Abstract
PURPOSE
To evaluate the healing response at the flap interface in corneas with LASIK ectasia that required penetrating keratoplasty (PK).
METHODS
Corneas of five patients who developed corneal ectasia after LASIK (range: 2.5 to 5 years postoperative) were collected after corneal transplant surgery. The corneas were bisected and processed for conventional histologic analysis and immunofluorescence.
RESULTS
Light microscopy showed a hypocellular fibrotic scar at the wound margin compared with the adjacent corneal stroma in all eyes. All corneas had positive staining for alpha-smooth muscle actin (SMA), a myofibroblast marker. In one eye, alpha-SMA cells were located in the fibrotic scar region in the area of the semicircular ring of haze along the margin of the LASIK flap corresponding to an area of epithelial ingrowth. In all other eyes, alpha-SMA positive cells were fewer and mainly located in the superficial stroma under the epithelial wound margin surface. Type III collagen was minimal or absent in the central zone and wound margin of all corneas except for the cornea with epithelial ingrowth present in the hypercellular fibrotic scar region. Chondroitin sulfate was stronger in the periphery of the flap wound coinciding with a higher presence of alpha-SMA–positive cells in that region. Positive staining for matrix metalloproteinase 9 (MMP-9) in the paracentral wound margin scar was seen.
CONCLUSIONS
A wound-healing process characterized by absence of significant fibrosis and myofibroblasts at the wound edge in the flap interface was noted in all keratectatic eyes. However, changes in the composition of collagen and the presence of MMP-9 at the wound edge several years after LASIK indicates active wound remodeling that may explain the ongoing loss of tissue and tendency of the cornea to bulge.
Ectasia after LASIK is a devastating complication of lamellar corneal surgery that results in a progressive deformation and thinning of the cornea associated with severe visual impairment.1 Multiple risk factors have been implicated in the development of corneal ectasia.2 Among them are abnormal or suspicious topography suggestive of pre-existing forme fruste keratoconus and an excessive resection of corneal tissue that makes the cornea mechanically unstable. However, in some cases none of these risk factors are identified. Additionally, this complication is seldom seen in deep lamellar keratoplasty. In such cases, the residual stromal bed thickness is usually no more than 10% to 20% of the corneal thickness (50 to 100 μm). We recently suggested that an increase in the amount of myofibroblasts induced by sutures at the edge of the flap in rabbit eyes may reduce the amount of steepening when intraocular pressure (IOP) is artificially increased up to 25 mmHg.3 We postulated that corneal ectasia may be related to the clinically observed lack of corneal wound-healing response at the edge of the flap, which allows the cornea to bulge.
Evaluations of the results and complications of LASIK surgery focus mainly on clinical outcomes with little emphasis on the wound-healing process.4–7 Most reports on wound healing consist mainly of clinical confocal microscopy evaluations or histopathological studies in animals, which do not necessarily mimic the human corneal wound-healing response.
The present study examines five human corneas that developed ectasia after LASIK, which required penetrating keratoplasty (PK). We describe changes that occur in the cellular and extracellular wound-healing components in the lamellar stromal LASIK wound interface. Some of these changes may affect the stability of the cornea after surgery.
PATIENTS AND METHODS
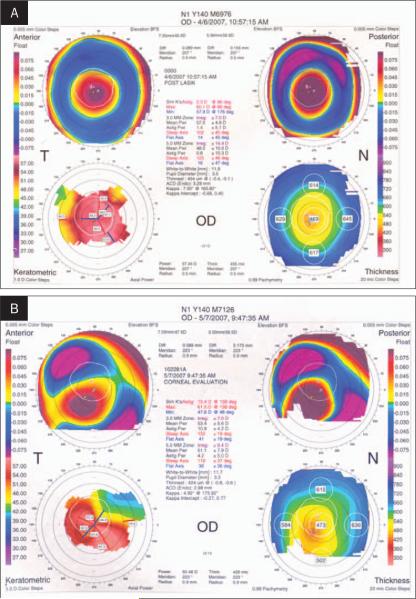
We examined five corneas of five patients with a history of unilateral ectasia after LASIK who failed conventional treatment (eg, spectacle or contact lens wear) and required PK. Four of the five patients had uneventful contralateral LASIK; one had bilateral ectasia and was successfully fitted with gas permeable contact lenses, which provided 20/30 best corrected contact lens visual acuity and good lens comfort. Preoperative data are summarized in Table 1. All patients had LASIK between 2.5 to 5 years prior to undergoing keratoplasty. All LASIK surgeries were performed elsewhere, and the preoperative LASIK corneal topographies were interpreted as normal according to review of previous records. The measured stromal beds from the sections varied between 290 and 340 μm. Preoperative PK topographies are shown in Figure 1. All PK surgeries were performed by one surgeon (S.E.). The specimens were received in the laboratory in Optisol-GS solution (Chiron Ophthalmics, Irvine, Calif) within 1 day of the surgical procedure.
TABLE 1.
Refraction and Corneal Thickness Before and After LASIK for Ectactic Eyes That Required Penetrating Keratoplasty
| Before LASIK |
After LASIK* |
||||
|---|---|---|---|---|---|
| Patient Age | Refraction (D) | Corneal Thickness (μm) | Refraction (D) | Flap Thickness (μm) | Bed Thickness (μm) |
| 28 | −6.50 Sph | 545 | −2.25 –6.25 × 45 | 155 | 303 |
| 33 | −5.25 –1.00 × 5 | 528 | −1.75 –3.25 × 32 | 138 | 296 |
| 27 | −4.75 –0.50 × 95 | 572 | −2.75 –7.75 × 134 | 161 | 348 |
| 34 | −6.25 –0.50 × 10 | 541 | −2.00 –6.75 × 16 | 133 | 321 |
| 31 | −5.75 –1.75 × 0 | 532 | −0.75 –4.25 × 113 | 155 | 292 |
Measurement of refraction was performed prior to corneal transplant surgery performed between 2.5 to 5 years after LASIK.
Note. Before LASIK, corneal thickness was measured by ultrasound. Measurement of flap and bed thickness of the corneal buttons after LASIK was performed using histochemistry and ultrasound pachymetry.
Figure 1.
Corneal topographies of two cases after LASIK. A) Case 1. Left eye shows severe central steepening with central 3-mm irregular astigmatism of 7.00 D. Minimal corneal thickness is 454 μm. B) Case 3. Right eye shows inferior steepening with asymmetric bowtie. Minimal corneal thickness is 424 μm.
Tissue Preparation
Upon receiving the tissue, the corneas were evaluated for gross abnormalities using a dissecting microscope. The corneal buttons were then oriented with the hinge superiorly, cut in half, and fixed in 10% neutral buffered formalin for 2 hours prior to conventional histological processing. The other half was embedded in optimal cutting temperature compound (Tissue-Tek; Miles Laboratories Inc, Elkhart, Ind) and 8-μm cryostat sections were prepared, air-dried, and then stored at −80°C until further use. They were evaluated with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) staining, and by immunohistochemical analysis.
Measurement of the corneal buttons' flap and bed thickness was performed using histochemistry and ultrasound pachymetry. Mean flap thickness (F) and total thickness (T) of the specimen was measured after staining with H&E. The F/T ratio was calculated. Total corneal thickness was measured using ultrasound pachymetry. This value was multiplied by the F/T ratio to obtain the flap thickness in microns. The bed thickness was calculated by subtracting the flap thickness from the total corneal thickness as measured by ultrasound.
Immunohistochemistry
Slides were air-dried for 30 minutes and then incubated with 5% goat serum for 45 minutes to block non-specific binding. Tissue sections were incubated with the following monoclonal antibodies: 1) to stain for corneal myofibroblasts, tissue sections were incubated with monoclonal mouse anti-alpha smooth muscle actin (alpha-SMA) (Sigma-Aldrich Corp, St Louis, Mo) at 1:500 dilution for 2 hours; 2) to determine proteoglycan synthesis and distribution in the corneal stroma, chondroitin sulfate and keratan sulfate were assayed with 1:300 dilution of mouse anti-chondroitin sulfate and 1:400 dilution of mouse anti-keratan sulfate (Sei-kagaku Corp, Chuo-Ku, Tokyo, Japan) for 1 hour; 3) as markers of scar tissue, stains for collagens I, III, IV, and VI were performed using antibodies (Sigma-Alrich Corp) at 1:200 to 1:400 dilutions for 2 hours; and 4) the presence of matrix metalloproteinases 1 and 9 (MMP-1, MMP-9) were detected using a monoclonal mouse anti-MMP-1 (1:300 dilution) and anti-MMP-9 (1:100 dilution) antibodies (Sigma-Aldrich Corp) for 2 hours.
In all cases, after rinsing with PBS, the secondary antibody fluorescein conjugated goat anti-mouse IgG (Santa Cruz Biotechnology Inc, Santa Cruz, Calif) was applied for 1 hour at room temperature. 4'6-diamidino-2-phenylindole (DAPI) nuclear counterstaining was performed for 30 minutes at room temperature according to the manufacturer's instructions. Negative controls were prepared without the primary antibody. Cover slips were mounted with VECTASHIELD mounting medium H:1000 (Vector Laboratories, Burlingame, Calif). All tissue sections were viewed and photographed using a Nikon Eclipse TE 200 fluorescence microscope equipped with a Nikon digital camera DXM 1200 (Nikon Inc, Melville, NY).
Cell Number Analysis
Photographs of the tissue sections were acquired using MetaVue version 5.0 (Universal Imaging Corp, Downingtown, Pa) and saved as a TIFF (Tagged Image File Format) file. The total number of DAPI-positive cells was measured in the flap and residual stromal bed, and the percentage of alpha-SMA–positive cells to the total DAPI-positive cells was calculated in a fixed area (10.000 μm2) for each sample using the image analysis program Image-Pro Plus 4.5 (Leeds Precision Instruments Inc, Minneapolis, Minn). Five fields were counted for each sample. Statistical analysis was performed using the Statistical Analysis System (SAS) software version 9.0 (SAS Institute, Cary, NC).
RESULTS
Gross examination of the corneal buttons showed a subtle paracentral LASIK flap identifiable using retroil-lumination techniques in four eyes. One cornea had a semicircular ring of haze that measured 60° along the wound margin of the LASIK flap corresponding to an old area of epithelial ingrowth.
Light Microscopy
Histological analysis revealed a 5- to 10-μm thick lamellar interface in all specimens. The scar was identified using H&E and PAS staining. The scar appeared slightly more cellular and fibrotic at the wound margin and less cellular and thinner in the paracentral and central portions compared with the normal adjacent corneal stroma. One corneal flap revealed the presence of epithelium at the margin, filling gaps in Bowman's layer, and ingrowth into the lamellar wound, known as epithelial facettes. Also, there was variable alignment of the cut end of Bowman's layer at the wound margin, and mild epithelial hyperplasia was seen over the wound margin in all eyes. Bowman's layer did not demonstrate any breaks, fissures, or iron staining along the entire cornea, which may be an indication of previous forme fruste keratoconus.
Expression of Alpha-Smooth Muscle Actin-positive Cells
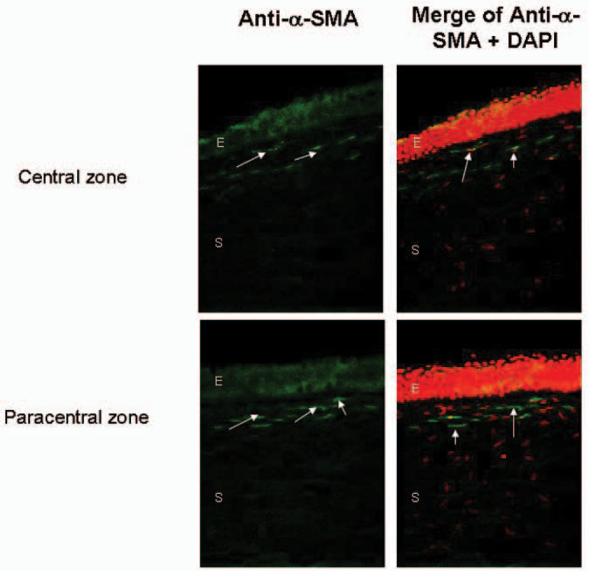
Alpha-SMA is a marker of stromal myofibroblasts, which are important in the process of wound healing. Small numbers of alpha-SMA–positive cells were found at the wound margin and in the paracentral or central regions in four corneas. They were mainly located in the superficial stroma under the epithelial wound margin surface (Fig 2). In one cornea, there was a strong, positive staining of alpha-SMA in the hypercellular fibrotic scar regions in the area of the semicircular ring of haze along the margin of the LASIK flap that clinically corresponded to epithelial ingrowth. The percentage of alpha-SMA–positive cells was compared with the total DAPI-positive cells at the flap edge as described. The peripheral flap interface in the cornea with a semicircular ring of haze along the wound margin of the LASIK flap corresponding to the epithelial ingrowth area had 14.3±1.2% of positive alpha-SMA cells compared with 2.3±3.1% of positive alpha-SMA cells in the other four corneas (P<.05). The total number of DAPI-positive corneal cells in the flap and stromal bed per mm2 for each case is shown in Table 2.
Figure 2.
Expression of alpha-smooth muscle actin (SMA) positive cells (arrows) is observed in the anterior 50 μm stroma under the epithelial wound margin surface in the central (upper panels) and paracentral zones (lower panels). All nuclei were counterstained with 4'6-diamidino-2-phenylindole (DAPI) (red cells). E=epithelium, S=stroma
TABLE 2.
4'6-diamidino-2-phenylindole (DAPI) Positive Stromal Cells at the Flap Edge and Anterior 50 μm Stromal Bed Using Immunohistochemistry After Penetrating Keratoplasty for Ectasia
| DAPI-Positive Stromal Cells (cells/mm2) |
||
|---|---|---|
| Age (y) | Flap Edge | Anterior 50 μm Stromal Bed |
| 28 | 26,312 | 16,820 |
| 33 | 24,312 | 19,132 |
| 27 | 23,211 | 16,543 |
| 34 | 25,390 | 21,328 |
| 31 | 27,531 | 17,176 |
Composition of the Extracellular Matrix
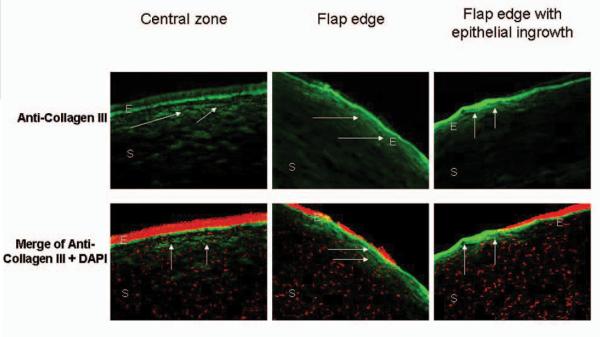
Weak positive collagen type III staining was found at the wound margin and paracentral or central regions in all corneas (Fig 3). Significant positive staining in the peripheral fibrotic scar region in the cornea with the epithelial ingrowth was noted.
Figure 3.
Low expression of collagen III (arrows) in ectatic human corneas after LASIK surgery is observed at the central zone (left panels) and flap edge (middle panels) of corneas with subtle flap edges. Significant expression of collagen III (arrows) was observed in the peripheral fibrotic scar region (right panels) in the cornea with epithelial ingrowth. 4'6-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining (red cells). E=epithelium, S=stroma
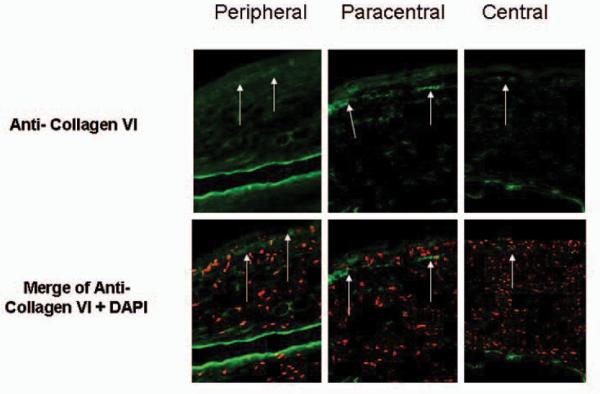
Collagen type I and V were noted throughout the entire lamellar scar (data not shown). Collagen VI was present in the interface at the wound margin of all corneas (Fig 4); however, its presence was reduced in the paracentral or central regions.
Figure 4.
Expression of Collagen VI (arrows) was observed in the interface of all samples in the paracentral and peripheral wound margin and less intensely in the central cornea (upper panels). 4'6-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining (red cells) (lower panels).
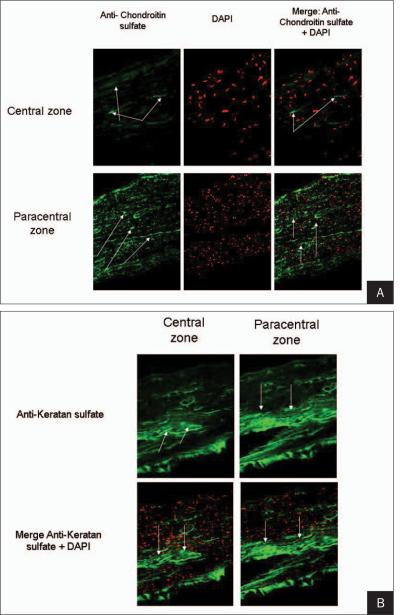
Chondroitin and keratan sulfate was stronger in the periphery of the flap wound (Fig 5).
Figure 5.
A) Increased expression of chondroitin sulfate (arrows) is observed in the central zone (upper panels) compared with the paracentral corneal flap edge (lower panels). Left panels show the expression of chondroitin sulfate. Middle panels demonstrate 4'6-diamidino-2-phenylindole (DAPI) nuclear counterstaining (red cells). Right panels show a merge of both stains. B) Increased expression of keratan sulfate (arrows) is observed in the central zone (left panels) compared with the paracentral corneal flap edge (right panels). Upper panels show the expression of keratan sulfate. Lower panels illustrate a merge of keratan sulfate and 4'6-diamidino-2-phenylindole (DAPI) nuclear counterstaining (red cells).
A significant expression of MMP-9 in the paracentral wound margin scar in all eyes was noted (Fig 6). No MMP-1 staining was observed in the paracentral or central corneas.
Figure 6.
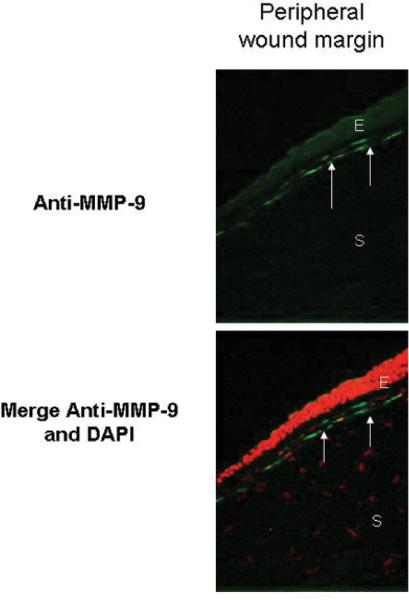
Significant expression of MMP-9 (arrows) is observed in the peripheral wound margin scar in all eyes analyzed (upper panel). Lower panel illustrates a merge of MMP-9 and 4'6-diamidino-2-phenylindole (DAPI) nuclear counterstaining (red cells). E=epithelium, S=stroma
DISCUSSION
The occurrence of iatrogenic keratectasia after LASIK is a concern in modern lamellar refractive surgery. Although the incidence is rare, its consequences may be devastating and induce severe myopia, irregular astigmatism, and loss of best spectacle-corrected visual acuity.
Corneal healing has been studied extensively in animal models and humans, especially in correlation with corneal transplantation, cataract surgery, and incisional refractive surgery.8,9 However, most of the cellular and extracellular details of LASIK wound healing have been evaluated using animal models.10–15 In the present study, we evaluated several key components of the corneal wound-healing process in fresh corneas of patients who developed ectasia after LASIK and required corneal transplantation.
Previous studies performed in rabbits and postmortem human corneas that underwent successful LASIK have shown a weak healing response along the central and paracentral flap surface, with the exception of the flap edge where distinct histologic signs of fibrotic scar formation have been observed.10
Our results are in agreement with previous studies done of successful LASIK buttons that describe a hypocellular primitive central and a hypercellular scar at the edge of the flap.10,16 We observed the presence of myofibroblasts in these corneas several years after the LASIK procedure. In the cornea with the epithelial ingrowth, myofibroblasts were located mainly at the flap edge. In the other four cases, they were present in fewer numbers in the anterior stroma. Three morphologically and functionally distinct types of keratocytes, derived from the quiescent undamaged keratocyte population after corneal stromal injury, have been documented: the migratory keratocyte (fibroblast) that migrates and repopulates the acellular wound zones; the activated keratocyte (protomyofibroblast) that synthesizes, deposits, and degrades extracellular matrix components; and the myofibroblast that initiates wound contraction.17 The reason for the presence of myofibroblasts several years after LASIK surgery is unknown. Using a rabbit model, we recently showed that the addition of sutures at the flap edge after LASIK reduces the amount of corneal steepening when the IOP was artificially increased.3 A significant increase in the amount of myofibroblasts was induced by the sutures, which may be responsible for this behavior. We postulated that the myofibroblasts at the flap edge may induce a stronger wound healing reaction, which may act as a belt to prevent the bulging effect of the central cornea when the IOP is increased.
The amount of myofibroblasts in the ectatic corneas in the present study was not sufficient to prevent corneal bulging. Our hypothesis is that their presence may be related to chronic movement of the flap that produces constant keratocyte activation and remodeling of the wound interface. We base our hypothesis on previous studies of uneventful LASIK eyes that have shown that the flap remained only weakly attached to the posterior stroma along most of its surface.10 Furthermore, Maguen et al18 suggest that the presence of fibronectin observed in LASIK eyes may not be a strong adhesive for corneal cells, which may explain the occurrence of flap dislocations many years after surgery.
In our group of eyes with ectasia after LASIK, the immunohistological evaluation of the flap margin demonstrated the presence of collagen III in a hypocellular scar, similar to that found in the paracentral and central areas of normal corneas. Collagen III lacks the insoluble connective tissue component important for resisting tensile stress. Also, the corneas contain a good amount of altered proteoglycans, such as chondroitin and keratin sulphate. Increased presence of MMP-10 has been found in the ectatic epithelium compared with corneas after uneventful LASIK.18 We observed significant MMP-9 and mild MMP-1 expression in the flap edge more than 3 years after the surgical procedure. The presence of MMP-9 may represent a marker of ectasia after LASIK and can lead to ongoing basement membrane remodeling with consequences in the stroma such as prolonged keratocyte apoptosis, resulting in decrease synthesis of extracellular components, corneal thinning, and ectasia.
In addition to a decrease in the population of keratocytes, a recent study demonstrated reduced expression of nidogen-1 and nidogen-2 around keratocytes in two patients with ectasia after LASIK, which may reflect an abnormal keratocyte state near the flap interface.18 Their influence on the biomechanical strength of the cornea remains to be clarified.
Corneal biomechanics embody many factors that include not only central corneal thickness but also viscosity, elasticity, hydration, regional corneal thickness, and many others not yet fully defined.19 The contribution of the above described changes in biomechanical properties that result in corneal thinning and ectasia needs to be further clarified.
Acknowledgments
This study was supported by LSU Translational COBRE Grant P20RR021970 (S. Esquenazi), National Institutes of Health; and R01EY04928 (Bazan), National Eye Institute, National Institutes of Health, Bethesda, Md.
Footnotes
The authors have no proprietary interest in the materials presented herein.
REFERENCES
- 1.Lifshitz T, Levy J, Klemperer I, Levinger S. Late bilateral keratectasia after LASIK in a low myopic patient. J Refract Surg. 2005;21:494–496. doi: 10.3928/1081-597X-20050901-12. [DOI] [PubMed] [Google Scholar]
- 2.Randelman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- 3.Abdelkader A, Esquenazi S, Shihadeh W, Bazan HE, He J, Gill S, Kaufman HE. Healing process at the flap edge in its influence in the development of corneal ectasia after LASIK. Curr Eye Res. 2006;31:903–908. doi: 10.1080/02713680600954278. [DOI] [PubMed] [Google Scholar]
- 4.Amm M, Wetzel W, Winter M, Uthoff D, Duncker GI. Histopathological comparison of photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Refract Surg. 1996;12:758–766. doi: 10.3928/1081-597X-19961101-07. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Kurosaka D, Bissen-Miyajima H, Tsubota K. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br J Ophthalmol. 2001;85:209–213. doi: 10.1136/bjo.85.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wachtlin J, Langenbeck K, Schründer S, Zhang EP, Hoffman F. Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg. 1999;15:451–458. doi: 10.3928/1081-597X-19990701-11. [DOI] [PubMed] [Google Scholar]
- 7.Esquenazi S, He J, Bazan HE, Bazan NG. Prevention of experimental diffuse lamellar keratitis using a novel platelet-activating factor receptor antagonist. J Cataract Refract Surg. 2004;30:884–891. doi: 10.1016/j.jcrs.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 8.Assil KK, Quantock AJ. Wound healing response to keratorefractive surgery. Surv Ophthalmol. 1993;38:289–302. doi: 10.1016/0039-6257(93)90078-l. [DOI] [PubMed] [Google Scholar]
- 9.Tuft SJ, Gartry DS, Rawe IM, Meek KM. Photorefractive keratectomy: implications of corneal wound healing. Br J Ophthalmol. 1993;77:243–247. doi: 10.1136/bjo.77.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, III, Edel-hauser HF. Histologic, ultrastructural and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch Ophthalmol. 2005;123:741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 12.Latvala T, Linna T, Tervo T. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int Ophthalmol Clin. 1996;36:21–27. doi: 10.1097/00004397-199603640-00005. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SE, Mohan RR, Hong JW, Lee JS, Choi R, Mohan RR. The wound healing response after laser in situ keratomileusis and photorefractive keratectomy: elusive control of biological variability and effect on custom laser vision correction. Arch Ophthalmol. 2001;119:889–896. doi: 10.1001/archopht.119.6.889. [DOI] [PubMed] [Google Scholar]
- 14.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 15.Spirn MJ, Dawson DG, Rubinfeld RS, Burris C, Talamo J, Edelhauser HF, Grossniklaus HE. Histopathological analysis of post-laser-assisted in situ keratomileusis corneal ectasia with intrastromal corneal ring segments. Arch Ophthalmol. 2005;123:1604–1607. doi: 10.1001/archopht.123.11.1604. [DOI] [PubMed] [Google Scholar]
- 16.Schmack I, Dawson DG, McCarey BE, Waring GO, III, Gross-niklaus HE, Edelhauser HF. Cohesive tensile strength of human LASIK wounds with histologic, ultrastructural, and clinical correlations. J Refract Surg. 2005;21:433–445. doi: 10.3928/1081-597X-20050901-04. [DOI] [PubMed] [Google Scholar]
- 17.Iskander NG, Peters NT, Anderson Penno E, Gimbel HV. Late traumatic flap dislocation after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1111–1114. doi: 10.1016/s0886-3350(01)00752-0. [DOI] [PubMed] [Google Scholar]
- 18.Maguen E, Maguen B, Regev L, Ljubimov AV. Immunohisto-chemical evaluation of two corneal buttons with post-LASIK keratectasia. Cornea. 2007;26:983–991. doi: 10.1097/ICO.0b013e3180de1d91. [DOI] [PubMed] [Google Scholar]
- 19.Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ. Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic and noncontact tonometry. Am J Ophthalmol. 2007;143:39–47. doi: 10.1016/j.ajo.2006.09.036. [DOI] [PubMed] [Google Scholar]