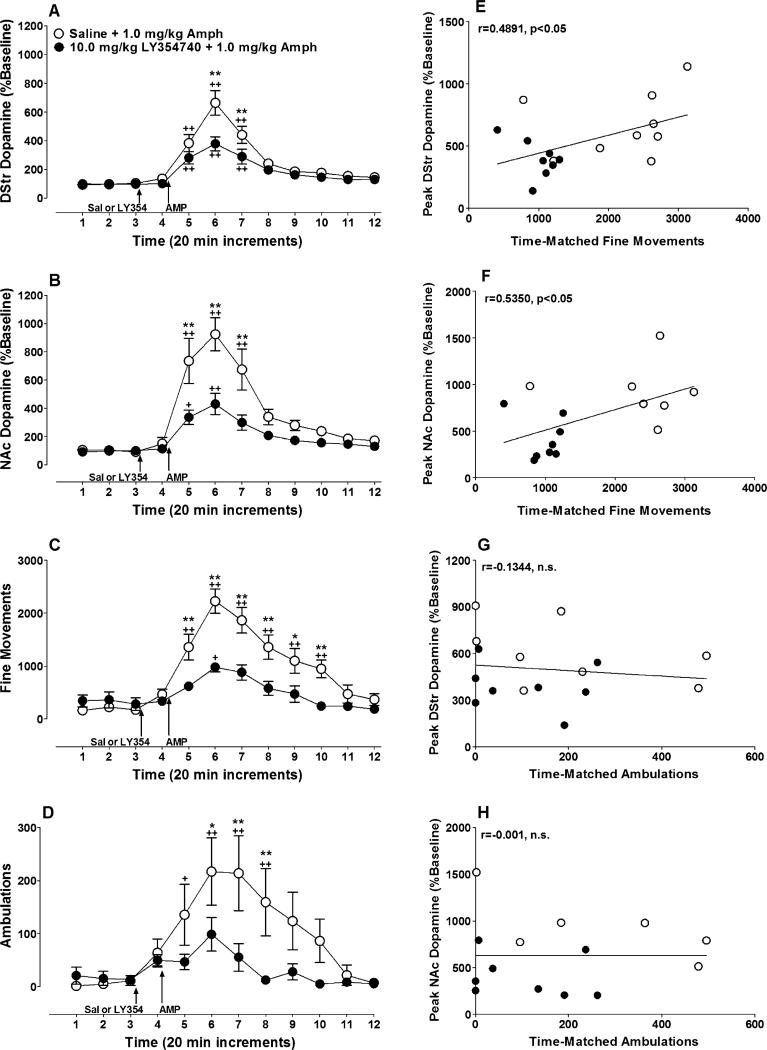
Pretreatment with LY354740 (10.0 mg/kg, IP) 20 min prior to amphetamine (1.0 mg/kg, IP) significantly attenuated amphetamine-induced dopamine release in the DStr (Fig. 2a; treatment × time interaction; F(11, 176)=3.56, p<0.001) and in the NAc (Fig. 2b; treatment × time interaction; F(11, 154)=6.84, p<0.0001).
Fig. 2.
LY354740 pretreatment attenuates amphetamine-induced increases in dopamine release and hyperlocomotion. Rats were given an IP injection of 10.0 mg/kg of LY354740 (filled circles) or 0.9% saline (open circles) 20 min prior to an injection of 1.0 mg/kg of amphetamine. Dopamine release was measured in the a DStr (LY354740 group, n=9; mean baseline dopamine=1.7±0.3 fmol/μL; saline group, n=9; mean baseline dopamine=1.1±0.3 fmol/μL) and b NAc (LY354740 group, n=9; mean baseline dopamine= 1.6± 0.3 fmol/μL; saline group, n=7; mean baseline dopamine=0.6± 0.2 fmol/μL). Locomotor activity also was measured for each animal, expressed as c fine movements (LY354740 group, n=9; saline group, n=10) or d ambulations (LY354740 group, n=9; saline group, n=10). Peak fine movements were significantly correlated to peak dopamine release in the e DStr (one outlier was omitted from the LY354740 group) and f NAc (one outlier was omitted from the LY354740 group), but peak ambulations were not significantly correlated to peak dopamine release in g and h either brain region (one outlier was omitted from each treatment group in these two graphs). In graphs A, B, C, and D, baseline dopamine release or locomotor activity is represented by the first three time points displayed. Asterisks denote significant differences between treatment groups at a given time point (* p<0.05, ** p<0.01) while plus signs indicate significant differences compared to baseline within a given treatment group (+p<0.05, ++p<0.01)
The increase in dopamine efflux by amphetamine was accompanied by activation of fine movements and ambulation (Fig. 2c, d). Consistent with previous reports (Cartmell et al. 1999, 2000), pretreatment with an mGlu2/3 agonist significantly attenuated the locomotor-activating effects of amphetamine in fine movements (Fig. 2c; drug × time interaction; F(11, 187)=7.1369, p<0.0001) and ambulation-dependent measures (Fig. 2d; drug × time interaction; F(11, 176)=3.622, p<0.0001). Examination of the relationship between peak dopamine release and peak locomotor activity by correlation analysis found significant positive relationships between fine movements and dopa-mine release in the DStr (Fig. 2e; r=0.4869, n=18, p<0.05) and NAc (Fig. 2f; r=0.5350, n=16, p<0.05). No significant correlation was found between ambulations and peak dopamine release in either the DStr (Fig. 2g; r=−0.0805, n=17, n.s.) or the NAc (Fig. 2h; r=−0.0264, n=15, n.s.).
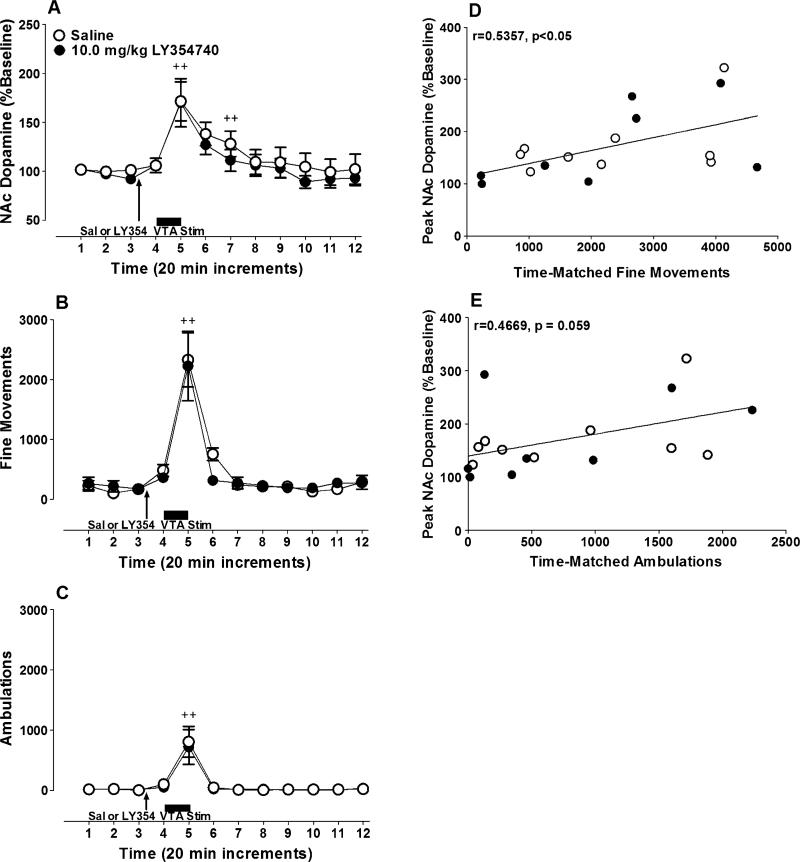
VTA stimulation significantly increased extracellular dopamine concentrations in the NAc in both the saline and LY354740 treatment groups (Fig. 3a; time main effect; F(11, 176)=11.15, n.s.), but the magnitude of this response was not significantly affected by LY354740 pretreatment (treatment main effect; F(1, 16)=0.45, n.s.; treatment × time interaction; F(11, 176)=0.19, n.s.). Similarly, VTA stimulation significantly increased locomotor activity in the saline-treated and LY354740-treated groups. This behavioral response was characterized by large increases in both fine movements (Fig. 3b;time maineffect; F(11, 165)=26.14, p<0.0001) and ambulations (Fig. 3c; F(11, 165)=14.79, p<0.0001), but pretreatment with 10.0 mg/kg of LY354740 did not significantly affect the magnitude of this response (fine movements: treatment main effect; F(1, 15)=0.13, n.s.; treatment × time interaction; F(11, 165)=0.41, n.s.; ambulations: treatment main effect; F(1, 15)=0.036, n.s.; treatment × time interaction; F(11, 165)=0.067, n.s). A significant correlation was found between peak NAc dopamine release and peak fine movements (Fig. 3d; r=0.5357, n=17, p<0.05), while a nonsignificant trend toward a correlation was found between peak NAc dopamine and peak ambulations (Fig. 3e; r=0.4669, n=17, p=0.059).
Fig. 3.
LY354740 pretreatment has no effect on dopamine release and locomotion elicited by electrical stimulation of the VTA. Rats were given an IP injection of 10.0 mg/kg of LY354740 (filled circles)or 0.9% saline (open circles) 20 min prior to electrical stimulation of the VTA. Dopamine release in the a NAc (LY354740 group, n=8; mean baseline dopamine=0.8±0.2 fmol/μL; saline group, n=9; mean baseline dopamine=0.6±0.2 fmol/μL) and locomotor activity were measured in each animal. Activity is expressed in terms of b fine movements (LY354740 group, n=8; saline group, n=9) or c ambulations (LY354740 group, n=8; saline group, n=9). d Peak fine movements were significantly correlated to peak NAc dopamine release. A nonsignificant trend toward a correlation was found between e peak ambulations and peak NAc dopamine release. In graphs A, B and C, baseline dopamine release or locomotor activity are represented by the first three time points displayed. Plus signs denote significant increases in dopamine release or activity compared to baseline (+ p<0.05; ++ p<0.01)
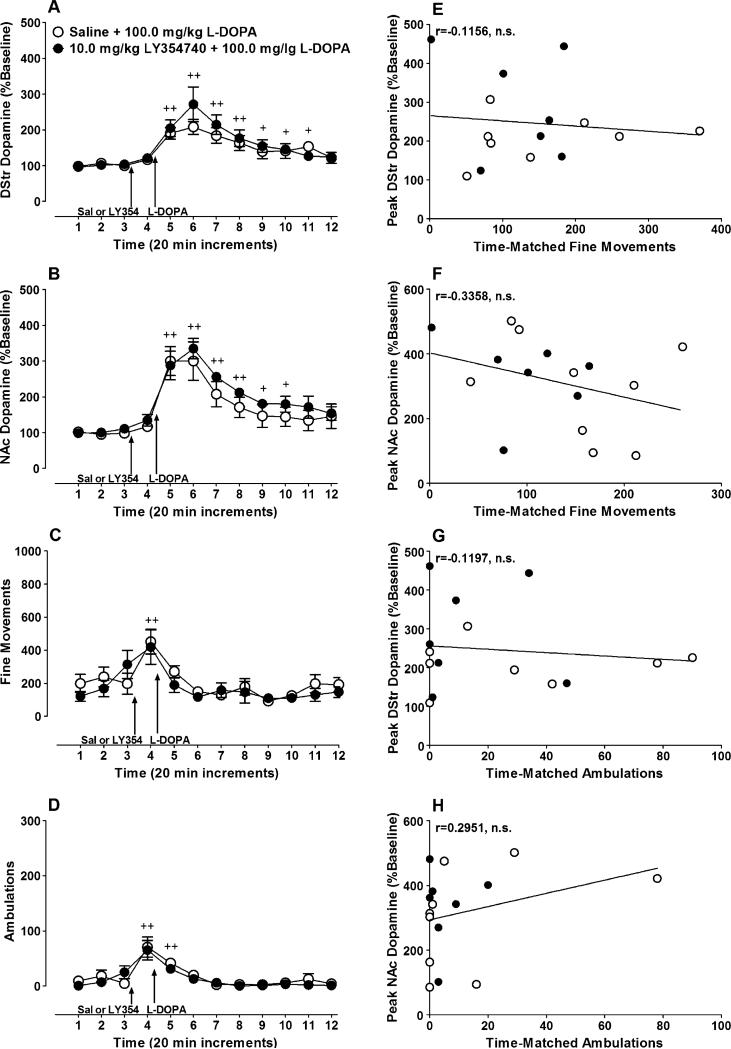
L-dopa (100 mg/kg) significantly increased dopamine release in the DStr (Fig. 4a time main effect: F(11,154)= 21.35, p<0.0001) and NAc (Fig. 4b; time main effect; F(11, 154)=27.08, p<0.0001). This increase was not affected by pretreatment with LY354740 (DStr, treatment main effect, F (1, 14)=0.29, n.s.; interaction effect F(11, 154)=1.25, n.s.; NAc, treatment main effect, F(1, 14)=0.75, n.s.; interaction effect, F(11, 154)=0.49, n.s). Small but significant increases in fine movements (Fig. 4c; time main effect, F(11, 220)= 5.92, p<0.0001) and ambulations (Fig. 4d; time main effect, F(11, 275)=11.54, p<0.0001) were observed in these experimental groups after injection of saline or LY354740, which may be attributable to injection stress; however, L-dopa did not significantly change locomotor activity compared to the baseline. Moreover, there were no differences between animals treated with saline or LY354740 in the magnitude of the locomotor responses in terms of fine movements (treatment main effect, F(1, 20)=1.23, n.s.; treatment × time interaction, F(11, 220)=1.07, n.s.) or ambulations (treatment main effect, F(1, 21)=0.582, n.s.; treatment × time interaction, F(11, 231)=0.568, n.s.). Finally, no significant correlations were found between peak dopa-mine release from the DStr and NAc and fine movements (DStr, Fig. 4e; r=−0.1156, n=15, n.s.; NAc, Fig. 4f: r=−0.3358, n=16, n.s.) or ambulations (DStr, Fig. 4g; r=−0.1197, n=15, n.s.; NAc, Fig. 4h: r=0.2951, n=16, n.s.).
Fig. 4.
LY354740 pretreatment has no effect on L-dopa-induced increases in dopamine release. Rats were given an IP injection of 10.0 mg/kg of LY354740 (filled circles) or 0.9% saline (open circles) 20 min prior to injecting 100.0 mg/kg of L-dopa. Dopamine release was measured in the a DStr (LY354740 group, n=8; mean baseline dopamine=1.2±0.1 fmol/μL; saline group, n=8; basal release=1.3± 0.3 fmol/μL) and b NAc (LY354740 group, n=7; mean baseline dopamine=0.7±0.2 fmol/μL; saline group, n=9; mean baseline dopamine=1.2±0.2 fmol/μL) for each animal. In addition, locomotor activity also was measured and is expressed as c fine movements (LY354740 group, n=9; saline group, n=14) or d ambulations (LY354740 group, n=9; saline group, n=14). No significant correlation was found between peak fine movements and dopamine release in e the DStr or f the NAc. Similarly, significant correlations were not found between g, h peak ambulations and dopamine release in either brain region. In graphs A, B, C and D, baseline dopamine release or locomotor activity is represented by the first three time points displayed. Plus signs denote significant increases in dopamine release or locomotor activity compared to baseline (+ p<0.05, ++ p<0.01). Note that the discrepancy in sample sizes between the DStr micro-dialysis graph and the related correlations is due to missing activity data for one subject. This missing data was caused by an unrecoverable computer error
Discussion
The present study demonstrates that the previously reported inhibitory effects of mGlu2/3-receptor agonists on amphetamine-induced increases in fine movements (Cartmell et al. 1999, 2000; Fell et al. 2008) correlates with an attenuated increase in extracellular levels of dopamine in the NAc and striatum. Furthermore, our data shows that this effect is not due to changes in mechanisms that govern activity dependent release of dopamine and dopamine synthesis or reuptake. Specifically, mGlu2/3 receptor activation did not attenuate the increase in extracellular levels of dopamine or locomotion induced by electrical stimulation of dopamine neurons. The increase in the extracellular levels of dopamine measured by microdialysis after electrical stimulation of dopamine neurons or fibers is thought to be due to the impulse flow dependent vesicular release of dopamine, which has escaped reuptake. Thus, a lack of effect of the mGlu2/3-receptor agonists on these levels indicates that dynamics of dopamine reuptake or vesicular storage, two processes that are disrupted by amphetamine (Sulzer et al. 1995), are not targeted by these agonists. Amphetamine may also influence dopamine synthesis (Patrick et al. 1981); however, we found that increasing extracellular levels of dopamine by L-dopa, which primarily is due to increasing pools of newly synthesized dopamine (Dluzen and Liu 1994), was not affected by mGlu2/3 receptor activation suggesting that these receptors do not influence packaging and release of newly synthesized dopamine.
Our finding that mGlu2/3 receptor activation attenuated amphetamine-induced hyperlocomotion and dopa-mine release is consistent with other studies showing that stimulation of these receptors reduces the acute behavioral effects of amphetamine (Cartmell et al. 2000;Fell etal. 2008; Rorick-Kehn et al. 2007; Uslaner et al. 2009; Woolley et al. 2008) and blocks the expression of amphetamine sensitization in locomotion, self-administration and release of glutamate and dopa-mine (Kim et al. 2005). In general, inhibition of amphetamine-induced effects by mGlu2/3 agonists may be attributed to postsynaptic processes that may involve modulation of the AC activity. Specifically, amphetamine's dopaminergic effects are mediated primarily by cAMP given that both D1 and D2 receptors, similar to mGlu2/3 receptors, are coupled to AC (Missale et al. 1998). The inhibitory effect on dopamine release, however, is most likely not governed by cAMP-mediated processes. Although disruption of the AC activity may influence the expression or kinetics of vesicular monoamine transporters or dopamine transporters (Guillot et al. 2008;Pageet al. 2004), such an effect also would have reduced the impact of electrical stimulation of dopamine neurons on extracellular levels.
Although amphetamine is thought to increase dopamine release independent of impulse flow by relocating vesicular dopamine into the cytosol and reversing the dopamine transporter (Sulzer et al. 2005), there is evidence that some portion of dopamine release by amphetamine in intact animals may be impulse flow dependent (Porras et al. 2002; Speckenbach and Kehr 1976) and depends on the activation of cortical feedback loops. For example, lesions of the prefrontal cortex reduce amphetamine-induced striatal dopamine release (Wilkinson et al. 1997; Dalley et al. 1999). Prefrontal cortex lesions or deactivation also reduce amphetamine-mediated behaviors that have traditionally been associated with accumbal or striatal dopamine neurotransmission including locomotion, prepulse inhibition, place preference conditioning and behavioral sensitization (Ventura et al. 2003; Bjijou et al. 2002; Lacroix et al. 2000; Wilkinson et al. 1997). Thus, a likely mechanism for the inhibitory effects of mGlu2/3 agonists on amphetamine-induced dopamine release observed here may involve reduced activation of cortical feedback loops. The dose of LY354740 used here inhibits baseline firing of spontaneously active prefrontal cortex neurons (Homayoun et al. 2005). This could transiently deactivate the cortical input to striatal or midbrain regions mimicking lesion-like conditions and, therefore, reduce amphetamine's effect on locomotion and dopamine release.
An alternative explanation for our neurochemical data may involve different storage pools of dopamine targeted by amphetamine. Amphetamine is thought to have the ability to release dopamine from both “newly synthesized” and “older” pools of dopamine. This idea stems from reports that dopamine release due to low doses of amphetamine are reduced by pretreatment with the tyrosine hydroxylase inhibitor α methyl para tyrosine (αMPT), but not by pretreatment with the catecholamine-vesicle-depleting agent reserpine. However, higher doses of amphetamine are sensitive to both αMPT and reserpine (Cadoni et al. 1995; Chiueh and Moore 1975). Thus, low doses of amphetamine may release dopamine preferentially from the newly synthesized cytosolic dopamine pool; whereas, higher doses release dopamine from new and older pools of dopamine. Here we observed that mGlu2/3 receptor activation significantly reduces amphetamine-induced dopamine release, but has no effect on dopamine release evoked by the dopamine precursor L-dopa, which is insensitive to reserpine treatment and thus is derived from a newly synthesized pool (Dluzen and Liu 1994). This result is consistent with a recent paper showing that activation of mGlu 2/3 receptors has no effect on the rate of dopamine synthesis in reserpine-treated rats (Fell et al. 2009). Therefore, one interpretation of these data may be that mGlu 2/3 activation preferentially affects amphetamine's ability to release old stores of dopamine. However, the functional significance of these putative old stores is unclear.
The impact of mGlu2/3 receptor activation on amphetamine may be due to interactions with serotonergic or noradrenergic systems (Lanteri et al. 2008). For example, behavioral effects of amphetamine are attenuated through the administration of 5HT2A antagonists such as M100907 and ritanserin (Sorensen et al. 1993; O'Neill et al. 1999), and more recent evidence suggests a functional interaction between mGlu2/3 receptors and 5HT2A receptors. Agonists of mGlu2/3 receptors decrease 5HT2A agonist-induced head-twitch responses (Gewirtz and Marek 2000) and excitatory postsynaptic potentials (Marek et al. 2000). Additionally, it was discovered recently that mGlu2/3 receptors can form heteroreceptor complexes with 5HT2A receptors and that agonists at mGlu2/3 receptors attenuate 5HT2A agonist-induced signaling in the cortex (Gonzalez-Maeso et al. 2008). Finally, the 5HT2A antagonist M100907 potentiates the inhibitory effect of mGlu2/3 activation on amphetamine-induced hyperlocomotion (Uslaner et al. 2009), lending further support to the idea that mGlu2/3 agonists influence amphetamine's effects partially through effects on serotonin signaling.
The influence of stress on amphetamine's effects may be an important consideration while interpreting the results because stress in various forms (i.e., restraint stress, tail pinch, foot shock, social defeat, etc.) is known to cause sensitization to amphetamine's effects on locomotion (Antelman et al. 1980; Diaz-Otanez et al. 1997; Herman et al. 1984; Pacchioni et al. 2002) and dopamine release (Pacchioni et al. 2002; Pacchioni et al. 2007) in a manner that mimics repeated exposure to the drug. Some aspects of the surgical procedure used in this study may have been stressful and thus, LY354740's ability to attenuate amphetamine-induced hyperlocomotion and dopamine release may be related to an interaction between the drug's known anxiolytic properties (Linden et al. 2005). Dopa-mine release in the striatum, however, is not sensitive to stress (Inglis and Moghaddam 1999) suggesting that, while reduction of amphetamine's behavioral effects may have been due, in part, to the anxiolytic properties of LY354740, its neurochemcial effects were not.
Recent binding studies from Seeman and co-workers (Seeman 2008; Seeman et al. 2008; Seeman and Guan 2009) have suggested that mGlu2/3 agonists including LY354740 have partial agonist actions at dopamine D2 receptors. However, this finding has not been replicated by other laboratories (Fell et al. 2009). Our findings also indirectly question a partial D2 agonistic property for mGlu2/3 agonists. Highly selective partial D2 agonists such as (−)-3PPP increase basal dopamine release in the NAc (Imperato et al. 1988;See 1994); whereas, selective mGlu2/3 agonists either have no effect or decrease dopamine levels at higher doses (Greenslade and Mitchell 2004; Hu et al. 1999; Karasawa et al. 2006; Moghaddam and Adams 1998). Of note, the attenuation in amphetamine-induced hyperlocomotion caused by mGlu 2/3 agonists is blocked by knocking out mGlu 2 receptors (Fell et al. 2008) and by administering mGlu 2/3 antagonists (Cartmell et al. 2000; Rorick-Kehn et al. 2007), indicating that this effect is mediated through mGlu 2/3 receptors and not D2 receptors.
Previous studies demonstrating that activation of mGlu 2/3 receptors attenuates the effects of amphetamine on horizontal locomotion have mostly used other agonists with a much higher affinity for mGlu2/3 receptors (Wright et al. 2001); whereas, 10.0 mg/kg of LY354740 did not have a significant effect on the ambulations of a much higher dose of amphetamine (Cartmell et al. 1999; Schlumberger et al. 2009a). Thus, it may be the case that the dose of amphetamine and affinity for the mGlu 2/3 receptor are important determinants in the ability of an mGlu2/3 agonist to reduce amphetamine's behavioral effects. In addition, a PET study in monkeys has reported that IV administration of 20.0 mg/kg of LY354740 increases amphetamine-induced [11C]raclopride displacement suggesting that, in contrast to the rodent microdialysis data, mGlu2/3 agonists increase dopamine release (van Berckel et al. 2006). Several methodological reasons may account for these differences. For example, monkeys involved in that study were anesthetized with a combination of the glutamatergic NMDA antagonist ketamine and isoflurane prior to the beginning of the PET scan. Although ketamine administration alone does not interfere with the measurement of dopamine release, preadministration of NMDA antagonists has been observed to cause significant increases in amphetamine's dopamine-releasing effects (Kegeles et al. 2000).
A large body of evidence based largely in lesion studies supports the idea that amphetamine-induced dopamine release in the DStr is related to increases in stereotypy, while dopamine release in the NAc is related to ambulation (for a brief review, please see Beninger 1983). The significant correlation we observed between NAc dopamine release and fine motor activation in this study appears to run in conflict with these data. There is, however, a wealth of data from intact animals demonstrating that these classic relationships between regional dopamine release and behavioral activation are loose and depend on the dose of the amphetamine used. For example, 2.0 mg/kg of amphetamine increases both ambulations and dorsal striatal dopamine release in a similar temporal pattern (Kuczenski and Segal 1989), while 8.0 mg/kg of amphetamine induces dopamine release in the striatum that is highly temporally dissociated with stereotypy (Kuczenski et al. 1997). In addition, it has been demonstrated that intra-accumbens infusion of 3 μM of amphetamine is able to induce a fivefold increase in dopamine release without a concomitant increase in ambulatory activity (Darracq et al. 1998; Darracq et al. 2001). Thus, the relationship between dopamine release in these brain regions and behavioral activation described previously is not a strict one, and therefore, the correlation we observed between amphetamine-induced increases in fine movements and dopamine release in both of these brain regions is not inconsistent with the previous literature.
In conclusion, mGlu2/3 receptor activation does not appear to influence basic mechanisms that regulate basal or activated release of dopamine, suggesting that any potential antipsychotic efficacy of mGlu2/3 agonists (Patil et al. 2007) is not mediated by modulation of the dopamine system. Although we observed that mGlu2/3 agonists attenuate amphetamine-induced dopamine release in correlation with reduced hyperlocomotion, this effect may be mediated through a mechanism that indirectly influences amphetamine's effects on striatal dopamine such as reduced activation of cortical feedback loops. Future studies using local administration of mGlu 2/3 receptor agonists in the frontal cortex via microinjection or lesions of the frontal cortex in combination with systemic administration of mGlu 2/3 receptor agonists will be useful in determining the role of cortical feedback loops in this effect.
Acknowledgements
This study was supported by the National Institute of Mental Health Grant MH48404. The authors thank Alicia Lisowitz for her technical assistance and Eli Lilly and Company for providing LY354740.
References
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res Rev. 1983;6:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, De Deurwaerdere P, Spampinato U, Stinus L, Cador M. D-amphetamine-induced behavioral sensitization: effect of lesioning dopaminergic terminals in the medial prefrontal cortex, the amygdala and the entorhinal cortex. Neuroscience. 2002;109:499–516. doi: 10.1016/s0306-4522(01)00508-5. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pinna A, Russi G, Consolo S, Di Chiara G. Role of vesicular dopamine in the in vivo stimulation of striatal dopamine transmission by amphetamine: evidence from microdialysis and Fos immunohistochemistry. Neuroscience. 1995;65:1027–1039. doi: 10.1016/0306-4522(94)00507-2. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The mGlu(2/3) receptor agonist LY379268 selectively blocks amphetamine ambulations and rearing. Eur J Pharmacol. 2000;400:221–224. doi: 10.1016/s0014-2999(00)00423-4. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Moore KE. D-amphetamine-induced release of “newly synthesized” and “stored” dopamine from the caudate nucleus in vivo. J Pharmacol Exp Ther. 1975;192:642–653. [PubMed] [Google Scholar]
- Dalley JW, Thomas KL, Howes SR, Tsai TH, Aparicio-Legarza MI, Reynolds GP, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the rat prefrontal cortex on CREB regulation and presynaptic markers of dopamine and amino acid function in the nucleus accumbens. Eur J Neurosci. 1999;11:1265–1274. doi: 10.1046/j.1460-9568.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of d-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darracq L, Drouin C, Blanc G, Glowinski J, Tassin JP. Stimulation of metabotropic but not ionotropic glutamatergic receptors in the nucleus accumbens is required for the D-amphetamine-induced release of functional dopamine. Neuroscience. 2001;103:395–403. doi: 10.1016/s0306-4522(00)00578-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Otanez CS, Capriles NR, Cancela LM. D1 and D2 dopamine and opiate receptors are involved in the restraint stress-induced sensitization to the psychostimulant effects of amphetamine. Pharmacol Biochem Behav. 1997;58:9–14. doi: 10.1016/s0091-3057(96)00344-9. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. The effect of reserpine treatment in vivo upon L-dopa and amphetamine evoked dopamine and DOPAC efflux in vitro from the corpus striatum of male rats. J Neural Transm Gen Sect. 1994;95:209–222. doi: 10.1007/BF01271567. [DOI] [PubMed] [Google Scholar]
- Dohovics R, Janaky R, Varga V, Saransaari P, Oja SS. Cyclic AMP-mediated regulation of striatal glutamate release: interactions of presynaptic ligand- and voltage-gated ion channels and G-protein-coupled receptors. Neurochem Int. 2003;43:425–430. doi: 10.1016/s0197-0186(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Perry KW, Falcone JF, Johnson BG, Barth VN, Rash KS, Lucaites VL, Threlkeld PG, Monn JA, McKinzie DL, Marek GJ, Svensson KA, Nelson DL. In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S, 2S, 5R, 6S-2-aminobicyclo[3.1.0]hexane-2, 6-bicaroxylate monohydrate ( LY354740) and (–)-2-oxa-4-aminobicyclo[3.1.0] hexane-4, 6-dicarboxylic acid ( LY379268). J Pharmacol Exp Ther. 2009;331:1126–1136. doi: 10.1124/jpet.109.160598. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (–)-(1R, 4S, 5S, 6S)-4-amino-2-sulfonylbicyclo[3.1.0] hexane-4, 6-dicarboxylic acid ( LY404039). J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenslade RG, Mitchell SN. Selective action of (–)-2-oxa-4-aminobicyclo[3.1.0]hexane-4, 6-dicarboxylate ( LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47:1–8. doi: 10.1016/j.neuropharm.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Stinus L, Le Moal M. Repeated stress increases locomotor response to amphetamine. Psychopharmacology (Berl) 1984;84:431–435. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 (mGlu2/3) receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2004;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Imperato A, Tanda G, Frau R, Di Chiara G. Pharmacological profile of dopamine receptor agonists as studied by brain dialysis in behaving rats. J Pharmacol Exp Ther. 1988;245:257–264. [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Kilbride J, Huang L, Rowan M, Anwyl R. Presynaptic inhibitory action of the group II meatbotropic glutamate receptor agonists, LY354740 and DCG-IV. Eur J Pharmacol. 1998;356:149–157. doi: 10.1016/s0014-2999(98)00526-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73:333–337. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Frantz KJ, Parent MB. In vivo microdialysis: a method for sampling extracellular fluid in discrete brain regions. In: Lajtha A, Baker D, Dunn S, Holt A, editors. Handbook of Neurochemistry and Molecular Neurobiology. Springer Science and Buisness Media; L.L.C., New York: 2007. pp. 221–239. [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Melega WP, Cho AK, Segal DS. Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther. 1997;282:591–596. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix L, Spinelli S, White W, Feldon J. The effects of ibotenic acid lesions of the medial and lateral prefrontal cortex on latent inhibition, prepulse inhibition and amphetamine-induced hyperlocomotion. Neuroscience. 2000;97:459–468. doi: 10.1016/s0306-4522(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noreadrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Lapin IaMR. Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav Brain Res. 1995;70:145–151. doi: 10.1016/0166-4328(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 2005;179:284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine (2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Heron-Maxwell CL, Shaw G. 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol Biochem Behav. 1999;63:237–243. doi: 10.1016/s0091-3057(98)00240-8. [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology (Berl) 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Gioino G, Assis A, Cancela LM. A single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effects of amphetamine: involvement of NMDA receptors. Ann N Y Acad Sci. 2002;965:233–246. doi: 10.1111/j.1749-6632.2002.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Page G, Barc-Pain S, Pontcharraud R, Cante A, Piriou A, Barrier L. The up-regulation of the striatal dopamine transporter's activity by cAMP is PKA-, CaMK II- and phosphatase-dependent. Neurochem Int. 2004;45:627–632. doi: 10.1016/j.neuint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Patrick RL, Berkowitz AL, Regenstein AC. Effects of in vivo amphetamine administration on dopamine synthesis regulation in rat brain striatal synaptosomes. J Pharmacol Exp Ther. 1981;217:686–691. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. Academic Press; San Diego: 1998. [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdere P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Richards G, Messer J, Malherbe P, Pink R, Brockhaus M, Stadler H, Wichmann J, Schaffhauser H, Mutel V. Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H] LY354740 binding in vitro and quantitative radioautography: correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPgammas binding. J Comp Neurol. 2005;487:15–27. doi: 10.1002/cne.20538. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Ross SM. Peirce's criterion for the elimination of suspect experimental data. J Eng Technol. 2003;20:38–41. [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Klein KU, Greco S, More L, Danysz W. Comparison of the mGlu(5) receptor positive allosteric modulator ADX47273 and the mGlu(2/3) receptor agonist LY354740 in tests for antipsychotic-like activity. Eur J Pharmacol. 2009a;623:73–83. doi: 10.1016/j.ejphar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav Pharmacol. 2009b;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- See RE. Differential effects of 3-PPP enantiomers on extracellular dopamine concentration in the caudate-putamen and nucleus accumbens of rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:605–610. doi: 10.1007/BF00169364. [DOI] [PubMed] [Google Scholar]
- Seeman P. Glutamate agonists for schizophrenia stimulate dopamine D2High receptors. Schizophr Res. 2008;99:373–374. doi: 10.1016/j.schres.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Seeman P, Caruso C, Lasaga M. Dopamine partial agonist actions of the glutamate receptor agonists LY 354, 740 and LY 379, 268. Synapse. 2008;62:154–158. doi: 10.1002/syn.20482. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC. Glutamate agonists for treating schizophrenia have affinity for dopamine D2High and D3 receptors. Synapse. 2009;63:705–709. doi: 10.1002/syn.20673. [DOI] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- Speckenbach W, Kehr W. Effect of (+) amphetamine on monoamine synthesis and metabolism after axotomy in rat forebrain. Naunyn Schmiedebergs Arch Pharmacol. 1976;296:25–30. doi: 10.1007/BF00498836. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, Hutson PH. Combined administration of an mGlu2/3 receptor agonist and a 5-HT 2A receptor antagonist markedly attenuate the psychomotor-activating and neurochemical effects of psychostimulants. Psychopharmacology (Berl) 2009;206:641–651. doi: 10.1007/s00213-009-1644-y. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Kegeles LS, Waterhouse R, Guo N, Hwang DR, Huang Y, Narendran R, Van Heertum R, Laruelle M. Modulation of amphetamine-induced dopamine release by group II metabotropic glutamate receptor agonist LY354740 in nonhuman primates studied with positron emission tomography. Neuropsychopharmacology. 2006;31:967–977. doi: 10.1038/sj.npp.1300902. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LS, Dias R, Thomas KL, Augood SJ, Everitt BJ, Robbins TW, Roberts AC. Contrasting effects of excitotoxic lesions of the prefrontal cortex on the behavioural response to D-amphetamine and presynaptic and postsynaptic measures of striatal dopamine function in monkeys. Neuroscience. 1997;80:717–730. doi: 10.1016/s0306-4522(97)00075-4. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H] LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther. 2001;298:453–460. [PubMed] [Google Scholar]