Abstract
The objective was to examine the time-course of the cannabinoid 1 receptor antagonist/inverse agonist rimonabant’s ability to antagonize in vivo cannabinergic agonist effects. We used two behavioral procedures sensitive to the effects of Δ9-tetrahydrocannabinol (Δ9-THC): rat drug discrimination (EXP-1) and suppression of fixed ratio responding (FR) for food reinforcement (EXP-2). Two training doses of Δ9-THC (1.8 and 3 mg/kg) served as discriminative cues in two groups discriminating Δ9-THC from vehicle; injections were i.p. 20 min before session onset. Tests assessed the dose-response functions of Δ9-THC and the time-course for rimonabant in its ability to block the discriminative stimulus effects of Δ9-THC. For antagonism testing, the training doses of Δ9-THC were used and the rimonabant dose was 1 mg/kg. Tests were 20, 60, 120, and 240 min post rimonabant administration; Δ9-THC was always administered 20 min prior to testing. For EXP-2, only one response lever was activated and every 10th (FR-10) press on that lever resulted in food delivery. Once the response rate stabilized, tests occurred with Δ9-THC, rimonabant and combinations of the drugs. The ED50 estimates for the dose-response functions were 0.38 (± 0.28–0.51) and 0.50 (± 0.40–0.63) mg/kg for the training doses of 1.8 and 3 mg/kg Δ9-THC, respectively. The time-course studies suggested functional half-life estimates of 128.4 (± 95.7–172.2) and 98.4 (± 64.2–150.7) min by rimonabant for the two groups in EXP-1, respectively. Similarly, the functional half-life of rimonabant was 118.9 (± 66.1 – 213.9) min in EXP-2. Thus, antagonism of Δ9-THC by rimonabant is relatively short lasting.
Keywords: Δ9-THC, rimonabant, time-course, drug discrimination, fixed-ratio responding, rats
1. Introduction
Rimonabant is a cannabinoid receptor 1 antagonist/inverse agonist originally launched as an anti-obesity medication. Although now withdrawn as a medication, the compound still has utility as a pharmacological tool in uncovering roles of the endocannabinoid system in normal and pathophysiological body functions (Le Foll et al., 2009). The half-life of orally administered rimonabant in man has been estimated to be 1 to 2 weeks, depending on body fat content – the greater the body fat, the slower the excretion. The slow elimination is thought to reflect preference for uptake by adipose tissue and subsequent sequestration (Huestis et al., 2007). In acute studies using food restricted rats that were lever pressing for food, the functional in vivo half-life after systemic administration was estimated to be around 15 h (McLaughlin et al., 2003). The time-course of rimonabant as an antagonist of cannabinergic induced receptor agonism has been less investigated. We used two behavioral operant procedures that are sensitive to the effects of Δ9-tetrahydrocannabinol (Δ9-THC): rat drug discrimination for examining conditioned effects and suppression of fixed ratio (FR) responding for examining unconditioned Δ9-THC effects. Two training doses of Δ9-THC (1.8 and 3 mg/kg) served as discriminative cues in two groups of rats discriminating between Δ9-THC and vehicle in the spirit of systematic replication (Sidman, 1960). Once trained to criterion, test sessions were interspersed between the regular training sessions to assess the dose-response functions of Δ9-THC as well as the time-course for rimonabant in its ability to block the discriminative stimulus effects of Δ9-THC. For these antagonism tests the training doses of Δ9-THC were used and the rimonabant dose was 1 mg/kg. For the studies examining FR responding, only one lever was activated and every 10th (FR-10) press on the lever resulted in food delivery. When the rate of responding had stabilized, tests with Δ9-THC, rimonabant and combinations of the two drugs occurred weekly, customarily on Tuesdays and Thursdays. On interim days, rate of responding was assessed when the rats were non-drugged while responding for food under the FR-10 schedule of reinforcement, which served as base-line rates of responding. A dose of 1 mg/kg rimonabant was chosen as such a dose regularly has been able to block the discriminative stimulus effects of Δ9-THC without too markedly affecting rate of responding (Järbe et al., 2001; Järbe et al., 2006b). The choice of 3 mg/kg dose of Δ9-THC as the reference in the FR procedure was based on dose-response studies previously conducted by us (Lamb et al., 2000) and elsewhere (De Vry and Jentzsch, 2004; De Vry et al., 2004).
2. Materials
2.1. Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) were individually housed in a colony room with an average temperature of 20°C and a 12-hour light/dark cycle (rats were trained and tested during the light phase). Animals (≈ 90 days old at the beginning of the studies) were experimentally naïve at the time of shaping the lever pressing response (see below). Post session supplemental feeding with Harlan Rat Chow® (# 2018) was restricted to approximately 12 to 14 g/day. All procedures were approved by the Animal Care and Use Committee of Temple University, Philadelphia, PA (EXP-1), and that of Northeastern University, Boston, MA (EXP-2), USA. The “Principles of Animal Laboratory Care” (National Institutes of Health, 1996) was followed.
2.2. Apparatus
Training and testing occurred in 8 chambers (ENV-001, Med. Associates, St Albans, VT) equipped with two non-retractable response levers, house-and lever lights, and a grid floor. Each chamber was enclosed within sound- and light-attenuating boxes with an exhaust fan and interfaced with a DOS/Windows compatible computer. Response contingencies were programmed using Med-PC software (v. 1.16; Med. Associates).
2.3. General procedure
Rats were trained to eat food pellets (45 mg, BioServe®) from a food receptacle located midway between the two response levers. The animals were shaped to lever press for food until they responded 10 times for each reinforcer (fixed-ratio 10 schedule of reinforcement; FR-10). Under our conditions, when the house light was off, and the stimulus lights above the response levers being lit, completion of 10 presses on the active lever resulted in the delivery of one (EXP-2) or two (EXP-1) 45 mg food pellets; followed by a 3-s (EXP-2) or 10-s (EXP-1) timeout period with only the house light on. At the end of the time-out period, the stimulus lights above the levers were lit, the house light was turned off, and the FR-10 schedule of reinforcement contingency reinstated. Sessions ended by all lights in the box being turned off.
2.3.1. EXP-1: Training
Once daily, beginning 20 min after i.p. injection, the two groups of rats were trained in this two-choice task to respond on drug-or vehicle-appropriate levers to receive food. For one group of rats, the training dose of Δ9-THC was 1.8 mg/kg (n = 12) and for the other group it was 3 mg/kg (n = 8). The position of drug-appropriate levers was randomly assigned among subjects so that it was to the right of the food cup for half the subjects and left for the other half. Throughout the session, the aforementioned FR-10 schedule of reinforcement was in effect. Presses on the incorrect lever were recorded, but had no programmed consequences. The order of drug or vehicle administrations was nonsystematic, with no more than two consecutive drug or vehicle sessions. Approximately an equal number of drug- and vehicle training sessions occurred throughout the study. To avoid the potential influence of odor cues left in a chamber by a preceding subject, the order in which drug and vehicle training sessions were conducted for animals trained in the same chamber was randomized (Extance and Goudie, 1981). Training took place Monday through Friday, and lasted 20 min. Training continued until and beyond animals reached the acquisition criterion of selecting the lever appropriate for the training condition on at least 8 out of 10 consecutive training days. Correct selection was defined as total presses before the first reinforcement (FRF) being equal to, or less than 14 (i.e., the incorrect lever not pressed more than 4 times before completing 10 responses on the lever appropriate for the prevailing training condition; FRF ≤ 14).
2.3.2. EXP-1: Testing
Once stable drug discriminations were achieved (see Results), test (T) sessions were conducted on average 3 times every two weeks; on interim days, regular drug (D) or vehicle (V) training sessions of 20 min duration took place. Such scheduling assured that each test was preceded by at least one drug and one vehicle maintenance session. Approximately two weeks before initial testing, animals began receiving two i.p. injections before the training sessions (i.e., vehicle and drug, or vehicle and vehicle) to accustom the animals to a double injection procedure such as that used for antagonism testing; the interval between the 1st and 2nd injection varied. Typically, the order of sessions was: D, V, T, V, D (week 1); V, T, V, D, T (week 2); V, D, T, D, V (week 3); and D, T, D, V, T (week 4). Tests were conducted only if responding during the preceding training sessions had been correct (FRF ≤ 14) during the initial six FR-10 cycles of the session. If incorrect, animals were retrained for at least three sessions where FRF ≤ 14 before additional testing took place. In test sessions food pellets were delivered for 10 presses on either lever for 6 reinforcement cycles or until 20 min had elapsed, whichever occurred first. There was one session per test day. Doses and drugs were examined in a mixed order. For each dose and interval tested, the percentage of responding on the drug-appropriate lever was calculated from the ratio of the number of presses on the Δ9-THC associated lever to the total number of lever presses in a test session (excluding responding during the time-out periods). Only data for animals receiving at least one reinforcer during the test session were considered for this measure, i.e., animals must have made a minimum of 10 presses on one of the two levers. Additionally, response rate (responses per second) across all subjects was calculated. This measure was based on the performance of all animals, including non-responders.
2.3.3. EXP-2
Initial shaping of bar pressing was as described above in general procedure. Animals were trained to press one lever for reward until the session ended after 20 min or after the animals had received 75 food pellets, whichever occurred first. Of the 8 separate rats utilized in EXP-2, 4 rats received food by pressing the right and the remaining 4 animals were rewarded by pressing the left lever and every 10th (FR-10) press on the lever resulted in food delivery. Presses on the non-contingent lever, a rare event, were recorded but had no programmed consequences. At the time of initial testing there was less than 15% variation in the day-to-day rate of responding, excluding performances during the first day after weekends and holidays. Tests with Δ9-THC, rimonabant and combinations of the two drugs occurred no more than twice weekly, customarily on Tuesdays and Thursdays. On interim days, base-line rate of responding was assessed when the rats were run non-drugged. Tests with combinations of Δ9-THC and rimonabant were studied first and completed before examining the effects of rimonabant alone in EXP-2. The first and last test of the phase examining the effects of the drug combinations consisted of challenge by 3 mg/kg of Δ9-THC alone (plus vehicle). Such arrangement allows for an appreciation of either variability and/or potential adaptation (pharmacological/behavioral) to the disruptive effects of Δ9-THC resulting from the intervening drug exposure while testing the drug combinations. Two weeks prior to examining the influence of drugs on the rate of responding, animals began receiving double injections (V + V) prior to the operant sessions. As in EXP-1, the interval between the two injections varied.
2.4. Statistics
Response rate was averaged (± S.E.M.) among rats and plotted as a function of dose and post session time since rimonabant administration. The effects of a drug on response rate were considered significant when the mean rate of responding was not within the 95% confidence limits (±95% C.L.) of the mean control response rate. For EXP-1, this was defined in individual rats as the mean response rate pertaining to the initial 6 reinforcement cycles calculated from vehicle training sessions in which the criteria for testing were met. Data points outside the ±95% C.L. of the vehicle rate preceding the tests in question were considered significant. For EXP-2, the response rate measure was equal to the total number of responses divided by the time taken to reach the imposed upper limit of 75 reinforcements or 20 min, whichever occurred first. Within-group comparisons in EXP-2 made use of one-way repeated measures ANOVA followed by the Holm-Sidak all pair-wise comparison procedure (α = 0.05). Responding during time-out periods was not included in the rate data in either of the studies.
Non-linear regression analyses of dose-generalization and antagonism data after log-X transformation were performed using Prism software (v. 5, GraphPad Software, San Diego, CA; www.graphpad.com) to provide estimates of the independent variables when the co-ordinates of X intersected with Y = 50 (EXP-1) or Y = 0.5 (EXP-2) and their 95% confidence limits (±95% C.L.; regression model: log dose or log time vs. response – variable slope with the top and bottom of the curves constrained to 100 and 0 in EXP-1 and 1 and 0 in EXP-2) Using the F-test, the Prism program estimates if slopes are parallel or not and if parallel, evaluates whether the intercepts are equal or not (a measure of potency).
2.5. Drugs
The levo isomer of Δ9-THC (6,6,9-trimethyl-3-pentyl-6a,7,810a-tetrahydro-6H-benzo[c]chromen-1-ol), dissolved in ethanol (200 mg/ml), was kindly provided by the National Institute on Drug Abuse (NIDA; Bethesda, Maryland, USA) and stored at −20°C until used. For preparing suspensions, appropriate amounts of Δ9-THC was withdrawn, the ethanol evaporated under a stream of nitrogen, the residue dissolved in a solution of propylene glycol and Tween-80, and stored at −20°C. Shortly before being used, the solute was slowly diluted with normal (0.9%) saline in a step-wise fashion after the solute had been sonicated for 20–30 min. Rimonabant, as the base (N-(piperidin-1-yl)-5-(4-chloro-phenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) was also provided by NIDA and stored refrigerated at 4°C before being dissolved in the propylene glycol/Tween-80 (v/v) mixture (final suspension 5%/3% for both drugs in EXP-1) before being diluted with saline (92%). For EXP-2, the suspensions for both Δ9-THC and rimonabant contained 2% dimethyl sulfoxide, 4% propylene glycol, 4% Tween-80 and 90% saline but otherwise followed above format. Doses were administered i.p. in a volume of 2 ml/kg. Suspensions were prepared fresh daily just prior to administration. Doses are expressed as the forms indicated.
3. Results
EXP-1
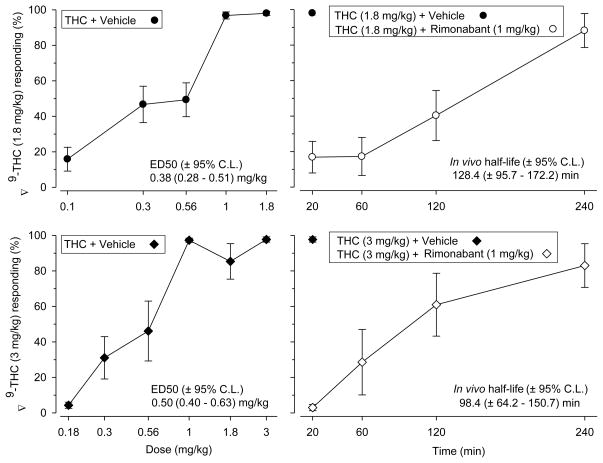
The first test session occurred after the animals had been discrimination trained for 80 (SD = 1.8 mg/kg) and 87 (SD = 3 mg/kg) sessions, respectively. Fig. 1 displays the discriminative stimulus data (% drug responding) obtained in the two groups trained to discriminate between Δ9-THC and vehicle. The left panels depict the dose-response data obtained 20 min post administration and the right panels the time-course data for the ability of 1 mg/kg rimonabant to block the discriminative stimulus effects of the training doses of Δ9-THC (1.8 and 3 mg/kg in the two groups, respectively). The figure also summarizes the main outcomes of the regression analyses. The ED50 estimates for the dose-response functions were 0.38 (± 0.28–0.51) and 0.50 (± 0.40–0.63) mg/kg for the training doses of 1.8 and 3 mg/kg Δ9-THC, respectively (filled symbols in the left panels). The F-test suggested no significant difference between the two ED50 estimates [F (1, 158) = 2.02; p > 0.05]. The time-course studies suggested functional in vivo half-life estimates of 128.4 (± 95.7–172.2) and 98.4 (± 64.2–150.7) min for the two training conditions, respectively (open symbols in the right panels). The F-test suggested no significant difference between the two functional in vivo half-life estimates [F (1, 76) = 1.05; p > 0.05]. Slope functions were parallel for both the dose [F (1, 158) = 2.42; p > 0.05] and time-course data [F (1, 76) = 0.20; p > 0.05] in the two groups comprising EXP-1.
Fig. 1.
(EXP-1): Generalization test results for two separate groups of rats discriminating between Δ9-THC and vehicle; training sessions began 20 min after i.p. administration. The two training doses of Δ9-THC were: 1.8 mg/kg (n = 12) and 3 mg/kg (n = 8). The generalization results represent the mean (± S.E.M.) percentage of lever presses on the Δ9-THC appropriate lever out of the total number of lever presses emitted during a test session (Y-axis); X-axis, left panels: doses examined in mg/kg; X-axis, right panels: time elapsed since injection of 1 mg/kg rimonabant; Δ9-THC was always administered 20 min before session onset. Data points are based on one (3 mg/kg Δ9-THC) to two (1.8 mg/kg Δ9-THC) observations for each rat and were obtained on separate test days. Test results are based on sessions of a maximum of 6 reinforcements (12 food pellets) or 20 min, whichever occurred first. Two of the 12 rats examined at the 240 min interval in the low-dose Δ9-THC trained group did not respond, hence % Δ9-THC (1.8 mg/kg) responding is based on 10 observations for that interval. Median dose- and functional in vivo time effect estimates are based on non-linear regression (Prism, v. 5).
The average (±S.E.M.) response rates were 1.21 (0.21), 1.72 (0.21), 1.50 (0.22), 2.13 (0.46) and 2.13 (0.45) responses per second, in ascending order, for the dose tests conducted in the 1.8 mg/kg Δ9-THC trained group; the 95% C.L. based on the corresponding vehicle rates obtained during non-drug training sessions were 1.02 (lower limit) and 2.00 (upper limit) with a mean (±S.E.M.) of 1.51 (0.22) responses per second. The rates for the tests over time with Δ9-THC (1.8 mg/kg)/rimonabant (1 mg/kg) combinations were 0.93 (0.13), 1.64 (0.28), 0.86 (0.16) and 1.63 (0.41) responses per second, listed in the order of shortest (20 min) to the longest test interval (240 min) examined. The 95% C.L. based on the corresponding vehicle rates were 1.25 (lower limit) and 2.07 (upper limit) with a mean (±S.E.M.) of 1.66 (0.19) responses per second. Thus, the rates observed at the 20 and 120 min time points were outside of the 95% C.L. for the low dose (1.8 mg/kg) Δ9-THC trained group (data not shown).
For the group trained with the higher Δ9-THC dose (3 mg/kg), the average (±S.E.M.) response rates were 0.83 (0.18), 1.27 (0.21), 0.90 (0.16), 1.62 (0.37), 0.87 (0.14) and 0.95 (0.10) responses per second, in ascending order, for the dose tests; the 95% C.L. based on the corresponding vehicle rates obtained during non-drug training sessions preceding these tests were 0.49 (lower limit) and 1.60 (upper limit) with a mean (±S.E.M.) of 1.05 (0.24) responses per second. The rates for the tests over time with Δ9-THC (3 mg/kg)/rimonabant (1 mg/kg) combinations were 0.52 (0.10), 0.89 (0.16), 1.12 (0.14) and 0.90 (0.34) responses per second, listed in the order of shortest (20 min) to the longest test interval (240 min) examined. The 95% C.L., based on the corresponding vehicle rates, were 0.68 (lower limit) and 1.43 (upper limit) with a mean (±S.E.M.) of 1.06 (0.16) responses per second. Thus, the rate observed at the 20 min time point was outside of the 95% C.L. for the high dose (3 mg/kg) Δ9-THC trained group (data not shown).
EXP-2
Fig. 2 shows the results from tests with Δ9-THC alone (together with a vehicle injection) and when combined with 1 mg/kg rimonabant in rats maintained on a straight FR-10 schedule of reinforcement. Δ9-THC (3 mg/kg) alone was the first and last tests conducted, with testing of the drug combinations occurring in irregular order between the two tests examining Δ9-THC alone. Tests occurring 20 and 60 min post rimonabant administration significantly blocked the Δ9-THC induced response rate decrease compared to both the first (12.61% of base-line rate) and second (21.72% of base-line rate) tests with Δ9-THC alone [F (6, 39) = 7.62; p < 0.001]. The difference in response rates between tests 1 and 2 with Δ9-THC alone was not significant. The non-linear regression analysis suggested a functional in vivo half-life of 118.9 (± 66.1 – 213.9) min post rimonabant administration. Compared to base-line rate of responding, only the test occurring 20 min post rimonabant administration resulted in a response rate falling in the range between the 95% C.L. of the control rate (mean vehicle control rate = 1.73 ±1.10 – 2.36 responses per second). All other tests resulted in response rates that were outside of the lower 95% C.L. of the control mean. The absolute mean (± S.E.M.) rates of responding were: 0.18 (0.06) and 0.30 (0.18) for the 1st and 2nd test with Δ9-THC alone, respectively. The rates for the tests over time with Δ9-THC (3 mg/kg)/rimonabant (1 mg/kg) combinations were 1.35 (0.27), 0.92 (0.23), 0.79 (0.22), 0.86 (0.26) and 0.35 (0.20) responses per second, listed in the order of shortest (20 min) to the longest test interval (480 min) examined. The average (± S.E.M.) base-line response rate was 1.73 (0.27).
Fig 2.
(EXP-2): Average (± S.E.M.) effects of i.p. administered 3 mg/kg Δ9-THC alone (1st and last bar) and in combination with 1 mg/kg rimonabant on the rate of responding in rats (n = 8) maintained on a FR-10 schedule of food reinforcement. Y-axis: response rate where 1 represents unity (dashed line), i.e., rate being equal to the base-line rate observed in each individual animal during preceding non-drug sessions. X-axis, time elapsed since injection (Δ9-THC was always administered 20 min before session onset) and dose in mg/kg (Δ9-THC, 3 mg/kg; rimonabant, 1 mg/kg). Data points are based on one observation for 7 to 8 individual animals. Functional in vivo half-life and the ±95% confidence limits (±95% C.L.) estimated by non-linear regression (Prism, v. 5). +) signifies that the response rate observed at 20 min post administration (Δ9-THC plus rimonabant) is significantly higher than the response rates observed with 3 mg/kg Δ9-THC alone (together with vehicle) as well as the response rate observed 8 h after rimonabant plus Δ9-THC administration; *) signifies that the response rate observed at 60 min post administration (Δ9-THC plus rimonabant) is significantly higher than the response rates observed with 3 mg/kg Δ9-THC alone (Holm-Sidak all pair-wise multiple comparison procedure).
Fig. 3 shows the effects of examining 1 mg/kg rimonabant alone (together with a vehicle injection) at different times after administration (ranging from 20 min to 8 h). ANOVA resulted in a main effect [F (4, 26) = 5.12; p = 0.004] and the response rates observed 4 and 8 h after administration were significantly higher than the response rate noted 2 h after rimonabant administration. Compared to the average (± S.E.M.) base-line rate of responding of 1.73 (0.27), all tests resulted in response rates falling within the range between the 95% C.L. of the control response rate (±1.10 – 2.36 responses per second). Hence, 1 mg/kg rimonabant by itself did not significantly affect the response rate compared to the base-line response rates. The absolute mean (± S.E.M.) rates of responding for the tests over time with rimonabant (1 mg/kg) alone were: 1.13 (0.19), 1.40 (0.29), 1.16 (0.28), 1.68 (0.25) and 1.97 (0.36) responses per second listed in the order of shortest (20 min) to the longest test interval (480 min) examined.
Fig. 3.
(EXP-2): Average (± S.E.M.) effects of 1 mg/kg rimonabant alone (plus vehicle injection) on the rate of responding in rats (n = 8) maintained on a FR-10 schedule of food reinforcement. Y-axis: response rate where 1 represents unity (dashed line), i.e., rate being equal to the base-line rate observed in each individual animal during preceding non-drug sessions. X-axis, time elapsed since injection (vehicle plus 1 mg/kg rimonabant). Data points are based on one observation for 7 to 8 individual animals. +) signifies that the response rate observed at 480 min post administration is significantly higher than the response rate observed at 120 min after rimonabant administration; *) signifies that the response rate observed at 240 min post administration is significantly higher than the response rate observed at 120 min after rimonabant administration (Holm-Sidak all pair-wise multiple comparison procedure).
4. Discussion
The current studies examined the ability of a fixed dose of rimonabant (1 mg/kg) to block Δ9-THC induced behavioral effects in rats as a function of elapsed time since the administration of the antagonist/inverse agonist. In both the drug discrimination as well as the simple FR responding assays, the functional in vivo half-life of rimonabant was around 2 h with maximum efficacy apparent at the shortest test interval that was examined (20 min post rimonabant administration). The blockade of the effects of Δ9-THC by rimonabant was much attenuated 4 to 8 h after antagonist administration. Thus, in the drug discrimination assays both groups exhibited close to 100% Δ9-THC appropriate responding and in the FR-10 assay the rate of responding approached the level(s) of the response rate decreases elicited by administration of Δ9-THC alone (together with vehicle). Such a relatively brief duration of action in the ability of rimonabant to block Δ9-THC induced cannabinoid receptor 1 activation is in contrast to its reported time course in suppressing food intake where the estimated functional in vivo half-life was around 15 h after systemic administration in rats (McLaughlin et al., 2003), and obviously quite different from the slow pharmacokinetic clearance reported for man after oral administration (Huestis et al., 2007). While McLaughlin’s study (experiment 3) and our EXP-2 have many similarities, one reason for the discrepancy in the functional half-lives may be that McLaughlin’s rats were obtaining 10 or more grams of food per session while our animals were restricted to a maximum of 3.4g during the operant sessions. In addition, our rats were kept at 80% of free-feeding weight while McLaughlin’s were kept at 85%. This suggests that the differences in the functional in vivo half-life estimates may be due to differences in the degree of satiation/motivation.
That the two Δ9-THC discrimination groups exhibited a similar functional in vivo antagonistic half-life in regard to rimonabant challenge is according to expectation given the relative nature of the procedure (Järbe, 1989). However, the systematic replication (Sidman, 1960) puts current data on a more solid basis and taken together with the similar functional half-life estimate of around 2 h derived from the FR procedure, would seem to suggest that both the conditioned (percent drug responding in EXP-1) and unconditioned (rate of FR responding in EXP-2) Δ9-THC induced effects were affected similarly by rimonabant challenge and furthermore imply mediation by (central) cannabinoid receptor 1 mechanisms. The degree by which current data can be extended also to other cannabinoid receptor 1 agonists is an open question as various ligands based on different chemical templates exhibit both similarities and differences in their interaction with the cannabinoid receptor 1 binding site(s) as described elsewhere (Järbe et al., 2010). For example, although rimonabant will block the discriminative stimulus effects of the cannabinoid receptor 1 agonist R-(+)-methanandamide (Järbe et al., 2001; Järbe et al., 2006b), surmountable antagonism has been difficult to demonstrate in rodents presumably because of the profound rate decreasing effects of the drug combination whereas surmountable antagonism with Δ9-THC and rimonabant mixtures readily occurs (Järbe et al., 2001; Järbe et al., 2006a; Järbe et al., 2009; Wiley et al., 1995). Also other behaviors induced by above cannabinoid receptor 1 agonists are differentially affected by rimonabant challenge (Baskfield et al., 2004; Järbe et al., 2002; Järbe et al., 2003). Although rimonabant was not tested singly in the current drug discrimination groups, previous data have consistently shown lack of substitution in Δ9-THC trained animals using drug discrimination procedures (Järbe et al., 2001; Mansbach et al., 1996; McMahon et al., 2008; Perio et al., 1996; Solinas et al., 2003; Vann et al., 2009; Wiley et al., 1995). For example, tests with 1 mg/kg rimonabant alone resulted in 1.07% Δ9-THC (3 mg/kg) appropriate responding and the associated rate of responding (mean ± S.E.M.) was 0.45 (± 0.11) responses per second; the corresponding vehicle rate was 0.89 (± 0.33) responses per second and the associated Δ9-THC (3 mg/kg) appropriate responding was 3.52% (Järbe et al., 2001). Although comparatively small in magnitude, the rate of responding was reduced in the combination tests at the early time interval in EXP-1 but the response rate data for the drug combination tests did not exhibit a slope and hence are not particularly suited for an analysis of regression.
There was a tendency for response rate decreases during the earlier test intervals (20 to 120 min) when examining rimonabant alone in the FR procedure, agreeing with previous data suggesting that rimonabant exerts intrinsic activity on operant responding which usually is more apparent at somewhat higher doses (De Vry and Jentzsch, 2004; Järbe et al., 2001). The mechanism for the intrinsic activity is unclear but may involve response competition resulting from the well known cannabinoid receptor 1 antagonist induced increases in the incidence of scratching and grooming in rats; see (Järbe et al., 2008) and/or a reduced motivation for food (Salamone et al., 2007). It has also been proposed that rimonabant acts as a partial cannabinoid receptor 1 agonist for the receptor mechanism(s) mediating the rate suppressant effects of cannabinergics in this procedure (De Vry and Jentzsch, 2004).
Our time-course results with rimonabant suggest a faster onset and shorter duration of action compared to previous data using rats discriminating between 3 mg/kg Δ9-THC and vehicle trained at 30 min post administration (Solinas et al., 2003). In the latter study, it was reported that 1 mg/kg rimonabant was ineffective in blocking the discriminative stimulus effects of 3 mg/kg Δ9-THC when the antagonist was injected i.p. 45 min prior to session onset, i.e., 15 min prior to the Δ9-THC administration. Complete blockade of the effects of Δ9-THC occurred in tests conducted 60 and 75 min post rimonabant administration with some recovery seen when examining the 3 and 6 h time intervals. However, not even at the 6 h interval did the average percentage of Δ9-THC appropriate responding reach the 50% level. Although there may be a number of reasons for the divergence in results between the two studies, one potential source might be the vehicle used. Thus, there is the added complexity arising from the lipophilic character of cannabinergic ligands when comparing results across different laboratories. Hence, different vehicles are used in different laboratories to prepare suspensions and may differ in their efficacy as carriers of these molecules. Solinas and colleagues stated that rimonabant “was suspended in 0.3% Tween-80” (Solinas et al., 2003), a vehicle which results in lower compound solubility than that used for the current studies and could very well explain the difference in onset and duration of action between the two studies. Unfortunately, there are no recent systematic comparisons of the more common currently used vehicles for cannabinoid compounds. Irrespective of the discrepancy, we conclude that antagonism of Δ9-THC by rimonabant is relatively short lasting for both a conditioned (EXP-1) as well as an unconditioned (EXP-2) behavior in rats.
Acknowledgments
Research supported by NIH/NIDA grants DA023142 (AM) and DA009064 (TUCJ). Portions of the data were presented at the 20th annual meeting of the International Cannabinoid Research Society Symposium July 23–27, 2010 at the Scandic Star, Lund, Sweden (Tai et al., 2010).
‘Role of the funding source’: Authors declare that the study sponsor did not have any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
“Disclosure Statement”: All authors declare that there is no actual or potential conflict of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baskfield CY, Martin BR, Wiley JL. Differential effects of delta-9-tetrahydrocannabinol and methanandamide in CB1 knockout and wild-type mice. J Pharmacol Exp Ther. 2004;309:86–91. doi: 10.1124/jpet.103.055376. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Partial agonist-like profile of the cannabinoid receptor antagonist SR141716A in a food-reinforced operant paradigm. Behav Pharmacol. 2004;15:13–20. doi: 10.1097/00008877-200402000-00002. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Extance K, Goudie AJ. Inter-animal olfactory cues in operant drug discrimination procedures in rats. Psychopharmacology (Berl) 1981;73:363–371. doi: 10.1007/BF00426467. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC. Discrimination learning with drug stimuli: Methods and applications. In: Boulten AA, Baker GB, Greenshaw AJ, editors. Neuromethods: Vol. 13. Psychopharmacology. Humana Press; Clifton, N.J: 1989. pp. 513–563. [Google Scholar]
- Järbe TUC, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist delta-9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, DiPatrizio NV, Li C, Makriyannis A. The cannabinoid receptor antagonist SR-141716 does not readily antagonize open-field effects induced by the cannabinoid receptor agonist (R)-methanandamide in rats. Pharmacol Biochem Behav. 2003;75:809–821. doi: 10.1016/s0091-3057(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Lamb RJ, Liu Q, Makriyannis A. Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog, in rats trained with Δ9-THC or (R)-methanandamide (AM-356) Psychopharmacology (Berl) 2006a;188:315–323. doi: 10.1007/s00213-006-0517-x. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, LeMay BJ, Olszewska T, Vemuri VK, Wood JT, Makriyannis A. Intrinsic effects of AM4113, a putative neutral CB1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol Biochem Behav. 2008;91:84–90. doi: 10.1016/j.pbb.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Li C, Liu Q, Makriyannis A. Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog. Psychopharmacology (Berl) 2009;203:229–239. doi: 10.1007/s00213-008-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Li C, Vadivel SK, Makriyannis A. Discriminative stimulus functions of methanandamide and Δ9-THC in rats: Tests with aminoalkylindoles (WIN55, 212-2 and AM678) and ethanol. Psychopharmacology (Berl) 2010;208:87–98. doi: 10.1007/s00213-009-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TUC, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of delta-9-THC and (R)-methanandamide in rats. Psychopharmacology (Berl) 2006b;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TUC, Makriyannis A, Lin S, Goutopoulos A. Effects of Δ9-tetrahydrocannabinol, (R)-methanandamide, SR 141716,and d-amphetamine before and during daily Δ9-tetrahydrocannabinol dosing. Eur J Pharmacol. 2000;398:251–258. doi: 10.1016/s0014-2999(00)00318-6. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Winston EN, Lowe JA., 3rd Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats. Psychopharmacology (Berl) 1996;124:315–322. doi: 10.1007/BF02247436. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Δ9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perio A, Rinaldi-Carmona M, Maruani J, Barth F, Le Fur G, Soubrie P. Central mediation of the cannabinoid cue: activity of a selective CB1 antagonist, SR 141716A. Behav Pharmacol. 1996;7:65–71. [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Tactics of Scientific Research – Evaluating Experimental Data in Psychology. Basic Books; New York: 1960. [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Tai S, Gifford R, Järbe TUC, Makriyannis A. Antagonism of Δ9-THC by rimonabant: time-course studies in rats. International Cannabinoid Research Society (ICRS); Burlington, VT: 2010. [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. Discriminative stimulus properties of delta-9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol. 2009;615:102–107. doi: 10.1016/j.ejphar.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR. Antagonism of the discriminative stimulus effects of delta-9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1–6. [PubMed] [Google Scholar]