Abstract
Background
Adolescent alcohol use may contribute to long-term changes in the receptors and neuroactive steroids that may mediate its effects and to subsequent alcohol abuse and dependence as an adult. Therefore, in the present study, ethanol preference and intake as an adult were examined after adolescent ethanol or saline administration. In addition, ethanol intake in the same groups was examined after administration of two neuroactive steroids with modulatory effects at GABAA receptors.
Methods
Two groups of male Long-Evans rats were administered 15 intraperitoneal (i.p.) injections of either ethanol (2 g/kg, 20% v/v) or saline between postnatal days 35–63. Starting on postnatal day 75, both groups were trained to consume 10% ethanol using a saccharin-fading procedure, and ethanol intake and preference were measured after a series of manipulations involving food deprivation, changes in the duration of access to ethanol, and changes in the concentrations of ethanol presented. Following these manipulations, pregnanolone (1–10 mg/kg) and dehydroepiandrosterone (DHEA, 1–100 mg/kg) were administered prior to preference sessions with an 18% ethanol solution.
Results
Adult ethanol preference and intake did not differ significantly in subjects treated with either saline or ethanol as adolescents during training, the substitution of other ethanol concentrations (3.2–32%), ad-lib feeding, or moderate food deprivation. Pregnanolone administration altered the intake of both adolescent-treated groups after the first injection of 3.2 mg/kg and after repeated injections with 10 mg/kg, a dose that produced sedation. In contrast, multiple doses of DHEA consistently decreased intake of an 18% ethanol concentration in both groups after repeated injections and three doses of DHEA (10, 32, and 56 mg/kg) administered with various ethanol concentrations dose-dependently shifted the ethanol-concentration curves for the volume and dosage of ethanol consumed downward.
Conclusions
These results indicate that chronic intermittent ethanol (CIE) administration of 2 g/kg during adolescence did not alter preference or overall consumption of ethanol in outbred rats trained to drink ethanol as an adult under the conditions tested, and that DHEA may be more effective than pregnanolone at significantly decreasing ethanol consumption.
Keywords: adolescence, ethanol intake, pregnanolone, dehydroepiandrosterone, rats
Despite epidemiological data in humans indicating that alcohol consumption during adolescence increases the risk of alcohol abuse and dependence as they grow older (Hawkins et al., 1997; Grant and Dawson, 1997), only meager evidence has been generated with most outbred animal models to support these data. For example, several studies in rodents have reported that ethanol administration during the early periods of life can lead to changes in ethanol intake as an adult (Hayashi and Tadokoro, 1985; Ho et al., 1989), while other rodent studies have shown little or no effect of early ethanol administration on intake (Siegmund et al., 2005) or its reinforcing effects (Tolliver and Samson, 1991; Slawecki and Betancourt, 2002) as an adult. Whether or not the differences in these effects were largely due to differences in the experimental conditions or other factors has not yet been determined; however, there remains a distinct need to examine the pharmacological and behavioral effects that may result from early ethanol administration. In the present study, outbred rats received chronic intermittent ethanol (CIE) administration of 2 g/kg during adolescence to determine if this regimen would alter subsequent ethanol preference and intake as an adult. This chronic intermittent dose, which was administered every other day for a total of 15 doses, was used because higher doses can produce weight loss during adolescent treatment (Slawecki and Betancourt, 2002; Silvers et al., 2003) or other effects that may not necessarily model effects consistently obtained during adolescent binging in humans such as the loss of the righting reflex or sedation. For example, an acute dose of 3.5 g/kg has been used to study ethanol-induced sleep time (VanDoren et al., 2000). A chronic intermittent schedule was used because this dosing regimen has been proposed to more highly mimic the binge administration often used by adolescents (White et al., 2000; Olsen et al., 2005; Silvers et al., 2006; Tokunaga et al., 2006).
Another purpose of the study was to determine the effects of pregnanolone and dehydroepiandrosterone (DHEA), positive and negative steroid modulators of the GABAA receptor complex (Paul and Purdy, 1992; Lambert et al., 1995), respectively, on ethanol intake in rats. Because neuroactive steroids may mediate some of the effects of ethanol (cf. Morrow et al., 1999), these compounds could provide insights into the mechanism of action of alcohol and possibility serve as new pharmacotherapies for alcohol abuse as suggested by some investigators (e.g., VanDoren et al., 2000; Ford et al., 2007). The interaction between the neuroactive steroids and alcohol is also particularly interesting because both classes of drug share numerous effects. Mechanistically, for example, neuroactive steroids and alcohol bind to specific, yet separate, sites on GABAA receptors and allosterically alter chloride flux similar to the barbiturates and benzodiazepines (e.g., Harrison et al., 1987; Grobin et al., 1998). In addition, both the neuroactive steroids (Rupprecht and Holsboer, 1999) and alcohol (Crews et al., 1996) bind to other ion channels and can allosterically alter their function. Behaviorally, alcohol and the neuroactive steroids that act as positive modulators at GABAA receptors can produce anxiolytic, hypnotic, anticonvulsant and amnestic effects similar to one another (Rupprecht and Holsboer, 1999; Kumar et al., 2004), while also substituting for one another in operant drug-discrimination procedures (Ator et al., 1993; Bowen et al., 1999; Engel et al., 2001). Nevertheless, there are differences between the different classes of drugs that allosterically modulate GABAA receptors and some of these differences translate to differences in their behavioral effects (De Vry and Slangen, 1986; Vanover et al., 1999). For example, benzodiazepines produce ethanol-lever responding in drug-discrimination procedures (suggesting that the two types of drug share discriminative stimulus effects), but ethanol does not necessarily produce benzodiazepine-lever responding (De Vry and Slangen, 1986). Furthermore, ethanol is often used illicitly in combination with other positive allosteric modulators of GABAA receptors (suggesting additive reinforcing effects), but ethanol and other positive modulators do not necessarily decrease ethanol intake when administered together (Leonard et al., 2006). One possibility that can account for these results is that ethanol acts at multiple receptors to produce effects that do not always overlap with drugs that act only as positive allosteric modulators of GABAA receptors. Another possibility is that GABAA receptor heterogeneity affects how GABAA receptors mediate these behavioral effects (Kumar et al., 2004). Regardless of the explanation, there is a clear need to examine these interactions on different behaviors in order to determine the behavioral specificity of these interactions.
Of particular relevance to ethanol’s effects at the GABAA receptor complex are the findings that antagonists of GABAA receptors such as picrotoxin (Boyle et al., 1993) and some drugs that can negatively modulate GABAA receptors such as the imidazobenzodiazepine RO15-4513 can decrease ethanol intake (Suzdak et al., 1986; Samson et al., 1987; Rassnick et al., 1993). If negative allosteric modulators of the GABAA receptor complex can reduce ethanol intake, then a neuroactive steroid such as DHEA, which has been shown to negatively modulate chloride flux at GABAA receptors (Imamura and Prasad, 1998) and GABA-induced membrane currents (Park-Chung et al., 1999), should also decrease ethanol intake. However, as mentioned above with regard to the effects of positive GABAA modulators, the behavioral effects of the different classes of negative modulators are not always alike or consistent across studies, particularly with respect to their capacity for blocking ethanol intake. For example, O’Dell et al. (2005) found that the 3β-hydroxysteroid epipregnanolone significantly attenuated ethanol self-administration in rats responding under a fixed-ratio (FR) 1 schedule for presentation of a 10% ethanol solution, whereas Ford et al. (2005) found that epipregnanolone had no effect on ethanol intake patterns in mice during a 2-hr session in which a 10% ethanol concentration was consumed. Given some of these disparities in the literature regarding the capacity of GABAA antagonists and negative allosteric modulators of GABAA receptors to reduce ethanol intake, the present study administered a range of DHEA doses to determine if it could reduce intake of an 18% ethanol solution in outbred rats. Furthermore, after the effects of DHEA on ethanol intake were determined under these experimental conditions, three doses of DHEA were administered with varying concentrations of ethanol to determine its effects on the volume and dosage of ethanol consumed.
Determining the effects of DHEA and pregnanolone in rats administered either ethanol or saline during adolescence also provided an opportunity to examine whether CIE administration might produce long-term or permanent changes in the effects of the neuroactive steroids on ethanol intake (i.e., permanently change the potency or effectiveness with which neuroactive steroids could affect ethanol intake). Certainly, if ethanol can alter the concentrations of specific neuroactive steroids in brain (Robel and Baulieu, 1995; VanDoren et al., 2000; Sanna et al., 2004) and change the sensitivity of GABAA receptors to other allosteric modulators after chronic treatment (Negro et al., 1993; Mehta and Ticku, 1998; Kang et al., 1998; Mehta and Ticku, 2001), there is reason to suspect that adolescent administration of ethanol might potentially have long-term consequences or effects on neuroactive steroid sensitivity. In addition, neuroactive steroids such as DHEA and allopregnanolone are elevated during adolescence (Fadalti et al., 1999) and as a result some investigators have suggested that neuroactive steroids may be involved in the developmental shift in the GABAA receptor composition during adolescence (Smith et al., 2007).
MATERIALS AND METHODS
Animals
Twenty-two male Long–Evans hooded rats were purchased at 25 days of age and served as subjects. Upon arrival, these subjects were housed 4 per cage and provided a diet of standard rodent chow ad libitum (Rodent Diet 5001, PMI Inc., St. Louis, MO, USA) until postnatal day (PD) 70. From PD 71 forward, the subjects were housed individually and maintained at 95% of their free-feeding weight during which a saccharin-fading procedure was conducted. Water was provided ad libitum in the home cage except during the experimental sessions. The home cage was made of polypropylene plastic and contained hardwood chip bedding. The colony room was maintained at 21 ± 2 C° with 50 ± 10% relative humidity on a 14L:10D light/dark cycle (lights on 06:00 h; lights off 20:00 h). Following training under the saccharin-fading procedure, the subjects were returned to ad libitum feeding conditions for 73 (minimum) to 89 (maximum) days during which an ethanol concentration-effect curve was established. After this curve was obtained, subjects were maintained at 95% of their free-feeding weight for the rest of the study. This was advantageous for several reasons. The first reason was that this relatively mild food deprivation reduced food consumption prior to the daily preference sessions, which could have affected absorption and thereby BECs. The second reason was that deprivation increased the comparability of the dosages of ethanol consumed across each set of manipulations by prohibiting the additional weight gain that would have resulted from ad-lib feeding. Lastly, deprivation tends to reduce the day-to-day variability in consumption and by reducing the baseline variability allows for the assessment of smaller effects. In general, changes in consumption or intake by a drug during restriction or in a highly motivated subject can be a strong indicator of a pharmacological effect.
Ethanol preference training and experimental sessions were conducted daily during the light cycle between the hours of 12:00 h and 14:00 h. Whenever subjects were on a restricted diet, their daily ration was provided between 16:00 and 17:00 h, several hours after their preference sessions were completed. All subjects used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, Louisiana State University Health Sciences Center, and in compliance with the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animal Resources (1996).
Adolescent ethanol and saline administration
While still housed 4 per cage, subjects were randomly divided into two groups, a group that received ethanol between PD 35 and 63 (adolescent ethanol group) and a group that received saline during the same postnatal period (adolescent saline group). The adolescent ethanol group (n=11) received 2 g/kg (20% v/v) of ethanol intraperitoneally (i.p.) every other day, whereas the adolescent saline group (n=11) received an equal volume of saline every other day, for a total of 15 injections.
Acquisition of ethanol drinking
Subjects were trained to consume ethanol orally using a modified saccharin/ethanol fading procedure (Samson, 1986; Leonard et al., 2006) starting on PD 75. Prior to each daily training session, animals were weighed and then returned to their home cage. Water bottles were removed and replaced for 30 min with 50-ml plastic centrifuge tubes containing a saccharin/ethanol solution and fitted with a rubber stopper and metal sipper tube. At the end of the 30-min session, the drinking tubes were removed, water bottles were replaced, and the volume of the solution consumed was determined by weighing the tubes (i.e., [(weight prior to the session - the weight after the session) - 0.4 ml]). The constant subtracted from the amount consumed served as a correction factor for spillage. All solutions were prepared fresh daily.
Rats initially received a 0.2% (w/v) saccharin sodium/0% ethanol (v/v) solution that was gradually replaced until a solution of 0% saccharin /10% ethanol was achieved. After a subject acquired stable saccharin/ethanol intake (± 20% of the mean for 3 consecutive days) or a maximum of 8 days elapsed, the next ethanol/saccharin solution was presented. Solutions were presented in the following order: 0.2% saccharin/0% ethanol, 0.15% saccharin/0.5% ethanol, 0.125% saccharin/1% ethanol, 0.1% saccharin/2% ethanol, 0.05% saccharin/5% ethanol, 0.01% saccharin/8% ethanol, and 0% saccharin/10% ethanol. Training, which took 40–57 sessions, and all subsequent testing was conducted 7 days per week.
Ethanol preference test
After a stable baseline of ethanol drinking was established with a 10% ethanol solution, a concentration-effect curve for ethanol and water consumption was established using the standard two-bottle preference test. During these tests, subjects were allowed simultaneous access to two drinking tubes daily for 60 min in their home cage, and the positions of the drinking tubes were reversed each day to avoid the development of a positional bias. One drinking tube contained tap water and the other contained varying concentrations of ethanol presented in the following order: 10%, 18%, 10%, 32%, 10%, 5.6%, 10%, 3.2%, 10% v/v. Immediately following the 60-min session, drinking tubes were removed, the water bottles were replaced, and the volume of water and ethanol consumed were determined. Each concentration of ethanol was presented daily until either stable consumption was observed (± 20% of the mean for 3 consecutive days) or a maximum of 8 days had elapsed. As indicated above, after the criterion was met for each concentration, the subjects were always returned to the 10% ethanol concentration (baseline) until one of the two criteria was met in order to assess any changes in baseline levels of ethanol intake. The data for these redeterminations of baseline intake were pooled for analysis. After completion of the ethanol concentration-effect curve under ad libitum feeding conditions, the subjects were again deprived to 95% of their free-fed weight at that time, and the ethanol concentration-effect curve was redetermined. During the redetermination of the curve and all subsequent experimental manipulations, the preference sessions were decreased in duration from 60 to 30 minutes, largely due to the fact that the majority of drinking occurred during that first 15 minutes of the session and there was little difference obtained in the overall amount consumed between these access durations.
Neuroactive steroid administration
Pregnanolone (5β-pregnan-3α-ol-20-one, Steraloids, Inc., Newport, RI) and dehydroepiandrosterone (DHEA; 5-androstene-3β-ol-17-one, Sigma-Aldrich, Inc, St. Louis, MO) were dissolved in a vehicle comprised of 45% (w/v) (2-hydroxypropyl)-γ-cyclodextrin (Sigma-Aldrich, Inc) and saline, and their effects on ethanol consumption were assessed using the two-bottle preference test as described above; however, the concentration of the ethanol solution that was presented daily was maintained at 18% (v/v) instead of 10% as consumption of the 18% concentration was similar to that for 10% while producing higher ethanol intake on a g/kg basis. Using the 18% ethanol concentration and a 30-minute session also facilitated comparisons with previous research in this laboratory examining the effects of GABAA modulators on ethanol intake (Leonard et al., 2006). Pregnanolone (1–10 mg/kg), DHEA (1–100 mg/kg) or vehicle were administered intraperitoneally (i.p.) 15 minutes prior to the daily 30-min sessions. Rats received non-contingent injections of each drug dose daily until one of the two criteria was met, i.e., either a stable level of intake was observed (± 20% of the mean for 3 consecutive days) or a maximum of 8 days had elapsed. After the testing of each dose was completed, subjects were always returned to the 18% ethanol concentration for the specified criterion to minimize any “carry-over” effects and to ensure that drug treatments had not altered baseline levels of ethanol intake permanently. Doses of pregnanolone were administered in the following mixed or semi-random order: 1 mg/kg, 3.2 mg/kg, vehicle, 10 mg/kg, 1.8 mg/kg, saline, and 5.6 mg/kg. Because DHEA’s effect on ethanol consumption persisted for some time after the injections were discontinued, injections of vehicle or saline were interspersed between doses of DHEA in the following order: 32 mg/kg, vehicle, 56 mg/kg, saline, 18 mg/kg, saline, 100 mg/kg, 1 mg/kg, vehicle, and 10 mg/kg. Saline was occasionally substituted for vehicle to help defray the inordinate expense of the vehicle and to prevent any toxic effects that might be associated with the repeated administration of high glucose-containing solutions (Frank et al., 1976). For pregnanolone and DHEA, the volume for both control (saline or vehicle) and drug injections was 0.1 ml/100 g body weight. To determine whether DHEA shifted the concentration-effect curve for ethanol, a concentration-effect curve for ethanol preference was also determined alone and in combination with 10, 32, and 56 mg/kg of DHEA as described above with a baseline concentration of ethanol maintained at 18%.
Blood collection and blood alcohol concentration (BEC) determinations
Venous blood samples of approximately 0.2 ml were collected by saphenous venepuncture immediately after the 30-min two-bottle preference test on the day each subject met criteria for the four different ethanol concentrations. These samples were then centrifuged (Eppendorf, Model #5418) at 14000 g for 4 min to collect plasma. These plasma samples were then harvested and stored at −80°C until blood alcohol levels (mg/dl) were quantified in duplicate using the MicroStat GM7 Analyzer (Analox Instruments, Inc., Lunenburg, MA). The intra-assay coefficient of variation was 2.5%, whereas the inter-assay coefficient of variation was 4.1%.
Data analyses
Data for the volume of ethanol or water consumed and for the dosage of ethanol consumed were analyzed using a two-way repeated measures ANOVA (treatment condition × type of solution, and treatment condition × ethanol concentration) followed by Holm Sidak post-hoc tests when significant main effects were detected (SigmaStat Statistical Software, SYSTAT Software, Inc. Point Richmond, CA, USA). The mean data for each subject were also grouped and analyzed for an effect of treatment and neuroactive steroid dose on ethanol intake and dosage using a two-way repeated measures ANOVA (treatment condition × dose of neuroactive steroid); however, when there was no effect of adolescent treatment, the data for both adolescent-treated groups were combined and analyzed using a one-way repeated measures ANOVA (Figures 4 and 5). When a significant effect of neuroactive steroid dose was detected, post-hoc Holm-Sidak tests were used to compare each dose with the respective control condition. To analyze the effects of the different doses of DHEA on the intake of the various ethanol concentrations (Figure 6), two-way repeated measures ANOVA tests were used to establish the significant interactions and one-way ANOVA tests were used to compare the effects of DHEA to the effects of ethanol alone on intake for each ethanol concentration. Significance was accepted at α level ≤0.05 for all statistical tests.
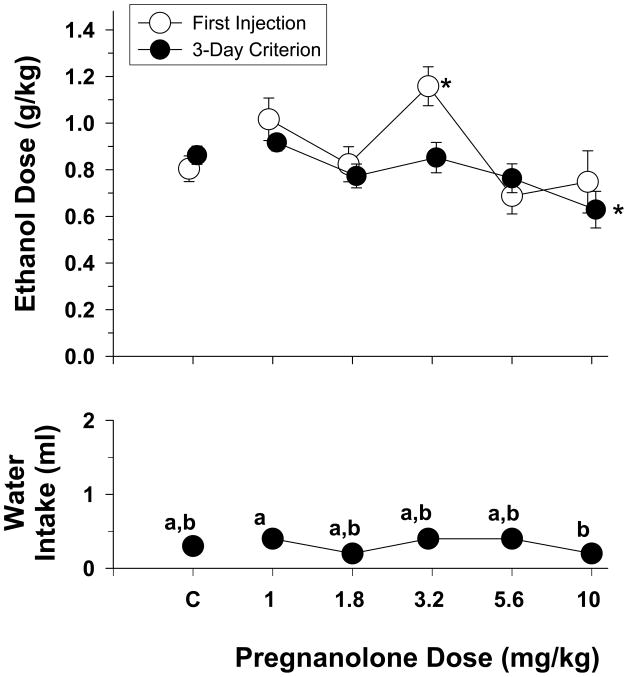
Fig. 4.
Effects of increasing doses of pregnanolone on the dosage of ethanol consumed during the presentation of different ethanol concentrations during 30-min two-bottle preference sessions in rats (n=22). The effects of pregnanolone on water intake are shown in the bottom panel. Unfilled circles with vertical lines indicate the mean and SEM obtained for the group after the first injection, whereas the filled circles with vertical lines indicate the mean and SEM obtained for the group after one of the two stability criteria was met (for additional details, see legend for Fig. 1). The adolescent-treated groups are not shown separately in this figure because there was no significant main effect for adolescent treatment and no significant interaction between adolescent treatment and the dose of pregnanolone. Asterisks indicate significant differences from control (“C”) sessions in which either vehicle or saline was administered prior to the presentation of the 18% ethanol solution. The letters in the bottom panel indicate significant differences among the different ethanol concentrations on water intake.
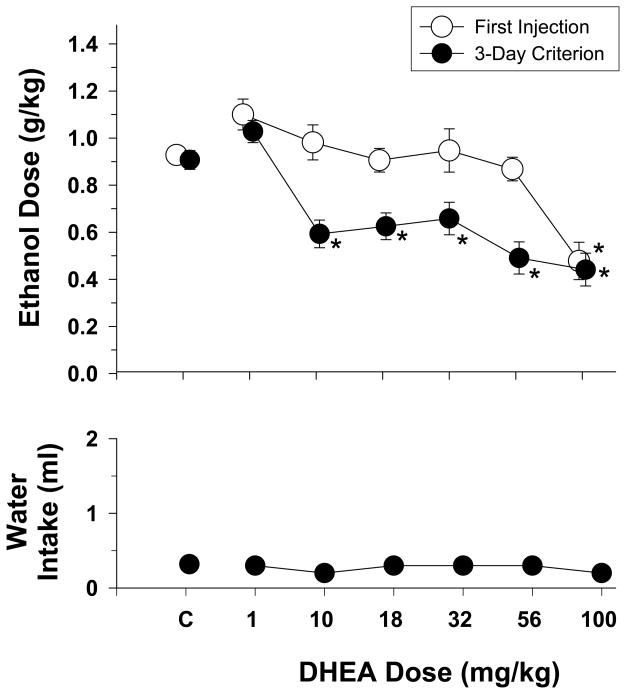
Fig. 5.
Effects of increasing doses of DHEA on the dosage of ethanol consumed during the presentation of different ethanol concentrations during 30-min two-bottle preference sessions in rats (n=22). The effects of DHEA on water intake are shown in the bottom panel. Unfilled symbols with vertical lines indicate the mean and SEM obtained for the group on the first day of injection, whereas the filled data points with vertical lines indicate the mean and SEM obtained for the group after one of the two stability criteria was met (for additional details, see legend for Fig. 1). The adolescent-treated groups are not shown separately in this figure because there was no significant main effect for adolescent treatment and no significant interaction between adolescent treatment and the dose of DHEA. Asterisks indicate significant differences from control (“C”) sessions in which either vehicle or saline was administered prior to the presentation of the 18% ethanol solution. No significant main effects were revealed for water intake.
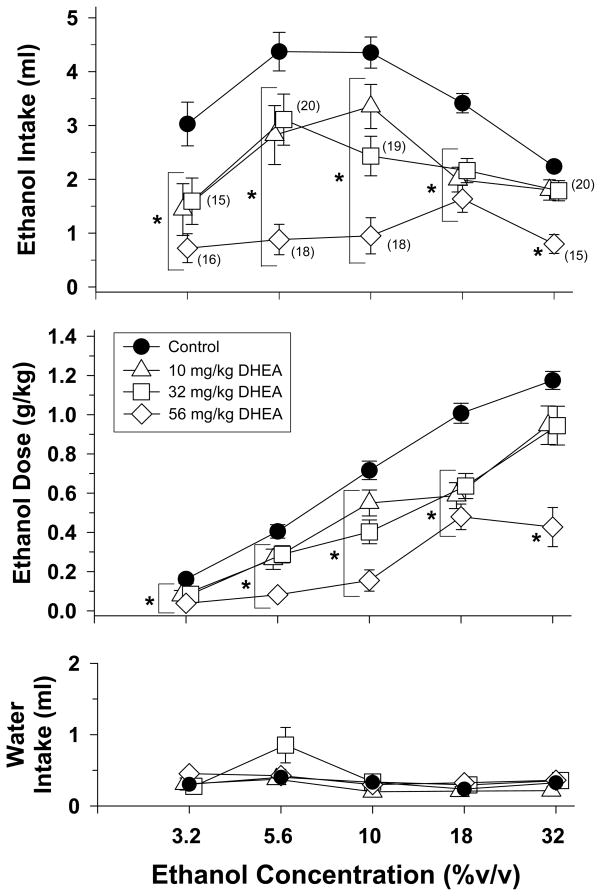
Fig. 6.
Effects of DHEA on the volume (top panel) and dosage (middle panel) of ethanol consumed by both adolescent-treated groups during 30-min two-bottle preference sessions with different concentrations of ethanol. The effects of DHEA on water intake are shown in the bottom panel. Filled circles with vertical lines (control) indicate the mean and SEM obtained for the group when they were drinking that concentration alone or the same concentration preceded by an injection of vehicle. Unfilled symbols with vertical lines indicate the mean and SEM obtained for the group when different doses of DHEA preceded that particular ethanol concentration. Each dose of DHEA was administered with each concentration of ethanol until one of the two stability criteria was met (for additional details, see legend for Fig. 1). Numerical values in parentheses and adjacent to a data point indicate the number of subjects represented by that point when it differed from the total number of subjects for that group (i.e., n=22). Asterisks alone or with vertical brackets indicate significant differences from control (filled circles).
RESULTS
Adolescent ethanol and saline administration
Prior to the initiation of ethanol and saline administration in the two groups of subjects on PD 35, the weights for the two groups were 146.64 ± 1.88 and 144.58 ± 2.31 g (mean ± SEM), respectively. On PD 63 at the end of ethanol and saline administration, the weights for the two groups were 340.18 ± 3.81 and 331 ± 5.14 g, respectively. When the grouped data were analyzed for an effect of treatment on body weight during the administration period, a two-way repeated measures ANOVA indicated that there was no effect of ethanol or saline treatment on body weights (F(1,20)=3.03, p=0.097), but there was a main effect of days due to the obvious weight gain during this period (F(25,500)=2573.66, p<0.001). The interaction between the adolescent treatments and the number of days of treatment was also not significant (F(25,500)=1.02, p>=0.434).
Acquisition of ethanol drinking
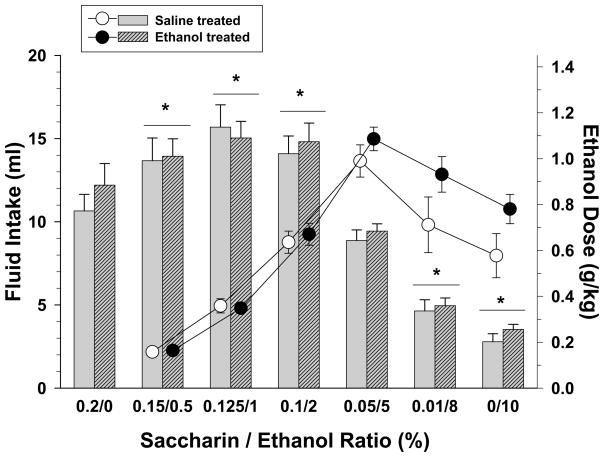
Figure 1 shows the volume of intake and the respective dosage of ethanol where appropriate for each of the saccharin/ethanol solutions by both groups during the 30-min training sessions. A two-way repeated measures ANOVA indicated that there was a significant main effect of solution (F(6,120)=148.08, p<0.001), but no effect of adolescent ethanol or saline treatment on the volume of intake during training (F(1,20)=0.2, p=0.657) and no interaction (F(6,120)=0.698, p=0.652). As shown, the fading procedure produced an inverted U-shaped curve for the volume of each solution consumed. More specifically, solutions comprised of relatively small ethanol concentrations produced the largest intake volumes for each group and the smallest dosages for ethanol intake, whereas solutions comprised of large ethanol concentrations produced the smallest intake volumes for each group and the largest dosages for ethanol intake. For example, compared to a solution containing only saccharin (i.e., 0.2/0), the mean fluid intake for the 10% ethanol concentration was 2.78 ± 0.49 ml for the group administered saline as adolescents, and 3.53 ± 0.31 ml for the group administered ethanol as adolescents. The average dosage produced by consuming the 10% ethanol solution was 0.58 ± 0.1 g/kg for the group administered saline as adolescents and 0.78 ± 0.06 g/kg for the group administered ethanol as adolescents. Similar to the analyses on the volume of each ethanol solution consumed during training, a two-way repeated measure ANOVA of the dosage of ethanol consumed indicated that there was a significant main effect of solution (F(5,100)=86.77, p<0.001), but no effect of adolescent ethanol or saline treatment (F(1,20)=2.01, p=0.171) and no interaction (F(5,100)=2.15, p=0.066).
Fig. 1.
Volume (bars) and dosage (circles) of ethanol consumed when varying solutions of saccharin and ethanol were presented to subjects that received either saline or ethanol as adolescents. Solutions were presented during 30-min training sessions and ranged from 0.2% saccharin with 0% ethanol (0.2/0) to 0% saccharin with 10% ethanol (0/10). Unhatched bars and unfilled circles with vertical lines indicate the means and SEM for subjects that received saline as adolescents (n=11), whereas hatched bars and filled circles with vertical lines indicate means and SEM for subjects that received ethanol as adolescents (n=11). Each solution was presented until one of two criteria was met; that is, until either intake did not vary by more than ± 20% for 3 days or a total of 8 days, in which case the last 3 of those 8 days were averaged for comparability. Asterisks indicate significant differences for both adolescent-treated groups from saccharin alone (0.2/0) as there was no significant main effect of adolescent treatment and no significant interaction between adolescent treatment and the various solutions.
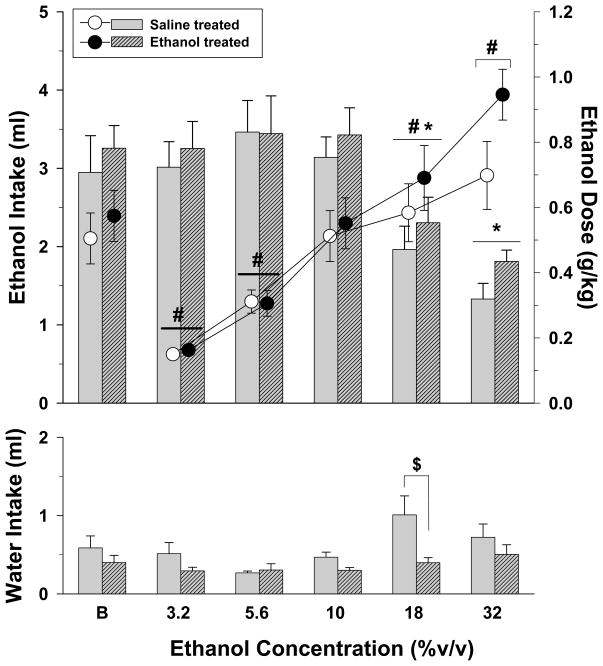
Ethanol preference and intake under ad libitum conditions
Shown in Figure 2 are the effects of varying concentrations of ethanol on the volume and dosage of ethanol consumed in rats fed ad libitum. During these 60-min drinking sessions, ethanol solutions were consumed preferentially over water at each of the five ethanol concentrations with preference ratios (ethanol intake/total fluid intake × 100) ranging from 71.68% to 89.9%. A two-way repeated measures ANOVA conducted on the data for ethanol intake in milliliters indicated that there was a significant effect of varying the ethanol concentration (F(5,100)=17.3, p<0.001), but no effect of adolescent saline or ethanol treatment at the different ethanol concentrations (F(1,20)=0.62, p=0.44) and no interaction of these factors (F(5,100)=0.21, p=0.959). Post-hoc analyses of the effects of ethanol concentration on ethanol intake indicated that both the 18 and 32% concentrations decreased intake in both of the adolescent-treated groups compared to their respective baseline levels of intake. A similar two-way analysis of ethanol concentration on the dosage of ethanol consumed also revealed a significant effect of ethanol concentration (F(5,100)=40.62, p<0.001), but no effect of adolescent treatment (F(1,20)=0.81, p=0.378) and no interaction between these two factors (F(5,100)=1.93, p=0.096). When compared to the dose of ethanol consumed under baseline conditions, both adolescent-treated groups consumed a significantly smaller dose of ethanol when the 3.2 and 5.6% ethanol concentrations were tested (p<0.05), and a significantly larger dose when the 18 and 32% ethanol concentrations were tested (p<0.05).
Fig. 2.
Volume (bars) and dosage (circles) of ethanol consumed when varying concentrations of ethanol were substituted for a 10% ethanol solution during a 60-minute two-bottle preference test. Water intake during the substitution of various ethanol concentrations is also shown in the bottom panel of the figure. Subjects from both adolescent-treated groups were fed ad libitum during these preference tests. Unhatched bars and unfilled circles with vertical lines indicate the means and SEM for subjects that received saline as adolescents (n=11), whereas hatched bars and filled circles indicate the means and SEM for subjects that received ethanol as adolescents (n=11). Each ethanol concentration was presented until one of two criteria was met; that is, until either intake did not vary by more than ± 20% for 3 days or a total of 8 days, in which case the last 3 of those 8 days were averaged for comparability. Asterisks indicate significant differences for both adolescent-treated groups from the volume consumed during baseline (B) conditions, whereas pound signs indicate significant differences for both adolescent-treated groups from the dosage of ethanol consumed during baseline (B) conditions. Significance is indicated for both groups because there was no significant main effect of adolescent treatment and no significant interaction between adolescent treatment and ethanol concentration. The dollar sign in the bottom panel indicates a significant difference between the saline- and ethanol-treated adolescent groups at the 18% ethanol concentration. This effect was revealed by post hoc tests conducted after a two-way repeated measures ANOVA revealed a significant interaction between water intake and ethanol concentration.
A two-way repeated measures analysis of water intake revealed small differences compared to ethanol intake in that there was a significant effect of adolescent treatment (F(1,20)=7.1, p=0.015) and ethanol concentration (F(5,100)=3.73, p=0.004); however, this was largely due to an increase in water intake in the group that received saline as an adolescent when an 18% ethanol solution was presented (i.e., p<0.05 for treatment within the 18% ethanol concentration). The interaction of these factors was not significant for water intake (F(5,100)=1.72, p=0.136).
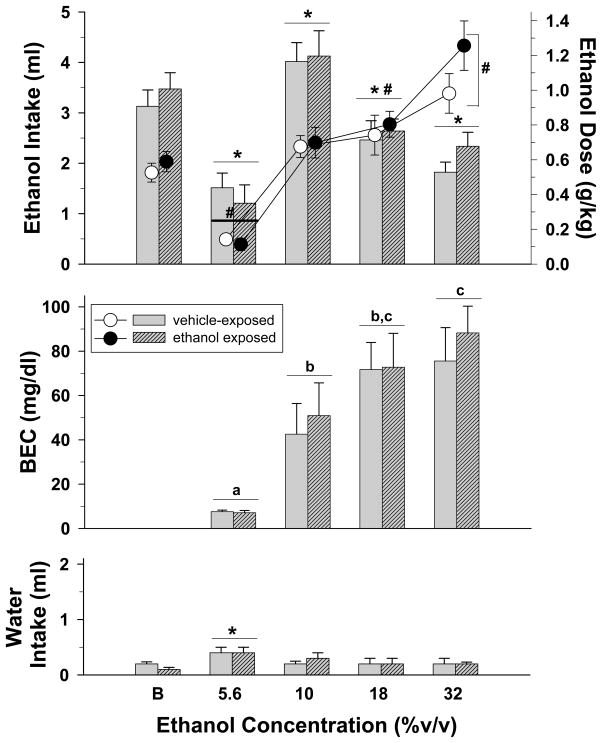
Ethanol intake under 95% food deprivation and blood ethanol concentrations (BECs)
Figure 3 shows the volume and dosage of intake of each concentration, and the corresponding BECs for both adolescent-treated groups during sessions in which both water and varying ethanol concentrations were available for 30 minutes. Under these baseline conditions, a two-way repeated measures ANOVA of the dosage data indicated that there was a significant main effect of ethanol concentration (F(4,80)=53.55, p<0.001), but no effect of adolescent treatment (F(1,20)=0.93, p=0.346) and no interaction (F(4,80)=1.39, p=0.246) of adolescent treatment with ethanol concentration. Subsequent comparisons using the Holm-Sidak method indicated that the dosage consumed was decreased significantly after substitution of the 5.6% concentration and increased significantly after the 18 and 32% ethanol concentrations compared to the baseline concentration of 10%. In terms of the volume of each concentration that was consumed in milliliters, there was a significant main effect for ethanol concentration (F(4,80)=32.14, p<0.001); the effect of adolescent treatment (F(1,20)=0.2, p=0.66) and the interaction was not significant (F(4,80)=0.69, p=0.604). As indicated by the inverted U-shaped curve, post-hoc tests also indicated that substituting each of the ethanol concentrations significantly altered the volume both groups consumed when compared to the baseline concentration of 10%.
Fig. 3.
Volume (bars) and dosage (circles) of ethanol consumed when varying concentrations of ethanol were substituted for a 10% ethanol solution during a 30-minute two-bottle preference test. Blood ethanol concentrations (BECs) and water intake during the substitution of various ethanol concentrations are also shown in the middle and bottom panels of the figure, respectively. Subjects from both adolescent-treated groups were mildly food deprived during these preference tests. Unhatched bars and unfilled circles with vertical lines indicate the means and SEM for subjects that received saline as adolescents (n=11), whereas hatched bars and filled circles indicate the means and SEM for subjects that received ethanol as adolescents (n=11). Each ethanol concentration was presented until one of two criteria was met; that is, until either intake did not vary by more than ± 20% for 3 days or a total of 8 days, in which case the last 3 of those 8 days were averaged for comparability. Blood to determine BEC was obtained on the final day of each concentration condition. Asterisks indicate significant differences for both adolescent-treated groups from the volume consumed during baseline (B) conditions, whereas pound signs indicate significant differences for both adolescent-treated groups from the dosage of ethanol consumed during baseline (B) conditions. Significance is indicated for both groups because there was no significant main effect of adolescent treatment and no significant interaction between adolescent treatment and ethanol concentration. Letters in the middle panel indicate significant differences among the different concentrations for both groups as there was no significant main effect of adolescent treatment and no interaction between adolescent treatment and ethanol concentration.
With respect to BEC, increasing concentrations of ethanol produced increasing BECs (F(3,60)=15.32, p<0.001), but the effect of adolescent treatment was again negative (F1,20)=0.39, p=0.539) and there was no interaction between ethanol concentration and adolescent treatment (F(3,60)=0.13, p=0.939). Not surprisingly, post-hoc analyses of the effect of concentration on BEC indicated that substitution of the 5.6% ethanol concentration produced BECs that were significantly less than the 10, 18, and 32% ethanol concentrations, and substitution of the 10% ethanol concentration produced BECs that were significantly less than the 32% ethanol concentration.
An analysis of the data for water intake revealed a significant effect of ethanol concentration (F(4,79)=6.52, p<0.001), but no effect of adolescent treatment (F(1,20)=0.18, p=0.673) and no interaction (F(4,79)=1, p=0.411). Further analysis of the effect of ethanol concentration indicated that water intake was increased in both adolescent-treated groups only for the 5.6% ethanol concentration when compared to the baseline concentration of 10% (Figure 3, bottom panel).
Effect of pregnanolone on ethanol intake
The data in the upper panel of Figure 4 show the effects of increasing doses of pregnanolone on the dosage of ethanol produced by consuming an 18% (v/v) concentration of ethanol. The dose-effect curves shown reflect the mean dosage after the first day of injection with each dose of pregnanolone as well as the mean dosage after the subjects in each group met criterion (i.e., 3 days in which intake did not vary by more than 20% or the last 3 days of the 8-day maximum for each dose combination). In this particular graph, the mean data for both adolescent treated groups were combined as there was no significant difference between the adolescent-treated groups in the effects of pregnanolone. When a one-way repeated-measures ANOVA was conducted on the data for the first day of injection, the analysis indicated that there was a significant effect of pregnanolone on the dose of ethanol consumed (F(5,105)=4.39, p=0.001); however, this effect was largely due to the 3.2-mg/kg dose of pregnanolone, which significantly differed from control administrations of vehicle or saline.. In contrast, the mean data obtained for the subjects when the criteria were met for each dose were less variable and tended to be more orderly. More specifically, there was a significant effect of pregnanolone dose on the ethanol dose consumed (F(6,105)=5.04, p<0.001) and post-hoc analyses using the Holm-Sidak method indicated that the 10-mg/kg dose significantly differed from control administrations of either vehicle or saline. Finally, as shown in the bottom panel of Figure 4, a one-way ANOVA on the volume of water consumed indicated that there was a significant effect of pregnanolone dose (F(5,105)=2.9, p=0.017). The only difference that was significant, however, was the difference between the effects of 1 and 10 mg/kg; that is, none of the doses of pregnanolone significantly altered the amount of water consumed when compared to control administrations of vehicle or saline (p>0.05).
Effect of DHEA on ethanol intake
Figure 5 shows the effects of increasing doses of DHEA on the mean dosage of ethanol consumed when subjects from both adolescent-treated groups were presented an 18% ethanol concentration. The data shown were obtained after the first day of injection with each dose of DHEA as well as after the subjects met criterion for that DHEA dosage. As in the previous figure for pregnanolone, the data for both adolescent-treated groups were combined and a one-way repeated-measures ANOVA was used to analyze the two dose-effect curves as there were no significant main effects for adolescent treatment. The one-way ANOVA indicated that there was a significant main effect of DHEA dosage after the first day of injection (F(6,126)=9.81, p<0.001), and after the subjects met criterion (F(6,126)=19.79, p<0.001). More specifically, compared to control injections, the highest dose of DHEA (100 mg/kg) significantly reduced ethanol intake after the first injection (p<0.05), whereas multiple doses (10 to 100 mg/kg) significantly reduced the dosage of ethanol consumed after the repeated injections necessary for the subject to meet the stability criteria (p<0.05). None of the doses of DHEA affected water intake (F(6,126)=0.6, p=0.733). Interestingly, ethanol intake also took 5 to 8 days to return to baseline levels after the final injection of each dose of DHEA (data not shown).
As shown in Figure 6, when 10, 32 and 56 mg/kg of DHEA were administered with varying concentrations of ethanol (3.2–32%), these doses shifted the ethanol-concentration effect curves for the amount of ethanol consumed (top panel) and the respective dosage of ethanol consumed (middle panel) downward significantly. The effects on both of these measures were indicated by significant interactions (i.e., F(12,218)=3.5, p<0.001) for ethanol intake and F(12,218)=5.74, p<0.001 for ethanol dose) after a two-way repeated measures ANOVA using a general linear model. Additional analyses of these data were comprised of one-way repeated measures ANOVA tests within each ethanol concentration to determine significant differences from ethanol alone (control). For the amount of ethanol consumed, all three doses of DHEA decreased intake for the concentrations of ethanol ranging from 3.2 to 18% (3.2% -F(3,50)=9.63, p<0.001, 5.6% - F(3,57)=18.74, p<0.001, 10% - F(3,56)=20, p<0.001, and 18% -F(3,63)=22.8, p<0.001), whereas 56 mg/kg of DHEA only decreased the intake of the 32% ethanol concentration (F(3,55)=16.23, p<0.001). The same pattern of effects was obtained on the respective dosages of ethanol consumed (3.2% - F(3,50)=9.69, p<0.001, 5.6% -F(3,57)=18.89, p<0.001, 10% - F(3,56)=20.36, p<0.001, 18% - F(3,63)=22.3, p<0.001, and 32% - F(3,55=15.97, p<0.001)
A two-way repeated measures ANOVA indicated that there was a significant interaction between DHEA and ethanol concentration on water intake (F(12,218)=1.97, p=0.028), but this effect was quite small overall and resulted from significant differences that were obtained when the 5.6% ethanol concentration was presented (F(12,218)=3.15, p=0.032).
DISCUSSION
As shown previously in this laboratory and others, ethanol intake can be established successfully in outbred male rats using a saccharin-fading procedure (e.g., Tolliver et al., 1988; Slawecki and Betancourt, 2002; Leonard et al., 2006). Furthermore, upon completion of training in this experiment, varying the ethanol concentration between 5.6 and 32% under either ad libitum or deprived feeding conditions produced concentration-dependent changes in ethanol intake, and deprived subjects reliably consumed ethanol in volumes that produced pharmacologically detectable BECs. For example, when subjects were food deprived to 95% of their free-feeding weight, increasing concentrations of ethanol produced an inverted U-shaped curve for intake and monotonically increased the curve for BECs from near 0 mg/dl for the 5.6% ethanol concentration to just over 80 mg/dl for the 32% ethanol concentration. Although subjects consumed somewhat less ethanol during each daily session when they were fed ad libitum, the consumption of ethanol under these conditions suggested that they were not just consuming ethanol for the calories. Another indication of the behavioral selectivity of this type of voluntary intake was that the subjects also maintained a clear preference for ethanol over water at each of the ethanol concentrations tested.
The present study also found that ethanol administration during adolescence did not affect ethanol intake, BECs or ethanol preference when compared to saline administration during adolescence. Although this finding is inconsistent with the general notion that adolescent experience with the effects of alcohol may increase the risk for alcohol abuse and dependence (Hawkins et al., 1997; Grant and Dawson, 1997), these negative data are consistent with several other studies in rats, which suggest that adolescent ethanol administration alone is not sufficient to produce high ethanol intake as an adult (Tolliver and Samson, 1991; Slawecki and Betancourt, 2002; Siegmund et al., 2005). In fact, in one of these studies, differences in the age of drinking onset could not be demonstrated until the subjects were stressed by either a forced swim or exposure to foot shock (Siegmund et al., 2005). Another concern regarding the negative findings of the present study could be that the 2-g/kg dose on alternate days was not large enough to produce the types of changes necessary to obtain the positive results reported in other studies with rats (Hayashi and Tadokoro, 1985) and mice (Ho et al., 1989) or that have led to the results reported in some epidemiological studies with humans (e.g., Hawkins et al., 1997; Grant and Dawson, 1997). However, 2 g/kg of ethanol is a behaviorally effective dose that has been shown to produce disruptions in behavior (Silvers et al., 2006) and a dose capable of increasing concentrations of specific neuroactive steroids in rat brain (VanDoren et al., 2000), while CIE administration is thought to be a reasonable model of a binge pattern of consumption frequently displayed by adolescents (White et al., 2000; Silvers et al., 2003). Another advantage of using a lower dose of ethanol than has been used previously for intermittent administration (White et al., 2000; Silvers et al., 2006) was that this dose did not produce any weight loss or other untoward effects in the ethanol-treated adolescent group. Finally, if the adolescent dosage alone were the critical factor for producing changes in adult preference and intake, studies such as the one by Slawecki and Betancourt (2002) where adolescent rats received ethanol vapor for 12 hours per day for 10 consecutive days, would have likely produced a positive effect, especially given that BECs in these adolescents averaged 250 mg/dl.
The administration of pregnanolone had little effect on ethanol intake in either adolescent-treated group and for these reasons the data obtained for the two groups were combined. When combined, analyses of the data indicated that 3.2 mg/kg of pregnanolone produced an increase in consumption on the first day of administration; however, this effect did not persist after the repeated injections that were required for a subject to meet the criteria used to indicate relative stability. In addition, 10 mg/kg of pregnanolone significantly decreased ethanol intake compared to vehicle when the subjects were allowed to meet criteria. These effects with pregnanolone are consistent with the literature in several ways, but they are also somewhat smaller than those that have been reported in the literature for allopregnanolone, the naturally occurring epimer of pregnanolone (Janak et al., 1998; Sinnott et al., 2002; Ford et al., 2005). For example, Janak et al. (1998) found that 3 mg/kg of allopregnanolone increased the number of lever presses for ethanol in rats responding under a fixed-ratio (FR) 4 schedule of reinforcement and that 10 mg/kg did not decrease overall responding significantly, but decreased the response rate during the initial minutes of the session indicating some nonspecific effects of this dose. Similarly, Ford et al. (2005) found that 3.2 mg/kg of allopregnanolone increased intake patterns for a 10% ethanol solution in mice during a 2-hr session, whereas 17 and 24 mg/kg decreased intake; 10 mg/kg was not different from vehicle in this study. In a third study by Sinnott et al. (2002) involving mice, both 3.2 and 10 mg/kg significantly increased intake of a 10% ethanol solution during the first hour of a 2-hr access period in male mice when administered over 3 consecutive days, whereas 10 mg/kg decreased access during the second hour of that period. Unlike the present study, however, doses of allopregnanolone were administered immediately before the access period, which could possibly have delayed the onset of the rate-decreasing effects of the 10-mg/kg dose.
The present data with rats are also consistent with a recent study from this laboratory (Leonard et al., 2006) indicating that all positive GABAA modulators do not produce additive effects on ethanol intake. In the study by Leonard et al. (2006), another positive GABAA modulator at the benzodiazepine binding site, flunitrazepam, did not affect ethanol intake at any dose tested when it was administered prior to 30-min ethanol preference sessions. These effects with flunitrazepam were also in direct contrast to the effects of noncontingent administrations of ethanol prior to the preference sessions, which dose-dependently decreased the intake of an 18% ethanol solution. Interestingly, the intake of ethanol in each subject decreased as the noncontigent dose of ethanol increased such that the total ethanol dose remained relatively stable, suggesting that the subjects were titrating the amount of ethanol they consumed to adjust for the noncontingent dose. This comparison between ethanol and flunitrazepam is not meant to suggest that benzodiazepines do not have the capacity to alter ethanol intake as they clearly can (Soderpalm and Hansen, 1998; Schmitt et al., 2002), but to suggest that positive GABAA modulators do not appear to uniformly produce effects that are identical to, or additive with, the effects that mediate ethanol intake (at least compared to ethanol itself). Moreover, this assessment likely includes the neuroactive steroids despite their capacity to alter ethanol intake in a dose-related manner.
Unlike the effects of pregnanolone on ethanol intake, DHEA doses ranging from 10 to 100 mg/kg significantly decreased intake of an 18% ethanol solution and three doses in this range (10, 32 and 56 mg/kg) significantly shifted the ethanol-concentration effect curves for the volume and dosage of ethanol consumed downward. To our knowledge, this is the first report of DHEA’s capacity to decrease ethanol intake in a preclinical animal model, although other steroids that can act as negative modulators at GABAA receptors have been shown to decrease ethanol self-administration (O'Dell et al., 2005), and there has been extensive research on the interaction of ethanol with the neuroactive steroids (Morrow et al., 2001 for review). In addition, the capacities of negative modulators of GABAA receptors for decreasing ethanol’s effects (particularly those that bind to the benzodiazepine binding site such as RO15-4513) have been highly publicized (e.g., Suzdak et al., 1986; Samson et al., 1987; Rassnick et al., 1993). With regard to the effects of the neuroactive steroids on ethanol intake, there does remain some confusion regarding the exact nature of their interaction with ethanol as both have not only been shown to interact with GABAA receptors, but ethanol has been shown to increase the plasma and brain concentrations of specific neuroactive steroids such as allopregnanolone (Barbaccia et al., 1999; VanDoren et al., 2000; Sanna et al., 2004). Also adding to the confusion surrounding the interaction of ethanol with the neuroactive steroids is the fact that ethanol can modulate both receptor subunit expression (Kumar et al., 2004) and the interaction of endogenous neuroactive steroids with specific GABAA receptor subtypes (e.g., Akk et al., 2007). A final complication surrounding the interaction of ethanol with the neuroactive steroids is that both are known to have effects at other ion channels (Crews et al., 1996; O'Dell et al., 2005).
To what extent these various mechanisms might be responsible for the effects of DHEA on intake will need to be determined with additional studies; however, there are some data indicating that 2 g/kg of ethanol in rats does not affect plasma or brain levels of DHEA or its sulfated congener, DHEAS (Morrow et al., 2001). According to Morrow et al. (2001), this is further evidence that ethanol may differentially regulate androstane steroids compared to pregnane steroids. Interestingly, the effects of pregnanolone and DHEA in the present study were not markedly different on the first day of injection even though pregnanolone increased intake at 3.2 mg/kg and DHEA decreased intake at 100 mg/kg, but they were very different after multiple injections were administered as indicated by the data obtained after one of the stability criterion was met. Moreover, ethanol intake often took multiple days to recover after the last injection of each of the doses of DHEA (data not shown), suggesting that its effect on intake may have had a slow onset and offset. If this is the case, these data could support the more general notion that DHEA was reducing ethanol intake by reducing caloric intake (e.g., Porter and Svec, 1995, rather than the notion that it was reducing ethanol intake by negatively modulating GABAA receptors, which is considered to be a relatively short-term, non-genomic effect of many neuroactive steroids (Losel et al., 2003). However, the fact that the highest dose of DHEA was able to reduce intake on the first day of injection argues against the notion of a slow onset, and multiple factors unrelated to its capacity to act as a negative modulator could account for the slow offset. For example, high concentrations of DHEA can be converted to DHEAS (Legrain et al., 2000), and DHEAS has been shown to enhance the desensitization of a specific population of GABAA receptors (Spivak, 1994). If that population of GABAA receptors is directly involved in mediating ethanol intake, then ethanol intake might require some time to recover after DHEA administration. Thus, even though DHEA had a slow offset in the current study, DHEA’s nongenomic capacity for negatively modulating GABAA receptors can not be ruled out as the main pharmacological mechanism responsible for its effect on ethanol intake.
Finally, the present study was able to examine the effects of adolescent ethanol administration on the subsequent sensitivity of adult rats to the effects of neuroactive steroids on ethanol intake. Because ethanol can increase the concentrations of specific neuroactive steroids (VanDoren et al., 2000; Sanna et al., 2004) and change the sensitivity of GABAA receptors to other allosteric modulators after chronic treatment (Negro et al., 1993; Mehta and Ticku, 1998; Kang et al., 1998; Mehta and Ticku, 2001), there is reason to suspect that adolescent administration of ethanol might potentially have had long-term consequences or effects on neuroactive steroid sensitivity; however, neither the effects of the pregnane steroid, pregnanolone, nor the androstane steroid, DHEA, significantly differed between ethanol- and saline-treated adolescent groups when they were tested with a range of doses of each drug as adults. These data are in contrast to several studies indicating that chronic ethanol administration can alter the modulatory effects of the neuroactive steroids at GABAA receptors. For example, Negro et al. (1993) found that more than 14 days of a 20% ethanol solution differentially increased the capacity of positive modulators to enhance muscimol binding in rat brain cortex. More specifically, this amount of ethanol administration increased muscimol binding produced by pregnanolone, but decreased muscimol binding produced by the barbiturate thiopental. In another study by Mehta and Ticku (1998), allopregnanolone further enhanced flunitrazepam and muscimol binding after high doses of ethanol (5 g/kg and higher) were administered three times per day for 6 days, and produced a greater inhibition of t-butylbicyclophosphorothionate (TBPS) binding in the hippocampus. Most of these data, however, were collected under very different experimental conditions than those in the present study. Unlike the present study, in these studies, there was frequently very little time between ethanol administration and testing, and the doses were substantially larger. In addition, not all of the published data show a positive effect. For example, Kang et al. (1998) found that the modulatory effects of the neuroactive steroid alphaxolone at GABAA receptors were not altered after 5–6 g/kg every other day for 60 doses.
In summary, CIE administration of 2 g/kg in rats during adolescence did not significantly alter ethanol intake or preference of adult subjects that were trained to drink using a sucrose-fading procedure. Compared to adolescent saline administration, adolescent ethanol administration also had no effect on ethanol intake under ad libitum or deprived feeding conditions, or on the intake-altering effects of two neuroactive steroids, pregnanolone and DHEA. More specifically, as adults, the neuroactive steroid pregnanolone had no effect on intake to criterion until a relatively high dose with sedative effects was administered, although there was an acute increase in intake at an intermediate dose similar to that reported previously.
In direct contrast to pregnanolone, DHEA decreased intake of an 18% ethanol solution over a range of doses and dose-dependently shifted the concentration-effect curve for ethanol intake and dosage downward. Such downward shifts in the concentration-effect curves for ethanol-maintained behavior in the presence of DHEA suggest an extinction-like pattern of responding, which is quite different from rightward or leftward shifts of a concentration-effect curve that reflect only changes in the sensitivity to ethanol. Moreover, an extinction-like pattern for ethanol intake in the presence of DHEA suggests that the androstane steroids may be as valuable, or more valuable, than the pregnane steroids as tools for understanding potential pharmacotherapies for alcoholism.
Acknowledgments
Supported by USPHS AA09803 (P.J.W.) and T32AA07577 (O.V.G.) from the National Institute on Alcohol Abuse and Alcoholism.
References
- Akk G, Li P, Manion BD, Evers AS, Steinbach JH. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the alpha1beta2gamma2L GABAA receptor. Mol Pharmacol. 2007;71:461–472. doi: 10.1124/mol.106.029942. [DOI] [PubMed] [Google Scholar]
- Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241:237–243. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases "GABAergic" neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- De Vry J, Slangen JL. Effects of training dose on discrimination and cross-generalization of chlordiazepoxide, pentobarbital and ethanol in the rat. Psychopharmacology (Berl) 1986;88:341–345. doi: 10.1007/BF00180836. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- Fadalti M, Petraglia F, Luisi S, Bernardi F, Casarosa E, Ferrari E, Luisi M, Saggese G, Genazzani AR, Bernasconi S. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr Res. 1999;46:323–327. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Gray JE, Weaver RN. Cyclodextrin nephrosis in the rat. Am J Path. 1976;83:367–374. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Tadokoro S. Learning retardation and enhanced ethanol preference produced by postnatal pretreatments with ethanol in adult rats. Jpn J Pharmacol. 1985;37:269–276. doi: 10.1254/jjp.37.269. [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6:511–515. doi: 10.1016/0741-8329(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22:2165–2173. [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Legrain S, Massien C, Lahlou N, Roger M, Debuire B, Diquet B, Chatellier G, Azizi M, Faucounau V, Porchet H, Forette F, Baulieu EE. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85:3208–3217. doi: 10.1210/jcem.85.9.6805. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Gerak LR, Gurkovskaya O, Moerschbaecher JM, Winsauer PJ. Effects of gamma-hydroxybutyric acid and flunitrazepam on ethanol intake in male rats. Pharmacol Biochem Behav. 2006;85:780–786. doi: 10.1016/j.pbb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Chronic ethanol administration alters the modulatory effect of 5alpha-pregnan-3alpha-ol-20-one on the binding characteristics of various radioligands of GABAA receptors. Brain Res. 1998;805:88–94. doi: 10.1016/s0006-8993(98)00649-0. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Unsulfated and sulfated neurosteroids differentially modulate the binding characteristics of various radioligands of GABA(A) receptors following chronic ethanol administration. Neuropharmacology. 2001;40:668–675. doi: 10.1016/s0028-3908(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Negro M, Casanova E, Chinchetru MA, Fernandez-Lopez A, Calvo P. Differential effect of chronic ethanol treatment on barbiturate and steroid modulation of muscimol-binding to rat brain cortex. Neurosci Lett. 1993;158:83–86. doi: 10.1016/0304-3940(93)90618-u. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Liang J, Cagetti E, Spigelman I. Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem Res. 2005;30:1579–1588. doi: 10.1007/s11064-005-8836-6. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Porter JR, Svec F. DHEA diminishes fat food intake in lean and obese zucker rats. Ann NY Acad Sci. 1995;774:329–331. doi: 10.1111/j.1749-6632.1995.tb17400.x-i1. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D'Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann N Y Acad Sci. 1995;774:82–110. doi: 10.1111/j.1749-6632.1995.tb17374.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, Mills FG. Oral ethanol reinforcement in the rat: effect of the partial inverse benzodiazepine agonist RO15-4513. Pharmacol Biochem Behav. 1987;27:517–519. doi: 10.1016/0091-3057(87)90357-1. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Waldhofer S, Weigelt T, Hiemke C. Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol Clin Exp Res. 2002;26:457–462. [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O'Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm AH, Hansen S. Benzodiazepines enhance the consumption and palatability of alcohol in the rat. Psychopharmacology (Berl) 1998;137:215–222. doi: 10.1007/s002130050613. [DOI] [PubMed] [Google Scholar]
- Spivak CE. Desensitization and noncompetitive blockade of GABAA receptors in ventral midbrain neurons by a neurosteroid dehydroepiandrosterone sulfate. Synapse. 1994;16:113–122. doi: 10.1002/syn.890160205. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol Clin Exp Res. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose- fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Suruk M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology (Berl) 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]