Abstract
The TSH receptor (TSHR) is the key molecule influencing thyroid growth and development and is an antigenic target in autoimmune thyroid disease. The TSHR exists in monomeric and multimeric forms, and it has been shown previously that multimeric complexes of the TSHR preferentially localize in lipid rafts. However, unlike other glycoprotein hormone receptors, the TSHR exists in several forms on the cell membrane due to intramolecular cleavage of its ectodomain, which causes the production of α- and β-subunits of various lengths. After cleavage and reduction of disulfide bonds, α-subunits consisting of the receptor ectodomain may be lost from the cell surface by receptor shedding, leading to accumulation of excess β-subunits within the membrane. Because cell surface expression of these various forms of the TSHR is critical to receptor signaling and autoimmune responses, we set out to model the influence of β-subunits on full-length TSHRs. To study this interaction, we generated three truncated ectodomain β-subunits linked to green fluorescent protein (named β-316, -366, and -409) as examples of native cleaved forms of the TSHR. These constructs were transfected into human embryonic kidney 293 cells in the presence and absence of the full-length receptor. Whereas the β-316 and β-366 forms showed cell surface expression, the expression of β-409 was primarily intracellular. Cotransfection of the β-subunits with a full-length hemagglutinin-tagged wild-type (WT) receptor (HT-WT-TSHR) in both transient and stable systems caused a significant decrease in surface expression of the full-length WT receptors. This decrease was not seen with control plasmid consisting of a plasma membrane-targeted protein tagged to red fluorescent protein. To ascertain if this response was due to homointeraction of the truncated β-constructs with the WT-TSHRs, we immunoprecipitated membranes prepared from the cotransfected cells using antihemagglutinin and then probed with anti-green fluorescent protein. These studies confirmed dimerization of the β-subunits with the WT full-length receptor, and this interaction was further observed in vivo by fluorescence resonance energy transfer. We then studied the functional consequences of this interaction on TSHR signaling by examining Gαs-mediated signals. The well-expressed truncated constructs, when coexpressed with full-length TSHR, did not alter constitutive cAMP levels, but there was a significant decrease in TSH-induced cAMP generation. Furthermore, we observed that truncated β-316 and β-366 had faster internalization rate, which may lead to a significant decrease in the expression of the full-length receptor on the cell surface, thus contributing to the decreased signaling response. However, the decrease in surface receptors may also be due to inhibition of newly formed receptors reaching the surface as result of receptor-receptor interaction. It is well known that under normal physiological conditions both cleaved and uncleaved TSHR forms coexist on the cell surface of normal thyrocytes. Our studies allow us to conclude, therefore, that multimerization of cleaved/ truncated forms of the β-subunits with the full-length TSHR has a profound influence on TSHR internalization and signaling. Hence, the degree of intramolecular cleavage must also modulate TSHR signaling.
Multimerization of cleaved/truncated forms of the β subunits with the full length TSHR has a profound influence on TSHR internalization and signaling.
The TSH receptor (TSHR) provides the major activation signals for thyroid growth and development. The TSHR is a seven-transmembrane, G protein-coupled glycoprotein receptor that undergoes complex posttranslational modification (1,2). These modifications include intramolecular cleavage of the ectodomain into two covalently bound subunits (A or α and B or β). The α-subunit is subsequently lost from the cell surface after further reduction of disulfide bonds by protein disulfide isomerase causing an accumulation of excess β-subunits on the cell membrane surface (2,3,4). We have demonstrated that TSHRs also constitutively dimerize and form higher order forms both in thyrocytes and transfected cells (5,6). TSH appears to reduce such higher order forms to monomers and, in addition, there is evidence that TSH also enhances constitutive intramolecular cleavage (7,8).
Because the TSHR β-subunit is present in excess (3:1) in thyrocytes (9), previous studies have focused on the function of these forms using transfected cell models (10,11). Studies of stably expressed truncated TSHRs have shown an increased internalization rate compared with full-length receptor (3). Complete removal of the ectodomain leads to loss of TSH signaling, making the receptor nonfunctional. However, some investigators have observed that truncated TSHR β-subunits showed enhanced constitutive cAMP generation whereas others have failed to observe this (11,12). In addition, these truncated receptors have never been examined for their potential to influence the remaining intact TSHRs and thus influence TSHR signaling at the surface of the cell.
Therefore, in this study we have examined the role of TSHR subunits on full-length TSHR trafficking and function by using the three truncated constructs and the various tagged full-length receptors (Fig. 1). We found aberrant homodimerization using different forms of truncated TSHR β-subunits that were similar to native cleaved receptors. We also found that such constructs markedly decreased wild-type (WT) TSHR signaling, most probably secondary to their more rapid internalization and also by their ability to hold the newly synthesized receptors by dimerization.
Figure 1.
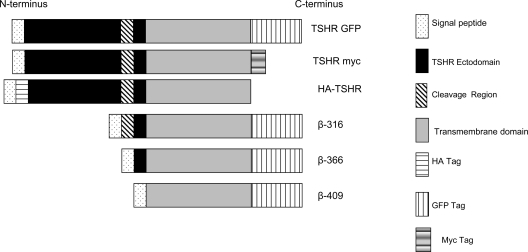
Constructs of WT and TSHR β-subunits. This schematic diagram illustrates the tagged WT full-length and truncated β-subunit constructs of the TSHR. The N-terminal HA-tagged full-length TSHR construct was used for interaction and functional studies, and the TSHR constructs tagged with c-myc and/or GFP at the C terminus were used for FRET studies. All truncated TSHR β-unit constructs were tagged with GFP at their C terminus.
Results
Trafficking of truncated constructs
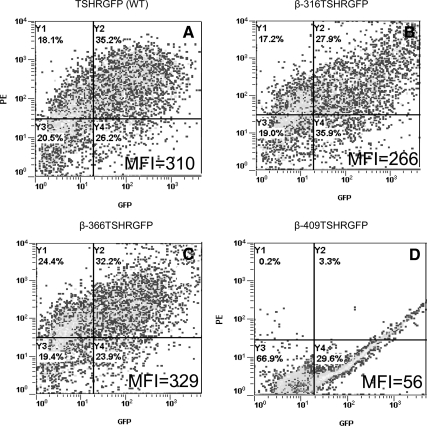
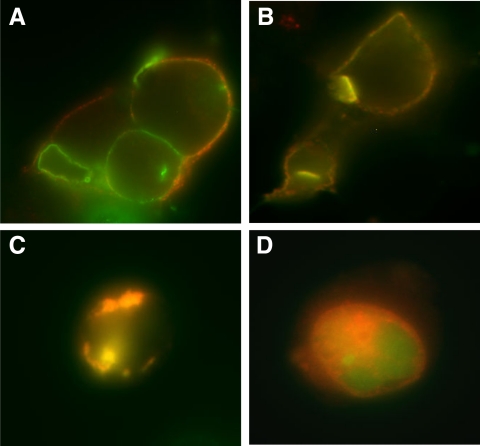
Before studying the interaction of the truncated β-subunits with full-length TSHR, we verified their expression and localization. The expression of the truncated constructs was assessed by flow cytometry after transfection of human embryonic kidney (HEK) 293 cells. The unfixed cells were stained for surface expression of the receptor by using TSHR-specific monoclonal antibody M1, which recognizes the epitope 381-385 on the extracellular domain of the receptor (13). The binding of the antibody to the receptor was detected by antimouse Fab′ conjugated to PE. Because the β-constructs had a green fluorescent protein (GFP) tag at their carboxyl terminus, the cells were analyzed simultaneously in GFP (FL1) and phycoerythin (PE) (FL2) channels. The percentage of positive cells for all three truncated constructs was similar to the WT receptor (TSHR GFP WT); however, the expression levels of the truncated constructs as measured by the mean fluorescent intensity in FL2 channel was different among the three constructs (Fig. 2). For the 409 construct we could only detect GFP expression in cells because of the loss of the antibody M1 recognition epitope in the 409 construct and the lack of a TM-specific antibody. A 30% positivity observed in the percent gated cells of double positive cells with β-316- and β-366-unfixed cells indicated equal efficacy of expression of these three truncated TSHRs, although the levels of expression of these constructs varied, The subcellular localization of β-316, β-366, and β-409 was also ascertained microscopically (Fig. 3) using a plasma membrane (PM) protein localization marker labeled with red fluorescent protein (RFP). PM localization of receptors was observed clearly in the WT, 316, and 366 transfected cells with surface expression of PM-RFP as evidenced by the presence of colocalization of GFP and RFP in these cells as yellow fluorescence (Fig. 3, A–C). In contrast, predominantly intracellular localization was observed with the 409 construct by the presence of yellow fluorescence within the cells in the overlaid image (Fig. 3D).
Figure 2.
Expression of TSHR subunits in HEK-293 cells. Expression and cellular localization for the full-length WT and the three truncated β-subunits was assessed by flow cytometry Cell surface expression of receptors was detected on unfixed cells with antibody M1 (residues 381-385) followed by staining with secondary antimouse PE (1:200). Due to the presence of GFP and PE, β-316 (panel B) showed 27% and β-366 showed 32% (panel C) of double-positive cells for surface expression that was similar to full-length TSHR (panel A). However, β-409 (panel D) showed a 29% GFP expression only, indicating just intracellular expression of the product. The FL2 mean fluorescence intensity (MFI) for each of the constructs is also indicated.
Figure 3.
Localization of TSHR subunits on the plasma membrane (PM). To study whether the truncated receptors localized to the PM, we transfected the GFP-tagged truncated receptors into HEK-293 cells. After 24 h of transfection, the cells were then transduced with a baculovirus construct that had a PM protein tagged to RFP. The cells were then fixed and observed with GFP and RFP excitation and emission filters (GFP excitation, 475 nm, and emission, 525 nm; RFP excitation, 550 nm, and emission, 600 nm). The images in panels A–D are colocalized images of GFP and RFP giving a yellow fluorescence. Panel A refers to TSHR-GFP WT receptor-expressing cells in which images in B, C, and D are β-316, -366, and -409, respectively. Note that β-409 is not colocalized to PMs, indicating intracellular entrapment of receptors.
The interaction between β-subunits and the full-length TSHR
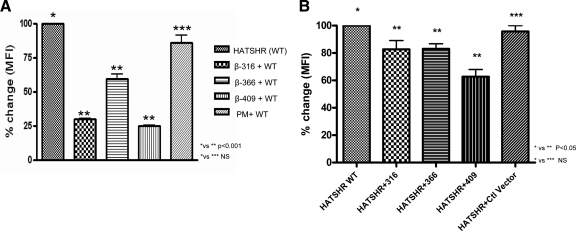
To study the interaction of TSHR β-subunits with full-length human TSHRs, we cotransfected HEK-293 cells with hemagglutinin (HA)-TSHR-WT and individually with the GFP-labeled TSHR β-subunit constructs. Cotransfection of β-constructs with HA-tagged WT TSHR resulted in a marked decrease in HA-TSHR-WT expression. A decrease in expression of up to 40%–80% of the HA-TSHR-WT was observed when transfected with each of the truncated subunit constructs (Fig. 4A). A similar significant decrease was observed when these constructs were transfected into stably expressing Chinese hamster ovary (CHO) HA-SHR (Fig. 4B). The degree of decreased expression was greatest with the shortest construct (β-409). In contrast, there was no significant decrease in HA-TSHR expression when a nonspecific PM protein labeled with RFP (Organelle Lights; Molecular Probes, Inc., Eugene, OR) was colocalized to the PM along with the HA-TSHR.
Figure 4.
Altered surface expression of HA-TSHR (WT) on transient and stable cells when cotransfected with the β-subunit constructs. A, HEK-293 cells were cotransfected with both the WT receptor (HA-TSHR) and the β-subunits. The expression of the WT TSHR, assayed with anti-HA, is shown as 100%. There was a marked decrease in surface expression of this WT TSHR when coexpressed with the truncated TSHR subunits. The decrease in the WT HA-TSHR surface expression due to the presence of the β-constructs ranged between 40–75% whereas only an 8% decrease was observed with nonspecific PM protein tagged with GFP. B, A CHO-HA-TSHR stable line was transfected with β-subunits, and surface expression of HA was assayed as described above. Significant decreases in surface expression of the WT receptor was observed when the truncated subunits were transfected. The data shown here are the average from two experiments. Ctl, Control; MFI, mean fluorescence intensity; NS, not significant (P > 0.05).
Dimerization of truncated β-constructs with full-length TSHR
To confirm whether the change in surface residence of the full-length TSHR was due to direct receptor- receptor interaction, we performed coimmunoprecipitation and Foster resonance energy transfer (FRET).
Coimmunoprecipitation
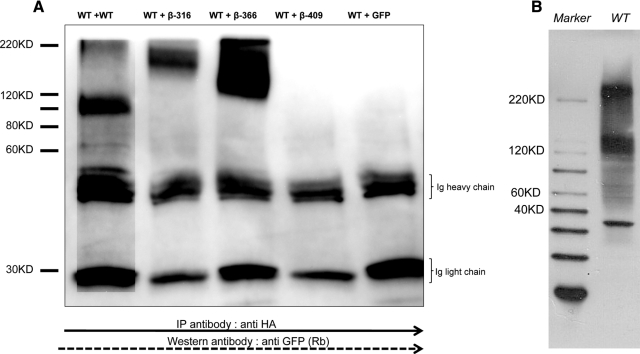
To confirm that the decreased expression of the full-length receptor observed by flow cytometry was due to homointeraction between the full-length receptor and the β-subunits, we performed coimmunoprecipitation studies. As a control, we used the combination of full-length HA-TSHR and full-length TSHR-GFP. Dimerization/multimerization of the full-length TSHR has previously been established by us and others to be primarily between the transmembrane domains of this receptor. This differential labeling of the constructs allowed us to detect HA-TSHR/β-GFP complexes with anti-GFP after precipitation with anti-HA. We were able to detect monomeric and multimeric forms of β TSHR complexes with the WT receptor. Despite the use of a reducing gel, most of the observed receptor complexes with the β-316 and β-366 constructs, which themselves were in the range of 143–176 kDa, were seen as higher order nonreducing complexes. However, we did not observe such complexes with the β-409 construct because β-409 was localized intracellularly and was absent from these surface membrane preparations. From the above studies we know that, except for the control WT+WT cells, we did not observe any monomeric forms in the β cotransfected cells (Fig. 5).
Figure 5.
Coimmunoprecipitation showing the interaction between the WT receptor and β-subunits. A, To study the potential interaction between WT TSH and the TSHR subunit constructs, we cotransfected HA-TSHR and each of the truncated β-subunit constructs. We used a TSHR-GFP vector along with HA-TSHR in a 1:1 ratio in HEK-293 cells as the positive control and a control DNA-GFP vector as a negative control. The cells were harvested after 48 h, and solubilized receptors were prepared as described in Materials and Methods. Individual preparations of protein were initially precleaned with protein G sepharose and then precipitated with anti-HA (2 μg/ml) for 3 h at 4C. This was followed by precipitation of the complex by sepharose G beads for another 2 h. The precipitate was then run on reduced gel and after transfer probed with anti-GFP. The lanes are as indicated: WT+WT, HA-TSHR cotransfected with TSHR-GFP; WT + β-316, WT + β-366, WT + β-409 and WT+ GFP vector. The WT+WT lane showed the presence of a small quanitity of oligomeric forms (>200 kDa) in addition to the glycosylated monomeric subunit of 120 kDa, whereas in the β-316 and β-366 subunit transfected cells we observed exclusively higher order forms of 145–200 kDa, which were resistant to reduction. We did not observe any higher order forms in the β-409 cells because these were solubilized membrane preparations in which this construct failed to express. The control transfected cells lacked the presence of any higher order forms. B, An immunoblot of the WT TSHR protein indicating the clear presence of oligomers. IP, Immunoprecipitation; KD, kilodalton; Rb, Rabit antibody.
FRET
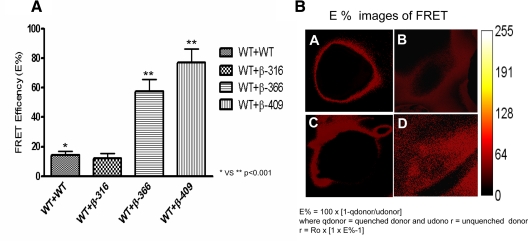
The results of the coimmunoprecipitations suggested a major interaction between truncated receptors and the full-length WT TSHRs. To ensure if these receptor-receptor interactions occurred in live cells, we performed FRET using double-transfected TSHR constructs tagged with GFP and Myc. In full-length WT cells, we achieved an average FRET efficiency of 15%. However, the FRET efficiency for β-366- and β-409-truncated constructs when cotransfected with TSHR-myc, and stained with anti-myc labeled with Cy3, was markedly greater with an increase up to approximately 75%. FRET efficiency for the β-316 construct was similar to that of the WT receptors. The energy transfer image (Ep) of β-409 clearly suggested higher energy transfer, but this was observed predominantly intracellularly, whereas with the β-316 and β-366 the transfer was more at the cell surface (Fig. 6). This finding was consistent with β-409 intracellular entrapment and simply indicated an intracellular interaction with the WT receptor in keeping with the earlier data.
Figure 6.
In vivo FRET of TSHR forms. FRET was performed on HEK-293 cells transiently transfected with the following combination of constructs: 1) TSHR-GFP + TSHR-myc; 2) β-316 GFP + TSHR-myc; 3) β-366GFP+TSHR-myc; and 4) β-409GFP + TSHR-myc. Myc was stained with anti-myc conjugated to cy3 after fixation with 4% parformaldehyde and permeabilization of cells with 0.5% saponin. FRET efficiency was calculated from five images of each setting acquired using the pFRET software as described in Materials and Methods. A, Illustrated in the graph is the calculated FRET efficiency (E%) of WT TSHR-GFP with the three truncated subunit constructs. FRET efficiencies were calculated using pFRET software. Note the high efficiency of energy transfer of the β-366 and β-409 constructs with the WT TSHR. B, This illustrates the E% images obtained for the FRET efficiency data shown in panel A. Upper left (A) is the WT control (TSHR-GFP + TSHR-myc), (B) TSHR-myc + β316-GFP, (C) TSHR-myc + β366-GFP and (D) TSHR-myc + β409-GFP.
Inhibition of Gαs-mediated receptor signaling associated with aberrant dimerization
We next examined the signaling consequences of the subunit-associated decrease in cell surface expression of the full-length TSHR in transfected and native thyrocytes. The total amount of cAMP generated by such cells depends on the affinity of the ligand for the receptor and the number of functional receptors available on the surface. As shown earlier, the surface expression of the full-length TSHR was compromised by interaction with the β-receptor constructs. However, the β-constructs had no ability to generate cAMP because they do not bind TSH (12).
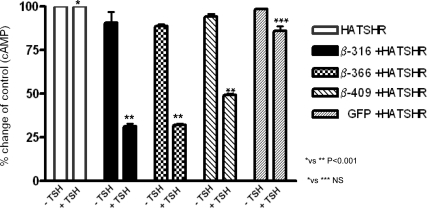
Constitutive activity of the WT HA-TSHR was 322 ± 43 fmol/well compared with 60 ± 5 fmol/well in untransfected HEK-293 cells. As we have previously reported (12), we did not observe any up-regulation of constitutive activity with any of the subunit constructs (data not shown). On stimulation with TSH (100 μU/ml) those cells transiently transfected with HA-TSHR developed intracellular cAMP concentrations of up to 1210 ± 31 fmol/well, a more than 4-fold increase. No significant change in the cAMP response to TSH was observed when GFP vector alone was cotransfected with the full-length receptor nor did we see any TSH-stimulated cAMP generation with the three truncated receptors when transfected alone. However, when cells were transfected with both truncated and full-length receptors and then exposed to TSH, we found that all three truncated constructs significantly reduced TSH-stimulated cAMP generation by the WT receptor but to variable degrees (Fig. 7).
Figure 7.
Effect of β-truncated constructs on cAMP generation. HEK-293 cells were transiently transfected with HA-TSHR and the respective β-constructs. After 48 h, the total intracellular cAMP generated was measured before and after 1 h of TSH stimulation (100 μU/ml). The basal constitutive cAMP of the WT HA-TSHR cells was 322 ± 43 fmol/well and was expressed as 100%. The β-subunit constitutive cAMP activities were low showing values of only 71–84 fmol/well. This low level likely reflects the degree of transient expression and needs further study by determining their specific constitutive activity (21). Cotransfection of the β-constructs with the full-length receptor compromised TSH responsiveness of the WT receptor as evidenced by the marked decrease in stimulated cAMP generation. The data shown here are an average of three independent experiments. NS, Not significant (P > 0.05).
Internalization of full-length TSHR enhanced by β-subunits
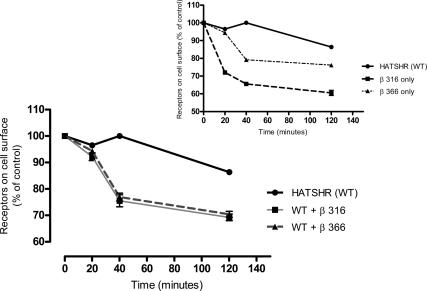
We found that the truncated constructs, β-316 and β-366, had a more rapid internalization compared with the full-length receptor (Fig. 8, inset). We then examined the effect of dimerization on the internalization of the full-length receptor by cotransfecting the WT and the truncated constructs individually and chasing the labeled cell surface full-length receptors at different time points. As shown in Fig. 8, the full-length receptor had enhanced constitutive internalization in the presence of the truncated constructs. Indeed approximately 30% of the receptors were internalized after 20 min in the cotransfected cells compared with less than 5% in the full-length-only transfected cells.
Figure 8.
Effect of β-truncated constructs on TSHR internalization. HEK-293 cells transfected with the expression vectors encoding the β-constructs and WT receptors were incubated with biotinylated TSHR-monoclonal antibody (9F4), which recognizes the TSHR ectodomain. After removal of the unbound Ig, cells were incubated at 37 C as indicated. Surface-bound antibody was quantified by measuring the fluorescent intensity of the bound Streptavidin-PE by the flow cytometry. Specific binding at each time point was normalized to the biotinylated TSHR monoclonal antibody (9F4) at 0 min. The inset illustrates the internalization rate of the WT TSHR and the truncated subunits alone. The main figure demonstrates that the subunit constructs markedly increased the internalization rate of the WT TSHR when cotransfected.
Discussion
The TSHR, due to the presence of unique cleavage sites in its extracellular domain (14), is unique in its structure when compared with other glycoprotein receptors, such as the LHR and FSHR. The large cleavage site of 50 amino acids (residues 316-367) in the ectodomain of the receptor undergoes nonspecific proteolytic degradation by unidentified matrix metalloproteases, leading to the formation of cleaved receptors, some of which may subsequently shed the α-subunits (15,16). Thus, it is possible that at any given time point, TSHRs exists in several different forms on the cell surface, and this will likely influence TSHR physiology including trafficking and signaling. Cleavage of the ectodomain would result in β-subunits of the receptor with an intact transmembrane domain capable of dimerizing with the WT receptors during trafficking and/or with the receptors on the cell surface. Therefore, this study investigated the possible influence of TSHR β-subunits on the physiology of the WT receptor and also began to examine their influence on the signalosome.
We modeled this study by using truncated receptor constructs that are akin to naturally cleaved receptors found on the surface of thyrocytes (39). We found that TSHR β-subunit expression was related to its degree of truncation and as emphasized by the compromised expression of the shortest β-409 construct, which was found mostly intracellularly (Fig. 2D and Fig. 3D). This observation was consistent with previous studies (10,13). The lower expression and complete intracellular entrapment of β-409 suggested that the degree of truncation of the TSHR influenced its normal trafficking to the surface although the other truncations (β316 and β366), which also removed the entire LRD region including five of the six glycosylation sites, still trafficked to the surface rather effectively. If truncated receptors lacking these major glycosylation sites on the TSHR can still reach the surface, it is clearly more than glycosylation that governs the cell surface expression of these cleaved receptors (17). The 30–40% surface expression of truncated β316 and β366 suggested that only changes in the distal part of the hinge region may be critical for trafficking of functional protein and may be critical for the binding of chaperones involved in proper folding and exit from the endoplasmic reticulum (ER). Alternatively, the relatively decreased expression of the truncated β316 and β366 constructs could be the result of enhanced receptor internalization. In studies by Misrahi and co-workers (3), only 55% of the truncated receptors remained on the cell membrane after 10 min compared with 75% of WT TSHRs. After 1 h, the membrane expression of the truncated receptors decreased further to 30%, whereas the WT TSHRs were still present on the surface. Our present data confirm these observations and suggest that such effects may be related to the altered binding kinetics of β-arrestin to these truncated receptors.
We wanted to study whether the modeled truncated receptors, which correspond to cleaved native TSHR subunits that are found in 3-fold excess over uncleaved receptors (9), would influence the normal physiology of the full-length WT receptors by homodimerization. To address this, when we first looked at their influence on expression of full-length WT receptors, we found that each of the β-subunits significantly reduced the expression of the WT receptors on the cell surface (Fig. 4, A and B). This decrease could be due to 1) Less surface trafficking of the full-length WT receptor due to homointeraction of properly folded WT protein with partially folded slow trafficking truncated receptors and/or 2) Faster internalization of such truncated/ WT multimers leading to an apparent down-regulation of the WT full-length receptors, or it could be both. In both scenarios there must be direct interaction of the truncated β-receptors with the WT receptors most likely via the transmembrane domains (7,18). To confirm whether this was indeed the case, we performed coimmunoprecipitation followed by FRET (Figs. 5 and 6). The coimmunoprecipitation and FRET studies indicated that the truncated receptors retained the ability to dimerize with the full-length receptor. However, the FRET studies also showed a dramatic increase in energy transfer for the 366 and 409 transfected constructs. This increased FRET may be due to a favorable structural change in the transmembrane domain resulting in an improved dipole orientation of the FRET tags leading to increased proximity of the donors and acceptors. It also suggested that the presence of the cleavage region, a 50-amino acid peptide, in the full-length receptor may have a tertiary structure or fold that results in a structural hindrance to the proximity of the transmembrane domains for energy transfer. This seems to be the case with lower FRET and the decreased immunoprecipitate observed in our study (Figs. 5 and 6).
Having established that the β-subunits of the TSHR were capable of homodimerizing with the full-length receptors, we next wanted to study whether this interaction had any physiological consequence on the signaling capacity of the receptors. The truncated receptors did not induce any increased constitutive activity, an observation consistent with our previous study (12) and with studies on chemical shedding of the ectodomain, which did not increase basal activity in other closely related glycoprotein hormone receptors (19). Others have reported an increase in constitutive activity with similar approaches that remains unclear (10,12). However, our studies showed that the truncated receptors were able to alter the ligand-stimulated Gαs signaling capacity of the full-length receptor. This may be a consequence of decreased full-length receptor expression at the surface or a consequence of structural changes secondary to the dimerization. The fact that the truncated receptors (β-316 and β-366) enhanced the basal internalization of the WT receptors makes the former an attractive explanation for alteration of signaling by receptors residing on the surface. Alternatively, this effect on signaling could be due to the ability of the truncated receptor, such as β-409, holding the newly synthesized receptors within the endoplasmic ER or Golgi and preventing their normal trafficking to the surface. A decrease in receptor signaling leading to congenital hypothyroidism has been observed where mutant receptor, which misfolded in the ER, prevented the full-length receptor from trafficking normally (20).
In vivo, the TSHRs are subjected to cleavage at the cell surface, as opposed to our transfection model system, where already synthesized β-subunits were expressed within the cell and subsequently transported to the membrane. This model system, therefore, allowed intracellular dimerization with β-subunits to occur de novo and may not reflect exactly the processing on the cell surface of thyrocytes. However, the strong receptor-receptor interactions indicated by the FRET studies coupled with the data on the increased internalization rate of truncated TSHRs, was strong evidence that this phenomenon most likely occurs at the normal thyrocyte cell surface. These data also strongly supported the hypothesis that spontaneously occurring intramolecular cleavage of the TSHR resulting in subunit generation must have physiological consequences for TSHR expression, signaling, internalization, and degradation. The ultimate effect of receptor-receptor interactions in our system was a reduction in signaling by the WT receptors. For example, both basal and stimulated cAMP levels of cells cotransfected with the WT TSHR and β-subunits were decreased. Hence, impaired signaling of the full-length receptors was a natural consequence of β-subunit multimerization so that the well known accumulation of such subunits, secondary to intramolecular cleavage and shedding of the α-subunits, and their binding to WT receptors, in the natural setting would reduce the TSHR signal. Therefore, subunit forms of the TSHR on thyrocytes may have an important role in dampening the ligand signal by homodimerization, and this modulation of TSHR signaling may be vital for the normal growth and function of thyrocytes.
Materials and Methods
Constructs
In this study we used three plasmid constructs of a truncated human TSHR (hTSHR), starting at 316, 366, and 409 amino acids of the hTSHR protein, respectively. The constructs were cloned into GFP-N3 vector, being C-terminally tagged with GFP, as we previously characterized (12).
The β-316 sequence commenced at the beginning of the cleavage region (the first cleavage site), thus retaining the whole 50-amino acid region, whereas β-366 was designed to omit the cleavage region completely but still retaining the portion of the receptor ectodomain. The β-409 sequence, the shortest construct almost completely deprived of the ectodomain, consisted of just the 409-417 amino acids of the ectodomain as well as transmembrane and intracellular domains of the TSHR (Fig. 1).
For WT TSHR control, we used three constructs: 1) N-terminally HA-tagged full-length human TSHR cloned into pCDNA3.1, described previously (8), and used for expression, immunoprecipitation, and cAMP generation; 2) Human full-length WT-TSHR cloned into GFP-plasmid (C-terminally tagged); and 3) Full-length WT-TSHR directly tagged with Myc again at the carboxyl terminus and detected with anti-myc-Cy3 (6,7). In addition, we used a membrane expressing control vector that expresses a PM protein tagged with RFP/GFP (Invitrogen, Carlsbad, CA).
Cell culture and transfection
Human embryonic kidney cells (HEK-293) were acquired from the American Type Culture Collection (ATCC, Manassas, VA) and were routinely cultured in DMEM supplemented with 10% fetal bovine serum and 100 U/ml of penicillin G sodium, 100 μg/ml of streptomycin sulfate (Invitrogen) in 100-mm cell culture dishes (Becton-Dickinson), until 80–90% confluent. Before subsequent experiments, the cells were washed twice with PBS), and then split using trypsin and reseeded in the desired density for transfection. Transient transfections and cotransfections were performed with Lipofectamine 2000 (Invitrogen) in 100-μl and 2-ml reaction volume, for 24-well and 100-mm plates, respectively. The cultures were assessed 48 h after transfection for transfection efficiency by expression of GFP before stimulation. A stable line expressing HA-TSHR in CHO cells and maintained in F12 medium with 10% fetal bovine serum and 100 U/ml of penicillin G sodium and 100 U of streptomycin sulfate, as described elsewhere (8), was used for modulation of cell surface expression by truncated β-subunit constructs. For FRET, cells were grown in delta-T dishes. To ensure cell adhesion, before seeding, the plates were initially pretreated with 0.1% gelatin for 30 min at room temperature. For immunoprecipitation experiments, 8 × 106 cells were seeded into 100-mm culture dishes and transfected and subsequently subjected to lysis to obtain the membrane fraction as described previously (6,7).
Flow cytometry
For flow cytometry [fluorescence-activated cell sorting (FACS)], 0.5 × 106 cells were cultured in 24-well culture plates and transfected with respective DNA. The cells were detached after 48 h with 1 mm EDTA/EGTA and collected by gentle pipetting and washed twice in PBS. To ascertain membrane expression of the β-constructs or the full-length receptor as required, we stained unfixed and nonpermealized cells with receptor-specific antibody M1 to residues 381-385 of the TSHR (RSR1, kindly supplied by Dr. B. Rees Smith, RSR Ltd, Cardiff, Wales, UK). The cells were incubated in 100 μl of 0.5 μg/ml RSR1 antibody in FACS buffer with for 1 h at room temperature. After that, the cells were spun down at 1200 rpm for 5 min at 4 C and washed three times with 1 ml of ice-cold FACS buffer. The cells were then incubated with antimouse Fab PE-conjugated antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at 1:200 dilution. HEK-293 cells expressing HA-tagged full-length TSHR were stained with 1 μg/ml of anti-HA mouse monoclonal antibody (Jackson ImmunoResearch) followed with antimouse conjugated with PE. Stained cells were then analyzed in the red channel (FL2) for PE staining and green channel (FL1) for GFP expression. Negative controls stained with primary and secondary isotype were also analyzed the same way.
Immunohistochemistry
For immunohistochemistry staining, 0.4 × 106 HEK-293 cells were seeded in chamber slides and transfected with the particular DNA either WT or the truncated β-subunit constructs. After 24–48 h of transfection, these cells were transduced with PM-RFP as per the manufacturer’s instructions. The PM-RFP-transduced cells were then fixed with 4% paraformaldehyde and mounted using Vectashield with 4′,6-diamidino-2-phenylindole and observed using the appropriate filter sets in a fluorescent microscope.
Coimmunoprecipitation
Forty eight hours after cotransfection with HA-TSHR and/or β-subunit DNA in 100-mm dishes, the cells were washed twice with ice-cold PBS and subjected to lysis using 2 ml of cell lysis buffer (120 mm HEPES; 100 mm NaCl; 1% Triton-X; 10% glycerol, pH 7.2) containing protease inhibitor cocktail (Complet Mini; Promega Corp., Madison, WI). After a 10-min incubation on ice, the cells were scraped and collected into precooled 1.5-ml tubes. The lysate was centrifuged at 15,000 × g for 10 min at 4 C, and the soluble fraction containing solubilized receptors was collected. For immunoprecipitation, 500 μg of the solubilized receptors was precleared with protein G for 30 min at 4 C, and the supernatant of solubilized receptors was precipitated with 2 μg/tube of anti-HA antibody in 600 μl of 1× RIPA buffer (25 mm Tris·HCl, pH 7.6; 150 mm NaCl; 1% Nonidet P-40; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate), at 4 C for 3 h. Further, antibody-receptor complexes were bound to 25 μl of equilibrated protein G sepharose beads by incubating for another 3 h at 4 C. The beads were spun down (500 × g, 1 min) and washed four times with 1 ml of ice-cold PBS containing protease inhibitors. The samples were then reduced with 5× Laemmeli buffers containing 100 mm dithiothreitol and incubated in a water bath for 1 h at 50 C. Samples were then electrophoresed on a 4–15% gradient polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride membranes, and the membranes were blocked using 5% skimmed milk in Tris-buffered saline-Tween 20 for 2 h at room temperature. The blot was probed further using anti-GFP antibody and developed using goat antirabbit horseradish peroxidase conjugate at 1:5000 dilution. After 1-h incubation and repeated washing steps, enhanced chemiluminescence was applied and blot was developed using the imager FluorChem HD2 (Alpha Innotech, San Leandro, CA).
FRET
FRET measurements were carried out by the method of sensitized emission (also commonly referred to as “two color ratiometric imaging”) on a standard wide-field Nikon SE 2000 microscope (Nikon, Melville, NY) using a 60× Plan Apo VC oil objective with an NA of 1.4. The hardware consisted of the excitation and emission fluorescence filter wheel sets, the motorized stage, and a 12-bit cool SNAPez monochrome camera with a 20-mHz readout. The motorized stage and filter wheels on the scope were controlled by software In Vivo (Media Cybernetics, Inc.,). In sensitized emission, the donor fluorophores, in this case GFP, was excited by its specific wavelength, and the signal was gathered using two emission filter sets tuned for either the donor or acceptor fluorescence. The bleedthrough is usually a problem when using fluorescent proteins, and this was taken care of in our FRET assay by subtracting out donor spectral bleedthrough and acceptor spectral bleedthrough from the images before FRET efficiency (E%) calculations using the pFRET software (obtained from Dr. Ammasi Periasamy, W.M Keck Center for Cellular Imaging, University of Virginia, Charlottesville, VA). A total of four images were acquired for each of the transfected cells to be processed by the pFRET algorithm (W.M Keck Center for Cellular Imaging, University of Virginia). The images acquired are 1) Dex-Dem: The donor excitation wavelength and donor emission filters; 2) Aex-Aem: The donor excitation wavelength and acceptor emission filters; 3) Dex-Aem: The donor excitation wavelength and acceptor emission filters were used. This was the FRET image. 4) Aex-Dem: The acceptor excitation wavelength and acceptor emission filters were used. All images were collected as 16-bit gray scale TIFF images. The software also generates the pFRET (corrected FRET), Ep (Efficiency), and r (FRET distance) images for each set of analysis. The Ep images thus generated are pseudocolored to reflect the energy efficiency. The energy transfer efficiency percent (E%) and FRET distance are calculated by the pFRET software as follows : E% = 100 × [1 − qdonor/udonor] where qdonor = quenched donor and udono r = unquenched donor and r = Ro × [1 × E% − 1]^(1/6) where Ro = Foster distance between the fluorophores.
To demonstrate dimerization of the β-subunits with the full-length receptor, HEK-293 cells were double transfected as described below: 1) TSHR-myc + TSHR-GFP tagged full-length receptors; 2) β-316-TSHR-GFP + full length TSHR-myc; 3) β-366 -TSHR-GFP + TSHR-myc; 4) β-409-TSHR-GFP + TSHR-myc. TSHR-myc in all these double-transfected cells was detected using anti-myc conjugated to Cy3 after fixing the cells with 4% parformalhyde and permeabilizing with 0.5% saponin in PBS. Single-transfected cells of 5) TSHR-GFP only and 6) TSHR-myc only were the internal controls in the FRET experiment. We have also previously shown that physically mixing single populations of TSHR-GFP and TSHR-myc stained with cy3 did not result in a positive FRET image (7).
Intracellular cAMP generation
HEK-293 cells were transfected in 24-well plates with the same combination of constructs as described above and allowed to reach 80–90% confluency in 48 h. The cells were then stimulated with bovine TSH (1000 μU/ml) in complete medium containing phosphodiestrase inhibitor 3-ispbutyl-1-methylxanthine (2 mm/well). The cells were lysed using commercial lysis buffer, and intracellular cAMP levels were measured by EIA (Amersham cAMP Biotrak System; GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Measuring internalization of receptors
HEK-293 cells were transfected with full-length HA-TSHR construct and β-316 and β-366 in equal ratios. The cells were harvested and washed twice 48 h after transfection and chilled on ice for 10 min. A biotinylated antibody (9F4) to the TSHR was added to the cells and incubated for 30 min on ice. The cells were then washed once with cold PBS and once with warm PBS (37 C), and tubes were transferred immediately to 37 C water bath and held for various time points. The surface-labeled cells at various time points were taken out and washed twice with FACS buffer containing 0.02% azide and labeled with strepavidin conjugated to phycoerythrin (PE). We chased the cell surface b9F4 and expressed the percentage of biotinylated 9F4 binding based on 9F4 binding at 0 min.
Statistical analysis
The analysis of significance for the data presented here was done using two-way ANOVA: Bonferroni’s multiple comparison test using Prism software (GraphPad Software, Inc., San Diego, CA).
Acknowledgments
We thank Zhong Yao (VAMC) for technical assistance and Professor Jerzy Sowinski (Department of Endocrinology, University of Medical Sciences, Poznan, Poland) for advice and support.
Footnotes
This work was supported by: National Institutes of Health Grant DK069713 from National Institute of Diabetes and Kidney and Digestive Diseases, the Veterans Affairs Merit Award program 4535-05-074 (to T.F.D.), and the David Owen Segal Endowment (to K.M.).
Disclosure Summary: We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this research paper.
First Published Online August 18, 2010
Abbreviations: CHO, Chinese hamster ovary; ER, endoplasmic reticulum; FACS, fluorescence-activated cell sorting; FRET, Foster resonance energy transfer; GFP, green fluorescent protein; HA, hemagglutinin; HEK, human embryonic kidney; PE, phycoerythin; PM, plasma membrane; RFP, red fluorescent protein; TSHR, TSH receptor; WT, wild type.
References
- Kursawe R, Paschke R 2007 Modulation of TSHR signaling by posttranslational modifications. Trends Endocrinol Metab 18:199–207 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- Quellari M, Desroches A, Beau I, Beaudeux E, Misrahi M 2003 Role of cleavage and shedding in human thyrotropin receptor function and trafficking. Eur J Biochem 270:3486–3497 [DOI] [PubMed] [Google Scholar]
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- Graves PN, Vlase H, Bobovnikova Y, Davies TF 1996 Multimeric complex formation by the thyrotropin receptor in solubilized thyroid membranes. Endocrinology 137:3915–3920 [DOI] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF 2001 Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem 276:45217–45224 [DOI] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF 2002 Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J Biol Chem 277:45059–45067 [DOI] [PubMed] [Google Scholar]
- Latif R, Ando T, Davies TF 2004 Monomerization as a prerequisite for intramolecular cleavage and shedding of the thyrotropin receptor. Endocrinology 145:5580–5588 [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Pichon C, Jolivet A, Misrahi M, Caillou B, Jamous M, Vannier B, Milgrom E 1992 Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA 89:3765–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Tong KP, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW 2000 The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517 [DOI] [PubMed] [Google Scholar]
- Zhang ML, Sugawa H, Kosugi S, Mori T 1995 Constitutive activation of the thyrotropin receptor by deletion of a portion of the extracellular domain. Biochem Biophys Res Commun 211:205–210 [DOI] [PubMed] [Google Scholar]
- Ciullo I, Latif R, Graves P, Davies TF 2003 Functional assessment of the thyrotropin receptor-β subunit. Endocrinology 144:3176–3181 [DOI] [PubMed] [Google Scholar]
- Jeffreys J, Depraetere H, Sanders J, Oda Y, Evans M, Kiddie A, Richards T, Furmaniak J, Rees Smith B 2002 Characterization of the thyrotropin binding pocket. Thyroid 12:1051–1061 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Tanaka K, Nagayama Y, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B 1997 Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology 138:2893–2899 [DOI] [PubMed] [Google Scholar]
- Couet J, Sar S, Jolivet A, Hai MT, Milgrom E, Misrahi M 1996 Shedding of human thyrotropin receptor ectodomain. Involvement of a matrix metalloprotease. J Biol Chem 271:4545–4552 [DOI] [PubMed] [Google Scholar]
- Couët J, de Bernard S, Loosfelt H, Saunier B, Milgrom E, Misrahi M 1996 Cell surface protein disulfide-isomerase is involved in the shedding of human thyrotropin receptor ectodomain. Biochemistry 35:14800–14805 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Nishihara E, Namba H, Yamashita S, Niwa M 2000 Identification of the sites of asparagine-linked glycosylation on the human thyrotropin receptor and studies on their role in receptor function and expression. J Pharmacol Exp Ther 295:404–409 [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S 2005 Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J 24:1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karges B, Gidenne S, Aumas C, Haddad F, Kelly PA, Milgrom E, de Roux N 2005 Zero-length cross-linking reveals that tight interactions between the extracellular and transmembrane domains of the luteinizing hormone receptor persist during receptor activation. Mol Endocrinol 19:2086–2098 [DOI] [PubMed] [Google Scholar]
- Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L 2005 Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet 14:2991–3002 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Chen CR, McLachlan SM, Rapoport B 2008 The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol 22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]