Abstract
Many of the long-term physiological effects of GH require hormone-mediated changes in gene expression. The transcription factor signal transducer and activator of transcription 5b (Stat5b) plays a critical role in the actions of GH on growth and metabolism by regulating a large number of GH-dependent genes by incompletely understood mechanisms. Here we have assessed the impact of GH-initiated and Stat5b-mediated signaling on the chromatin landscape of hormone-regulated genes in the liver of pituitary-deficient young adult male rats. In the absence of GH there was minimal ongoing transcription at the Socs2, Cish, Igfals, and Spi 2.1 promoters, minimal occupancy of Stat5b at proximal promoter sites, and relatively closed chromatin, as evidenced by low levels of core histone acetylation. In contrast, transcriptionally silent Igf1 promoter 1 appeared poised to be activated, based on binding of coactivators p300 and Med1/Trap220, high levels of histone acetylation, and the presence of RNA polymerase II. GH treatment led to a 8- to 20-fold rise in transcriptional activity of all five genes within 30–60 min and was accompanied by binding of Stat5b to the proximal Socs2, Cish, Igfals, and Spi 2.1 promoters and to seven distal Igf1 Stat5b elements, by enhanced histone acetylation at all five promoters, by recruitment of RNA polymerase II to the Socs2, Cish, Igfals, and Spi 2.1 promoters, and by loss of the transcriptional repressor Bcl6 from Socs2, Cish, and Igfals Stat5b sites, but not from two Igf1 Stat5b domains. We conclude that GH actions induce rapid and dramatic changes in hepatic chromatin at target promoters and propose that the chromatin signature of Igf1 differs from other GH-and Stat5b-dependent genes.
GH induces rapid changes in hepatic chromatin at target gene promoters, and we propose that the chromatin signature of Igf1 differs from other GH-activated genes.
Pituitary-derived GH plays a central role in many physiological processes in humans and other mammalian species (1,2,3). GH actions are critical for normal statural growth during childhood (3) and are important for regulating glucose, protein, and fat metabolism, and for tissue maintenance and repair throughout the lifespan (3). GH also has been implicated in the negative aspects of accelerated aging and in the development and propagation of certain cancers (4,5,6,7,8).
Because of both its biological and medical importance, much is now known about the physiology of GH actions in the whole organism and about the biochemical and molecular mechanisms linking the earliest events of GH-initiated intracellular signaling pathways to longer-term changes in cellular economy and tissue homeostasis (1,2). We now know that a single GH molecule binds with high affinity to the extracellular domains of two GH receptors, leading to the rapid and transient activation of Jak2, a receptor-linked intracellular tyrosine protein kinase, which by phosphorylating key tyrosine residues in the intracellular part of the GH receptor, promotes the activation of several signaling pathways that regulate the early cellular responses to GH (1,2). The intermediate and longer-term effects of GH are mediated by the actions of several hormone-stimulated transcription factors, which directly control gene expression and indirectly subsequent protein synthesis (1,2). Among the critical transcription factors that mediate these actions of GH are members of the signal transducers and activators of transcription (Stat) family, a group of seven related proteins that collectively function as effectors for many cytokines, growth factors, and hormones (9,10,11,12).
Stats are found as latent molecules in the cytoplasm of responsive cells before hormone or cytokine stimulation and then are recruited to phosphorylated tyrosine residues on activated receptors, where they are phosphorylated, usually by a Jak kinase, on a tyrosine near their COOH terminus (9). The modified Stats then dissociate from the receptor-docking site, dimerize by reciprocal interactions of a Src homology 2 domain on one Stat molecule with the phosphorylated tyrosine on the other (9), and enter the nucleus, where they bind as dimers to specific DNA sites in chromatin (9,10,11,12). A combination of observations, including inactivating genetic lesions in humans with growth deficiency (13,14,15) and targeted gene knockouts in mice (16,17), have implicated Stat5b as the Stat responsible for many of the longer-term physiological actions of GH. Several additional studies have demonstrated potential regulation of a cohort of genes by GH via Stat5b (18,19,20,21,22,23), including IGF-I (24,25), a secreted protein that mediates most of the effects of GH on growth (26,27,28).
IGF-I is a conserved single-chain 70-amino acid peptide with a complicated and conserved gene organization that in mammals consists of six exons and five introns, and spans more than 80 kb of chromosomal DNA (29). Transcription of IGF-I is governed by the actions of two promoters with variable transcription start sites, and gene activation leads to multiple IGF-I mRNAs through a combination of dual promoter usage, alternative RNA splicing, and differential RNA polyadenylation (reviewed in Ref. 29). Moreover, although Stat5b-dependent, the IGF-I gene lacks promoter-associated Stat5b response elements, and transcriptional activation in response to GH appears to be dependent on a series of dispersed distant Stat5b-binding domains (30,31,32).
Recent observations from our laboratory have focused on early events mediated by GH on IGF-I gene regulation, and we have found that GH treatment causes acute changes in chromatin structure at both IGF-I promoters, concomitant with transcriptional activation in the liver (33). Here we assess the early steps in GH action from a chromatin perspective at other hormone-regulated promoters in an in vivo model of GH deficiency, the hypophysectomized rat, and find that GH causes rapid increases in core histone acetylation and alterations in histone lysine 4 methylation in the liver that correlate with the rate and extent of GH-induced transcriptional activation. Remarkably, unlike IGF-I promoter 1, in which RNA polymerase II forms a preinitiation complex before GH, and the coactivator, p300, and cofactor, Med1/Trap220, are associated with DNA in chromatin at the promoter before gene activation, the other genes analyzed lack any evidence of being poised for transcription in the absence of hormonal stimulation. Thus, we conclude that GH-mediated signaling not only leads to acute changes in hepatic chromatin architecture but also that there are at least two distinct in vivo chromatin signatures for GH- and Stat5b-activated promoters.
Results and Discussion
Acute activation of gene transcription by GH
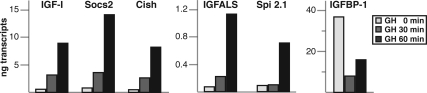
Previous studies have found that GH rapidly stimulates gene expression in cultured cell lines (19), in hepatocytes in primary culture (34,35,36), and in the liver in vivo (22,37,38). For a cohort of GH-responsive genes it has been established that Stat5b functions as a critical mediator of GH-activated target gene expression (22). Most prior experiments have relied on qualitative assays to establish gene regulation by GH or have assessed steady-state mRNA levels as indices of gene activation (18,19,21,22). We recently developed a real-time PCR-based assay for quantitatively measuring gene transcription by analysis of nascent nuclear transcripts (see Ref. 33 for details) and have applied it here to five genes induced by GH in a Stat5b-dependent way, Igf1, Socs2, Cish, Igfals, and Spi 2.1 (22,24,39,40,41,42,43), and to one, Igfbp1, in which Stat5b mediates acute transcriptional inhibition (44,45). As seen in Fig. 1, the transcription rates of all five GH-stimulated genes were low in hepatic nuclei from hormone-deficient rats (< 0.5 ng), whereas activity of Igfbp1 was 75–100 times higher. Systemic hormone treatment led to the rapid induction of transcription for the genes encoding IGF-I, Socs2, Cish, and IGFALS, with an approximately 5-fold rise within 30 min, and a 16- to 20-fold increase by 60 min after GH, although the overall abundance of nascent nuclear transcripts varied, with Igfals being 10-fold less than the other three genes. Transcription of Spi 2.1 also was stimulated by GH, but with a slower rate of rise (no change by 30 min, 8-fold increase by 60 min), and a lower overall abundance on par with that of Igfals. In contrast to the other five genes, transcription of Igfbp1 was acutely reduced by GH to approximately 20% of starting values by 30 min to levels comparable to those of GH-stimulated Igf1, Socs2, and Cish and remained diminished at 60 min (Fig. 1). Taken together, these results define a broad range of acute transcriptional responses to GH in the liver.
Figure 1.
GH rapidly induces gene transcription in vivo. Results are shown of time course studies of gene transcription in liver from GH-deficient male rats treated with a single ip injection of recombinant rat GH (1.5 mg/kg) for 0, 30, or 60 min. The graphs depict accumulation of nascent nuclear transcripts for IGF-I, Socs2, Cish, IGFALS, Spi2.1, and IGF-binding protein 1 (IGFBP-1), measured by RT-PCR, and quantified by using genomic DNA to generate standard curves (33). Transcript levels from a negative control GAPDH averaged approximately 0.1 ng (data not shown) and did not change after GH treatment. Results are representative of three independent experiments. Information on the PCR primers may be found in Table 1.
GH induces binding of Stat5b to target gene promoters
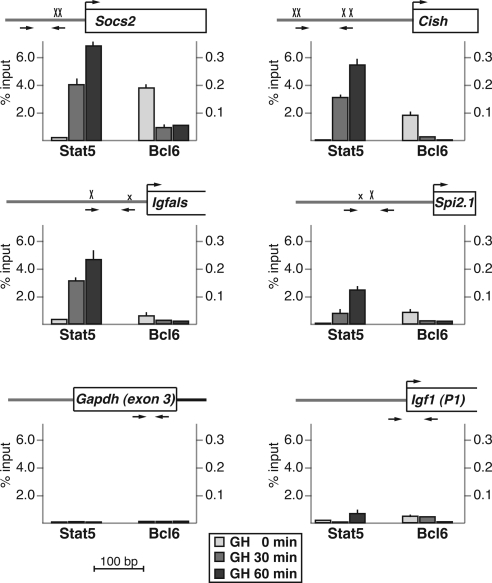
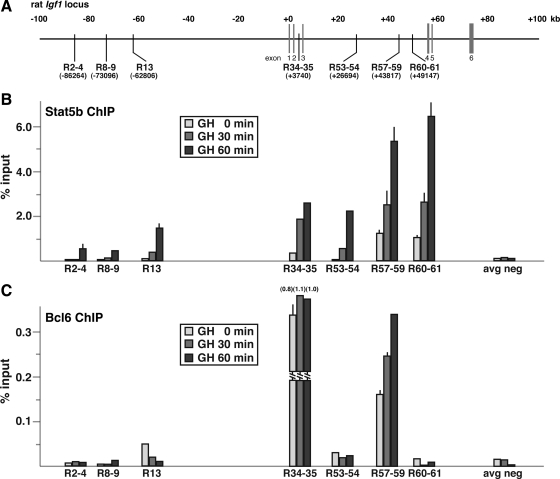
Previous studies have mapped putative Stat5 DNA elements to the proximal promoters of Socs2, Cish, Igfals, and Spi 2.1, primarily by functional assays and in vitro DNA-protein binding experiments (22,42,43,46), although only a few of these studies were performed with the cognate rat genes (43) (see notes on gene annotations in Materials and Methods). To test a potential role for Stat5b in mediating GH-activated transcription of these genes in vivo, we examined the impact of hormone treatment on recruitment of Stat5b to each proximal promoter by quantitative chromatin immunoprecipitation (ChIP) experiments, using rat hepatic chromatin as the starting material. Results demonstrate that in the absence of GH, very little Stat5b is found on any of these promoter regions, but that within 30–60 min after a single systemic GH injection, robust increases were seen, particularly at Socs2 and Cish (∼20-fold rise by 30 min, ∼30-fold by 60 min to ∼6% of input) (Fig. 2) but also to a lesser extent at Igfals (10-fold increase at 30 min, 15-fold at 60 min to ∼4.5% of input), and Spi 2.1 (increase to 2.5% of input at 60 min) (Fig. 2). There thus appears to be a rough correspondence between the rate and extent of GH-induced Stat5b binding and induction of gene transcription for Socs2, Cish, and Spi 2.1, but not for Igfals, where recruitment of Stat5b is substantially greater than the amount of transcriptional activation. In contrast, little Stat5b could be detected at Igf1 promoter 1 (Fig. 2), in agreement with our previous analyses (33), primarily because Stat5b is recruited to a dispersed series of at least seven putative binding domains that are found throughout the rat Igf1 locus (Fig. 3, A and B, and Ref. 32). As seen in Fig. 3B, peak recruitment of Stat5b to these seven DNA regions in response to GH varied in magnitude over an approximately 10-fold range, with values at domains R57-59 and R60-61 being as high as at the Socs2 and Cish promoters, and others (R2-4, R8-9, R13) being less than at the Spi 2.1 promoter. Because it has not been established yet which Stat5b domains might be responsible for activating the two Igf1 promoters, the precise biochemical mechanisms leading to the rapid and robust induction of Igf1 gene transcription by GH via Stat5b remain to be discovered.
Figure 2.
GH induces recruitment of Stat5b to the promoters of hormone-responsive genes and promotes loss of Bcl6 from a subset of genes. Results are shown of ChIP experiments by qPCR for Stat5b and Bcl6 using hepatic chromatin harvested from GH-deficient male rats treated with a single ip injection of recombinant rat GH (1.5 mg/kg) for 0, 30, or 60 min. Each graph depicts the strength of binding of Stat5b and Bcl6 to the region bounded by the PCR primers (horizontal arrows) on the map above (see Table 2 for primer DNA sequences and locations). The maps also display the proximal promoter and exon 1 (as a box; a bent arrow indicates the transcription start site) of each gene (Socs2, Cish, Igfals, Spi 2.1, Igf1); an X indicates each potential Stat5b-binding site (a smaller x marks imperfect sites). Results depict the mean ± sd of three independent experiments (the lack of an error bar indicates that the sd is too small to be seen). The negative control is from Gapdh exon 3.
Figure 3.
Variable binding of Bcl6 to GH-inducible Stat5b domains within the Igf1 locus. Results are shown of ChIP experiments by qPCR for Stat5b and Bcl6 using hepatic chromatin harvested from GH-deficient male rats treated with a single ip injection of recombinant rat GH (1.5 mg/kg) for 0, 30, or 60 min. A, Schematic of rat Igf1 locus, showing locations of previously documented GH-inducible Stat5b-binding domains. The graphs illustrate results of binding of Stat5b (panel B) or Bcl6 (panel C) to each Stat5b domain as a function of GH treatment and represent the mean ± sd of three independent experiments (the lack of an error bar indicates that the sd is too small to be seen). The negative control is from IGF-I exon 1. The location and DNA sequences of PCR primers may be found in Table 2. avg neg, Average negative.
GH promotes loss of binding of Bcl6 to a subset of Stat5b sites in chromatin
Recent publications have identified the transcriptional repressor Bcl6 (47,48) as a GH- and Stat5b-regulated gene (49,50) and have shown that hormone treatment acutely reduces Bcl6 gene expression, leading to a decline in its protein abundance (49,50). Bcl6 also has been found by ChIP to be located at the Stat5-binding element in the proximal Socs2 promoter in both cultured 3T3F442A adipocytes and in rat liver (49,50). To assess the impact of GH on Bcl6 DNA binding in hepatic chromatin, we performed a series of quantitative ChIP experiments targeting the 11 Stat5b domains identified in the five GH-activated genes. In the absence of GH, Bcl6 was readily detected at the Socs2 and Cish promoters (Fig. 2), and at Igf1 Stat5b domains R34-35 and R57-59 (Fig. 3), and to a lesser extent at the Spi 2.1 promoter (Fig. 2) and at Igf1 R13 (Fig. 3), but was minimally present at the Igfals promoter, at Igf1 promoter 1 (Fig. 2), or at the other Stat5b-binding domains in the rat Igf1 locus, R2-4, R8-9, R53-54, and R60-61 (Fig. 3). Bcl6 DNA recognition sites resemble those of Stat5 but appear to have more stringent requirements (47,51,52), although the optimal DNA sequences appear to vary somewhat, at least when results of binding site selection strategies from different investigative groups are compared (47,51,52). By inspection and comparison of the DNA sequences, we have not been able to distinguish the features that promote Bcl6 binding at Stat5 elements from those that do not (data not shown).
GH caused rapid loss of binding of Bcl6 to DNA in hepatic chromatin at the Socs2, Cish, and Spi 2.1 promoters concomitant with recruitment of Stat5b (Fig. 2), with the result for the former gene agreeing with previous observations (49,50), and at Igf1 R13 (Fig. 3), but surprisingly, hormone treatment did not reduce the abundance of Bcl6 at the other two Stat5b domains in the Igf1 locus, R34-35 and R57-59, despite accumulation of Stat5b, and the amount of Bcl6 at the latter location even increased after GH (Fig. 3). Based on these results, our observations raise a question about the postulated role of Bcl6 as a repressor of GH- and Stat5b-activated genes (49,50). Clearly additional studies will be needed to determine the spectrum of Bcl6 target sites that overlap with Stat5b domains in GH-regulated genes and to define the effects of Bcl6 on GH actions. Because ChIP studies do not reveal the fraction of potential sites occupied by a DNA-binding protein at an individual cell level, it is conceivable that both Bcl6 and Stat5b are bound to DNA in chromatin at the same time at the R34-35 and R57-59 regions in the Igf1 locus but possibly in different cells or on different chromosomes in the same cell. Whatever the explanation, these results show that GH-mediated signaling does not unfailingly promote loss of Bcl6 from DNA sites in chromatin.
Recruitment of RNA polymerase II to GH-activated promoters
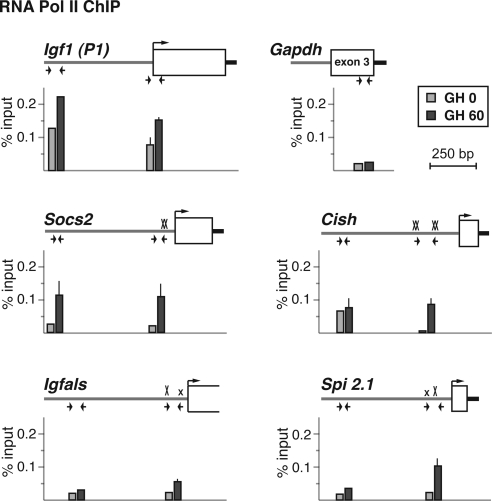
For many eukaryotic protein-coding genes, recruitment of RNA polymerase II (pol II) to the proximal promoter is the rate-limiting step for gene activation (53), although recent studies have suggested an alternative control point, in which previously recruited RNA pol II, poised at the promoter, is activated (53,54). In human hepatocytes, RNA pol II is found at more than one third of inactive promoters (55), and we showed recently that it was present at Igf1 promoter 1 in liver nuclei from GH-deficient rats in the absence of ongoing transcription, but that hormone stimulation was needed to recruit RNA pol II to promoter 2 (33). As seen in Fig. 4, analysis by quantitative ChIP revealed that RNA pol II was 2- to 10 times more abundant at Igf1 promoter 1 in hepatic nuclei from GH-deficient rats than at the Socs2, Cish, Igfals, or Spi 2.1 promoters. Treatment with GH led to accumulation of RNA pol II at all five GH-activated promoters, although at 60 min after hormone injection its abundance remained highest at Igf1 promoter 1 (Fig. 4). Thus, GH-mediated signaling leads to recruitment of RNA pol II to responsive promoters, although it is already present at fairly high levels before GH administration at Igf1 promoter 1.
Figure 4.
RNA polymerase II is present at Igf1 promoter 1 in the absence of GH but is recruited by GH treatment to other hormone-activated promoters. The graphs present results of ChIP experiments by qPCR showing binding of RNA Pol II at Igf1 promoter 1 and at the Socs2, Cish, Igfals, and Spi 2.1 promoters in hepatic chromatin of pituitary-deficient rats before (light gray bars) or 60 min after (dark gray bars) a single systemic GH injection. The map above each graph illustrates the 5′-end of each gene. Horizontal arrows denote the locations of qPCR primers (see Table 2 for DNA sequences), boxes represent exons, and a bent arrow indicates the transcription start site. An X marks each Stat5b site (smaller x’s are imperfect sites). The negative control is from Gapdh exon 3. Results represent the mean ± range of two independent experiments (the lack of an error bar indicates that the range is too small to be seen).
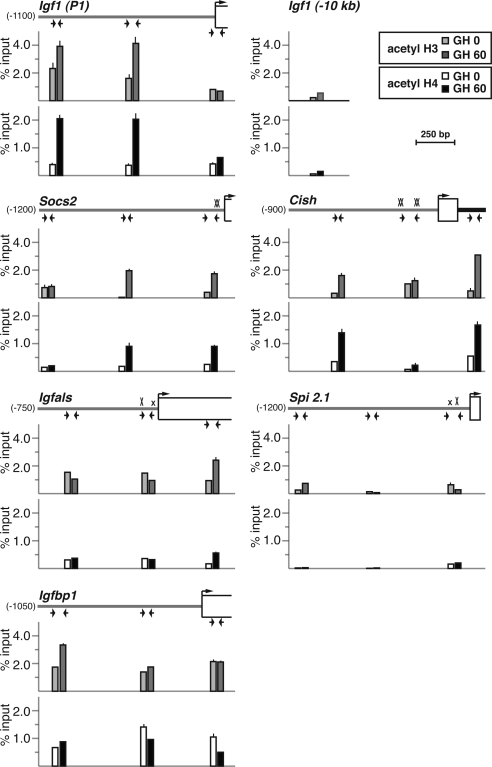
GH induces histone acetylation at transcriptionally activated promoters
Chromatin modifications at promoters that make chromosomal DNA more accessible to transcription factors and other regulatory proteins typically accompany signal-mediated gene activation (56), and we have shown recently that GH treatment leads to the acute enhancement of core histone acetylation at Igf1 promoters 1 and 2 in the liver (33). To discern the epigenetic effects of GH on other hormone-regulated genes, we examined their proximal promoters by quantitative ChIP for changes in histone lysine acetylation (H3 K9 and 14; H4 K5, 8, 12, and 16). GH treatment led to a rapid increase of core histone acetylation at Igf1 promoter 1 from a relatively high baseline (Fig. 5), as seen previously (33). GH also acutely enhanced histone acetylation at several sites in the Socs2 and Cish promoters and to a modest degree at a single location within the Igfals transcription unit (Fig. 5). By contrast, levels of histone H3 and H4 acetylation at the Spi 2.1 promoter were as low as a negative control, a site located approximately 10 kb 5′ to Igf1 exon 1 (Fig. 5). H3 and H4 acetylation at the Igfbp1 promoter remained relatively constant before and after GH treatment (Fig. 5), demonstrating that there was no correlation between acute transcriptional inhibition and loss of core histone acetylation, at least at this gene.
Figure 5.
GH induces histone H3 and H4 acetylation at hormone-responsive promoters. The graphs depict results of ChIP experiments by qPCR assessing acetylation of histone H3 (top) and H4 (bottom) at Igf1 promoter 1 and at the Socs2, Cish, Igfals, Spi 2.1, and Igfbp1 promoters in hepatic chromatin of pituitary-deficient rats before (light bars) or 60 min after (dark bars) a single systemic GH injection. The map shown above each pair of graphs illustrates the 5′-end of each gene. Horizontal arrows denote the locations of qPCR primers (see Table 2 for DNA sequences), a bent arrow indicates the transcription start site, and boxes represent exons. An X marks each Stat5b site (smaller x’s are imperfect sites). Depicted results represent the mean ± sd of three independent experiments (the lack of an error bar indicates that the sd is too small to be visualized). The negative control is from a region located approximately 10 kb 5′ to IGF-I exon 1.
Additional studies by ChIP-chip assay confirmed results observed by quantitative ChIP for histone H3 acetylation at Igf1, Socs2, and Spi 2.1 promoters (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). These results further revealed more extensive GH-stimulated acetylation beyond the Igf1 and Socs2 promoters, and into the respective transcription units, while demonstrating that histone H3 is minimally acetylated in chromatin throughout the entire Spi 2.1 locus (Supplemental Fig. 1). Collectively, the studies in Fig. 5 and Supplemental Fig. 1 show a rough correspondence between the extent and breadth of GH-stimulated histone acetylation at these gene promoters and the amount of gene transcription acutely induced by GH, suggesting that these modifications may provide a useful index for transcriptional potency.
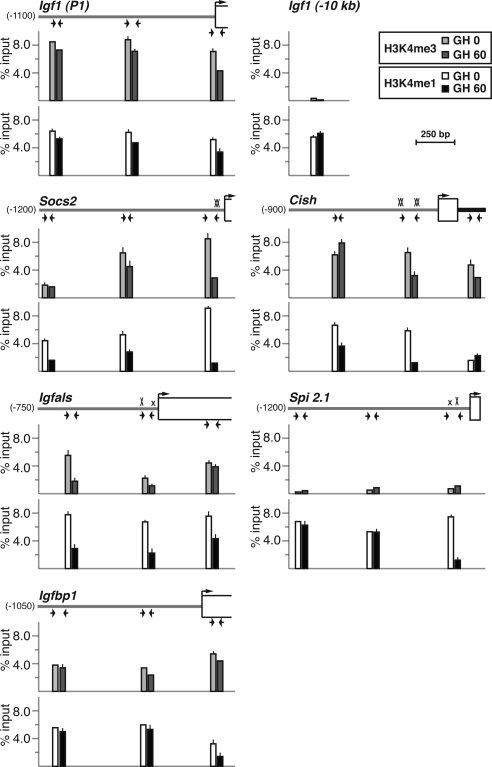
GH-induced changes in histone lysine methylation coincide with Stat5b-binding domains in promoters of hormone-activated genes
Whole genome studies of methylation of core histones have led to the hypothesis that high levels of trimethylation of histone H3 lysine 4 (H3K4me3) are associated with active promoters (56,57,58). In contrast, monomethylated histone H3 at lysine 4 (H3K4me1) has been proposed to be part of the epigenetic signature of enhancers (56,57,58,59). Analysis of H3K4me3 and H3K4me1 by quantitative ChIP in hepatic chromatin at the five GH-activated promoters revealed high levels of both modifications at Igf1 promoter 1, with small decreases in H3K4me1 and H3K4me3 after GH (Fig. 6), in good agreement with our previous results (33). In contrast, at the promoters of the other four genes induced by GH, hormone treatment stimulated acute declines in the abundance of both H3K4me3 and H3K4me1 that were most pronounced in chromatin that overlaid the Stat5b-binding domains in each proximal promoter (Fig. 6). The only exception was the Spi 2.1 promoter, which had levels of H3K4me3 that were as low as a negative control, a region located approximately 10 kb 5′ to Igf1 exon 1 (Fig. 6). Of note, there was a good correlation between the amount of H3K4me3 at the Stat5b-binding domain before GH treatment and subsequent transcriptional activation by GH for the four genes with Stat5b elements in their proximal promoters. By contrast to these observations, the abundance of H3K4me3 and H3K4me1 in liver chromatin at the Igfbp1 promoter remained fairly constant before and after GH, with only a mild decrease being seen within the transcription unit after systemic hormone administration. Thus, GH appears to be able to selectively promote the recruitment and/or activation of histone demethylases to some hormone-stimulated genes, including Socs2, Cish, Igfals, and Spi 2.1, leading to dramatic acute chromatin modifications in their promoters, but has a much more limited effect at others, such as Igf1. One of the enzymes recruited or activated by GH is likely to be LSD1, the only known H3K4me1-specific demethylase (60,61), and others may be one or more of several H3K4me3 demethylases (61). Future experiments will need to definitively identify these enzymes, to establish how GH-mediated signaling promotes their localized activity and to determine their functional roles in GH-regulated gene transcription.
Figure 6.
Assessing changes in histone lysine methylation after GH treatment. The graphs present results of ChIP experiments by qPCR measuring levels of histone H4 lysine 4 methylation [H3K4me3 (top), H3K4me1 (bottom)] at three locations in Igf1 promoter 1 and in the Socs2, Cish, Igfals, Spi 2.1, and Igfbp1 promoters in hepatic chromatin of pituitary-deficient rats before (lighter bars) or 60 min after (darker bars) a single systemic GH injection. The map shown above each pair of graphs illustrates the 5′-end of each gene. Horizontal arrows denote the locations of qPCR primers (see Table 2 for DNA sequences), a bent arrow indicates the transcription start site, and boxes represent exons. An X marks each Stat5b site (smaller x’s are imperfect sites). The unchanging control is from a region located approximately 10 kb 5′ to IGF-I exon 1. Results depict the mean ± range of two independent experiments (the lack of an error bar indicates that the range is too small to be seen on the graphs).
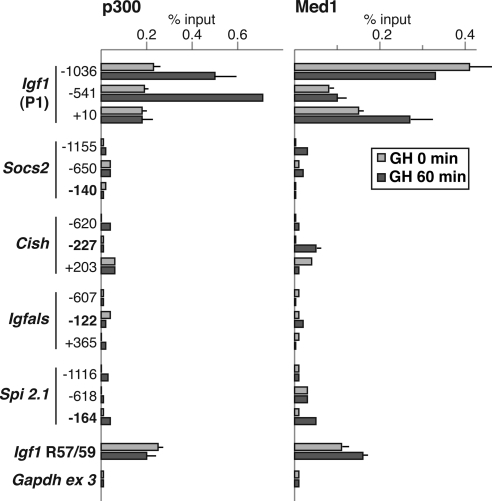
Analyzing GH-regulated transcriptional coactivators at hormone-stimulated promoters
Several previous studies have shown that the acetyltransferase and coactivator p300 is able to enhance Stat5a- and Stat5b-mediated gene transcription (62,63) and can physically bind to these proteins (62). The multicomponent protein complex term Mediator also has been implicated as a bridge between enhancer- and promoter-based transcription factors (64) and has been found to associate with several transcription factors (64). Based on these observations, and on our previous demonstration that p300 is present in hepatic chromatin at Igf1 promoter 1 (33) and at some Stat5b domains within the Igf1 locus (32), we examined the five GH-activated promoters for p300 and for the Med1/Trap220 subunit of the Mediator complex by quantitative ChIP. Both p300 and Med1 were broadly distributed over 1000 bp of Igf1 promoter 1 in liver chromatin from GH-deficient rats, and levels of p300 increased by 2- to 3-fold after acute GH administration (Fig. 7 and Ref. 33). By contrast, little p300 was found at the other GH-regulated promoters either before or after hormone treatment, with values tending to be similar to a negative control region, Gapdh exon 3, even at Stat5b-binding domains within each promoter (Fig. 7). In contrast, Med1 was detected but at low levels at the Cish and Spi 2.1 proximal promoters (Fig. 7). As a positive control, both p300 and Med1 were readily found at the Igf1 locus Stat5b-binding domain, R57-59 (32) (Fig. 7). Thus, based on these results we conclude that although p300 and Mediator may be involved in regulation of Igf1 gene transcription in response to GH, at least at promoter 1, it is likely that other as yet undefined coactivators and histone acetyltransferases are responsible for comparable functions at other GH-activated genes. Moreover, despite data from cell culture model systems and overexpression paradigms about the role of p300 as a coactivator for Stat5b (62,63), our in vivo experiments do not support this hypothesis, at least for the Socs2, Cish, Igfals, or Spi 2.1 promoters. Future studies will need to identify the critical transcriptional cofactors for these genes.
Figure 7.
Selective binding of transcriptional cofactors p300 and Med1 to Igf1 promoter 1. The graphs present results of ChIP experiments by qPCR assessing binding of p300 (left) and Med1 (right) at three locations in Igf1 promoter 1 and in the Socs2, Cish, Igfals, and Spi 2.1 promoters in hepatic chromatin of pituitary-deficient rats before (light gray bars) or 60 min after (dark gray bars) a single systemic GH injection. The R57-59 Stat5b binding domain of Igf1 serves as a positive control and Gapdh exon 3 serves as a negative control. Numbers indicate locations of PCR primers (bolding denotes Stat5b-binding regions; see Table 2 for DNA sequences). Results represent the mean ± range of two independent experiments (the lack of an error bar means that the range is too small to be seen on the graphs).
Summary and perspectives
GH exerts powerful physiological effects on somatic growth and intermediary metabolism through activation of multiple intracellular signal transduction pathways (1,2,3). GH actions also play important roles in tissue repair (65,66) and have been implicated in deleterious aspects of aging (5,6) and in the development of several cancers (7,8). Many of the intermediate and long-term effects of GH are a consequence of hormone-regulated alterations in gene expression, and a significant fraction of these changes are mediated by the GH-activated transcription factor, Stat5b (20,21,22,45). Remarkably, as characterized here, GH exerts dramatic and variable epigenetic actions on genes that are acutely regulated through Stat5b. For four of the five GH-activated genes studied, Socs2, Cish, Igfals, and Spi 2.1, which share a very similar genomic architecture, with two to four closely spaced Stat5b-binding sites located in their proximal promoters (see diagrams in Figs. 2 and 4–6), the epigenetic effects of GH are similar and differ primarily in degree of responsiveness. GH rapidly stimulates recruitment of Stat5b to proximal promoter sites on all four genes, and as yet uncharacterized chromatin-modifying enzymes enhance core histone acetylation and reduce histone H3K4 methylation. RNA pol II is recruited to each proximal promoter in response to GH, leading to transcriptional activation, although the coactivators that facilitate communication between Stat5b and RNA pol II remain unknown.
Our results also show that Igf1 is different than the other GH-stimulated and Stat5b-dependent genes studied here. Igf1 gene transcription is directed by two tandem promoters, with promoter 1 being responsible for approximately 65% and promoter 2 for about 35% of IGF-I mRNAs produced in response to GH in the liver (33,67,68). Igf1 also lacks Stat5b sites in DNA near its two promoters and contains instead a series of at least seven dispersed GH-inducible Stat5b-binding units that are located as far away as 87 kb from the promoters and are found in both flanking DNA and introns (32). As defined in this manuscript, the epigenetic profile of Igf1 promoter 1 varied substantially from the other four GH-activated genes. Unlike the other four promoters studied here, Igf1 promoter 1 appears poised to be activated (33), because in the absence of hormone, RNA pol II already has been recruited to promoter 1 (Fig. 4), core histones have been highly acetylated (Fig. 5), and transcriptional coactivators p300 and Med1 have been recruited. Also, after GH, histone acetylation increases more extensively than at the other promoters (Fig. 5 and Supplemental Fig. 1), and H3K4 methylation declines minimally (Fig. 6). Despite these differences in its epigenetic promoter profile, the rate and extent of transcriptional activation of Igf1 promoter 1 by GH is quantitatively similar to Socs2 and Cish, as is the degree of new histone acetylation induced by GH, and the amount of histone H3K4 trimethylation present before GH-mediated signaling, further supporting the idea that histone modifications may exert some control over signal-mediated transcriptional responsiveness. Surprisingly, Igf1 promoter 2 more closely resembles the Socs2 and Cish promoters in its overall epigenetic profile than it does Igf1 promoter 1, particularly with regard to recruitment of RNA pol II by GH, lack of promoter-associated p300, and relatively high H3K4 trimethylation (33).
In these studies we only examined a single gene that was repressed by GH, Igfbp1. In previous analyses, we found that Stat5b could directly inhibit activity of the Igfbp1 promoter, despite not binding to promoter DNA and not interfering with the DNA-binding capabilities of the critical activating transcription factor, FoxO1 (45). We now find that despite a profound drop in Igfbp1 gene transcription after GH (Fig. 1), very few chromatin changes could be detected acutely at the Igfbp1 promoter, consisting primarily of modest declines in histone H4 acetylation (Fig. 5) and H3K4 monomethylation (Fig. 6). Thus, the mechanisms involved in transcriptional inhibition by GH at a chromatin level remain unknown.
Finally, although these studies show that GH orchestrates rapid and dramatic alterations in the organization of chromatin at the promoters of several target genes in the liver, they also raise a number of important questions. From a physiological perspective, it is unknown whether the patterns of chromatin changes defined here in the liver after a single injection of a large dose of GH resemble the epigenetic effects after exposure to physiological GH levels. It is also unknown whether the histone modifications observed at each GH-activated gene are reset after every hormone pulse under normal physiological conditions, or whether the patterns of chromatin changes are unique to the liver, or are found in other GH-responsive tissues and cell types. Clearly, additional studies are needed to address these and other questions that will define the dynamics of the GH-regulated transcriptome under physiological and pathological conditions.
Materials and Methods
Materials
The following were purchased: recombinant rat GH (National Hormone and Pituitary Program, National Institutes of Diabetes and Digestive and Kidney Diseases, National institutes of Health); SYBR green platinum quantitative PCR (qPCR) mix and Qia-Quick PCR purification kit, (QIAGEN, Valencia, CA); okadaic acid (Alexis Biochemicals, San Diego, CA); SuperScript III reverse-transcriptase system and protein A-sepharose beads (Invitrogen, Carlsbad, CA); whole genome amplification kit (Sigma, St. Louis, MO); BCA protein assay kit (Pierce Biotechnologies, Rockford, IL); restriction enzymes, buffers, ligases, and polymerases (Roche Applied Sciences, Indianapolis, IN; and BD Biosciences-Clontech, Palo Alto, CA). The following antibodies were purchased from commercial vendors: Stat5 (C17), RNA Polymerase II (H-224), p300 (C-20), Med1/Trap 220 (C-19), and Bcl6 (N-3) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); acetylhistone H3 (recognizes acetyl K9 and 14), acetylhistone H4 (recognizes acetyl K5, 8, 12, and 16) (Millipore Corp., Billerica, MA); histone H3 trimethyl K4 and histone H3 monomethyl K4 [Abcam (Cambridge, UK]. Oligonucleotides were synthesized at the Oregon Health & Science University DNA Services Core. All other chemicals were reagent grade and were purchased from commercial suppliers.
Animal studies
Male Sprague Dawley rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN), after being hypophysectomized by a transauricular route at age 7 wk. Rats were housed at the Oregon Health & Science University Animal Care Facility on a 12-h light, 12-h dark schedule with free access to food and water and received care according to NIH guidelines. GH deficiency was confirmed by failure to grow during a 2-wk observation period. Rats subsequently were injected ip with either vehicle (10 mm NaHCO3) or recombinant rat GH (1.5 mg/kg) and were killed 30 or 60 min later. The liver was removed, and chromatin and nuclear RNA were isolated from individual animals as outlined below. For gene expression analysis and ChIP experiments, two to three independent series of rats were used. The OHSU Committee on Animal Care and Use approved all studies involving rats.
RNA isolation and analysis
Hepatic nuclear RNA was isolated as described (69). RNA integrity was assessed by agarose gel electrophoresis, and concentrations were determined by spectrophotometry at 260 nm. Nuclear RNA (5 μg) was reverse transcribed with random hexamers in a final volume of 50 μl using an RT-PCR kit, diluted to 5 ml, and 1 μl of cDNA was used as template for PCR (the equivalent of 1 ng of nuclear RNA input). qPCR was performed as described elsewhere (33). Primer sets were designed to amplify transcribed segments and were located just downstream of promoters, except for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was located in exon 3 (Table 1). A series of seven rat genomic DNA standards ranging from 0.016 to 250 ng was prepared by serial dilution. PCR amplification was performed with the Bio-Rad Chromo4 Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA). For each primer set, a standard curve was constructed by plotting the cycle threshold vs. the amount of input DNA (log10 scale), and the slope and correlation coefficient for each curve were determined (see Ref. 33). Primer sets were judged to be acceptable if the slope of the linear part of the curve approximated −3.3 (theoretical maximum efficiency of amplification) and the r2 coefficient exceeded 0.985. The cycle threshold for each cDNA sample was extrapolated to the standard curve to determine the quantity of nascent transcript (ng). Results for GAPDH were used to normalize samples at each time point.
Table 1.
DNA sequences of oligonucleotide primers for qPCR of nuclear RNA
| Gene | Location (5′ end) | Top strand (5′ to 3′) | Bottom strand (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|---|
| Igf1 | Intron 1 (+697) | ACTTCTATAGGGCTTCCACGCT | CCCGCGTTTATATCCATGCTTCCT | 93 |
| Socs2 | Exon 1 (+63) | TTAACTCTTGCCAAGTCTCGCC | CGGAGGAAAGGCTCATGCTTCA | 78 |
| Cish | Intron 1 (+203) | TGCGGGATTTGAGAGGGCTTGA | TGTGTGTGTGTGTGTGTGTCCT | 87 |
| Igfals | Intron 1 (+595) | CTTAACTCAGGGTGGGCTGGT | ACCCTCCGGTGCCAGAAATTGTTA | 70 |
| Spi 2.1 | Intron 1 (+405) | TTGCGGAATCATGCTGGGTGAA | ATTTGGGATCTAGGCGGGAAGT | 100 |
| Igfbp1 | Exon 1 (+90) | GAAAGTCGTGACTACTGAGCCA | ACAACAGTTAGGAACTCCGGCA | 93 |
| Gapdh | Exon 3 | ACTCTACCCACGGCAAGTTCAA | ATGGGTTTCCCGTTGATGACCA | 73 |
ChIP assays
All steps followed previously published protocols (32,33,70). For each time point a fragment (∼600 mg) of rat liver was minced and incubated at 20 C in 30 ml of DMEM with 1% formaldehyde on a rotating platform for 15 min, followed by addition of 4.5 ml of 1 m glycine and incubation for an additional 5 min. After centrifugation for 5 min at 200 × g at 20 C, the pellet was washed in PBS and suspended in lysis buffer (1 ml of 50 mm Tris-HCl; 5 mm EDTA; 1% sodium dodecyl sulfate, pH 8.1; plus protease inhibitors), and incubated at 4 C for 15 min, followed by sonication on ice with a Branson microtip sonicator (setting 10) using a total of 10 rounds of 10 pulses for 1 sec each, interspersed with 30-sec incubations on ice. The supernatant was then isolated after centrifugation at 14,000 × g for 10 min at 4 C (these steps generated DNA fragments of average size of 500 ± 100 bp), and the protein concentration (measured by BCA assay) was adjusted to 1 mg/ml in immunoprecipitation (IP) buffer (50 mm Tris-HCl; 5 mm EDTA; 150 mm NaCl; 0.1% sodium dodecyl sulfate; 1% Triton X-100, pH 8.1; plus protease inhibitors). Aliquots of 1 ml were stored at −80 C until use. IPs were performed as described elsewhere (32,33,70), and DNA was isolated using the QiaQuick PCR purification kit, suspended in 50 μl of 10 mm Tris-HCl, 1 mm EDTA, pH 8.1, and used as template in qPCR reactions. Primers were designed following guidelines from PrimerQuest (Integrated DNA Technologies, San Diego, CA) to yield amplicons ranging in length from 70–113 bp (see Table 2 for primers). PCR experiments were performed with a Bio-Rad Chromo4 Real-Time PCR detection system. Reactions (20 μl final volume) contained 1X SYBR Green mix, 200 nm primers, and ChIP-enriched DNA and were performed in eight-well strips in a 96-well format. Standard curves were included in each experiment for each primer set, and contained 0.01–1.25 ng of genomic DNA. Values are expressed as percent input necessary to achieve the identical cycle threshold. Results represent the mean of two to three independent qPCR experiments from independent IPs.
Table 2.
DNA sequences of oligonucleotide primers for ChIP assays
| Gene and domain | Location (5′-end) | Top strand (5′ to 3′) | Bottom strand (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|---|
| Igf1 | ||||
| 5′ | −10,054 | CCAGCCGTTAAACACCAAGAAG | TGCTAGTCCCAGTACAGCATCT | 94 |
| Promoter 1 | −1,036 | AGCAGGTCTGGCTCATTTCCAT | AGATCAGATGCTGCGCTTTCCA | 78 |
| Promoter 1 | −541 | TAGTGTGTGCCTCCCATACTGCTT | TGAGCTCTGGGACAGTCTGAAA | 105 |
| Promoter 1 | +10 | AAACGCCTCTGTGCTCCAGTTT | TCTCTCTCCCTCTTCTGGCAAAGT | 104 |
| R2-4 | −86,264 | TGAATCTCCAGGGATCACACCA | CTCTTGTTGATTTCATCTTTGACCCA | 84 |
| R8-9 | −73,096 | GTGAGTCTGTGTTAGTCAGG | GGTTATGTAAGTCTGATAGAG | 93 |
| R13 | −62,806 | TGCATTGGCTACCAGGAACTCT | GAGACAGTTGGAAGGAAGGATAGA | 76 |
| R34-35 | +3,740 | GGTGAAACCGCTCACCTTGG | TTCTAAGAAGCAGACAGAGG | 103 |
| R53-54 | +26,694 | TGGCACATGCCATTGACCAGAT | AAGGGCAGACCCATCTTTCTGA | 102 |
| R57-59 | +43,817 | GGCCAATTCATTCGGCAACTGT | TCCTTTCTGAGAACTGGAGACCGT | 87 |
| R60-61 | +49,147 | CCCAACTGAGAAGTAGTAGGCT | GAAACAGTCCCGACAACCTATC | 105 |
| Socs2 | ||||
| Promoter | −1,155 | CCCAAAGTCACAGCTTCAACTAA | ACTATTCTGTTGCATCACTGCTCA | 73 |
| Promoter | −650 | TGAGAGCACTCTGTGCAGCTTT | CGCCAGCATCCTAGAAGGTCAA | 70 |
| Promoter | −140 | TCCCTTGAAGCCTGCGCATTA | AACTTTCCAGGAATCCGCCTCA | 86 |
| Cish | ||||
| Promoter | −620 | AGTTCTTCGGTTCTCTTAAGCGCC | ACACTGCATGAAGGTGGGTGAT | 71 |
| Promoter | −227 | CTTGGAAATCTGTCAAAGGTG | GGAAGCCGCATCTTCCTAGA | 113 |
| Intron 1 | +203 | TGCGGGATTTGAGAGGGCTTGA | TGTGTGTGTGTGTGTGTGTCCT | 87 |
| Igfals | ||||
| Promoter | −607 | ACCTTCCCAGACCTGCCTATTT | CTGGGTTTCAGGAAGGACAGTT | 96 |
| Promoter | −122 | CCAGGTGTTCCTAGAAGAGG | TTGTCCAAGGCCCAGTCTCAG | 95 |
| Exon 1 | +365 | AGGGTTAAAGGAGCGAGAGTGT | AGCAAGGGCCGTCTACAATACA | 106 |
| Spi 2.1 | ||||
| Promoter | −1,116 | AAAGGCTACCACTCTGTCCTCA | AGATCCCAAACTCCTGTAGCCA | 92 |
| Promoter | −618 | GGCCTGTAAAGAGTTGTGTTGG | TGTTCACCTTACCAATGCCTATCT | 74 |
| Promoter | −164 | AAGGTGTGCTCAGGAGATCAGT | TCGGACAACCTGTGCTTTGTGT | 101 |
| Igfbp1 | ||||
| Promoter | −990 | CCCTACAACCTTTGCCTTCTCT | TGTCCAGCCCTTCAGTGTCTTCA | 94 |
| Promoter | −401 | AGGAGAGGAAACAACTGTGGGT | TTAGACCAAGAAGGCTTCAGGC | 83 |
| Exon 1 | +46 | ACACAAACCCAGCGAGCATTGA | ATAGTAGCGGAAGTGGTGGTCACA | 76 |
| Gapdh | ||||
| Exon 3 | ACTCTACCCACGGCAAGTTCAA | ATGGGTTTCCCGTTGATGACCA | 73 |
ChIP-chip analysis
Chromatin from liver tissue of hypophysectomized rats at 60 min after systemic GH injection was used to prepare input and acetyl H3-ChIP-enriched DNA. One round of whole genome amplification was performed on each sample, per protocol from Sigma, to provide a total DNA yield of more than 4 μg and concentration of more than 250 ng/μl. Agarose gels confirmed that most of the amplified DNA was 200–500 bp in length, and no dominant individual DNA bands were present. Labeling and hybridization of amplified DNA to the rat 385K genomic tiling array set 17 of 37 (containing DNA from chromosomes 7 and 6) was performed by Roche-NimbleGen (Madison, WI). Results were analyzed by calculating the enrichment of binding for each feature in the tiling array by dividing raw figures for acetyl H3-ChIP hybridization by input DNA hybridization, and mean and sd for the data set were determined. The z scores (or sd scores) for individual features were calculated. Peaks of enrichment were defined as four consecutive features with z scores greater than +1.0 and were plotted as vertical bars. The complete ChIP-chip dataset may be found at the European Molecular Biology Laboratory MIAMExpress database.
Notes on gene annotation
Information on several of the genes investigated in this study is either incomplete or incorrect in public databases. To identify exon 1 and the proximal promoter of rat Socs2, we mapped regions of high similarity with the mouse gene. We find that mouse and rat exon 1 are each 240 bp in length and are more than 95% identical. Also, the proximal promoters are 90% identical over 250 bp, and the two adjacent Stat5-binding sites are identical in sequence and relative locations beginning 67 bp 5′ to the start of exon 1. For rat Igfals, we relied on published data from Delhanty and Baxter (71) and assigned the 5′-end of exon 1 to their most 5′-transcription start site mapped by primer-extension analysis (71). Exon 1 is thus 601 bp in length. For rat Spi 2.1, we used information from Yoon et al. (34) and Bergad et al. (43) to guide annotation of the proximal promoter and exon 1 (81 bp) and to distinguish Spi 2.1 from many related genes on rat chromosome 6. Data on rat Igf1 are derived from publications from our laboratory (68,72). Information on rat Cish and Igfbp1 is from the Ensemble database.
Supplementary Material
Acknowledgments
We thank members of our laboratory for advice throughout the course of these studies.
Footnotes
This work was supported by National Institutes of Health Grants K08 DK077897 and K12 HD057588 (to D.J.C.) and R01 Grant DK69703 (to P.R.).
Current address for D.J.C.: Department of Pediatrics, Mt. Sinai School of Medicine, One Gustave L. Levy Place, Box 1616, New York, New York 10028.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 11, 2010
Abbreviations: ChIP, Chromatin immunoprecipitation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; H3K4me1, monomethylated histone H3 at lysine 4; H3K4me3, trimethylation of histone H3 at lysine 4; IP, immunoprecipitation; pol II, polymerase II; qPCR, quantitative PCR; Stat, signal transducer and activator of transcription.
References
- Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ 2006 New insights into growth hormone action. J Mol Endocrinol 36:1–7 [DOI] [PubMed] [Google Scholar]
- Lanning NJ, Carter-Su C 2006 Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7:225–235 [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Hwa V 2009 The growth hormone cascade and its role in mammalian growth. Horm Res 71(Suppl 2):36–40 [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A 2003 The endocrine regulation of aging by insulin-like signals. Science 299:1346–1351 [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ 2008 Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18:455–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD 2010 Extending healthy life span–from yeast to humans. Science 328:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim YH, Yee D 2004 Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res 14:261–269 [DOI] [PubMed] [Google Scholar]
- Pollak M 2008 Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928 [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell Jr JE 2002 Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3:651–662 [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW 2008 Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev 22:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Plumlee C 2008 Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol 19:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R 2009 STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG 2003 Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349:1139–1147 [DOI] [PubMed] [Google Scholar]
- Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG 2005 Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol Metab 90:4260–4266 [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V 2007 Defects in growth hormone receptor signaling. Trends Endocrinol Metab 18:134–141 [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, Waters MJ 2005 In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo JS, McEachin RC, Cui TX, Duggal NK, Hai T, States DJ, Schwartz J 2006 Profiles of growth hormone (GH)-regulated genes reveal time-dependent responses and identify a mechanism for regulation of activating transcription factor 3 by GH. J Biol Chem 281:4132–4141 [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ 2006 Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20:1333–1351 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Vidal OM, Merino R, Rico-Bautista E, Fernandez-Perez L, Chia DJ, Woelfle J, Ono M, Lenhard B, Norstedt G, Rotwein P, Flores-Morales A 2007 In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol Endocrinol 21:293–311 [DOI] [PubMed] [Google Scholar]
- Wauthier V, Waxman DJ 2008 Sex-specific early growth hormone response genes in rat liver. Mol Endocrinol 22:1962–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfle J, Billiard J, Rotwein P 2003 Acute control of insulin-like growth factor-1 gene transcription by growth hormone through STAT5B. J Biol Chem 278:22696–22702 [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang H 2005 Identification of a distal STAT5-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem 280:10955–10963 [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P 1989 Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10:68–91 [DOI] [PubMed] [Google Scholar]
- LeRoith D 2008 Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev 5(Suppl 2):739–743 [PubMed] [Google Scholar]
- Rotwein P, Chia DJ 2010 Gene regulation by growth hormone. Pediatr Nephrol 25:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotwein P 1999 Molecular biology of IGF-I and IGF-II. In: Rosenfeld R, Roberts CJ, eds. The IGF system. Totowa, NJ: Humana Press; pp 19–35 [Google Scholar]
- Eleswarapu S, Gu Z, Jiang H 2008 Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology 149:2230–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laz EV, Sugathan A, Waxman DJ 2009 Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol Endocrinol 23:1242–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DJ, Varco-Merth B, Rotwein P 2010 Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem 285:17636–17647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DJ, Young JJ, Mertens AR, Rotwein P 2010 Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol 24:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JB, Berry SA, Seelig S, Towle HC 1990 An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem 265:19947–19954 [PubMed] [Google Scholar]
- Le Cam A, Pantescu V, Paquereau L, Legraverend C, Fauconnier G, Asins G 1994 cis-Acting elements controlling transcription from rat serine protease inhibitor 2.1 gene promoter. Characterization of two growth hormone response sites and a dominant purine-rich element. J Biol Chem 269:21532–21539 [PubMed] [Google Scholar]
- Paul C, Seiliez I, Thissen JP, Le Cam A 2000 Regulation of expression of the rat SOCS-3 gene in hepatocytes by growth hormone, interleukin-6 and glucocorticoids mRNA analysis and promoter characterization. Eur J Biochem 267:5849–5857 [DOI] [PubMed] [Google Scholar]
- Tollet-Egnell P, Flores-Morales A, Stavréus-Evers A, Sahlin L, Norstedt G 1999 Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology 140:3693–3704 [DOI] [PubMed] [Google Scholar]
- Flores-Morales A, Ståhlberg N, Tollet-Egnell P, Lundeberg J, Malek RL, Quackenbush J, Lee NH, Norstedt G 2001 Microarray analysis of the in vivo effects of hypophysectomy and growth hormone treatment on gene expression in the rat. Endocrinology 142:3163–3176 [DOI] [PubMed] [Google Scholar]
- Woelfle J, Chia DJ, Rotwein P 2003 Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278:51261–51266 [DOI] [PubMed] [Google Scholar]
- Woelfle J, Rotwein P 2004 In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab 286:E393–E401 [DOI] [PubMed] [Google Scholar]
- Ooi GT, Cohen FJ, Tseng LY, Rechler MM, Boisclair YR 1997 Growth hormone stimulates transcription of the gene encoding the acid-labile subunit (ALS) of the circulating insulin-like growth factor-binding protein complex and ALS promoter activity in rat liver. Mol Endocrinol 11:997–1007 [DOI] [PubMed] [Google Scholar]
- Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR 1998 Binding of STAT5a and STAT5b to a single element resembling a γ-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol Endocrinol 12:675–687 [DOI] [PubMed] [Google Scholar]
- Bergad PL, Shih HM, Towle HC, Schwarzenberg SJ, Berry SA 1995 Growth hormone induction of hepatic serine protease inhibitor 2.1 transcription is mediated by a Stat5-related factor binding synergistically to two γ-activated sites. J Biol Chem 270:24903–24910 [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Luo JM, Murphy LJ 1990 Transcriptional regulation of rat insulin-like growth factor-binding protein-1 expression by growth hormone. Mol Endocrinol 4:1199–1204 [DOI] [PubMed] [Google Scholar]
- Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, Rotwein P 2007 Signal transducer and activator of transcription (Stat) 5b-mediated inhibition of insulin-like growth factor binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol 21:1443–1457 [DOI] [PubMed] [Google Scholar]
- Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE 1999 STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol 158:111–116 [DOI] [PubMed] [Google Scholar]
- Chang CC, Ye BH, Chaganti RS, Dalla-Favera R 1996 BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci USA 93:6947–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM 1997 Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276:589–592 [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin G, Huo JS, Barney D, Wang Z, Livshiz T, States DJ, Qin ZS, Schwartz J 2009 Computational and functional analysis of growth hormone (GH)-regulated genes identifies the transcriptional repressor B-cell lymphoma 6 (Bc16) as a participant in GH-regulated transcription. Endocrinology 150:3645–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RD, Laz EV, Su T, Waxman DJ 2009 Male-specific hepatic Bcl6: growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol Endocrinol 23:1914–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata N, Miki T, Ohashi K, Suzuki K, Fukuda T, Hirosawa S, Aoki N 1994 Recognition DNA sequence of a novel putative transcription factor, BCL6. Biochem Biophys Res Commun 204:366–374 [DOI] [PubMed] [Google Scholar]
- Seyfert VL, Allman D, He Y, Staudt LM 1996 Transcriptional repression by the proto-oncogene BCL-6. Oncogene 12:2331–2342 [PubMed] [Google Scholar]
- Fuda NJ, Ardehali MB, Lis JT 2009 Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT 2008 Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322:1845–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA 2007 A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130:77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ, Chang HY 2009 Genome-wide views of chromatin structure. Annu Rev Biochem 78:245–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B 2007 Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B 2009 Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AG, Bilenky M, Tam A, Zhao Y, Zeng T, Thiessen N, Cezard T, Fejes AP, Wederell ED, Cullum R, Euskirchen G, Krzywinski M, Birol I, Snyder M, Hoodless PA, Hirst M, Marra MA, Jones SJ 2008 Genome-wide relationship between histone H3 lysine 4 mono- and tri-methylation and transcription factor binding. Genome Res 18:1906–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forneris F, Battaglioli E, Mattevi A, Binda C 2009 New roles of flavoproteins in molecular cell biology: histone demethylase LSD1 and chromatin. FEBS J 276:4304–4312 [DOI] [PubMed] [Google Scholar]
- Lan F, Nottke AC, Shi Y 2008 Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol 20:316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzner E, Jähne R, Wissler M, Stoecklin E, Groner B 1998 p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol 12:1582–1593 [DOI] [PubMed] [Google Scholar]
- Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, Honjo T, Ikuta K 2001 The IL-7 receptor controls the accessibility of the TCRγ locus by Stat5 and histone acetylation. Immunity 15:813–823 [DOI] [PubMed] [Google Scholar]
- Conaway RC, Sato S, Tomomori-Sato C, Yao T, Conaway JW 2005 The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem Sci 30:250–255 [DOI] [PubMed] [Google Scholar]
- Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C 2008 Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev 129:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustina A, Mazziotti G, Canalis E 2008 Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 29:535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe Jr WL, Roberts Jr CT, Lasky SR, LeRoith D 1987 Differential expression of alternative 5′ untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci USA 84:8946–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LJ, Kajimoto Y, Bichell D, Kim SW, James PL, Counts D, Nixon LJ, Tobin G, Rotwein P 1992 Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol 11:301–313 [DOI] [PubMed] [Google Scholar]
- Bichell DP, Kikuchi K, Rotwein P 1992 Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6:1899–1908 [DOI] [PubMed] [Google Scholar]
- Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P 2006 Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 281:3190–3197 [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, Baxter RC 1997 Cloning and characterization of the rat gene for the acid-labile subunit of the insulin-like growth factor binding protein complex. J Mol Endocrinol 19:267–277 [DOI] [PubMed] [Google Scholar]
- Shimatsu A, Rotwein P 1987 Mosaic evolution of the insulin-like growth factors. Organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem 262:7894–7900 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.