Abstract
Aging or acute loss of estrogens or androgens increases the levels of reactive oxygen species, activates nuclear factor-κB (NF-κB), and promotes the phosphorylation of p66shc, a redox enzyme that amplifies mitochondrial reactive oxygen species generation and stimulates apoptosis. We report that in mesenchymal progenitor and osteoblastic cell models, H2O2 activated a protein kinase C (PKC)β/p66shc/NF-κB signaling cascade and that p66shc was an essential mediator of the stimulating effects of H2O2 on the apoptosis of osteoblastic cells as well as their ability to activate NF-κB. 17β-Estradiol (E2) or the nonaromatizable androgen dihydrotestosterone abrogated the effects of H2O2 on p66shc and NF-κB activation by attenuating the phosphorylation of the redox-sensitive cytoplasmic kinase PKCβ. Additionally, both E2 and dihydrotestosterone prevented H2O2-induced apoptosis by a mechanism that involved attenuation of p66shc resulting from decreased phosphorylation of PKCβ. Consistent with a kinase-mediated mechanism of sex steroid action, the effects of E2 were reproduced by a polymeric form of estradiol that is not capable of stimulating the nuclear-initiated actions of ERα. These results demonstrate that p66shc is an essential mediator of the effects of oxidative stress on osteoblastic cell apoptosis, NF-κB activation, and cytokine production. The ability of either estrogen or androgen to attenuate the effects of oxidative stress on osteoblastic cell apoptosis, NF-κB activation, and cytokine production results from their common property to suppress PKCβ-induced p66shc phosphorylation via a mechanism that does not require stimulation of the nuclear-initiated actions of sex steroids.
Estrogens or androgens attenuate the effects of oxidative stress on osteoblast apoptosis and cytokine production via actions initiated outside the nucleus.
Similar to humans, female or male C57BL/6 mice exhibit a progressive loss of bone strength and mass with age. These changes are temporally associated with increased osteoblast and osteocyte apoptosis and decreased osteoblast numbers and bone formation rate (1). Moreover, the age-dependent changes at the tissue and cellular level are temporally associated with increased levels of reactive oxygen species (ROS) in the bone marrow and a corresponding increase in bone lysates of the phosphorylation status of p66shc, an adapter protein that amplifies mitochondrial ROS generation and influences apoptosis and lifespan in mice (2,3). Proapoptotic signals, including ROS, activate protein kinase C (PKC)β, which in turn phosphorylates p66shc at serine 36. Phosphorylated p66shc translocates to the inner mitochondrial membrane and acts as a redox enzyme to amplify oxidative stress by generating H2O2. Increased H2O2, in turn, causes opening of the mitochondrial permeability transition pore and apoptosis.
One of the many consequences of increased ROS production is the activation of redox-sensitive cytoplasmic kinases of the nuclear factor-κB (NF-κB) pathway and of the activity of NF-κB itself, leading to the increased transcription of NF-κB target genes (4,5). In unstimulated cells, NF-κB proteins are sequestered in the cytoplasm because of their tight association with IκB proteins. Phosphorylation and degradation of IκB disrupt this association and allows the translocation of NF-κB proteins into the nucleus. ROS-induced posttranslational modifications, such as oxidation of critical cysteins, enhance the activity of several of the cytoplasmic kinases that promote IκB phosphorylation and degradation, including IκB kinase and the PKC family of serine/threonine kinases. Additionally, ROS-induced modifications control key steps in the nuclear phase of the NF-κB program, including recruitment of coactivators, chromatin remodeling, and DNA binding (6,7).
The same increases in oxidative stress and p66shc phosphorylation observed with advancing age in bone of C57BL/6 mice are caused by the removal of the gonads in female or male mice (1,8). Moreover, these changes are reversed in the gonadectomized animals by the administration of antioxidants such as N-acetyl-l-cysteine, ascorbate, and catalase, as effectively as with replacement with estrogens or androgens. This evidence strongly suggests that the age-related oxidative stress plays a protagonist role in the pathogenesis of involutional osteoporosis whereas age-related changes in other organs and tissues, such as the ovaries, are contributory (9). Importantly, we have recently shown that the ability of estrogens to diminish the generation of ROS and decrease the phosphorylation of p66shc as well as to regulate osteoblast apoptosis and number are fully preserved in a mouse model bearing an estrogen receptor α (ERα) knock-in mutation that prevents binding to DNA (ERαNERKI/−) (10). Hence, the DNA-binding function of the ERα is dispensable for the antioxidant properties of estrogens.
Based on the above lines of evidence, in the work presented herein, we have investigated the biological significance of p66shc in osteoblasts and sought molecular details of the regulation of its phosphorylation status by estrogens or androgens.
Results
p66shc is indispensable for H2O2-induced apoptosis and NF-κB activation in osteoblasts
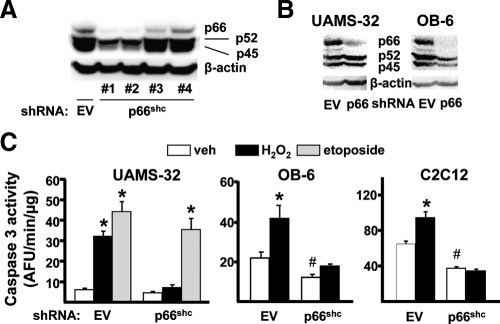
Based on evidence that p66shc phosphorylation in bone is associated with increased osteoblast apoptosis in vivo, as well as evidence that p66shc in other cell types amplifies the generation of H2O2 in mitochondria, and thereby promotes apoptosis, we investigated whether p66shc is indeed functionally involved in the stimulation of osteoblast apoptosis by H2O2. To accomplish this, we silenced p66shc using short hairpin (sh) RNAs introduced via lentiviral transduction. A panel of four different shRNAs was screened for the ability to suppress p66shc expression in C2C12 cells. The lentivirus expressing shRNA clone 3 reduced p66shc protein levels by more than 60% without affecting the expression levels of the p52 or p45 shc isoforms (Fig. 1A). UAMS-32 and OB-6 cells, two established osteoblastic cell lines of bone marrow origin (11), were also silenced for p66shc using shRNA clone 3 (Fig. 1B). Strikingly, p66shc silencing decreased the rate of basal apoptosis, as determined by caspase 3 activity in OB-6 and C2C12 cells and greatly attenuated apoptosis induced by H2O2 in all three cell types (Fig. 1C). Apoptosis induced by etoposide was not affected in UAMS-32 cells silenced for p66shc. These results demonstrate that p66shc is an important determinant of the basal rate of osteoblast apoptosis in culture and indispensable for H2O2-induced cell death.
Figure 1.
H2O2-induced osteoblast apoptosis requires p66shc. A, Shc protein levels by Western blot in C2C12 cells transduced with empty viral particles (EV) shRNA or one of four clones expressing shRNA directed against p66shc mRNA. B, Shc protein levels of the three shc isoforms by Western blot in scramble or p66shc clone 3 silenced cells. C, Caspase 3 activity in indicated cells, cultured in the presence of vehicle (veh), 50 μm H2O2, or 50 μm etoposide for 6 h. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. respective veh; #, P < 0.05 vs. veh in EV-silenced cells.
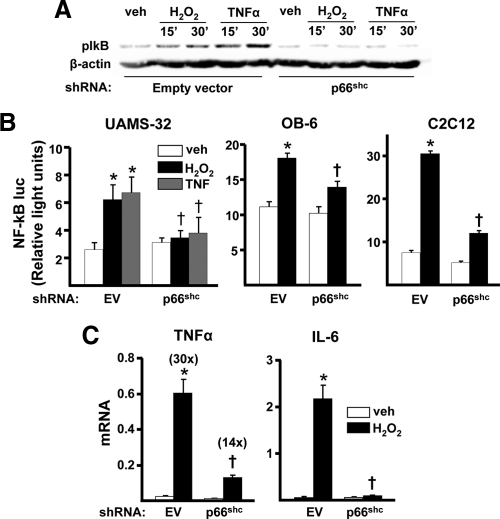
Because of the evidence that oxidative stress also leads to the activation of NF-κB, we next examined whether p66shc is involved in this effect, as well. Silencing of p66shc attenuated H2O2-induced phosphorylation of IκB in UAMS-32 cells (Fig. 2A). Silencing p66shc in UAMS-32, OB-6, or C2C12 cells also abrogated the stimulating effect of H2O2 on the transcriptional activity of an NF-κB reporter construct (Fig. 2B). Moreover, p66shc silencing attenuated the stimulating effect of H2O2 on the NF-κB target genes IL-6 and TNFα in UAMS-32 cells (Fig. 2C). Together, these results indicate that p66shc is an essential mediator of the effects of H2O2 on both the apoptosis of osteoblastic cells and the ability of H2O2 to activate NF-κB and stimulate the production of inflammatory cytokines.
Figure 2.
H2O2-induced NF-κB activation is stimulated by p66shc. A, IκB phosphorylation determined by Western blot in empty viral particles (EV) or p66shc -silenced UAMS-32 cells incubated with 100 μm H2O2 or 10 ng/ml TNFα for the indicated time. B, Luciferase activity in EV- or p66shc-silenced cells transfected with a NF-κB-luc reporter construct after treatment with vehicle (veh), H2O2, or TNFα, as above, for 24 h. C, mRNA levels of TNFα and IL-6 by qRT-PCR in EV- or p66shc-silenced UAMS-32 cells treated with veh or 100 μm H2O2 for 1 h. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. veh; †, P < 0.05 vs. equivalent treatment in EV-silenced cells.
H2O2-induced apoptosis and NF-κB activation require the activation of PKCβ
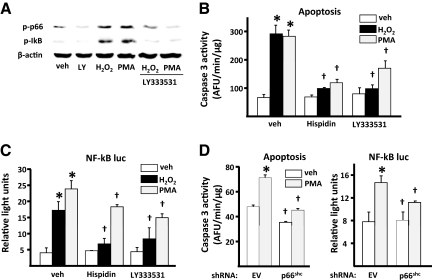
Oxidative stress activates PKCβ, which in turn induces phosphorylation of p66shc and triggers mitochondrial accumulation of the protein (12). To examine whether PKCβ is also required for H2O2-induced stimulation of osteoblast apoptosis and/or activation of NF-κB, we used hispidin or Ly333531, two specific inhibitors of this kinase. Ly333531 prevented H2O2-induced phosphorylation of p66shc in UAMS-32 cells (Fig. 3A), and hispidin had a similar effect (data not shown). In agreement with the mediating role of PKCβ in the induction of p66shc phosphorylation in response to H2O2, phorbol ester [phorbol 12-myristate-13-acetate (PMA)], a strong activator of PKC, also stimulated p66shc phosphorylation, and Ly333531 prevented this effect. PMA, similar to H2O2, stimulated the phosphorylation of IκB and Ly333531 (Fig. 3A) or hispidin (data not shown) attenuated this effect, indicating that PMA-induced stimulation of IκB phosphorylation is mediated, in part, by PKCβ. In agreement with the evidence that PKCβ activates p66shc and p66shc amplifies H2O2 generation and thereby stimulates apoptosis (2), cells exposed to H2O2 or PMA for 6 h exhibited a 2-fold increase in caspase 3 activity. Moreover, both H2O2-and PMA-induced osteoblast apoptosis was abrogated in the presence of hispidin or Ly333531 (Fig. 3B). Further, H2O2 or PMA stimulated NF-κB activation, and the effects of either agent were abrogated by hispidin and Ly333531 (Fig. 3C). The activating effect of PMA on apoptosis or NF-κB transcriptional activity was abrogated in cells in which p66shc was silenced (Fig. 3D).
Figure 3.
PKCβ activity is required for H2O2-induced apoptosis and NF-κB activation. A, IκB and p66shc phosphorylation determined by Western blot in UAMS-32 cells pretreated without or with 0.5 mm LY333531 (LY) for 1 h, followed by 100 μm H2O2 or 100 μm PMA for 6 h. B, Caspase 3 activity in cells described in panel A, cultured in the presence of vehicle (veh) or 100 μm H2O2 for 6 h. C, Luciferase activity in cells transfected with the NF-κB-luc reporter construct after pretreatment with 10 μm hispidin or LY333531 followed by H2O2 or PMA, as above, for 24 h. D, Caspase 3 and luciferase activity in empty vector (EV) or p66shc-silenced UAMS-32 cells, as in panels B and C, treated with veh or 10 μm PMA. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. veh; †, P < 0.05 vs. equivalent treatment in veh-treated or EV-silenced cells.
H2O2-induced activation of NF-κB in osteoblastic cells is antagonized by sex steroids
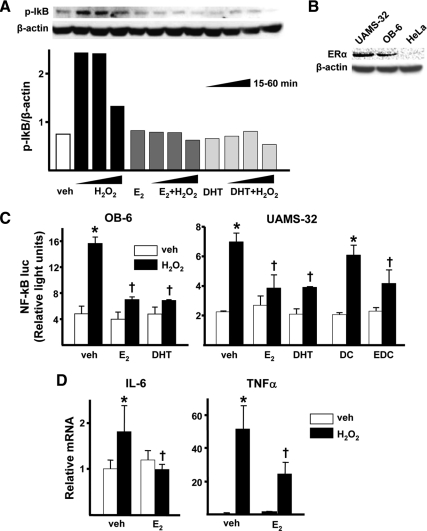
Exposure of cells to 100 μm H2O2 for 15 or 30 min increased IκB phosphorylation in OB-6 cells, and the magnitude of this effect decreased by 60 min (Fig. 4A). 17β-Estradiol (E2) or dihydrotestosterone (DHT) at 10−8 m had no effect on IκB phosphorylation in cells not exposed to H2O2, but either steroid completely abrogated H2O2-induced phosphorylation of IκB. Identical results were obtained with UAMS-32 cells (data not shown). Both OB-6 and UAMS-32 cells express the ERα, as demonstrated by Western blot (Fig. 4B). The ERα-negative HeLa cells were used as a control. In line with its effects on IκB phosphorylation, H2O2 stimulated NF-κB-mediated transcription, as determined by the activity of an NF-κB-luc reporter construct transfected into OB-6 or UAMS-32 cells (Fig. 4C). E2 or DHT did not affect basal NF-κB-luc activity, but both steroids prevented the stimulatory effect of H2O2 on NF-κB-luc activity. Importantly, an estradiol dendrimer conjugate (EDC), which is not capable of stimulating the nuclear-initiated actions of ERα but can activate kinases (13,14), was as effective as E2 or DHT in attenuating H2O2-induced NF-κB-mediated transcription in UAMS-32 cells. Furthermore, and in agreement with the results using the NF-κB reporter construct, H2O2 increased the expression of the endogenous NF-κB target genes IL-6 and TNFα in UAMS-32 cells, and E2 completely prevented the increase of IL-6 whereas it blunted by 50% the increase of TNFα expression induced by H2O2 (Fig. 4D).
Figure 4.
Sex steroids antagonize H2O2-induced activation of NF-κB. A, IκB phosphorylation determined by Western blot in OB-6 cells pretreated with vehicle (veh), E2 (10−8 m) or DHT (10−8 m) for 1 h and treated without or with H2O2 (100 μm) for the indicated time. B, ERα protein levels determined by Western blot in the indicated cell lines. C, Luciferase activity in cells transfected with the NF-κB luc reporter construct after pretreatment with veh, E2, DHT (as above), 10−8 m dendrimer control (DC) or estradiol dendrimer conjugate (EDC) for 1 h followed by 50 μm H2O2 for 24 h. D, mRNA levels of TNFα and IL-6 by qRT-PCR in UAMS-32 cells pretreated with veh or 10−8 m E2 for 1 h followed by 100 μm H2O2 for 5 h. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. respective veh; †, P < 0.05 vs. equivalent treatment in veh-treated cells.
Sex steroids prevent PKC-induced p66shc activation
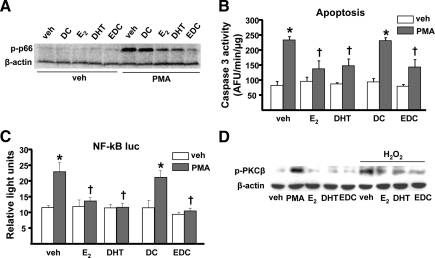
Finally, to determine whether estrogens or androgens prevent H2O2-induced p66shc phosphorylation by interfering with PKC activity, we examined whether E2 or DHT could modulate PMA-induced p66shc phosphorylation in UAMS-32 cells. As depicted in Fig. 5A, either E2 or DHT prevented the stimulatory effect of PMA on p66shc phosphorylation. Importantly, EDC had a similar effect to that of E2. In line with these results, all three steroids prevented PMA-induced apoptosis (Fig. 5B) as well as NF-κB activation (Fig. 5C). Additionally, PMA or H2O2 promoted the phosphorylation of PKCβ, and E2, EDC, or DHT prevented H2O2-induced PKCβ phosphorylation (Fig. 5D).
Figure 5.
PKC-induced p66shc activation is prevented by sex steroids. A, p66shc phosphorylation determined by Western blot in UAMS-32 cells pretreated with vehicle (veh), 10−8 m of each dendrimer control (DC), E2, DHT, or estradiol dendrimer conjugate (EDC) for 1 h and treated with veh or 100 μm PMA for 1 h. B, Caspase 3 activity in UAMS-32 cells pretreated as in panel A and treated with PMA for 6 h. C, Luciferase activity in cells transfected with the NF-κB-luc reporter construct after pretreatment as in panel A followed by PMA for 24 h. D, PKCβ phosphorylation determined by Western blot in UAMS-32 cells pretreated as in panel A and treated with or without H2O2 for 1 h. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. respective veh; †, P < 0.05 vs. equivalent treatment in veh-treated cells.
Discussion
Evidence obtained primarily from mechanistic studies in the mouse model provides a paradigm shift from the traditional “estrogen-centric” account of the pathogenesis of involutional osteoporosis to one in which age-related mechanisms intrinsic to bone and oxidative stress are protagonists, and age-related changes in other organs and tissues, such as ovaries, accentuate them (9). In addition, genetic studies by our group have elucidated that constant dynamic balance between ROS production and defense against ROS is indispensable for bone homeostasis at any age (15). Thus, an increase in oxidative stress, caused by the somatic conditional deletion of the forkhead box O transcription factors, which defend against oxidative stress by activating genes involved in free radical scavenging and apoptosis, recapitulates in young mice the adverse effects of aging on bone, including an increase in the phosphorylation of p66shc and osteoblast and osteocyte apoptosis and a decrease of bone mass. Conversely, targeted overexpression of forkhead box O3 in mature osteoblasts decreases p66shc phosphorylation and osteoblast apoptosis and increases osteoblast number, bone formation rate, and bone mass.
In the work presented herein, we have sought mechanistic evidence causally linking p66shc to the adverse effects of both oxidative stress and sex steroid deficiency on bone homeostasis. In addition, we have searched for a mechanism that could explain recent in vivo evidence that the ability of estrogens to diminish the generation of ROS and decrease the phosphorylation of p66shc as well as to regulate osteoblast apoptosis and number are fully preserved in a mouse model bearing an ERα knock-in mutation that prevents binding to DNA (ERαNERKI/−) (10). The results of the present work strongly support the conclusion that amplification of ROS production by p66shc is indeed required to increase osteoblast apoptosis and that p66shc is also required for ROS-induced activation of NF-κB in cells of the bone marrow stromal/osteoblastic lineage. In addition, the results of the present studies demonstrate that the antioxidant properties of estrogens or androgens result, at least in part, from the ability of these hormones to attenuate p66shc phosphorylation via a nongenotropic mechanism of action that involves PKCβ.
Specifically, the work of the present report has revealed that H2O2 activates a PKCβ/p66shc/NF-κB signaling cascade in cells of the osteoblast lineage and that p66shc is an essential mediator of the stimulating effects of H2O2 on the apoptosis of osteoblastic cells, as well as the ability of H2O2 to activate NF-κB. PMA, a potent inducer of PKC, has similar effects to H2O2 on both apoptosis and NF-κB activation, and the effects of PMA are abrogated by specific PKCβ inhibitors, identically to the situation with H2O2. These findings strongly support the conclusion that the H2O2-induced changes studied in this work are indeed dependent on PKC.
The results of the present report have also revealed that both E2 and DHT abrogate the effects of ROS on p66shc and NF-κB activation by attenuating the phosphorylation of the redox-sensitive cytoplasmic kinase PKCβ. Additionally, both E2 and DHT prevent H2O2-induced apoptosis by a mechanism that involves attenuation of p66shc resulting from decreased phosphorylation of PKCβ. Consistent with a kinase-mediated mechanism of sex steroid action, the effects of E2 are reproduced by a polymeric form of estradiol that is not capable of stimulating the nuclear-initiated actions of ERα. Collectively, this set of data indicates that estrogens and androgens antagonize the effects of oxidative stress on stromal/osteoblastic cells by a cell-autonomous mechanism of action. Moreover, this latter set of data provides a mechanistic explanation for the observations that the ability of estrogens to diminish the generation of ROS and decrease the phosphorylation of p66shc, as well as to regulate osteoblast apoptosis and number, are preserved in the ERαNERKI/− mice (10). Specifically, the results of the present report are consistent with the view that binding of ERα to DNA is not required for the antagonistic effect of estrogens on the production and actions of ROS; instead, these effects result from the modulation of cytoplasmic kinases (1,16).
We and others have shown that estrogens or androgens suppress IL-6 or TNFα production by osteoblastic cells, as well as by macrophages and lymphocytes (17,18,19,20). These previous findings, along with evidence that mice lacking TNFα or IL-6 are resistant to ovariectomy-induced bone loss (21,22), had lent credence to the hypothesis that increased production of these cytokines contributes to the adverse effects of estrogen deficiency on bone by stimulating osteoclastogenesis. Nonetheless, the results of more recent genetic studies by us and others have elucidated that the effects of estrogens on osteoclastogenesis and osteoclast lifespan are, by and large, cell autonomous (23,24). Thus, Nakamura et al. (24) showed that female mice in which the ERα is specifically deleted in mature osteoclasts using the cathepsin K promoter (ERαΔOc/ΔOc) exhibit decreased bone volume due to an increase in the number of osteoclasts, and this effect results from the loss of a cell-autonomous antiapoptotic effect of estrogens on mature osteoclasts. Similarly, we have selectively deleted the ERα from the monocyte/macrophage cell lineage in mice using the promoter of the lysozyme M gene (ERαLysM−/−) and found that these mice exhibit a 2-fold increase in osteoclast progenitors in the marrow and the number of osteoclasts in cancellous bone, along with a decrease in cancellous bone mass. After loss of estrogens the ERαLysM−/− mice fail to exhibit the expected increase in osteoclast progenitors, the number of osteoclasts in bone, and further loss of cancellous bone. However, they lose cortical bone indistinguishably from their littermate controls. In agreement with the in vivo findings of that earlier study, mature osteoclasts from ERαLysM−/− are resistant to the proapoptotic effect of E2. Nonetheless, the effects of estrogens on osteoclasts are unhindered in ERαNERKI/− mice. Moreover, EDC is as effective as E2 in inducing osteoclast apoptosis in cells with the wild-type ERα. From these earlier results we have concluded that estrogens attenuate osteoclast generation and lifespan via cell-autonomous effects mediated by DNA-binding-independent actions of ERα. Elimination of these effects is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts. However, additional effects of estrogens on osteoblasts, osteocytes, and perhaps other cell types are required for their protective effects on the cortical compartment, which constitutes 80% of the skeleton. In support of the contention that estrogens exert cell-autonomous effects in cells of the osteoblastic lineage and that these account for the protective actions of estrogens on bone, specific deletion of ERα in mesenchymal progenitor cells expressing Prx1 or in mature osteoblasts expressing collagen1α1, in mice, decreases cortical bone mass and increases osteoblast apoptosis, respectively (Almeida M., C. A. O’Brien, and S. C. Manolagas, unpublished observations).
Considering the above, down-regulation of the production of cytokines like IL-6 and TNFα by osteoblastic cells or macrophages and lymphocytes remains a plausible mechanism of the protective effects of estrogens in cortical, but not on trabecular, bone. Such down-regulation may result from the attenuating effect of the hormones on the NF-κB activity through the mechanism reported here, from protein-protein interactions of the estrogen-activated ERα with members of the NF-κB-signaling pathway (25,26) or other molecular mechanisms (27,28). Intriguingly, recent work by Chang et al. (29), has raised the possibility that NF-κB activation in osteoblasts attenuates the bone-forming function of these cells. Therefore, activation of NF-κB after sex steroid deficiency may not only promote osteoclast activation and bone resorption secondary to increased cytokine production, but may also compromise the ability of osteoblasts to refill the cavities dug by osteoclasts, thus further tilting the balance between resorption and formation in favor of the former.
Several lines of evidence suggest that the attenuating effect of estrogens or androgens on cytokine production is the result of the antioxidant properties of these hormones. In support of this notion, TNFα signaling has been implicated in the increased bone resorption and loss of bone caused by depletion of thiol antioxidants in mice. Thus, the loss of bone caused by administration of buthionine sulfoximine, an agent that increases oxidative stress in bone (1,8) by depleting cells of glutathione (30), as well as the loss of bone caused by ovariectomy, are prevented by the administration of soluble TNFα receptors that block TNFα action. Similarly, the effects of buthionine sulfoximine and ovariectomy are abrogated in mice in which the TNFα gene had been deleted (31).
In conclusion, the results described in this paper indicate that p66shc is an essential mediator of the effects of oxidative stress on apoptosis, NF-κB activation, and cytokine production by osteoblastic cells. Estrogens or androgens attenuate all these effects by suppressing PKCβ-induced p66shc phosphorylation via a mechanism that does not require stimulation of the nuclear-initiated actions of the ERα.
Materials and Methods
Chemicals and reagents
H2O2, hispidin, PMA, etoposide, DHT, and E2 were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human TNFα and LY333531 were purchased from R&D Systems (Minneapolis, MN) and Axon Medchem BV (Groningen, The Netherlands), respectively. EDC and DC were provided by John A. Katzenellenbogen, University of Illinois.
Cell culture
OB-6 and UAMS-32 cells, two osteoblastic cell lines derived in our laboratory from the murine bone marrow (11), were cultured in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT), penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (292 μg/ml). C2C12 cells were cultured in DMEM supplemented with 10% fetal bovine serum, antibiotics as above, and 1% sodium pyruvate. For the quantification of osteoblast apoptosis, cells were treated for 6 h with vehicle or H2O2 (5 × 10−5 m). Apoptosis was determined by measuring caspase 3 activity as described previously (32).
Western blot and quantitative RT-PCR (qRT-PCR) analysis
The phosphorylation status of p66shc, IκB, and PKCβ was analyzed by immunoblotting, using a mouse monoclonal antibody recognizing Ser36-phosphorylated p66shc (Abcam, Cambridge, MA), a rabbit polyclonal antibody recognizing Ser32 phosphorylated IκBα (Cell Signaling Technology, Danvers, MA), and a rabbit polyclonal antibody recognizing T642-phosphorylated PKCβ1 (Abcam). Protein levels of p66shc were analyzed using a rabbit polyclonal antibody (BD Biosciences, Palo Alto, CA), and of ERα and β-actin using the respective mouse monoclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Quantification of the intensity of the bands in the autoradiograms was performed using a VersaDoc imaging system (Bio-Rad Laboratories, Inc., Hercules, CA). Total RNA was extracted from cultured cells and reverse transcribed as previously described (33). Primers and probes for the different genes were manufactured by the TaqMan Gene Expression Assays service (Applied Biosystems, Foster City, CA). The mRNA was calculated by normalizing to the housekeeping gene ribosomal protein S2 using the ΔCt method (34).
Gene silencing
A set of four lentiviral clones expressing shRNAs directed against p66shc mRNA was obtained from the RNAi Consortium (made available by Sigma). One clone that suppressed p66shc expression by more than 80% was selected for use in these studies. The sequence of the p66shc shRNA construct selected was ccggcctgaccatcagtactaaatctcgagattgtagtactgatggtcaggtttttg (only the top strand is shown). As a negative control, cells were transduced with empty viral particles, containing no shRNA insert. Transduction with lentiviral particles was accomplished by seeding cells in 12-well plates at a density of 25,000 cells per well. Hexadimethrine bromide was added to the culture to a final concentration of 8 μg/ml, and the cells were then transduced with lentiviral particles added at a multiplicity of infection of 20. The cells were then incubated for 48 h and placed in medium containing puromycin (10 μg/ml) for 3 d. For gene expression assays, the cells were plated in 12-well plates at a density of 150,000 cells per well.
Transient transfections and luciferase assay
Cells were plated on a 48-well plate and 16 h later transfected with 0.1 μg of green fluorescent protein, 0.1 μg of NF-κB-luciferase reporter plasmid (CLONTECH Laboratories Inc, Mountain View, CA), and 0.2 μg of pcDNA using Lipofectamine Plus (Invitrogen). Cells were treated 24 h later with the different compounds for another 24 h. Luciferase activity was determined using the Dual-LuciferaseReporter assay system (Promega Corp., Madison, WI), according to the manufacturer’s instructions. Light intensity was measured with a luminometer, and luciferase activity was divided by the Renilla activity (control reporter) to normalize for transfection efficiency.
Statistical analysis
All data are reported as the mean ± sd. Group mean values were compared, as appropriate, by Student’s two-tailed t test or one-way ANOVA with Bonferroni’s multiple comparison test. P ≤ 0.05 was considered significant.
Acknowledgments
We thank A. Warren (UAMS) for technical assistance and Beth Bailey (UAMS) for help with the preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant P01 AG13918, the Department of Veterans Affairs Merit Review Grant (to S.C.M.), and Tobacco Settlement funds provided by the University of Arkansas for Medical Sciences. E.A. is supported by a Ph.D fellowship from the University of Pisa, Italy.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 4, 2010
Abbreviations: DHT, Dihydrotestosterone; E2, estradiol; EDC, estradiol dendrimer conjugate; ER, estrogen receptor; NF-κB, nuclear factor-κB; PKC, protein kinase C; PMA, phorbol 12-myristate-13-acetate; qRT-PCR, quantitative RT-PCR; ROS, reactive oxygen species; sh, short hairpin.
References
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2007 Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG 2005 Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG 1999 The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313 [DOI] [PubMed] [Google Scholar]
- Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM 2006 Redox-sensitive kinases of the nuclear factor-κB signaling pathway. Antioxid Redox Signal 8:1791–1806 [DOI] [PubMed] [Google Scholar]
- Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J 2010 NF-κB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85:473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Piette J 2009 Redox regulation of nuclear post-translational modifications during NF-κB activation. Antioxid Redox Signal 11:2209–2222 [DOI] [PubMed] [Google Scholar]
- Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F 2009 Role of hydrogen peroxide in NF-κB activation: from inducer to modulator. Antioxid Redox Signal 11:2223–2243 [DOI] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ 2003 A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC 2010 From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Martin-Millan M, Ambrogini E, Bradsher III R, Han L, Chen XD, Roberson PK, Weinstein RS, O'Brien CA, Jilka RL, Manolagas SC 2010 Estrogens attenuate oxidative stress, osteoblast differentiation and apoptosis by DNA binding-independent actions of the ERα. J Bone Miner Res 25:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL 1999 Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74:357–371 [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R 2007 Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315:659–663 [DOI] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS 2006 Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 20:491–502 [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini E, Almeida M, Martin-Millan M, Paik J, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC 2010 FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab 11:136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC 1992 Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91 [DOI] [PubMed] [Google Scholar]
- Bellido T, Jilka RL, Boyce BF, Girasole G, Broxmeyer H, Dalrymple SA, Murray R, Manolagas SC 1995 Regulation of interleukin-6, osteoclastogenesis and bone mass by androgens: the role of the androgen receptor. J Clin Invest 95:2886–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV 1991 Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 88:5134–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Weitzmann MN, Kimble RB, Rizzo M, Zahner M, Milbrandt J, Ross FP, Pacifici R 1998 Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of egr-1 and its interaction with Sp-1. J Clin Invest 102:1850–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R 2001 Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960–13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F 1994 Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 13:1189–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC 2010 The estrogen receptor α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Stein B, Yang MX 1995 Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κ B and C/EBP β. Mol Cell Biol 15:4971–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galien R, Garcia T 1997 Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res 25:2424–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G 2006 Cross-talk between nuclear receptors and nuclear factor κB. Oncogene 25:6868–6886 [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD 2005 Transcription factor cross-talk: the estrogen receptor and NF-κB. Trends Endocrinol Metab 16:46–52 [DOI] [PubMed] [Google Scholar]
- Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY 2009 Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med 15:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A 1991 Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci USA 88:9360–9364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger CJ, Lean JM, Davies JT, Chambers TJ 2005 Tumor necrosis factor-α mediates osteopenia caused by depletion of antioxidants. Endocrinology 146:113–118 [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T 1999 Prevention of osteocyte and osteoblasts apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC 2007 Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]