Several studies indicate FK506, a novel compound (Fig. 1), to be a potent immunosuppressive agent (1). Like cyclosporine, FK506 is isolated from a fungus, Streptomyces tsukubaensis, and is hydrophobic. Although cyclosporine is a peptide of larger molecular weight than FK506, both are cyclic compounds, with cyclosporine being more rigid than FK506. Recent in vitro studies suggest that FK506 possesses immunosuppressive properties similar to those of cyclosporine, implying that the mechanism of action for these two drugs may be similar as well. The immunosuppressive effect of FK506 is several-hundred–fold greater than that of cyclosporine (2, 3). As previously demonstrated for cyclosporine, the inhibitory effect of FK506 is seen in mixed leukocyte culture and on secondary proliferation of alloreactive T cells harvested from MLC or propagated from organ transplant biopsies (4). The published data further show that immunosuppression by FK506 may be mediated through an inhibition of interleukin-2 release (2, 4), again emphasizing the similarities between cyclosporine and FK506.
FIGURE 1.
FK506.
In this report we describe the characteristics and kinetics of cellular uptake and intracellular binding of FK506 by human peripheral blood lymphocytes. PBL were isolated from the blood of four healthy donors and depleted of their monocyte content as described previously (5).
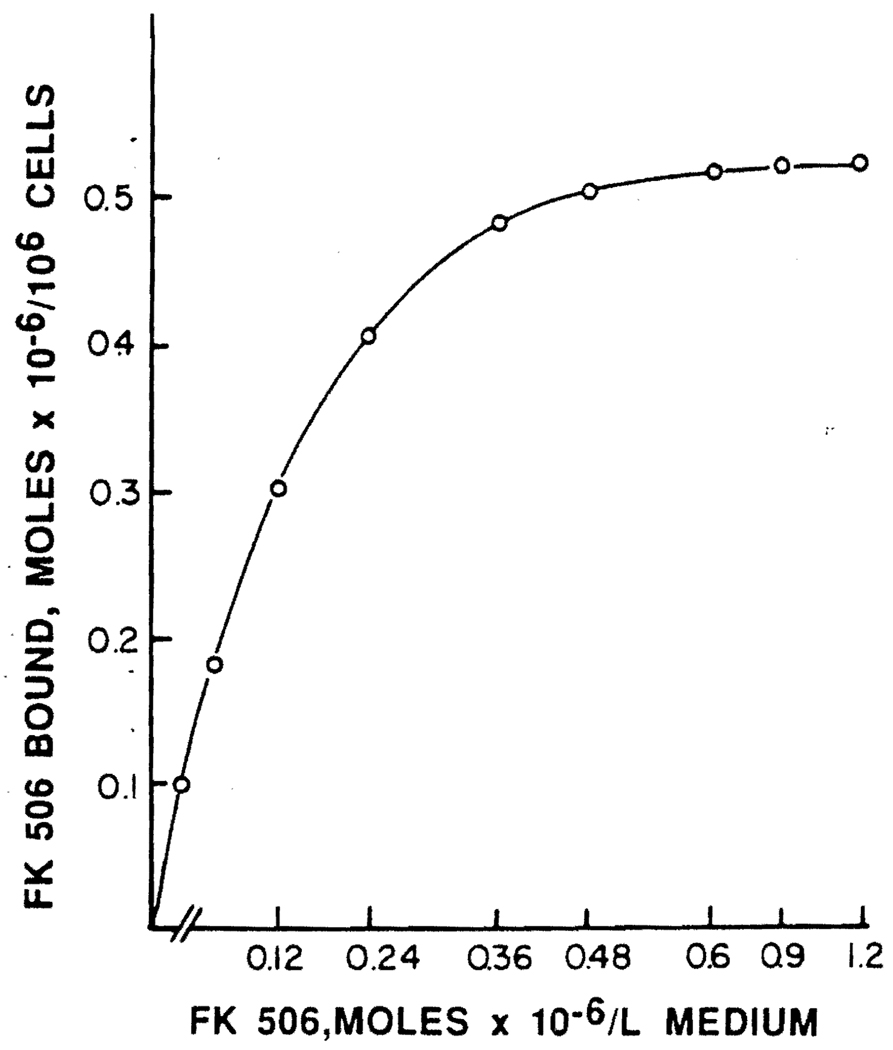
Binding and uptake of FK506 by PBL is shown in Figure 2. The uptake of FK506 by PBL is a saturable process, with saturation occurring at an approximately 0.5 µM concentration of the drug. Scatchard analysis of the binding data is consistent with two distinct classes of binding sites—one with a Kd = 3.9±1.8×10−8 M for the high affinity sites, and the second with a Kd = 5.2±0.8×10−6 M for the low affinity sites. This analysis further reveals that there are 5.6±1.0×l04 and 2.5±0.9×106 high- and low-affinity binding sites, respectively, per cell. LeGrue et al. (7) have reported that binding of cyclosporine by normal PBL also exhibits two classes of binding sites with a Kd of 2–6×10−9 M for the high-affinity site and a Kd of about 10−7 M representing a low-affinity site. They have further indicated that only B cells possess high-affinity sites. Our data, together with this information, seem to further delineate the similarities between FK506 and cyclosporine.
FIGURE 2.
Binding of FK506 by human peripheral blood lymphocytes. Normal human lymphocytes (5×106) were incubated for 60 min at 37°C in 250 µl of 1% BSA-RPMI 1640 medium containing the indicated concentrations of FK506. The cells were chilled at 0°C for 10 min and washed twice with cold saline, resuspended in phosphate buffer (0.2 M, pH 7.4), and the suspension was used for FK506 determination using the Fujisawa method (6). Results are in µmol/106 cells.
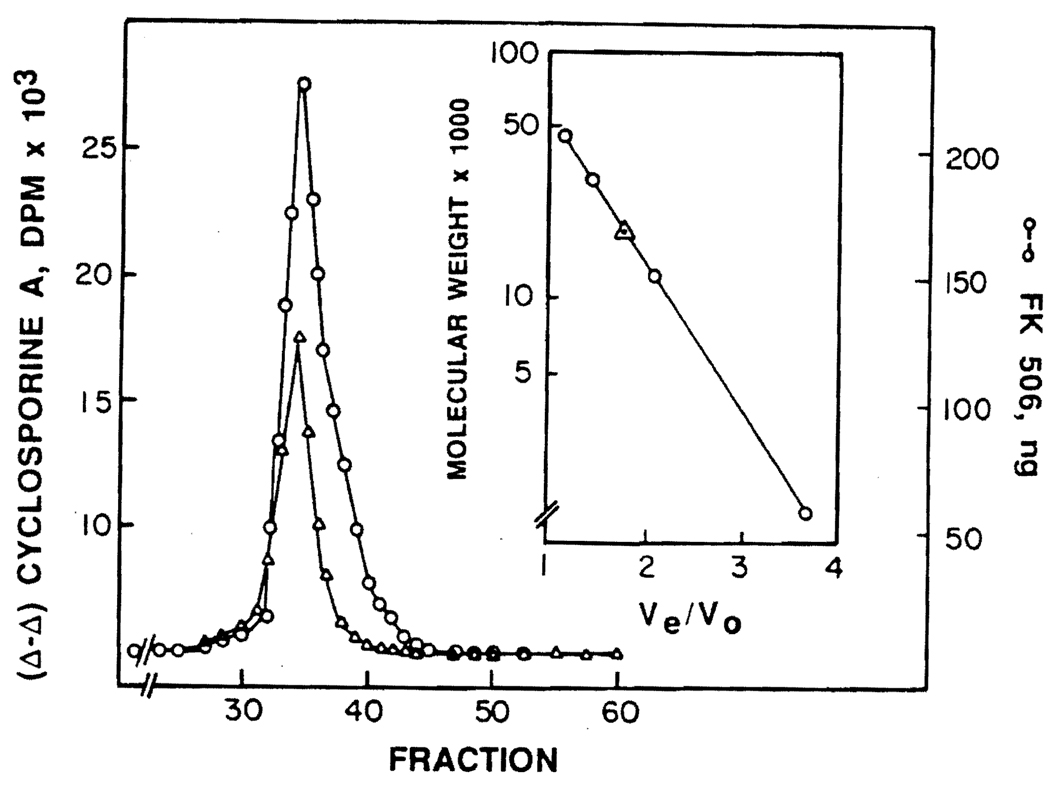
Merker and Handschumacher (8) have studied the intracellular localization of cyclosporine by a murine thymoma cell line (BW5147), and have shown that cyclosporine binds to a cytosolic protein (termed cyclophilin) with an apparent molecular weight of 15,000–20,000 daltons. More recently, Fabre et al. (9) have shown the presence of a similar protein in a human Burkitt lymphoma cell line (RAJI cells). The dissociation constant for the binding of cyclosporine to cyclophilin has been reported to be approximately 2.2 µM. We have investigated the binding of FK506 to cytosolic proteins obtained from PBL. The elution profile of FK506 and cyclosporine on a Bio-Gel P-60 exclusion column that had been calibrated with ovalbumin, carbonic anhydrase, cytochrome C, and vitamin B12 is shown in Figure 3. A graph of the log molecular weight versus the ratio of the elution volume to the void volume for the molecular weight markers is shown in the insert. These data suggest that both FK506 and cyclosporine elute in association with a protein with an apparent molecular weight of approximately 18,000–19,000 daltons. Whether this is incidental or there is in fact only one protein that binds both drugs is not clear at present. But, if a single protein, such as cyclophilin, is involved in the binding of both these drugs, then the question of whether two separate binding sites are involved in this process remains to be investigated. Recently, we have demonstrated that human PBL exposed to FK506 for periods varying from 1 hr to 40 hr are able to take up about 20% more cyclosporine relative to control cells not exposed to FK506 (10). Similarly, FK506 exposed cells show considerably increased sensitivity to cyclosporine (2–4) in terms of mixed lymphocyte reaction and primed lymphocyte tests. This evidence seems to suggest that binding of one drug, for instance FK506, modifies the cellular response to another drug (cyclosporine), resulting in a greater uptake of cyclosporine by the cell.
FIGURE 3.
Elution profile of the intracellular proteins of PBL on Bio-Gel P-60. Normal human lymphocytes (100×106) cells were incubated for 60 min at 37°C in 4 ml 1% BSA-RPMI 1640 medium containing 2 µg FK506 and 1.5 µg (3H) cyclosporine (specific activity 300 dpm/ng). The cells were chilled at 0°C for 10 min, washed twice with cold saline, and resuspended in Tris buffer (20 mM, pH 7.2) containing 2-mercaptoethanol (5 mM) and sodium azide (0.02%). Cells were disrupted by sonic oscillation (9), the cellular debris was removed by centrifugation at 40,000 g for 30 min, and the supernate was passed through a Bio-Gel P-6 column to remove protein-free FK506 and cyclosporine. The drugs were then chromatographed on a Bio-Gel P-60 column (40 cm × 1.5 cm) using the same Tris buffer. Utilizing the calibration curve of the molecular weight markers—(a) ovalbumin, (b) carbonic anhydrase, (c) cytochrome C and (d) vitamin B12—as described in the insert, it appears that both cyclosporine (∆) and FK506 (●) are associated with a protein or proteins with a molecular weight around 18,000–19,000 daltons.
In summary, kinetics of binding and uptake of FK506 by PBL, and the apparent common association of FK506 and cyclosporine with an intracellular protein, enhances further the similarities between these two immunosuppressive agents.
Acknowledgments
We gratefully acknowledge advice from Professor Adriana Zeevi throughout this work, and the technical assistance of Mrs. Jean Chao and Ms. Jacquelin Random.
REFERENCES
- 1.Sawada S, Suzuki G, Kanase y, Takaku F. Novel immunosuppressive agent, FK506: in vivo effects on the cloned T cell activation. J Immunol. 1987;139:1797. [PubMed] [Google Scholar]
- 2.Ochiai T, Nakajima K, Nagata M, et al. Effect of a new immunosuppressive agent, FK506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. 1987;19:1284. [PubMed] [Google Scholar]
- 3.Zeevi A, Duquesnoy R, Eiras G, Todo S, Makowka L, Starzl T. In vitro immunosuppressive effects of FR900506 on liver T cells aleoactivation. Surg Res Commun. 1987;1:315. [PMC free article] [PubMed] [Google Scholar]
- 4.Zeevi A, Duquesnoy R, Eiras G, et al. Immunosuppressive effect of FK-506 on in vitro lymphocyte alloactivation: synergism with cyclosporine A. Transplant Proc. 1987;19:40. [PMC free article] [PubMed] [Google Scholar]
- 5.Jerrells TR, Dean JH, Richardson GL, Herberman RB. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the Sephadex G-10 column method with other commonly used techniques. Immunol Methods. 1980;32:11. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Tamura K, Kaizu T, Tanaka H. A highly sensitive method to assay FR 900506 in plasma. Presented at a satellite workshop (FK900506: a potential breakthrough in immunosuppression) of the European Transplant Society; June 10, 1987; Gothenburg, Sweden. [Google Scholar]
- 7.LeGrue SJ, Friedman AW, Kahan BD. Binding of cyclosporine by human lymphocytes and phospholipid vescules. J Immunol. 1983;131:712. [PubMed] [Google Scholar]
- 8.Merker MM, Handschumacher RE. Uptake and nature of the intracellular binding of cyclosporin A in a murine thymoma cell line, BW5147. J Immunol. 1984;132:3064. [PubMed] [Google Scholar]
- 9.Fabre I, Fabre G, Lena N, Cano J-P. Kinetics of uptake and intracellular binding of cyclosporine�A in Raji cells, in vitro. Biochem Pharmacol. 1986;35:4261. doi: 10.1016/0006-2952(86)90704-5. [DOI] [PubMed] [Google Scholar]
- 10.Sanghvi A, Warty V, Zeevi A, et al. FK-506 enhances cyclosporine uptake by peripheral blood lymphocytes. Transplant Proc. 1987;19:45. [PMC free article] [PubMed] [Google Scholar]