Abstract
Androgens suppress TGF-β responses in the prostate through mechanisms that are not fully explored. We have recently reported that 5α-dihydrotestosterone (DHT) suppresses the ability of TGF-β to inhibit proliferation and induce apoptosis of prostatic epithelial cells and provided evidence that such suppression was fueled by transcriptional down-regulation of TGF-β receptor II (ΤβRII). We now show that androgen receptor (AR) activated by DHT suppresses the TGF-β-induced phosphorylation of Sma- and Mad-related protein (Smad)3 in LNCaP cells overexpressing TβRII under the control of a cytomegalovirus promoter, which is not regulated by DHT, suggesting that transcriptional repression of TβRII alone does not fully account for the impact of DHT on TGF-β responses. Instead, we demonstrate that such suppression occurs through loss of total Smad3, resulting from transcriptional suppression of Smad3. We provide evidence that DHT down-regulates the promoter activity of Smad3 in various prostate cancer cell lines, including NRP-154+AR, DU145+AR, LNCaP, and VCaP, at least partly through androgen-dependent inactivation of Sp1. Moreover, we show that overexpression of Smad3 reverses the ability of DHT to protect against TGF-β-induced apoptosis in NRP-154+AR, supporting our model that loss of Smad3 by DHT is involved in the protection against TGF-β-induced apoptosis. Together, these findings suggest that deregulated/enhanced expression and activation of AR in prostate carcinomas may intercept the tumor suppressor function of TGF-β through transcriptional suppression of Smad3, thereby providing new mechanistic insight into the development of castration-resistant prostate cancer.
Androgens suppress TGF-beta responses (including apoptosis) in prostate epithelial cells by loss of Smad3 expression through a Smad3 promoter-dependent mechanism involving reduced Sp1 binding.
The function of TGF-β as a tumor suppressor has been reported in a variety of tissues (1,2), and aberrant TGF-β signaling is believed to be pivotal to the development and progression of a variety of tumors. TGF-β signaling is initiated upon binding of TGF-β ligands to two transmembrane serine/threonine kinase receptors, namely, TGF-β receptor type II (TβRII) and type I (TβRI), which form a heterotetrameric receptor complex. TβRII is a constitutively active kinase that activates TβRI by transphosphorylation. Activated TβRI, in turn, activates Sma- and Mad-related protein (Smad)s 2 and 3 by phosphorylating their two C-terminal serine residues (3,4,5). The activities of these receptors and Smads seem to be intimately regulated by numerous other effectors such as epidermal growth factor, phosphotidylinositol-3 kinase, Akt, mammalian target of rapamycin, and the androgen receptor (AR) (6,7,8).
AR is a member of the nuclear hormone receptors and plays critical roles in development, growth, maintenance, and function of androgen target tissues such as the prostate. The most potent naturally occurring androgen, DHT, which is directly generated from testosterone by 5α-reductase, associates with AR to commence signal transduction. This ligand binding releases inhibitory heat shock proteins from AR, enabling AR to translocate to the nucleus where it can function as a transcriptional factor by associating to androgen response elements (AREs) or as a transcriptional coregulator through binding to and influencing the activity of other transcription factors (9,10,11,12,13).
Multiple levels of cross talk between AR and TGF-β have been reported (6,14,15,16,17,18). In rodents, androgen deprivation leads to rapid elevation of TGF-β, TβRI, TβRII, Smad3, and Smad4 and activation of Smad2, concomitant with the onset of apoptosis (19,20,21,22). These and other studies suggest that androgens promote cell survival, in part by blocking TGF-β-induced cell death through mechanisms that remain poorly understood (6,23,24,25,26,27).
Previously, our group showed that DHT can repress the ability of TGF-β to control gene expression through the binding of AR to Smad3, affecting the association of Smad3 to Smad-binding elements (6). More recently, we reported that androgen-bound AR partially protects cells from TGF-β-induced apoptosis and that DHT suppresses the levels of TβRII through a transcriptional mechanism (16). We now report an additional mechanism by which DHT inhibits TGF-β signaling, namely by selectively suppressing the expression of Smad3. We show that down-regulation of Smad3 levels occurs through transcriptional repression, which is mediated, at least in part, through suppression of Sp1/Sp3. We suggest that through this mechanism DHT selectively suppresses Smad3-dependent TGF-β signaling over that of a Smad2-dependent one.
Results
DHT suppresses the levels of Smad3 rat and human prostate cell lines
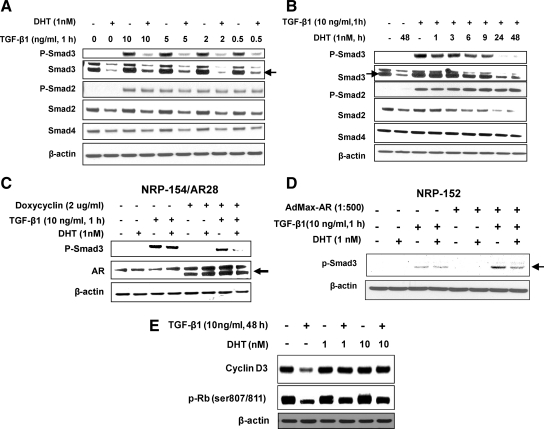
We recently reported that DHT triggers a robust attenuation of TGF-β responses in a variety of prostate epithelial cell lines including NRP-154, a tumorigenic rat prostate cell line that is exquisitely sensitive to TGF-β-induced apoptosis (28). Although the inclusion of 0.1 nm DHT alone did not enhance growth and viability of NRP-154+AR cells (NRP-154 cells expressing AR by infection with AdMax-AR adenovirus), short-term cotreatment of these cells with 0.1 nm DHT stably rescued them from TGF-β1-induced apoptosis. In that study we provided evidence that DHT suppresses TGF-β-induced apoptosis through down-regulation of TβRII levels, driven by a transcriptional mechanism. We showed that DHT also suppressed TGF-β-induced expression of plasminogen activator inhibitor-1 (PAI-1), down-regulation of cyclin Ds and Bcl-xl, and activation of caspase-3 (16). Consistent with loss of TβRII levels by DHT, here we show that DHT effectively intercepts TGF-β1 from activating Smad3. This occurred even with a 20-fold molar excess of TGF-β1 required to maximally activate Smad3, as assessed by Western blot analysis of phospho-Smad3 (Ser 423/425) (Fig. 1A). However, when the above blot was reprobed for total Smad3, we unexpectedly discovered that the total levels of Smad3 were also substantially suppressed by DHT, and such suppression directly correlated with loss of phospho-Smad3 (Fig. 1A). These effects were rather rapid, as loss of phospho-Smad3 and total Smad3 were observed between 6 and 9 h of DHT treatment (Fig. 1, A and B), suggesting that repression of phospho-Smad3 levels by DHT may result from reduced expression of both Smad3 (Fig. 1A) and TβRII (16). Similar results were obtained with NRP-154/AR28, a doxycycline-inducible NRP-154 clone stably expressing AR (Fig. 1C), and in NRP-152±AR, a nontumorigenic rat prostatic epithelial cell line transiently expressing AR (Fig. 1D). We also observed slight losses in total protein levels of Smads 2 and 4 by AR+DHT; however, total and phospho-Smad2 was not significantly down-regulated by DHT unless cells were cotreated with TGF-β1 for at least 24 h (Fig. 1, A and B, and Supplemental Fig. 1A published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). These results suggest that DHT selectively suppresses Smad3-dependent targets over Smad2-dependent ones, consistent with our findings that DHT more completely inhibits Smad3-dependent targets (cyclin D3) than ones dependent on Smad2 or Smad2 and Smad3 [retinoblastoma protein (Rb) phosphorylation, survivin down-regulation] (29). In the case of cyclin Ds, silencing either Smad2 alone or Smad3 alone reversed cyclin D down-regulation by TGF-β (data not shown). In contrast, silencing both Smads 2 and 3 was necessary to effectively reverse TGF-β down-regulation of survivin expression (29). Consistent with our findings in NRP-154 cells, DHT reversed the ability of TGF-β to down-regulate expression of cyclin Ds but did not appear to significantly reverse the ability of TGF-β to down-regulate survivin expression (Fig. 1E) (16).
Figure 1.
Androgen inhibits TGF-β1-induced Smad3 activation and Smad3-mediated TGF-β responses in NRP154, rat prostatic epithelial cell line. NRP-154 cells were infected for 2 h with AdMax adenoviral for expression of AR (AdMax-AR, 1:500), and after 24 h the infected cells were treated ± 1 nm DHT for 48 h (A) or 1–48 h (B) before TGF-β1 (1 h) (0.5 to 10 ng/ml) (A) or 10 ng/ml (B) treatment. Cell lysates were then analyzed for expression or total Smads 2, 3, and 4 as well as active Smads [phospho-Smad2 (Ser465/467) and phospho-Smad3 (Ser423/425)] by Western blot. C and D, Effect of DHT on suppression of phospho-Smad (Ser423/425) in NRP-154/AR28 (clone 28 cells stably overexpressing AR) ± doxycyclin (2 μg/ml) (C), and in NRP-152 cells infected 2 h with 1:500 AdMax-AR (NRP-152+AR) or control virus (D); DHT and TGF-β treatments were as in panel A. E, NRP-154+AR cells were pretreated with DHT for 24 h and then treated with TGF-β1 48 h TGF-β1 (10 ng/ml) after which lysates were examined for levels of cyclin D3 and Rb phosphorylation by Western blot analysis. Data shown are representative of two or three independent experiments (A–D). In cases in which multiple bands appeared, the correct bands are indicated by the arrows (→ or ←), as confirmed by use of Smad short hairpin RNAs (29) or overexpression of AR (in panel C).
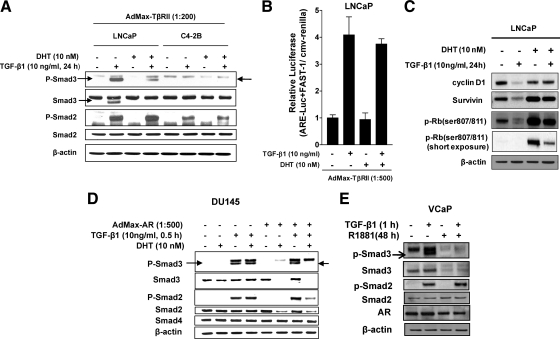
We performed similar experiments as above in a variety of human prostate cancer cell lines, including LNCaP, -2B, DU145, and VCaP cells (Fig. 2). Previously, we were not able to detect the induction of PAI-1 protein expression by TGF-β1 treatment in the C4-2B cell line (16), a bone metastatic, androgen-refractory derivative of LNCaP (30), even when TβRII was overexpressed. Endogenous Smad3 was undetectable in LNCaP cells unless cells received exogenous TGF-β1. Very interestingly, exogenous TGF-β1 strongly induced Smad3 expression in LNCaP but not in C4-2B cells. On the other hand, Smad2 was expressed at detectable levels in both cell lines. We found that DHT diminished both phospho- and total Smad3 levels in LNCaP cells, whereas neither total Smad2 nor phospho-Smad2 was regulated by androgen in either cell line (Fig. 2A). Consistent with this result, DHT failed to suppress TGF-β-activation of a Smad2-specific promoter-reporter construct, ARE-luc/FAST-1 (activin response element reporter/forkhead activin signal transducer-1), in LNCaP cells (Fig. 2B). Similarly, in these cells DHT completely reversed Smad3-mediated TGF-β responses (loss of cyclin D1 expression) (Fig. 2C and data not shown), but incompletely suppressed TGF-β responses mediated by both Smads 2 and 3 [survivin, deposphorylation of Rb (29)] (Fig. 2C). These results support that DHT selectively suppresses Smad3-mediated TGF-β responses in LNCaP cells.
Figure 2.
Androgens suppress expression of Smad3 and TGF-β-regulated gene expression in human prostate cancer cell lines. A, D, and E, Effect of DHT or R1881 on expression and activation of Smads in LNCaP, C4-2B (A), DU145 (D), and VCaP cells (E) and on TGF-β1 regulation of cyclin D1, survivin and phospho-Rb (807/811) expression in LNCaP cells (C). Before treating cells with androgens, LNCaP and C4-2B cells were infected overnight with 1:200 AdMax-TβII, and DU145 cells were infected overnight with 1:500 AdMax-AR. The infected cells were pretreated ± 10 nm DHT for 48 h before TGF-β1 (1 h, 10 ng/ml) treatment. Samples were subjected to Western blotting as in Fig. 1. B, Effect of androgen on ARE-luc/FAST-1 (activin response element/forkhead activin signal transducer-1) activity in LNCaP cells. Cells were transiently transfected with a total of 1 μg of DNA including ARE-luc construct, FAST-1 construct, and CMV-Renilla (using lipofectamine plus transfection reagent) followed by infection with AdMax-TβRII (for 16 h). Cells were then treated with or without DHT and after 24 h treated with TGF-β1 (10 ng/ml) for an additional 24 h. Data shown are relative values of firefly luciferase normalized to Renilla luciferase. Each bar represents the average of triplicate determinations ± se. Results are representative of two to three experiments (A–E). The correct bands are indicated by arrows, as confirmed by Smad small interfering RNA experiments (8).
In DU145+AR (expressing AR by adenovirus infection) and VCaP (positive for endogenous AR) (31), we similarly observed that DHT or the metabolically stable androgen, R1881, abrogates Smad3 activation along with suppressing total Smad3 levels (Fig. 2, D and E). Distinctly, the ability of AR to repress levels of Smad2 in DU145 was much greater than that in NRP-154, showing clear suppression of both phospho- and total Smad2 (Fig. 2D). In contrast, Smad4 was not noticeably regulated by DHT in DU145 cells (Fig. 2D), similar to NRP-154 cells (Fig. 1B). Collectively, our data here suggest that androgens repress the levels of Smad3 and, in certain cell lines and/or conditions, may also repress levels of Smad2.
Transcriptional repression of Smad3 by DHT
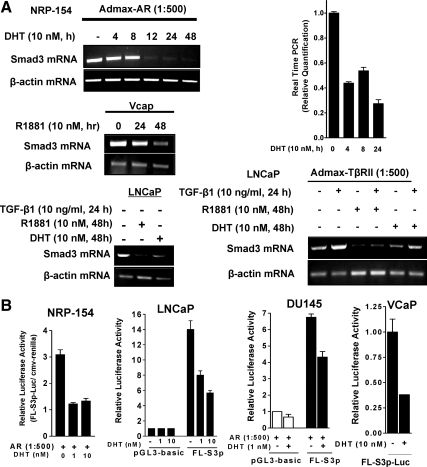
The relatively rapid (6–9 h) down-regulation of Smad3 protein by DHT (Fig. 1B) in NRP-154 cells suggested a transcriptional mechanism. We therefore examined whether DHT suppressed the mRNA levels of Smad3 in NRP-154 cells, as measured by RT-PCR. Consistent with the above results, a time course experiment revealed that DHT significantly decreased the mRNA levels of Smad3, with substantial loss by 12 h of treatment (Fig. 3A). Real-time PCR analysis indicated that loss of Smad3 mRNA occurred as early as 4 h, consistent with a transcriptional mechanism. Significantly, androgens (DHT and R1881) also suppressed Smad3 mRNA by 24 h in both VCaP and LNCaP cells, which express endogenous AR. As expected, the metabolically stable androgen R1881 suppressed levels of Smad3 mRNA more effectively than did DHT. Owing to our observation that Smad3 protein was detectable in LNCaP+TβRII cells only after TGF-β1 treatment, the ability of DHT to suppress expression of Smad3 mRNA was also examined in LNCaP+TβRII cells treated in the presence or absence of TGF-β1 under the same condition as in Fig. 2A. Consistent with Fig. 2A, Smad3 mRNA was induced about 3-fold by TGF-β1, and both DHT and R1881 suppressed the levels of Smad3 mRNA either in the presence or absence of TGF-β1 addition (Fig. 3A). These data suggest that androgens suppress the expression of Smad3 through changes in mRNA levels rather in than protein stability or translational control.
Figure 3.
Transcriptional regulation of Smad3 by DHT in NRP-154, LNCaP, DU145, and VCaP cells. A, Androgens repress Smad3 mRNA levels in prostate cancer cell lines. NRP-154 cells were plated in 100-mm dishes with modified GM3 medium containing 1% DC-FBS. After overnight for attachment, cells were and infected with AdMax-AR (1:500) for 2 h and then treated with DHT (10 nm, 4–48 h). VCaP cells were incubated with 10 nm of R1881 for 24 or 48 h. For LNCaP cells, the effect of DHT or R1881 (10 nm, 48 h) on Smad3 mRNA levels was examined either in noninfected cells, or in cells infected with AdMax-TβRII (1:500, 24 h), followed by TGF-β treatment for an additional 24 h. cDNA was made from 5 μg of total RNA (purified by RNeasy) by RT Superscript and semiquantitative PCR Smad3 expression (for NRP-154, LNCaP, and VCaP), or real-time PCR using TaqMan Real Time PCR kit and 7500-Real Time PCR System (Applied Biosystems) (for NRP-154 cells). B, Androgens repress activity of the Smad3 promoter in prostate cancer cell lines. Cells were transfected with a total 1 μg of DNA including either control luciferase vector (pGL3-basic) or full-length (1892 bp) Smad3 promoter-luciferase (FL-S3p-luc) construct and CMV-Renilla, followed by DHT treatment for 48 h. For NRP-154 and DU145 cells, cells were transfected in the presence of AR (AdMax-AR, 1:500). Data shown are relative values of firefly luciferase normalized to Renilla luciferase. Each bar represents the average of triplicate determinations ± se.
To test the relevance of our findings to human prostate tissues, we used the Oncomine database to compare the mRNA levels of Smad3 and AR in a cohort of 102 freshly collected human prostate tissues (normal, primary tumors, metastases) (32). This analysis supports our model that elevated AR activity, as reflected by higher levels of AR mRNA, significantly promotes the down-regulation of Smad3 mRNA (Supplemental Fig. 2).
We thus hypothesized that loss of Smad3 mRNA by DHT occurs by a transcription mechanism. To test this hypothesis, we cotransfected rat and human prostate cell lines (i.e. NRP-154+AR, LNCaP, DU145+AR, and VCaP) with a full-length (1892 bp) human Smad3 promoter-luciferase construct (FL-S3p-luc) (33) along with cytomegalovirus (CMV)-Renilla. Cells were then treated with the indicated concentrations of DHT, and changes in relative luciferase activity were measured 24 h later (Fig. 3B). DHT (1 and 10 nm) suppressed the activity of FL-S3p-luc by 60% in NRP-154+AR cells (Fig. 3B); similar results were observed in LNCaP, VcaP, and DU145+AR (Fig. 3B).
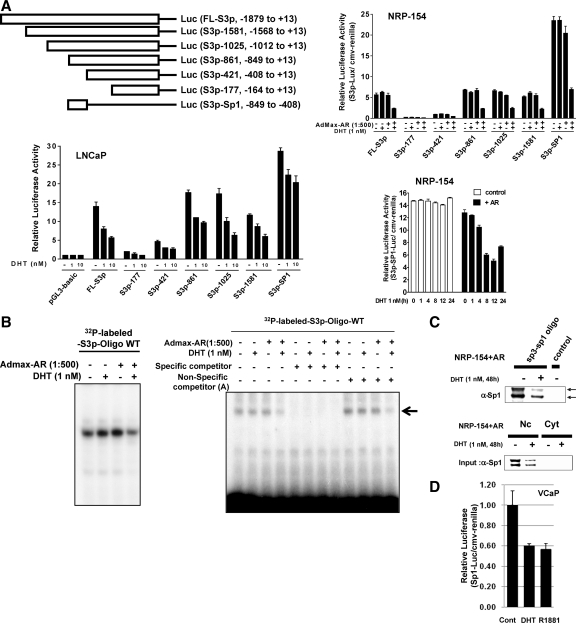
To identify the specific promoter region(s) of Smad3 responsible for transcriptional suppression by DHT, the NRP-154 (±AR) and LNCaP cells were transiently transfected with various 5′-end truncations of the Smad3 promoter linked to the firefly luciferase reporter (34). Surprisingly, DHT suppressed the activity of all those Smad3-promoter constructs despite differences in the magnitude of repression between various cell lines (Fig. 4A). This unexpected observation led us to examine the involvement of transcription factors essential for basal transcription of the Smad3 promoter.
Figure 4.
Transcriptional regulation of Smad3 by DHT may occur through mechanisms involving Sp1. A, Deletion map of Smad3-promoter constructs [full-length (FL) promoter = 1892 bp]. NRP-154 or LNCaP cells were transfected with either pGL3-basic [control (cont)] or Smad3 promoter-luciferase (S3p-luc) constructs (FL) or deletions in the presence/or absence of AR (AdMax-AR, 1:500), and incubated with/without DHT for 48 h or the indicated times. Data shown are relative values of firefly luciferase normalized to Renilla luciferase. Each bar represents the average of triplicate determinations ± se. B, Nuclear extracts from samples treated with/without DHT in NRP-154±AR cells were incubated with radiolabeled S3p-Oligo (human Smad3 promoter, nucleotides −612 to −584). For competition assay, nuclear lysates were preincubated for 20 min with either specific competitor (nonlabeled S3p-Oligo) or nonspecific competitors before incubation with the radiolabeled S3p-Oligo. Reactions were then resolved in 6% polyacrylamide-Tris-borate-EDTA gels. C, Separately, nuclear extracts from NPR-154 cells were incubated with biotinylated-S3p-oligo and for DNPAs (Nc, Nuclear extract; Cyt, cytosolic fraction). D, VCaP cells were transfected with Sp1-luciferase and incubated with/without either DHT or R1881 for 48 h. Data shown are relative values of firefly luciferase normalized to Renilla luciferase. Each bar represents the average of triplicate determinations ± se. WT, Wild type.
It has been shown that Sp1 is a critical transcription factor for transcriptional initiation of the Smad3 promoter (34). The Smad3 promoter also contains multiple Sp1/Sp3 binding sites, particularly clustering between −849 and −408 (34). As shown in Fig. 4A, the Smad3 promoter construct represented by this region alone (S3p-Sp1-luc) displayed the best repression by DHT in NRP-154+AR, with changes occurring as early as 4 h of DHT treatment and showing maximum inhibition by 12 h (Fig. 4A, bottom panel). Therefore, we speculated that Sp1 may play a potential role in androgen-mediated repression of the Smad3 promoter. To test this possibility, we performed an EMSA to assess the ability of DHT to alter the binding of proteins in NRP-154-AR cells to the Sp1-rich region of the Smad3 promoter (nucleotides −612 and −584). Nuclear lysates from NRP-154-AR cells retarded the migration of 32P-labeled 29-mer oligonucleotide designed from this segment, as shown by EMSA, corresponding to a single band (likely a single protein complex) representative of Sp1 binding. Nuclear lysates of NRP-154+AR cells treated with DHT showed less binding to DNA relative to the no DHT control, and the specificity of these bands was demonstrated by blocking with the same unlabeled oligonucleotide as competitor but not with three unrelated oligonucleotides (Fig. 4B and data not shown). These data suggests that DHT suppresses the binding of nuclear protein(s) to this region of the Smad3 promoter. To further test our hypothesis that Sp1 binds to this segment of the Smad3 promoter and that DHT suppresses such binding, we conducted a DNA pull-down experiment, in which this oligonucleotide was biotinylated and then used to pull down Sp1 protein from nuclear lysates of NRP-154+AR cells treated with or without 1 nm DHT (Fig. 4C). As is clear, Sp1 from NRP-154+AR cells bound to this oligonucleotide and DHT treatment reduced such binding. Importantly, the total levels of Sp1input were also decreased by treatment of cells with DHT (Fig. 4C).
To further define the physiological significance of the above androgenic effect, we examined whether androgen could suppress the biological activity of Sp1 in VCaP cells (expressing endogenous AR) and account for loss in Smad3 promoter activity (in Fig. 3B). This was achieved by transfecting VCaP cells with a luciferase reporter construct containing four tandem copies of the Sp1 response element upstream of firefly luciferase (Fig. 4D). This experiment revealed that DHT and R1881 each suppressed Sp1-luciferase activity by about 40% in VCaP cells (Fig. 4D), comparable to that of the full-length Smad3 promoter construct (Fig. 3B). Taken together, these data strongly support that Sp1 plays an important role in androgen-mediated suppression of the Smad3 promoter.
Loss of Smad3 by DHT mediates the ability of DHT to protect against TGF-β1-induced cell death
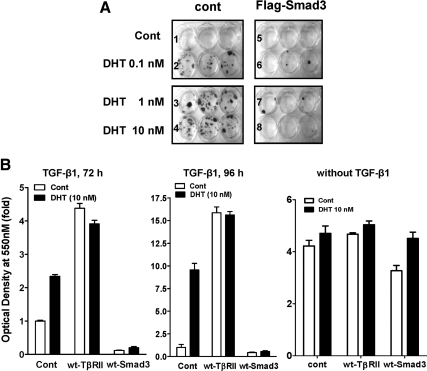
We previously showed that DHT protected NRP-154+AR cells against TGF-β-induced apoptosis. To test the significance of the down-regulation of Smad3 by androgen in blocking TGF-β-induced apoptosis, we examined whether overexpression of Smad3 could reverse the ability of DHT to protect cells against TGF-β-induced apoptosis or growth arrest. This was done by coinfecting NRP-154 cells with AdMax-AR combined with either AdMax-Flag-Smad3 or empty control adenovirus. In the absence of overexpressed Smad3, treatment of NRP-154+AR cells alone with DHT (0.1 nm to 10 nm) rescued cells from TGF-β-induced apoptosis (Fig. 5A). However, the protection by androgen was effectively reversed upon overexpression of Smad3 (P < 0.01 and P < 0.05) (Fig. 5, A and B). These results clearly support the physiological significance of the down-regulation of Smad3 after androgenic treatment. In contrast, overexpression of TβRII (by AdMax-TβRII) suppressed TGF-β-induced cell death (Fig. 5B). As a further control, DHT did not enhance basal cell growth or offer a survival benefit to NRP-154-AR cells in the absence of exogenous TGF-β or Smad3 (Fig. 5B). Overall, these results support that down-regulation of Smad3 expression is involved in the mechanism by which DHT represses TGF-β-induced cell death. Overexpression of Smad3 in the absence of TGF-β caused slight growth suppression over the course of the experiment, and interestingly such suppression was reversed by DHT (Fig. 5B); this is consistent with our previous report that DHT suppresses Smad3 responses also through a mechanism involving the physical interaction between AR and Smad3 (6).
Figure 5.
Enforced expression of Smad3 reverses the protective effect of DHT on TGF-β1-induced cell death. A, NRP-154+AR cells were infected with either control virus or AdMax-Flag-Smad3 (1:500) and treated in GM3 medium with TGF-β1 (10 ng/ml) in the absence or presence of three different doses of DHT. After 5 d of TGF-β1 treatment, medium was replaced with GM2.1 medium with/without DHT and further cultured for 1 wk. B, NRP-154 cells were transiently infected with Admax-AR (1:8000) along with either Admax-cont, Admax-Smad3 (1:500), or AdMax-TβRII and then treated with TGF-β1 (5 ng/ml for 72 h or 96 h) or without TGF-β in the absence/presence of DHT (10 nm). After TGF-β1 treatment, cells were washed and cultured in growth medium, GM2, for an additional 6 d. Cells were then washed/fixed and stained with crystal violet, after which stained colonies were photographed (A) or dye was eluted to measure cell viability by reading the optical density at 550 nm (B). Each bar represents the average of triplicate determinations ± se. cont, Control; wt, wild type.
Discussion
Here we report the first evidence that ligand-bound AR (DHT-AR) functions to down-regulate expression of Smad3, and that this suppression occurs through a transcriptional mechanism. Our data support that DHT represses the Smad3 gene promoter through a mechanism involving loss of the binding of Sp1 to this promoter. DHT also represses the expression of Smad2, although not as robust as that of Smad3 (Figs. 1 and 2, and Supplemental Fig. 1). Suppression of p-Smad2 occurs only after 24 h of TGF-β treatment, suggesting that DHT down-regulates Smads 2 and 3 through distinct mechanisms. Interestingly, in our previous report (16) we showed that DHT (by 3–6 h) down-regulates the expression of TβRII in NRP-154+AR cells, and we expected that such loss would instead lead to a concomitant reduction in the levels of phospho-Smad2. These and other results (data not shown) suggest that TGF-β receptors are not rate limiting for the phosphorylation of Smad2 in NRP-154+AR cells. By deduction, the levels of Smads and/or their transport to TGF-β receptors are the more likely rate-limiting candidates or cellular control points in these cells. This is consistent with our observations that silencing Smad3 suppresses TGF-β-induced apoptosis in NRP-154 cells (29,35), and overexpression of Smad3 (but not TβRII) reverses the protective effect of DHT (Fig. 5). Our findings here, in conjunction with previous reports (6,16,25,36), suggest that DHT can interrupt TGF-β responses at multiple levels, including transcriptional repression of Smad3 and TβRII, and suppression of the binding of Smad3 to Smad-binding element on target genes by a direct physical interaction of AR with Smad3 (6,16). In addition, DHT has been recently reported to repress the TGF-β1 promoter through the interaction of AR to six AREs in the TGF-β1 promoter (36). These findings are consistent with in vivo studies on rodents, in which androgen withdrawal induces the expression of TGF-β ligands and receptors, Smads 2, 3, and 4, and promotes the activation of Smad2 (23,37). Such redundancy on suppressing TGF-β signaling is likely to have an important homeostatic role in maintaining normal prostate growth and function. However, this redundancy may also play a role in enhancing malignant progression by loss of cytostatic responses to TGF-β.
The ability of DHT-AR to selectively down-regulate Smad3 levels is likely to significantly impact on TGF-β-induced apoptosis, as we demonstrated in NRP-154 cells (16). This is supported by our findings that silencing Smad3 with short hairpin RNA significantly dampens TGF-β-induced apoptosis in NRP-154 cells and interferes with the ability of TGF-β to activate Rb (29). The significance of the down-regulation of Smad3 as a mechanism by which DHT represses TGF-β responses is more evident in LNCaP cells, in which TβRII levels is not down-regulated by DHT (16), and silencing Smad3 but not Smad2 blocks TGF-β-induced PAI-1 promoter activity (38); DHT similarly represses the PAI-1 promoter induced by TGF-β or by constitutively active Smad3 (6). The ability of DHT to block the cytostatic activity of TGF-β in LNCaP cells can be illustrated at the level of the expression of cyclin Ds and survivin, where DHT reverses the ability of TGF-β to down-regulate the expression of cyclin Ds 1, 2, and 3 and survivin (29). Although Smad3 has been reported to mediate most of the cytostatic responses of TGF-β in a variety of nonprostatic and prostatic tumor cell lines (including the NRP-154 luminal cell line), we showed that in the NRP-152 nontumorigenic basal epithelial cell line, Smad2 was the more critical mediator of apoptosis and tumor suppression by TGF-β (35). We hypothesized that the critical requirement of Smad2 vs. Smad3 for the cytostatic responses to TGF-β is a general property of basal vs. luminal cells (35). The potential refractory nature of the suppressive activity of DHT on Smad2 in prostate basal epithelial cells may account for their relative resistance to androgen withdrawal-induced apoptosis (39), while expressing significant levels of AR (40) and accounting for androgen-induced regrowth of the prostate after androgen restoration (41,42,43).
Although our data suggest that repression of Sp1 plays a role the in the mechanism by which DHT inhibits the Smad3 promoter, the mechanism by which DHT represses Sp1 is not clear. Preliminary evidence from our laboratory supports that DHT represses the activity and/or stability of Sp1 protein as well as Sp1 mRNA levels, although the latter appears to occur only by 24 h. A potential model by which DHT promotes Sp1 protein loss may be through physical association of Sp1 with AR (44), which may serve as a scaffold for posttranslational modification and/or degradation of Sp1 by p42/p44 MAPKs (45) and cdk2 (46).
A surprising result we obtained in this study was that overexpression of TβRII suppressed rather than enhanced induction of apoptosis by TGF-β (Fig. 5B). The mechanistic basis for such protection is not clear. One possibility is that overexpressed TβRII may sequester TGF-β ligand away from TβRI and/or enhance ligand degradation. Another possibility is that TβRII may have a TGF-β ligand-independent survival function when overexpressed in prostate cancer cells. Irrespective of the mechanism, our results suggest that strategies (particularly enforced overexpression) to restore TβRII level may not be as effective in restoring the tumor-suppressive function of TGF-β in malignant tissues as strategies for restoring Smad3 levels.
Prostate cancers ultimately become hormone refractory, a phenomenon suggested to occur mainly through an AR-dependent and gonadal androgen-independent mechanisms (13,47,48), although the key mechanisms remain unresolved (49). Examination of Smad3 protein levels in a variety of prostate epithelial cell lines (our unpublished results) and prostate tissues (21), and Smad3 mRNA levels from profiling studies with human prostate tissues (Oncomine Research database, Supplemental Fig. 2, and Ref. 32) reveal that expression of Smad3 protein and mRNA levels are significantly down-regulated during prostate carcinogenesis and tumor progression. We hypothesize that the enhanced activity of AR or its constitutive activation during prostate cancer development and progression contributes to a concomitant transcriptional loss of Smad3. Consistent with this, Brodin et al. (21) showed that castration-induced androgen withdrawal significantly induces Smad3 protein levels in both normal and tumor rat prostate tissues, with greater induction occurring in tumors. Moreover, induction of Smad3 in both normal and tumor prostates occurred as early as 24 h after androgen withdrawal, consistent with a transcription mechanism. However, that study did not address the mechanistic basis for such regulation. Our current study suggests that AR transcriptionally down-regulates Smad3 and cooperates with loss of TβRII to repress cytostatic effects of autocrine/paracrine TGF-β. We have previously reported that DHT inhibits the ability of TGF-β to down-regulate Bcl-xL/cyclin D levels (16), to dephosphorylate Rb (29), to suppress survivin expression, and to activate caspase-3 (29)(Supplemental Fig. 3). These findings thus provide new mechanistic insight on the cross talk of AR with TGF-β and the acquisition of hormone-refractory prostate cancer, in which constitutive activation of AR facilitates loss of Smad3, leading to loss of cytostatic effects of TGF-β. We thus propose that the Smad3 promoter may be a new therapeutic target of castration-resistant prostate cancer.
Materials and Methods
Materials
Sources were as follows: recombinant human TGF-β1; anti-phospho-Smad3 (Ser433/435, catalog no. 9514) and anti-phospho-Smad2 (catalog no. 3101) antibodies (Cell Signaling Technology, Danvers, MA); anti-Smad3 (sc-8332), anti-Smad4 (sc-7966), anti-TβRII (sc-1700), and anti-AR (sc-815) antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-Flag M1 (F-3040), and anti-β-actin (A-5441) antibodies (Sigma Chemical Co., St. Louis, MO); anti-Smad2 (catalog no. S66220) antibody (Transduction Laboratories, Lexington, KY); anti-Sp1 (catalog no. 07-645) antibody (Upstate Biotechnology, Inc., Lake Placid, NY); PNGase F (New England Biolabs, Beverly, MA); pGL3-basic-luciferase (Promega Corp., Madison, WI); characterized fetal bovine serum (FBS) and dextran-charcoal (DC)-stripped FBS (HyClone Laboratories, Inc., Logan, UT). The Smad3 promoter-luciferase constructs were developed previously (34). LNCaP, DU145 and VCaP cell lines were obtained directly from American Type Culture Collection (Manassas, VA), the C4-2B obtained from Dr. Leland Chung’s laboratory via Dr. J. T. Hsieh, and NRP-154 cell line was established in our laboratory from the Lobund Wistar rat and authenticated (50). For our studies, all cell lines were expanded, aliquoted, and stored in liquid nitrogen immediately upon delivery, and then maintained in tissue culture for less than 3 months to retain cell line authenticity.
Cell culture
The NRP-154 prostatic epithelial cell lines (50,51) were maintained in GM2.1 culture medium as described previously (52). DU145, human prostate cancer cell line, was maintained in DMEM/F12 medium supplemented with 5% FBS. LNCaP, C4-2B, and VCaP human prostate cancer cell lines were maintained in DMEM/F12 containing 10% FBS in poly-d-lysine-coated 75 cm2 culture flask (6). Unless indicated, all experiments in NRP-154 and NRP-152 cells were performed in GM3.1 medium (DMEM/F12 supplemented with, 0.1 μm dexamethasone and 15 mm HEPES and with either 1% FBS or with 1% DC-stripped FBS when indicated, and experiments involving DU145, LNCaP, C4-2B, and VCaP cells were performed in DMEM/F12 supplemented with 1% or 10% DC-stripped FBS and 15 mm HEPES. Experiments with Admax-TβRII-infected LNCaP and C4-2B cells were performed in DMEM/F12 supplemented with 1% DC-stripped FBS, 15 mm HEPES, and 20 ng/ml EGF.
Development of doxycycline-inducible cell lines, NRP-154/AR28
NRP-154 cells were plated with GM2.1 medium overnight in six-well plates and then transfected with 1.7 μg AR-pcDNA4 + 0.3 μg pcDNA6. Cells were re-plated 24 h later in 100-mm dishes with GM2.1. Zeocin (750 μg/ml) and Blasticidin (5 μg/ml) were added, and medium was replaced every 3 d with GM2.1 containing Zeocin and Blasticidin. About 1 wk later, individual resistant colonies were isolated and tested for AR expression, with or without doxycycline (2 μg/ml) for 24 h. AR-pcDNA4 was developed by subcloning AR from pCDNA3-AR (6) into pcDNA4. pcDNA4 and pcDNA6 were obtained from Invitrogen (Carlsbad, CA).
Western blot analysis
Samples were analyzed by immunoblotting as described previously (16). In brief, NRP-154 and NRP-152 cells were plated overnight at a density of 2 × 105 cells/2 ml of 1% DC-GM3 media/well in six-well plates, infected for 2 h by AdMax-AR (1:500), and washed once with PBS followed by replacing with 2 ml 1% DC-GM3. Separately, DU145, LNCaP, C4-2B, and VCaP cells were seeded at a density of either 2–5 × 105 cells/2 ml of 1% or 10% DC-DMEM/F12 (+15 mm HEPES), followed by 24 h infection with adenovirus. After treatment, cells were lysed at 4 C with RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with 1 mm sodium orthovanadate 1 mm EDTA, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, Complete Mini-EDTA-free Protease Inhibitor Mixture (Roche, Indianapolis, IN), and 1 mm phenylmethylsulfonyl fluoride.
RT-PCR
Reverse transcript (RT) was performed as described (8). Taq Polymerase Master Mix (Promega) was used for PCR amplification of rat and human Smad3, using 29 cycles (rat) or 30 cycles (human) with the following temperature gradients: 95 C for 15 sec, 55 C (rat)/60 C (human) (for Smad3) for 30 sec, and 72 C for 2 min. β-Actin, amplified as above for 21 cycles, served as an internal control. The PCR primers (Operon Biotechnologies, Inc., Alameda, CA) applied to detect Smad3 expression were 5′-CCTGGGCAAGTTCTCCAGAG-3′ (forward) and 5′-CCATCGCTGGCATCTTCTGTG-3′ reverse) for rat Smad3, and 5′-GTCCATCCTGCCTTTCACTC-3′(forward) and 5′-CACTGGAACAGCGGATGCTT-3′ (reverse) for human Smad3. Real-time PCR was performed using TaqMan Real Time PCR kit from Applied Biosystems (Foster City, CA), optimal rat Smad3 primers with internal control designed by Applied Biosystems (undisclosed oligo sequences) and 7500-Real Time PCR System (Applied Biosystems).
Preparation of nuclear and cytosolic fractions
After treatment, cells were washed with ice-cold PBS and collected with 300 μl of ice-cold buffer 1 [10 mm HEPES (pH 7.5), 10 mm KCl, 1.5 mm MgCl2, 1 mm sodium orthovanadate, 1 mm EDTA, Complete Mini-EDTA-free Protease Inhibitor Mixture (Roche, Indianapolis, IN) and 1 mm phenylmethylsulfonyl fluoride] followed by centrifugation at 4000 rpm at 4 C for 5 min. Cell pellets were resuspended in 100 μl buffer 1, and cell suspensions were mixed gently by inverting tubes after the addition of 100 μl buffer 1 containing 0.15% Nonidet P-40. Samples were placed on ice for 15 min and centrifuged at 4000 rpm at 4 C for 2 min. The supernatant (cytosolic fraction) was collected, and the pellet was washed twice with 300 μl Buffer 1 without detergent. Cell pellet was lysed in 100 μl Buffer 2 [20 mm HEPES (pH 7.5), 20% glycerol, 0.42 m NaCl, 1 mm EDTA, 1 mm sodium orthovanadate, Complete Mini-EDTA-free Protease Inhibitor Mixture, and 1 mm phenylmethylsulfonyl fluoride] and placed on ice for 1 h. Samples were then clarified at 14,500 rpm for 20 min (at 4 C). Nuclear or cytosolic fractions were quantified and subjected to EMSA or DNA pull-down (DNAP) assay.
Transient transfection and luciferase assay
Cells were transfected by calcium phosphate coprecipitation (NRP-154) or Invitrogen’s Lipofectamine plus reagent (LNCaP, DU145, VCaP) as before (6). Luciferase activity was measured using Promega’s Dual Luciferase Assay Kit (Promega) and a ML3000 Microtiter Plate Luminometer (Molecular Devices Corporation, Sunnydale, CA).
Adenovirus
Adenovirus vectors that direct the expression of hemagglutinin-TβRII (AdMax-hemagglutinin-TβRIIWT) and AR (AdMax-AR) were constructed using the AdMax system (Microbix Biosystems, Mississauga, Ontario, Canada) as described previously (7,16).
DNA pull-down assay
DNA pull-down assay was performed as described previously (16). Biotin-labeled S3p oligonucleotide (wild type, 29-mer 5′-CCC CGG CGG CGA GGG GGC GGT GAC AGC AC-3′) was dimerized with its complement. Nuclear protein (100 μg) was incubated with oligo-conjugated beads, and reaction volume was adjusted up to 500 μl with 1× DNAP containing Complete EDTA-free Protease inhibitor Mixture (Roche, Mannheim, Germany), 1 mm sodium orthovanadate, 1 mm phenymethylsulfonyl fluoride, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, and 1 mm dithiothreitol (DTT). Polydeoxyinosinic-deoxycytidylic acid (5 μg) was added to the reaction tube, which was then incubated for 4 h at 4 C with gentle mixing on a rotator. Beads were washed three times on ice with DNAP containing 1 mm DTT, eluted with 45 μl of 1× sodium dodecyl sulfate buffer by treating for 5 min at 85 C. Eluates were subjected to Western blot analysis.
EMSA
EMSA was performed as described previously with some modifications (6). An oligonucleotide corresponding to an Sp1-rich region of the Smad3 promoter (nucleotides −612 to −584) 5′-CCC CGG CGG CGA GGG GGC GGT GAC AGC AC-3′ (S3p-Oligo) was dimerized with its complement, labeled with [γ-32P]ATP using T4 polynucleotide kinase (Promega), and then purified by ethanol precipitation. Aliquots (5 μg) of nuclear lysates were incubated with/without unlabeled dimerizedS3p-Oligo or unrelated oligonucleotide dimers (as specific and nonspecific competitors, respectively) in binding buffer (10 mm Tris, pH 7.5; 50 mm KCl; 1 mm DTT; 0.25% Tween-20; 1 μg of polydeoxyinosinic-deoxycytidylic acid) for 20 min at room temperature, and these reactions were then incubated with 50,000 cpm of [32P]S3p-Oligo for additional 20 min at room temperature. Complexes were resolved in 6% polyacrylamide gels with 0.5× Tris-borate-EDTA buffer (Invitrogen, Carlsbad, CA).
Acknowledgments
We thank Malcolm Whitman (Department of Developmental Biology, Cell Biology, Harvard Medical School/Harvard School of Dental Medicine, 188 Longwood REB 505, Boston, MA 02115) for ARE-luc and Myc-FAST-1, and Harvey Lodish (Whitehead Institute, Suite 601, 9 Cambridge Center, Cambridge, MA 02142) for pCMV5-TβRII plasmids.
Footnotes
This work was supported by the Flow Cytometry Core Facility of the Case Comprehensive Cancer Center (P30 CA43703) and by National Cancer Institute Grants R01CA092102, R01CA102074 (to D.D.), and R01CA134878.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 25, 2010
Abbreviations: AR, Androgen receptor; ARE, androgen response element; CMV, cytomegalovirus; DC, dextran-charcoal; DHT, dihydrotestosterone; DNAP, DNA pull down; DTT, dithiothreitol; FBS, fetal bovine serum; PAI-1, plasminogen activator inhibitor-1; Rb, retinoblastoma protein; Smad, Sma- and Mad-related protein; TβRI and TβRII, TGF-β type I and type II receptors.
References
- Roberts AB, Sporn MB 1990 The transforming growth factor β. New York: Springer-Verlag [Google Scholar]
- Roberts AB, Wakefield LM 2003 The two faces of transforming growth factor β in carcinogenesis. Proc Natl Acad Sci USA 100:8621–8623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L 2000 The Smad pathway. Cytokine Growth Factor Rev 11:5–13 [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen YG 2000 Controlling TGF-β signaling. Genes Dev 14:627–644 [PubMed] [Google Scholar]
- Itoh S, ten Dijke P 2007 Negative regulation of TGF-β receptor/Smad signal transduction. Curr Opin Cell Biol 19:176–184 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D 2002 The androgen receptor represses transforming growth factor-β signaling through interaction with Smad3. J Biol Chem 277:1240–1248 [DOI] [PubMed] [Google Scholar]
- Danielpour D, Song K 2006 Cross-talk between IGF-I and TGF-β signaling pathways. Cytokine Growth Factor Rev 17:59–74 [DOI] [PubMed] [Google Scholar]
- Song K, Krebs TL, Danielpour D 2006 Novel permissive role of epidermal growth factor in transforming growth factor β (TGF-β) signaling and growth suppression. Mediation by stabilization of TGF-β receptor type II. J Biol Chem 281:7765–7774 [DOI] [PubMed] [Google Scholar]
- Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E 1992 The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol 41:665–669 [DOI] [PubMed] [Google Scholar]
- Ohara-Nemoto Y, Nemoto T, Sato N, Ota M 1988 Characterization of the nontransformed and transformed androgen receptor and heat shock protein 90 with high-performance hydrophobic-interaction chromatography. J Steroid Biochem 31:295–304 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Burd CJ, Morey LM, Knudsen KE 2006 Androgen receptor corepressors and prostate cancer. Endocr Relat Cancer 13:979–994 [DOI] [PubMed] [Google Scholar]
- Kang HY, Lin HK, Hu YC, Yeh S, Huang KE, Chang C 2001 From transforming growth factor-β signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc Natl Acad Sci USA 98:3018–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C 2002 Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem 277:43749–43756 [DOI] [PubMed] [Google Scholar]
- Song K, Wang H, Krebs TL, Kim SJ, Danielpour D 2008 Androgenic control of transforming growth factor-β signaling in prostate epithelial cells through transcriptional suppression of transforming growth factor-β receptor II. Cancer Res 68:8173–8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SA, Zarnegar M, Sharma M, Yang F, Peehl DM, ten Dijke P, Sun Z 2001 SMAD3 represses androgen receptor-mediated transcription. Cancer Res 61:2112–2118 [PubMed] [Google Scholar]
- Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA 2008 Stromal transforming growth factor-β signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res 68:4709–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT 1989 Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol 3:1515–1522 [DOI] [PubMed] [Google Scholar]
- Kim IY, Ahn HJ, Zelner DJ, Park L, Sensibar JA, Lee C 1996 Expression and localization of transforming growth factor-β receptors type I and type II in the rat ventral prostate during regression. Mol Endocrinol 10:107–115 [DOI] [PubMed] [Google Scholar]
- Brodin G, ten Dijke P, Funa K, Heldin CH, Landström M 1999 Increased smad expression and activation are associated with apoptosis in normal and malignant prostate after castration. Cancer Res 59:2731–2738 [PubMed] [Google Scholar]
- Desai KV, Kondaiah P 2000 Androgen ablation results in differential regulation of transforming growth factor-β isoforms in rat male accessory sex organs and epididymis. J Mol Endocrinol 24:253–260 [DOI] [PubMed] [Google Scholar]
- Danielpour D 2005 Functions and regulation of transforming growth factor-β (TGF-β) in the prostate. Eur J Cancer 41:846–857 [DOI] [PubMed] [Google Scholar]
- Kundu SD, Kim IY, Yang T, Doglio L, Lang S, Zhang X, Buttyan R, Kim SJ, Chang J, Cai X, Wang Z, Lee C 2000 Absence of proximal duct apoptosis in the ventral prostate of transgenic mice carrying the C3(1)-TGF-β type II dominant negative receptor. Prostate 43:118–124 [DOI] [PubMed] [Google Scholar]
- Lucia MS, Sporn MB, Roberts AB, Stewart LV, Danielpour D 1998 The role of transforming growth factor-β1, -β2, and -β3 in androgen-responsive growth of NRP-152 rat prostatic epithelial cells. J Cell Physiol 175:184–192 [DOI] [PubMed] [Google Scholar]
- Zhu ML, Partin JV, Bruckheimer EM, Strup SE, Kyprianou N 2008 TGF-β signaling and androgen receptor status determine apoptotic cross-talk in human prostate cancer cells. Prostate 68:287–295 [DOI] [PubMed] [Google Scholar]
- van der Poel HG 2005 Androgen receptor and TGFβ1/Smad signaling are mutually inhibitory in prostate cancer. Eur Urol 48:1051–1058 [DOI] [PubMed] [Google Scholar]
- Hsing AY, Kadomatsu K, Bonham MJ, Danielpour D 1996 Regulation of apoptosis induced by transforming growth factor-β1 in nontumorigenic rat prostatic epithelial cell lines. Cancer Res 56:5146–5149 [PubMed] [Google Scholar]
- Yang J, Song K, Krebs TL, Jackson MW, Danielpour D 2008 Rb/E2F4 and Smad2/3 link survivin to TGF-β-induced apoptosis and tumor progression. Oncogene 27:5326–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW 2000 LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate 44:91–103 [DOI] [PubMed] [Google Scholar]
- Korenchuk S, Lehr JE, Mclean L, Lee YG, Whitney S, Vessella R, Lin DL, Pienta KJ 2001 VCaP, a cell-based model system of human prostate cancer. In Vivo 15:163–168 [PubMed] [Google Scholar]
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH 2004 Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 22:2790–2799 [DOI] [PubMed] [Google Scholar]
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann II G, Aronica E, Crino PB 2004 mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 56:478–487 [DOI] [PubMed] [Google Scholar]
- Lee JY, Elmer HL, Ross KR, Kelley TJ 2004 Isoprenoid-mediated control of SMAD3 expression in a cultured model of cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 31:234–240 [DOI] [PubMed] [Google Scholar]
- Yang J, Wahdan-Alaswad R, Danielpour D 2009 Critical role of Smad2 in tumor suppression and transforming growth factor-β-induced apoptosis of prostate epithelial cells. Cancer Res 69:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Gao S, Wang Z 2008 Transcriptional regulation of the TGF-β1 promoter by androgen receptor. Biochem J 416:453–462 [DOI] [PubMed] [Google Scholar]
- Nishi N, Oya H, Matsumoto K, Nakamura T, Miyanaka H, Wada F 1996 Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostates. Prostate 28:139–152 [DOI] [PubMed] [Google Scholar]
- Wang H, Song K, Krebs TL, Yang J, Danielpour D 2008 Smad7 is inactivated through a direct physical interaction with the LIM protein Hic-5/ARA55. Oncogene 27:6791–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English HF, Santen RJ, Isaacs JT 1987 Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11:229–242 [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL 1991 Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology 129:3187–3199 [DOI] [PubMed] [Google Scholar]
- Danielpour D 1999 Transdifferentiation of NRP-152 rat prostatic basal epithelial cells toward a luminal phenotype: regulation by glucocorticoid, insulin-like growth factor-I and transforming growth factor-β. J Cell Sci 112:169–179. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K 1994 Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol 25:42–46 [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K 1994 The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 24:114–118 [DOI] [PubMed] [Google Scholar]
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA 2001 Androgen suppression of GnRH-stimulated rat LHβ gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol 15:1906–1917 [DOI] [PubMed] [Google Scholar]
- Milanini-Mongiat J, Pouysségur J, Pagès G 2002 Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem 277:20631–20639 [DOI] [PubMed] [Google Scholar]
- Banchio C, Schang LM, Vance DE 2004 Phosphorylation of Sp1 by cyclin-dependent kinase 2 modulates the role of Sp1 in CTP:phosphocholine cytidylyltransferase α regulation during the S phase of the cell cycle. J Biol Chem 279:40220–40226 [DOI] [PubMed] [Google Scholar]
- Litvinov IV, Vander Griend DJ, Antony L, Dalrymple S, De Marzo AM, Drake CG, Isaacs JT 2006 Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci USA 103:15085–15090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemers HV, Regan KM, Schmidt LJ, Anderson SK, Ballman KV, Tindall DJ 2009 Androgen modulation of coregulator expression in prostate cancer cells. Mol Endocrinol 23:572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sawyers CL, Scher HI 2008 Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol 8:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D, Kadomatsu K, Anzano MA, Smith JM, Sporn MB 1994 Development and characterization of nontumorigenic and tumorigenic epithelial cell lines from rat dorsal-lateral prostate. Cancer Res 54:3413–3421 [PubMed] [Google Scholar]
- Bonham MJ, Danielpour D 1996 Improved purification and yields of RNA by RNeasy. Biotechniques 21:57–60 [DOI] [PubMed] [Google Scholar]
- Song K, Wang H, Krebs TL, Danielpour D 2006 Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J 25:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]