Abstract
Nuclear receptor coactivator 1 [NCOA1/steroid receptor coactivator (SRC)-1] and NCOA3 (SRC-3/AIB1/ACTR) constitute two thirds of the SRC (steroid receptor coactivator) family. Although in vitro experiments have suggested overlapping functions between NCOA1 and NCOA3, their in vivo functional relationship is poorly understood. In this study, NCOA1 and NCOA3 double knockout mice were generated to determine the compensatory roles of NCOA1 and NCOA3 in development. NCOA1−/− mice survived normally, whereas most NCOA3−/− embryos were viable at embryonic d 13.5 (E13.5). In contrast, the majority of double-knockout (DKO) embryos died by E13.5. NCOA1 and NCOA3 are expressed in the labyrinth, and labyrinths of NCOA1+/−;NCOA3−/− and DKO placentas were small compared with wild-type and single-knockout labyrinths. DKO labyrinths exhibited low densities of maternal blood sinuses and fetal capillaries and displayed fetomaternal blood transfusion. At the interface between maternal and fetal circulations, layer I sinusoidal trophoblast giant cells showed a reduced density of microvilli. Layer III syncytiotrophoblasts appeared to accumulate large lipid droplets and have reduced density and deepened invaginations of the intrasyncytial bays. The endothelial layer in DKO labyrinth showed abnormal morphologies and had large lipid droplets. Furthermore, disruption of NCOA1 and NCOA3 increased labyrinth trophoblast proliferation and their progenitor gene expression but decreased their differentiation gene expression. NCOA1 and NCOA3 deficiencies also affected the expression of several genes for placental morphogenesis including TGFβ-, peroxisome proliferator-activated receptor-β-, and peroxisome proliferator-activated receptor-γ-regulated genes and for glucose transportation including GLUT1 and Cx26. These findings demonstrate that NCOA1 and NCOA3 cooperatively regulate placental morphogenesis and embryo survival.
This study demonstrates that knockout of both NCOA1 and NCOA3 in mice causes more severe placental malformation and embryonic lethality than a single knockout.
The p160 steroid receptor coactivator (SRC) family comprises three homologous members: nuclear receptor coactivator (NCOA)1 (SRC-1), NCOA2 (SRC-2, transcriptional intermediary factor 2 or GRIP1), and NCOA3 (SRC-3, AIB1, or ACTR) (1,2). These coactivators do not directly bind to DNA; rather they are recruited to specific gene promoters by interacting with nuclear hormone receptors such as progesterone receptor, estrogen receptor α, and peroxisome proliferator activated-receptor γ (PPARγ) and other transcription factors such as E2F1, PEA3, and AP1 (3,4,5,6,7,8,9). These NCOAs execute their transcriptional functions by recruiting histone acetyltransferases, such as cAMP response element-binding protein-binding protein and p300, and methyltransferases, such as coactivator-associated arginine methyltransferase-1, to the chromatin. Collectively, these enzymes remodel the chromatin to facilitate assembly of basal transcription factors and initiation of mRNA transcription (reviewed in Refs 1 and 2). Due to the limited cellular concentrations of these NCOAs, alteration of their cellular levels and/or transcriptional activities by transcriptional regulation and/or posttranslational modifications may have dramatic effects on the expression of their target genes (1,3).
Our knowledge about the in vivo function of the SRC gene family has been greatly extended by generation and characterization of knockout mouse lines. NCOA1 knockout (NCOA1−/−) mice exhibit normal viability and somatic growth but display partial hormone resistance to steroid and thyroid hormones (10,11), delayed Purkinje cell development (12), reduced estrogen-dependent vasculoprotection (13), and multiple metabolic abnormalities (14). NCOA2−/− mice exhibit severely impaired reproductive function and display metabolic disorders (14,15,16). NCOA3−/− mice exhibit partial penetrance of prenatal and neonatal lethality, growth retardation, reduced reproductive capability, delayed mammary gland development, and metabolic abnormalities (2,17). NCOA3−/− mice also display reduced estrogen-dependent vasculoprotection and lower levels of circulating IGF-I and IGF-binding protein 3 (18,19). These different phenotypes of individual SRC-knockout mice suggest that each SRC family member has unique physiological functions, which might be explained by their different spatiotemporal expression patterns and/or functional specificities.
Among SRC family members, however, their homologous structural domains and interactions with a group of common nuclear receptors have suggested possible compensatory functions. Cell-based transfection assays have also demonstrated that overexpression of any SRC family member will coactivate most nuclear receptor-mediated transcriptional activation (1,3,4,20,21,22). Furthermore, we and others have previously shown that NCOA2−/− mice are hypofertile, NCOA1+/−;NCOA2−/− mice are infertile, and NCOA1−/−;NCOA2−/− mice are lethal (15,23), suggesting that NCOA1 can partially compensate for the loss of NCOA2 function. In addition, double-knockout (DKO) mice exhibit defective adaptive thermogenesis due to a failure of PPARγ function in adipogenesis and mitochondrial uncoupling, which are less severe in single-knockout mice (24). However, the functional relationship and potential redundancy between NCOA1 and NCOA3 during embryonic development remain uninvestigated.
During embryonic development, normal placental morphogenesis is essential for embryo survival and growth. The murine placenta is derived from ectotrophoblasts, and it contains trophoblast giant cells, spongiotrophoblasts, and the labyrinth with maternal blood sinuses and fetal vascular networks. The maternal blood from the arteries in the decidual tissue flows into the maternal blood sinuses in the labyrinth, where nutrients and oxygen are provided from the maternal circulation to the fetal circulation, whereas metabolic wastes are exported in an opposite direction (25,26,27). The fetomaternal exchange interface or circulation barrier consists of four layers of cells, layer I sinusoidal trophoblast giant cells (S-TGCs), also known as mononuclear trophoblasts, lining the maternal blood sinus, layers II and III syncytiotrophoblasts controlling the quantity and quality of the material exchanges, and the endothelial cell layer of the fetal capillary (25,28,29,30,31). Normal differentiation and morphogenesis of these cell layers are required for maintaining embryo survival and growth. Inappropriate development of or damage to this circulation interface may result in incomplete perfusion of the embryo or transfusion of maternal and fetal blood cells and subsequent possible immune rejection (32,33). Although many genes including PPARβ (PPARδ), PPARγ, Muc1, PBP, AIB3, FGFR2, P38, Gcm1, Cx26, RXRα, and RXRβ have been shown to play essential roles in placental morphogenesis and function (34,35,36,37,38,39,40,41,42,43,44), the molecular mechanisms by which these genes regulate placental development remain largely unclear.
In this study, we generated NCOA1 and NCOA3 double-mutant mice to investigate the functional relationship between NCOA1 and NCOA3 in development. We found that NCOA1 and NCOA3 coordinately function to support labyrinth morphogenesis of the placenta. Double knockout of NCOA1 and NCOA3 alters the expression of multiple key genes important for placental development, resulting in aberrant labyrinth morphogenesis of the placenta, which may lead to embryonic lethality.
Results
Double knockout of NCOA1 and NCOA3 resulted in higher penetrance of embryonic lethality
At the age of 3 wk, the survival rates of NCOA1+/−, NCOA1−/−, and NCOA3+/− mice were similar to that of wild-type (WT) mice regardless of their strain background (Table 1). However, NCOA3−/− mice exhibited a relatively high penetrance of lethality, and their survival rate was dependent on the genetic background. In fact, of the four genetic backgrounds tested (C57BL/6J, C57BL/6J-129SvEv, 129SvEv, and FVB), the number of the NCOA3−/− offspring was considerably lower than expected (Table 1). Among the progenies derived from NCOA3+/− breeding pairs, the survival rate of NCOA3−/− mice with either C57BL/6J or FVB strain background was significantly lower than the survival rate of NCOA3−/− mice with a mixed C57BL/6J-129SvEv strain background (P < 0.001). Among the compound mutant mice, the observed number of NCOA1+/−;NCOA3+/− , and NCOA1−/−;NCOA3+/− mice with the 129SvEv strain background was moderately reduced than the number predicted according to Mendelian ratio. The survival rates of NCOA1+/−;NCOA3−/− and NCOA1−/−; NCOA3−/− (DKO) mice with the 129SvEv strain background were further reduced to 16% and 7%, respectively. Furthermore, no NCOA1+/−;NCOA3−/− and DKO mice were obtained from a relatively small population of pups derived from NCOA1+/−;NCOA3+/− parents with the FVB strain background; and only 4% of the predicted number of DKO mice were observed from a large population of pups derived from NCOA1−/−;NCOA3+/− parents (Table 1).
Table 1.
Genotype and strain background-dependent survival of NCOA1 and NCOA3 mutant mice
| Genetic strains | Parents | No. of Progenies with designated genotypes
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I+/+III+/+a | I+/+III+/− | I+/+III−/− | I+/−III+/+ | I+/−III+/− | I+/−III−/− | I−/−III+/+ | I−/−III+/− | I−/−III−/− | ||
| C57BL/6J | I+/+III+/− | 192b (210c; 91%d) | 438 (420; 104%) | 23f (210; 11%) | —e | — | — | — | — | — |
| C57BL/6J-129SvEv | I+/+III+/− | 229 (236; 97%) | 479 (472; 101%) | 109f (236; 46%) | — | — | — | — | — | — |
| 129SvEv | I+/−III+/− | 36 (31c) | 65 (62) | 6 (31) | 55 (62) | 81 (123) | 10 (62) | 29 (31) | 33 (62) | 2 (31) |
| I−/−III+/− | — | — | — | — | — | — | 93 (93) | 161 (186) | 7 (93) | |
| Sum | 36 (31; 116%) | 65 (62; 105%) | 6f (31; 19%) | 55 (62; 89%) | 81g (123; 66%) | 10f (62; 16%) | 122 (124; 98%) | 194g (248; 78%) | 9f (124; 7%) | |
| FVB | I+/+III+/− | 59 (62) | 126 (123) | 6 (62) | — | — | — | — | — | — |
| I+/−III+/− | 11 (10) | 17 (20) | 6 (10) | 19 (20) | 27 (39) | 0 (20) | 12 (10) | 11 (20) | 0 (10) | |
| I−/−III+/− | — | — | — | — | — | — | 168 (168) | 304 (336) | 7 (168) | |
| Sum | 70 (72; 97%) | 143 (143; 100%) | 12f (72; 17%) | 19 (20; 95%) | 27 (39; 69%) | 0f (20; 0%) | 180 (178; 101%) | 315 (356; 88%) | 7f (178; 4%) | |
NCOA1+/+;NCOA3+/+;
the number of mice at 3 wk;
the expected number of mice;
the survival percentage;
no progeny generated;
P < 0.001 by χ2 test between obtained and expected numbers of mice;
P < 0.05.
To define the lethal stages, we further investigated the viability of embryos with the FVB strain background. The NCOA1+/−, NCOA1−/−, NCOA3+/−, and NCOA1+/−;NCOA3+/− embryos survived normally, showing no significant difference from their expected numbers (Table 2). The numbers of NCOA3−/− embryos at E12.5 and E13.5 were slightly reduced but were not statistically significant from expected numbers (Table 2). The NCOA1+/− NCOA3−/− embryos showed normal viability at E9.5, but their survival rates were reduced at E12.5 and E14.5. The survival rate of DKO embryos showed a reduced trend at E9.5 although it failed to reach statistical difference. At E12.5, approximately 48% of DKO embryos were still alive. However, only 18% and 7% of predicted numbers of DKO embryos were alive at E13.5 and E14.5, and no DKO embryos were found at E16.5 (Table 2). These results indicate that NCOA1 and NCOA3 function cooperatively to support embryo survival.
Table 2.
The survival of NCOA1 and NCOA3 DKO embryos with FVB strain background
| Parents | Days post coitus (dpc) | No. of progenies with defined genotypes
|
|||||
|---|---|---|---|---|---|---|---|
| I+/−III+/+a | I+/−III+/− | I+/−III−/− | I−/−III+/+ | I−/−III−/− | I−/−III−/− | ||
| I+/−III+/− | 9.5 | 11 | 14 | 11 | 9 | 25 | 5 |
| vs. | (10b; 110%c) | (20; 70%) | (10; 110%) | (10; 90%) | (20; 125%) | (10; 50%) | |
| I−/−III+/− | 12.5 | 48 | 59 | 13e | 40 | 66 | 21f |
| (44; 109%) | (88; 67%) | (44; 30%) | (44; 91%) | (88; 75%) | (44; 48%) | ||
| 13.5 | 10 | 29 | 6 | 11 | 32 | 2g | |
| (11; 91%) | (21; 138%) | (11; 55%) | (11; 100%) | (21; 152%) | (11; 18%) | ||
| 14.5 | 12 | 26 | 4g | 15 | 23 | 1f | |
| (14; 86%) | (27; 96%) | (14; 29%) | (14; 107%) | (27; 85%) | (14; 7%) | ||
| I−/−III+/− | 16.5 | —d | — | — | 21 | 20f | 0e |
| (21; 100%) | (42; 48%) | (21; 0%) | |||||
| Parents | dpc | I+/+III+/− | I+/+III−/− | ||||
|---|---|---|---|---|---|---|---|
| I+/+III+/− | 12.5 | 47 | 36 | ||||
| vs. | (47; 100%) | (47; 77%) | |||||
| I+/+III−/− | 13.5 | 13 | 7 | ||||
| (13; 100%) | (13; 54%) |
NCOA1+/−;NCOA3+/+;
the expected number of mice;
the survival percentage;
no progeny generated;
P < 0.001 by χ2 test between obtained and expected numbers of embryos;
P < 0.01;
P < 0.05.
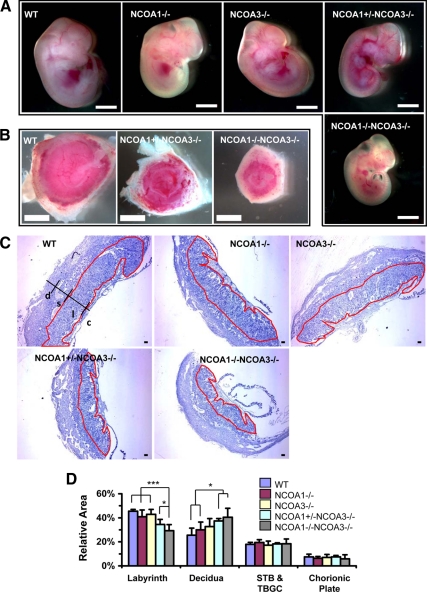
Disruption of NCOA1 and NCOA3 restricted fetal and placental growth
The sizes of WT, NCOA1−/−, NCOA3−/−, and most NCOA1+/−;NCOA3−/− embryos appeared similar at E12.5. However, all of the DKO embryos appeared much smaller than other genotype embryos (Fig. 1A). Interestingly, the diameters of some NCOA1+/−;NCOA3−/− and all DKO placentas were much shorter than WT, NCOA1−/−, and NCOA3−/− placentas (Fig. 1B and data not shown). In general, the DKO placentas of relatively bigger, surviving embryos were bigger and healthier than the DKO placentas of relatively smaller, dying embryos during E12.5 to E13.5. On hematoxylin and eosin (H&E)-stained sagittal sections through the central region of placenta, the relative area (%) of the trophoblast giant cell and spongiotrophoblast layers to total placental area as well as the relative area (%) of the chorionic plate to total placental area were similar among all examined genotype groups (Fig. 1, C and D). However, the percentage of NCOA1+/−;NCOA3−/− labyrinth area to the total placental area was significantly reduced when compared with WT, NCOA1−/−, and NCOA3−/− labyrinth areas. The percentage of DKO labyrinth area to the total placental area was further reduced, and it was significantly lower than the percentage of NCOA1+/−;NCOA3−/− labyrinth area (Fig. 1, C and D). Due to the reduced labyrinth areas, the percentages of maternal decidua areas were accordingly increased in NCOA1+/−;NCOA3−/−, and DKO placentas vs. WT and NCOA1−/− placentas (Fig. 1, C and D). These results demonstrate that NCOA1 and NCOA3 cooperatively regulate embryo placental growth. Disruption of NCOA1 and NCOA3 selectively affects the labyrinth growth.
Figure 1.
Disruption of NCOA1 and NCOA3 in mice restricted fetal and placental growth. A, E12.5 mouse embryos with indicated genotypes. Scale bar, 2 mm. B, E12.5 mouse placentas with indicated genotypes. Scale bar, 2 mm. Panel C, H&E-stained middle sagittal sections of E12.5 mouse placentas. The labyrinth area in each panel is circulated by red line. c, chorionic plate; l, labyrinth; s, spongiotrophoblast; d, decidua. Scale bar, 100 μm. Panel D, The relative areas of placental components for the indicated genotype groups. For each of the middle sagittal section of placenta, the labyrinth area, deciduas area, STB and TBGC area, chorionic plate area, and total placental area were separately measured, after which the percentages of a specific area to total area was calculated. For each group four to eight placentas were measured. *, P < 0.05; ***, P < 0.001, compared by unpaired t test between any two groups. STB, Spongiotrophoblast; TBG, trophoblast giant cell.
Expression patterns of NCOA1 and NCOA3 in the placenta
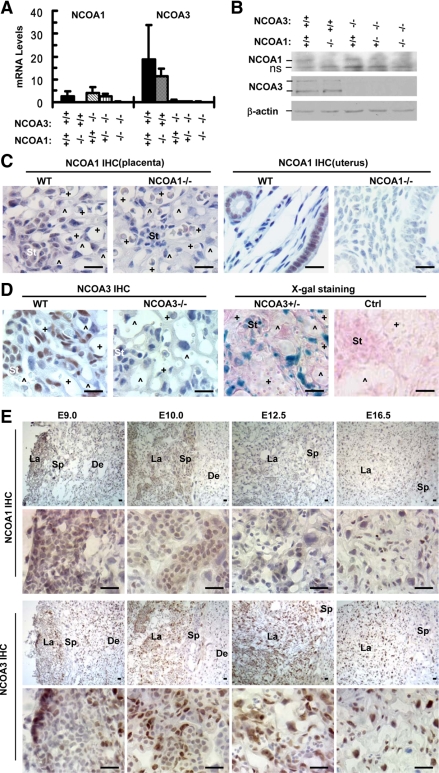
Real-time RT-PCR was performed to measure placental levels of NCOA1 and NCOA3 mRNAs at E12.5. NCOA1 mRNA was expressed in the placentas of WT, NCOA3−/−, and NCOA1+/−;NCOA3−/− mice, whereas NCOA3 mRNA was present at higher levels in WT and NCOA1−/− placentas. The specificity of these assays was validated by the minimal background readings from NCOA1−/− and NCOA3−/− placentas (Fig. 2A). NCOA1 and NCOA3 proteins were also detected by Western blot in placentas at E12.5 (Fig. 2B). The RT-PCR and Western blot assays suggest the mRNA and protein levels of either NCOA1 in NCOA3−/− placentas or NCOA3 in NCOA1−/− placentas are not significantly different than WT.
Figure 2.
The distribution patterns of NCOA1 and NCOA3 in the placenta. A, qPCR analysis of NCOA1 and NCOA3 mRNAs in E12.5 placentas with the indicated genotypes. RNA samples of two placentas were pooled, and three pools were assayed for each group. B, Western blot analysis of NCOA1 and NCOA3 proteins in E12.5 placentas with indicated genotypes. The weak NCOA1 bands and high background of the image were due to the low NCOA1 levels and elongated exposure time. β-Actin served as a loading control (Ctrl). The two NCOA3 bands were two NCOA3 splicing isoforms. ns, A non-specific band detected in all samples including NCOA1 null placentas. C, Detection of NCOA1 by IHC in the placental labyrinth of E12.5 WT embryos. The uterine sections of adult mice served as a positive control, whereas NCOA1−/− placental and uterine samples served as negative controls. D, Detection of NCOA3 expression by IHC and X-gal staining (blue) in E12.5 labyrinths with indicated genotypes. The sections were also counterstained with nuclear fast red. E, Detection of NCOA1 and NCOA3 by IHC using cryosections prepared from E9.0, E10.0, E12.5, and E16.5 placentas of WT embryos. Image annotations: St, stem-like trophoblast; +, fetal capillary; ^, maternal sinusoid; De, decidua; La, labyrinth; Sp, spongiotrophoblast; scale bars in panels C, D, and E, 20 μm.
Furthermore, immunohistochemistry (IHC) was performed to determine cell type-specific distributions of NCOA1 and NCOA3 in the placenta. When compared with uterine luminal and glandular epithelial cells, NCOA1 immunoreactivity was relatively weak in the placenta at E12.5 (Fig. 2C). In the labyrinth, the NCOA1 immunostaining signal in the placenta was mainly detected in all of the S-TGCs (layer I), syncytiotrophoblasts (layers II and III) and endothelial cells, and a subset of stem-like trophoblasts. In other parts of the placenta, NCOA1 was detected in all of the spongiotrophoblasts and subsets of the trophoblast giant cells and chorionic plate cells (Fig. 2C, Supplemental Fig. 1A, and Table 3). NCOA3 immunoreactivities were much stronger than NCOA1 in the placenta at E12.5 and were mainly observed in layer III syncytiotrophoblasts and endothelial cells in the labyrinth and in subsets of trophoblast giant cells, layer I S-TGCs, stem-like trophoblasts, and chorionic plate cells (Fig. 2D, Supplemental Fig. 1A, and Table 3). These results demonstrate that expression patterns of NCOA1 and NCOA3 are largely overlapping in the placental cells, particularly in layer III syncytiotrophoblasts and endothelial cells in the labyrinth. In addition, the NCOA1 immunoreactivity in NCOA3−/− labyrinth appeared slightly stronger than WT, whereas the NCOA3 immunoreactivity was similar in NCOA1−/− and WT labyrinths (Supplemental Fig. 1B). These data suggest that the slightly increased NCOA1 could compensate for the loss of NCOA3 function in the labyrinth.
Table 3.
NCOA1 and NCOA3 expression patterns in E12.5 mouse placenta
| Cell types | NCOA1 | NCOA3 |
|---|---|---|
| Decidua cells | − | + |
| Trophoblast giant cells | − or +a | − to ++b |
| Spongiotrophoblasts | + | − |
| Labyrinth | ||
| Stem-like trophoblasts | − to ++ | − to ++ |
| Endothelial cells | + | ++ |
| Layer I trophoblasts | − | − or + |
| Layer II trophoblasts | ++ | − |
| Layer III trophoblasts | + | ++ |
| Chorionic plate cells | − or + | − or + |
Negative levels or medium levels of NCOA1 were observed in different individual trophoblast giant cells;
negative levels, medium levels, or high levels of NCOA3 were observed in different individual trophoblast giant cells.
We have previously reported that the NCOA3 mutant allele carried a knock-in β-galactosidase (β-gal) sequence, which served as an indicator of NCOA3 promoter activity (17). Therefore, X-gal staining was carried out with NCOA3+/− (NCOA3+/β-gal) placentas to define NCOA3 expression pattern. The β-gal activity was detected at E12.5 in the placenta, and the expression pattern of β-gal activity was consistent with the pattern revealed by NCOA3 immunostaining, with most β-gal-positive cells located in the labyrinth (Fig. 2D and data not shown).
We further examined the expression patterns of NCOA1 and NCOA3 by IHC in WT placenta at different embryonic stages. We found that NCOA1 expression in the decidua was high at E9.0 but was markedly reduced at E10.0 and thereafter. NCOA1 expression in the labyrinth was high at E9.0 and E10.0, but much lower at E12.5 and thereafter (Fig. 2E and data not shown). NCOA3 expression in the decidua was high at E9.0, but it decreased to low levels at E10.0 and thereafter. NCOA3 expression appeared at the edge of certain labyrinth areas at E9.0, increased at E10.0 and E12.5, and then slightly decreased at E16.5 (Fig. 2E). These results suggest that the expression of NCOA1 and NCOA3 is regulated in a developmental stage-dependent manner in the placenta.
NCOA1 and NCOA3 contribute to labyrinth morphogenesis in a gene dosage-dependent manner
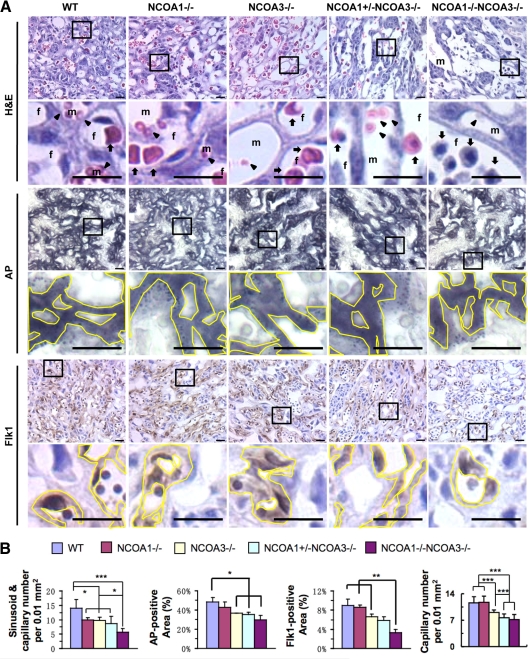
The labyrinth layer contains complex networks of maternal blood sinuses and fetal capillaries. The fetal capillary system in the labyrinth layer receives nutrition and oxygen from, and exports metabolic wastes to, the maternal blood sinusoids. We performed several assays to characterize the effects of NCOA1 and NCOA3 compound deficiencies on labyrinth morphogenesis. First, we stained the E12.5 placenta sections with H&E and counted the number of fetal capillaries and maternal blood sinusoids per unit labyrinth area (Fig. 3A). We found that the capillary and blood sinusoid density was reduced to about 30% in NCOA1−/−, NCOA3−/−, and NCOA1+/−;NCOA3−/− labyrinths and more drastically reduced to about 65% in DKO labyrinth compared with WT (Fig. 3B). Next, we performed histochemistry to detect alkaline phosphatase (AP) activity that was specifically expressed in S-TGCs lining the luminal wall of the blood sinusoid and measured the AP-positive area (Fig. 3A). We found that the percentages of AP-positive areas were significantly reduced in NCOA3−/−, NCOA1+/−;NCOA3−/−, and DKO labyrinths vs. WT (Fig. 3B). In association with the AP-positive area of layer I S-TGCs, the NCOA1+/−;NCOA3−/− , and DKO labyrinths exhibited dilated sinusoids with large lumens (Fig. 3A).
Figure 3.
Compound NCOA1 and NCOA3 mutations reduced maternal blood sinusoid and fetal vascular densities in the placental labyrinth. A, Histology and immunostaining of the labyrinth regions of E12.5 placentas with the indicated genotypes. Sections were stained by H&E, histochemically stained for AP activity (black), or immunostained by using an Flk1 antibody (brown) as indicated. The boxed areas were enlarged in the panels below. The immunostained slides were counterstained with hematoxylin. Arrows point at fetal erythrocytes. Arrowheads point at maternal erythrocytes. Yellow lines mark the AP-positive areas (black) or Flk1-positive areas (brown). f, fetal capillary; m, maternal sinusoid. Scale bar, 20 μm. B, Quantitative analyses of placental images shown in panel A. The density of sinusoids and capillaries in the labyrinth was measured by using the ImageTool software. The AP-positive area, Flk1-positive area, and total labyrinth area were measured by using the i-Solution software. The relative densities of sinusoids and capillaries and the relative AP-positive and Flk1-positive areas were normalized to the total labyrinth area. For each group, three to eight placentas were analyzed. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared by unpaired t test between any two groups.
Lastly, we performed immunostaining for the Flk1 endothelial marker and measured the relative area of the Flk1-positive endothelial cells and counted the number of capillaries per unit area in the labyrinth (Fig. 3A). We found that the percentage of Flk1-positive or endothelial area to total labyrinth area was significantly reduced in DKO labyrinths vs. that in WT, NCOA1−/−, and NCOA3−/−. The capillary density was reduced in NCOA3−/− labyrinths vs. WT and NCOA1−/−, and more drastically reduced in NCOA1+/−;NCOA3−/− and DKO (Fig. 3B). Immunostaining of another endothelial marker, PECAM, also showed a reduction in percentage of endothelial areas in NCOA1+/−;NCOA3−/−, and DKO labyrinths (Supplemental Fig. 2). Taken together, the overall consensus of these results supports the notion that both NCOA1 and NCOA3 gene products contribute to the labyrinth morphogenesis in a gene dosage-dependent manner.
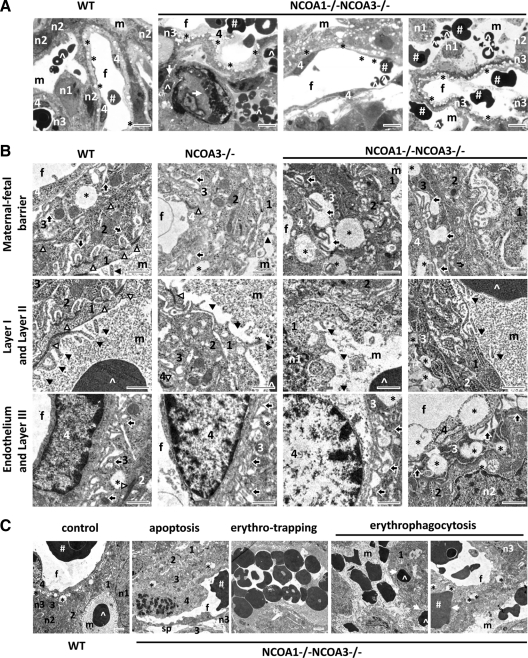
Double knockout of NCOA1 and NCOA3 resulted in abnormal structure of the four-cell-layer fetomaternal interface and blood transfusion
In the placental labyrinth, there are four layers of cells constituting the fetomaternal circulation barrier. Layer I consists of S-TGCs, layers II and III consist of syncytiotrophoblasts with multiple nuclei, and layer IV is the endothelium of the fetal capillary. In the WT labyrinth, S-TGCs had large nuclei, and they were loosely attached to the underlying syncytial layers via desmosomal adhesions. The tight junctions appeared between individual S-TGCs and between S-TGC and layer II syncytiotrophoblast (Fig. 4, A and B, and data not shown). Under transmission electron microscope, we observed numerous microvilli emanating from the apical cytoplasmic membrane of WT S-TGCs and intruding into the blood sinusoids (Fig. 4B). However, in DKO labyrinths, the junctions between S-TGCs were much looser, and the density of their microvilli appeared to be reduced (Fig. 4B).
Figure 4.
Abnormal morphogenesis observed in the labyrinths of E12.5 DKO mouse embryos. A, Toluidine blue-stained semithin sections of placental labyrinths from E12.5 embryos with the indicated genotypes. B, Subcellular ultrastructures of placental labyrinths from E12.5 embryos with the indicated genotypes. C, Several abnormalities observed in DKO labyrinths at E12.5. The image of a WT sample served as a control. Image annotations: 1, layer I mononuclear trophoblast; 2, layer II syncytiotrophoblast; 3, layer III syncytiotrophoblast; 4, fetal endothelial layer; #, fetal erythrocytes; f, fetal vessels; ^, maternal erythrocytes; m, maternal blood sinusoids; solid arrowhead, microvilli; n1, the nucleus of layer I trophoblast; n2, the nucleus of layer II trophoblast; n3, the nucleus of layer III trophoblast; open arrow, erythrophagocytosis; open arrowhead, tight cell-cell junction; solid arrow, intrasyncytial bay; *, lipid droplet; sp, big empty space between layer III trophoblasts and the endothelial layer of fetal capillaries; scale bars in panel A, 8 μm; scale bars in panel B, 1 μm; scale bars in panel C, 2 μm.
Although the morphology of layer II syncytiotrophoblasts in DKO labyrinths was similar to WT, the morphology and structure of layer III syncytiotrophoblasts in DKO labyrinths were abnormal. First, both the size and the number of cytoplasmic lipid droplets, which appeared as large vacuoles in images taken from both semithin and ultrathin sections, were drastically increased by visual assessment (Fig. 4, A and B). Second, the cytoplasmic membrane invaginations, also designated as intrasyncytial bays formed on the cellular side adjacent to the endothelium (45,46), appeared less numerous in DKO cells vs. WT but were extended more deeply into the cytoplasmic area (Fig. 4B). Third, due to the deeper invaginations and reduced density of the intrasyncytial bays, a large empty space was frequently seen between layer III trophoblasts and the endothelial layer of fetal capillary (Fig. 4B). These results demonstrate that double knockout of NCOA1 and NCOA3 causes multiple morphological defects in layer III syncytiotrophoblasts.
The endothelial cells of the WT fetal capillaries were thin and smooth. Large lipid droplets were rare in these WT endothelial cells. Most of the DKO endothelial surface, however, was rough. Some DKO endothelial nuclei were thickened and irregular in shape (Fig. 4B). Large lipid droplets were frequently observed in some DKO endothelial cells. In addition, apoptotic endothelial cells, as judged by nuclear condensation, were also frequently observed in the double-mutant endothelial cells (Fig. 4C).
In the DKO labyrinth layer, many maternal erythrocytes were trapped in narrow blood sinusoids (Fig. 4, A and C). Some fetal erythrocytes were endocytosed by nearby trophoblasts, and a number of maternal erythrocytes were observed in the cytoplasm of DKO S-TGCs (Fig. 4, A and C). These observations indicate an active erythrophagocytic activity of these trophoblasts, a hallmark of placental circulation defects. More importantly, a small number of maternal erythrocytes were found together with the nucleated fetal erythrocytes in the lumen of fetal vessels, and some of the fetal erythrocytes were also found in the maternal blood sinuses (Fig. 4A). These results indicate that placental transfusion between maternal and fetal circulations had occurred in DKO placentas.
We also examined the cellular and subcellular structures of NCOA1−/− and NCOA3−/− labyrinths at E12.5. The morphologies of NCOA1−/− and WT S-TGCs, syncytiotrophoblasts, and endothelial cells were similar (data not shown). The morphologies of NCOA3−/− S-TGCs, syncytiotrophoblasts, and endothelial cells were partially affected with respect to parameters described above for DKO mice. The degree of phenotypic abnormality of NCOA3−/− labyrinth cells in terms of these parameters was intermediate between WT and DKO mice (Fig. 4B).
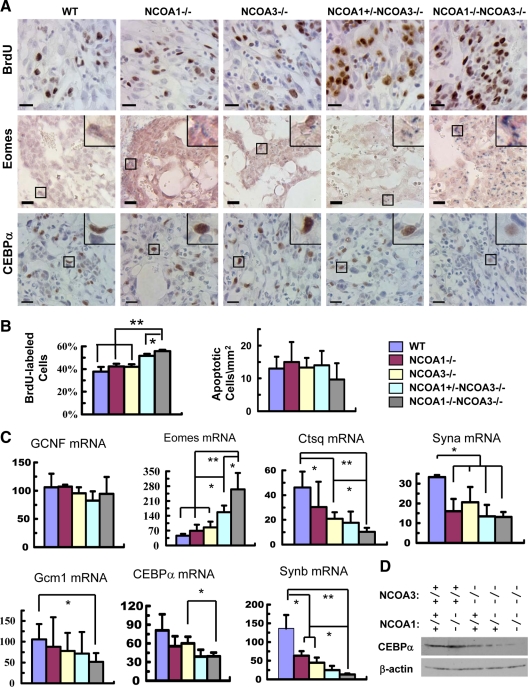
Genetic ablation of NCOA1 and NCOA3 increased labyrinth trophoblast proliferation and decreased their differentiation gene expression
In vivo bromodeoxyuridine (BrdU) incorporation assays revealed that 38% of trophoblasts in the WT labyrinth at E12.5 were labeled by BrdU within 1 h. Similar percentages of trophoblasts in NCOA1−/− and NCOA3−/− labyrinths were also labeled by BrdU. However, the BrdU-labeled trophoblasts were significantly increased in NCOA1+/−;NCOA3−/− and DKO labyrinths, which were 52% and 56%, respectively (Fig. 5, A and B). Phosphorylation of serine 10 in histone H3 (p-S10-H3) is a marker for G2/M phases of the cell cycle (47). In agreement with the BrdU-labeling results, the percentage of p-S10-H3-positive trophoblasts in DKO labyrinths was also significantly increased when compared with labyrinths of other genotypes (data not shown). Conversely, the number of apoptotic cells detected by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling assays in the labyrinths was low and comparable among all genotypes (Fig. 5B). These results indicate that ablation of both NCOA1 and NCOA3 increases labyrinth trophoblast proliferation in the placenta.
Figure 5.
Compound knockout of NCOA1 and NCOA3 increased proliferation and decreased differentiation of the labyrinth trophoblasts at E12.5. A, Detection of the BrdU-labeled proliferative cells (brown) by IHC (upper panels), the stem-like trophoblasts by in situ hybridization of Eomes mRNA (blue) (middle panels), and the CEBPα protein (brown) in layer III trophoblasts by IHC (lower panels) in the labyrinth layer of E12.5 placentas with the indicated genotypes. The insets at upper right corners are amplified images of the boxed areas. Scale bars, 20 μm. B, The percentage of BrdU-labeled labyrinth trophoblasts in total labyrinth trophoblasts (left panel) and the number of apoptotic cells per mm2 area of labyrinth with indicated genotypes. Apoptotic cells were detected by TUNEL assay. The cell numbers of three samples for each group were counted by using the ImageTool software. C, Relative mRNA levels of several labyrinth trophoblast marker genes assayed by qPCR. *, P < 0.05; and **, P < 0.01, compared by t test between any two groups (n = 3 for each group). D, Western blot analysis of CEBPα in E12.5 placentas of mouse embryos with the indicated genotypes. β-Actin served as a loading control. GCNF, Germ cell nuclear factor.
Morphological examination revealed that all cell types and basic tissue structures were present in DKO placentas, suggesting that disruption of NCOA1 and NCOA3 does not completely block cell lineage determination. To further assess the roles of these two genes in cell differentiation, we analyzed cell type-specific markers in E12.5 placentas. The 4311 gene is a marker of spongiotrophoblasts (48,49). Comparable levels and distributions of the 4311 mRNA were detected in spongiotrophoblasts by in situ hybridization in E12.5 placentas with all NCOA1 and NCOA3 genotypes (data not shown), suggesting that NCOA1 and NCOA3 are not required for spongiotrophoblast differentiation. The expression of germ cell nuclear factor, which is broadly expressed in the labyrinth (50), was also expressed at similar levels in placentas of all examined NCOA1 and NCOA3 genotypes (Fig. 5C).
However, the expression of several labyrinth trophoblast markers was significantly altered in the double-mutant placentas. The undifferentiated labyrinth progenitors display clusters of epithelial-like cells and specifically express Eomesodermin (Eomes) (51,52,53,54). Real-time RT-PCR analysis revealed that Eomes mRNA levels were 4.7-fold higher in DKO placentas (P < 0.01), 2.4-fold higher in NCOA1+/−;NCOA3−/− placentas (P < 0.05), and 0.8-fold higher in NCOA3−/− placentas (P < 0.05) vs. WT placentas. Eomes expression was not significantly changed in NCOA1−/− placentas (Fig. 5C). Analysis by in situ hybridization further demonstrated that the number of Eomes-positive trophoblasts in DKO labyrinths was markedly increased (Fig. 5A). Of note, the increased Eomes expression is consistent with the reduced vascular density and the increased number of clustered epithelial-like progenitors in the labyrinths of DKO placentas, suggesting that NCOA1 and NCOA3 may play a role to accelerate the differentiation of labyrinth trophoblast progenitors.
In the three layers of trophoblasts between maternal blood sinuses and fetal vessels, layer I S-TGCs specifically express cathepsin Q (Ctsq) (31). The levels of Ctsq expression were reduced by 50% in NCOA3−/− and NCOA1+/−;NCOA3−/− placentas (P < 0.05) and as much as 80% in DKO placentas vs. WT placentas (Fig. 5C). Layer II syncytiotrophoblasts specifically express syncytin A (Syna) (50). The levels of Syna expression were significantly reduced in NCOA1−/−, NCOA3−/−, NCOA1+/−;NCOA3−/−, and DKO placentas (Fig. 5C). Layer III syncytiotrophoblasts specifically express glial cells missing homolog 1 (Gcm1), CCAAT/enhancer binding protein α (CEBPα) and Syncytin B (Synb) (50). Interestingly, Gcm1 expression was not affected in NCOA1−/−, NCOA3−/−, and NCOA1+/−;NCOA3−/− placentas, but significantly reduced in NCOA1−/−;NCOA3−/− placentas (Fig. 5C). Similarly, the mRNA level of CEBPα was significantly reduced in DKO placentas vs. NCOA3−/−, which had similar levels of CEBPα as WT placenta (Fig. 5C). More importantly, IHC and Western blot analyses also revealed decreases in both the number of CEBPα-positive trophoblasts and the CEBPα protein in individual NCOA1+/−;NCOA3−/− and DKO trophoblasts (Fig. 5, A and D). The expression levels of Synb were correlated with the NCOA1 and NCOA3 allele numbers, following a descending order: WT more than NCOA1−/−, NCOA3−/− more than NCOA1+/−;NCOA3−/− more than DKO placentas (Fig. 5C).
These results demonstrate that disruption of NCOA1 and NCOA3 increased the proliferation rate of labyrinth trophoblasts, which correlated well with the increased expression of trophoblast progenitor marker genes and the decreased expression of trophoblast differentiation marker genes.
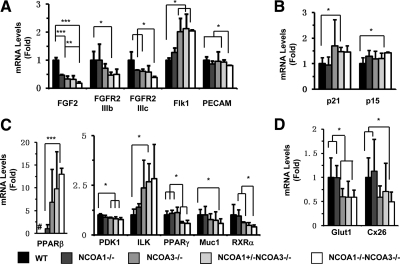
Knockout of NCOA1 and NCOA3 altered the expression of genes involved in multiple signaling pathways and functions
The severely reduced fetal vascular density in DKO labyrinths suggested a possible role of NCOA1 and NCOA3 in regulation of angiogenic factor expression. Therefore, we measured the expression levels of several genes involved in regulation of angiogenesis in E12.5 placentas. We found that knockout of both NCOA1 and NCOA3 failed to alter the mRNA levels of HIF-1a, VEGFa, VEGFb, VEGFc, PDGF, and TGFβ (data not shown). However, the levels of Flk1 mRNA in endothelial cells were significantly increased in NCOA3−/−, NCOA1+/−;NCOA3−/−, and DKO placentas. The transcriptional levels of PECAM were only slightly reduced in DKO placentas (Fig. 6A). Furthermore, the FGF2 expression was decreased in NCOA1−/−, NCOA3−/−, NCOA1+/−;NCOA3−/−, and DKO placentas. FGFR2-IIIb and FGFR2-IIIc expression levels were also reduced in NCOA1+/−;NCOA3−/−, and DKO placentas, respectively (Fig. 6A). The expression levels for two of the TGFβ-responsive genes, p21 and p15, were slightly increased in NCOA1+/−;NCOA3−/− and/or DKO placentas (Fig. 6B). Nevertheless, the amounts of total and active forms of Akt, ERK1/2, and p38 MAPK were not significantly changed in the double knockout placentas as determined by Western blotting using total and active form-specific antibodies (data not shown), suggesting that the activities of the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) and fibroblast growth factor (FGF)2/FGF receptor (FGFR)2 signaling pathways are still maintained.
Figure 6.
Relative mRNA expression levels of genes affected by NCOA1 and/or NCOA3 deficiencies in the placenta at E12.5. A, Genes that regulate angiogenesis. B, TGFβ-responsive genes important for endothelial differentiation. C, Nuclear receptors and their target genes in the placenta. D, Genes important for glucose transportation. Notes: the # in the left part of panel C indicates that the PPARβ mRNA was undetectable in WT placenta; therefore, the relative expression level of PPARβ mRNA in NCOA1−/− placenta was set to 1 unit. The relative expression levels of all other genes in WT placenta were set to 1 unit. *, **, and ***, P < 0.05, P < 0.01, and P < 0.001 by unpaired t test between any two groups (n = 3 for each group).
Because the function of the PPAR family is essential for placental development and NCOA1 and NCOA3 are known coactivators of PPARs, we investigated the effects of NCOA1 and NCOA3 loss of functions on the expression and function of PPAR family members. PPARα was comparably expressed in the placentas of all genotypes (data not shown). Interestingly, the expression of PPARβ (PPARδ) was undetectable in WT placentas and very low in NCOA1−/− placentas but was moderately increased in NCOA3−/− and NCOA1+/−;NCOA3−/− placentas and drastically increased in DKO placentas vs. NCOA1−/− placentas (Fig. 6C). However, the high expression of PPARβ failed to stimulate its target gene expression. The 3-phosphoinositide-dependent protein kinase 1 (PDK1) is a PPARβ target (55), and its expression was significantly reduced in NCOA3−/−, NCOA1+/−;NCOA3−/−, and DKO placentas (Fig. 6C). Integrin-linked kinase (ILK), another target gene of PPARβ (55), was slightly increased in NCOA1+/−;NCOA3−/− placentas. In addition, the expression of PPARγ was significantly reduced in NCOA1+/−;NCOA3−/−, and DKO placentas when compared with WT or NCOA1−/− placentas. Accordingly, the expression levels of Muc1, a PPARγ target in the placenta (39), were reduced in the DKO placentas. In addition, the expression levels of RXRα, a heterodimeric partner of PPAR family, were reduced in DKO placentas (Fig. 6C). However, RXRβ expression was unchanged in DKO placentas (data not shown).
Finally, we examined the expression levels of the glucose transporter 1 (Glut1), the glucose transporter 3 (Glut3), and the gap junction gene, Cx26, which play essential roles in glucose transportation in the three-layer trophoblast interface between maternal-fetal circulations (44,56,57). We found that the expression levels of both Glut1 and Cx26 were significantly decreased in DKO placentas (Fig. 6D), whereas Glut3 expression had no change (data not shown). In addition, Glut1 expression was also decreased in NCOA3−/− placentas (Fig. 6D).
Discussion
Since NCOA1 was first discovered as a transcriptional coactivator for steroid receptors (3), more than 285 nuclear receptor coregulators (coactivators and corepressors) have been identified. Approximately, 160 of these coregulators have been likely associated with some pathological state (58), indicating the physiological importance of these regulators in human health. Nevertheless, as most of these coregulators were characterized by biochemical experiments and one coregulator can work with multiple nuclear receptors, it has been a daunting challenge to determine the specific and compensatory functions of these coregulators in development and organ physiology. Our laboratory and that of others have used knockout mouse lines to investigate the in vivo functions of the SRC family. These studies have demonstrated that the SRC family members play pleiotropic roles in development, somatic growth, reproduction, and metabolism and have both unique and overlapping physiological roles, which might be dependent on their spatiotemporal expression patterns and functional specificities (1,14,23,24,59). To define their redundant function during development, we generated compound NCOA1 and NCOA3 knockout mice in this study and found that NCOA1 and NCOA3 cooperatively regulated placental labyrinth morphogenesis. Knockout of both NCOA1 and NCOA3 resulted in a failure of placental morphogenesis and an extremely high penetrance of embryonic lethality at middle gestation.
The three SRC family genes cooperatively support embryonic and postnatal survival
The in vivo evidence supporting the compensatory function among the three SRC family members in embryonic and postnatal survival comes from SRC DKO mouse models. We and collaborators have shown that both NCOA1−/− and NCOA2−/− mice have normal viability, whereas the majority of NCOA1−/−;NCOA2−/− mice die at neonatal stage (23). Furthermore, about half of the NCOA3−/− mice with a mixed C57BL and 129SvEv strain background are viable at weaning age (17), whereas most of the NCOA2−/−;NCOA3−/− mouse embryos with the same strain background die before birth (our unpublished data). In this study, we show that more than 50% of NCOA3−/− embryos with a FVB strain background are alive at E13.5 and 17% of NCOA3−/− mice are viable at weaning age. However, the majority of the DKO embryos die by E13.5, and very few DKO mice can survive to weaning age. Taken together, these findings indicate that the three SRC gene family members function cooperatively to support embryonic and postnatal survival.
Interestingly, the survival rates of NCOA3 mutant mice are modulated by genetic background. The survival rate of NCOA3−/− mice with a mixed C57BL and 129SvEv background is much higher than those mice with a relatively pure C57BL or FVB strain background. A previous study also reported a 30% survival rate at weaning age for DKO mice with a mixed C57BL and 129SvEv background (24). Similarly, the survival rate of PPARδ mutant mice is also related to their genetic background (37,60,61). However, the relationships between genetic background-related viabilities of SRC and PPARδ mutant mice are unclear, although PPARδ expression increases significantly in NCOA3−/− and DKO mice. We speculate that the different strain-dependent survival phenotypes might be related to variable expression levels of different coactivators and nuclear receptors.
NCOA1 and NCOA3 cooperatively support labyrinth morphogenesis of the placenta
We found a number of morphogenic defects in the labyrinths of NCOA1 and NCOA3 mutant placentas. These defects include small labyrinth, reduced fetal vascular density, and dilated maternal blood sinuses. Further morphological analysis also revealed that the density of microvilli of most S-TGCs was reduced, the intrasyncytial bays of layer III syncytiotrophoblasts were reduced in number and deeply invaginated in shape, the size and the number of cytoplasmic lipid droplets were significantly increased, and the endothelial nuclei were thickened. The worst of these defects was always observed in DKO labyrinths. Because the interface between maternal blood sinuses and fetal capillaries consists of three layers of thin trophoblasts and one layer of endothelial cells, reduction in densities of blood sinuses and capillaries may decrease the material exchange area between maternal and fetal circulations and cause inefficient material exchange between the two circulating systems.
The severe phenotype observed in DKO labyrinths correlates well with the overlapping distribution pattern of NCOA1 and NCOA3 in the labyrinth, especially in layer III syncytiotrophoblasts. These observations suggest that NCOA1 and NCOA3 may cooperatively function to support labyrinth development. Indeed, we found that the expression of Eomes, a marker of stem-like labyrinth trophoblast precursors, was higher and in more cells in NCOA1+/+;NCOA3−/− and DKO labyrinths. The labyrinth trophoblasts also exhibited higher proliferation rate. Alternatively, the expression levels of labyrinth trophoblast differentiation markers Ctsq, Syna, CEBPα, and Symb were reduced. These results suggest that NCOA1 and NCOA3 play important roles in regulation of labyrinth trophoblast proliferation and differentiation. The inappropriate trophoblast differentiation and poor labyrinth morphogenesis are likely responsible for DKO embryo lethality. However, we also observed expression of SRC-1 and SRC-3 in the developing heart of WT embryos and small liver and heart phenotypes in DKO embryos. Therefore, we cannot exclude the possible contribution from abnormal development of other organs to the lethal phenotype.
NCOA1 and NCOA3 are involved in the labyrinth morphogenesis regulated by the PPAR family members
Previous studies showed that several nuclear receptors and coactivators played important roles in placental morphogenesis. PPARγ-null mice exhibited developmental defects in the placental labyrinth and embryonic lethality at middle gestation (34). When placental function was rescued by a tetraploid approach, PPARγ-null fetuses could survive to birth, suggesting an essential role of PPARγ in labyrinth morphogenesis (34). PPARδ and retinoid X receptor (RXR) were also shown to have crucial roles in regulating trophoblast invasion, differentiation, and metabolism as well as nutrient transportation in the placenta (34,37,38,41,60). In agreement with the essential roles of PPAR and RXR in placental development, knockout of PBP and AIB3, two of the PPAR and RXR coactivators, resulted in placental defects similar to that observed in PPARγ-null mice (35,40). Both NCOA1 and NCOA3 were shown to be coactivators of PPARα, PPARβ (PPARδ), and PPARγ (24,62,63). In adipose tissue, NCOA1 and NCOA3 deficiency suppressed the expression of PPARγ target genes, including aP2, LPL, CD36, and UCP1 (24).
Here, we discovered multiple lines of evidence supporting the involvement of NCOA1 and NCOA3 in PPAR/RXR-regulated placental development. First, the placental defects observed in NCOA1 and NCOA3 compound-mutant placentas were similar to that observed in PPARγ, PBP, and AIB3 knockout placentas. Second, the expression levels of both PPARγ and RXRα were reduced in NCOA1 and NCOA3 compound-mutant placentas. The expression of Muc1, a PPARγ target gene, was also reduced in DKO placentas. Although it was unclear why PPARβ expression levels were significantly up-regulated in DKO placentas, the expression of its target gene PDK1 was reduced. These data suggest that NCOA1 and NCOA3 are required for these nuclear receptors to activate their target genes in the placenta. However, NCOA1 and NCOA3 are not required for all PPAR-mediated target gene expression. For example, the expression of ILK, another PPARβ target gene, was either increased or no significant change in NCOA1+/−;NCOA3−/− or DKO placentas. This might be a consequence of SRC-independent transcriptional activation by the increased PPARβ. Third, PPARβ was shown to stimulate proliferation of certain cell types (64). The increased PPARβ expression resulting from DKO of NCOA1 and NCOA3 may be partially responsible for the increased trophoblast proliferation and the decreased trophoblast differentiation observed in DKO labyrinths. Fourth, during adipogenesis C/EBPβ and C/EBPδ activate PPARγ expression, and then PPARγ activates C/EBPα expression (65,66). It was also reported that NCOA3 serves as a coactivator for C/EBP to up-regulate PPARγ during adipogenesis (67). In DKO placentas, C/EBPβ expression had no change (data not shown), but PPARγ and C/EBPα expression was significantly reduced. Therefore, NCOA1 and NCOA3 may serve as C/EBPβ coactivators to sequentially up-regulate PPARγ and C/EBPα expression in the placenta.
NCOA1 and NCOA3 compound mutation causes fetomaternal blood transfusion in the placenta
Separate studies have reported that 13.8% or 5% of human fetal deaths are attributed to fetomaternal blood transfusion, also known as fetomaternal hemorrhage or Kline’s hemorrhage (68,69,70). Pathological lesions leading to fetomaternal transfusion include abnormal morphogenesis or damage of the placental barrier, intervillous thrombosis, and placental infarction, presumably caused by local obstruction of the maternal circulation (71,72). However, the genetic basis and molecular mechanisms responsible for this disease have not been explored. In this study, we observed multiple circulation system problems in the labyrinth of DKO placenta. First, many maternal erythrocytes were trapped in some maternal blood sinuses, suggesting these sinuses were blocked. Second, active erythrophagocytosis of both fetal and maternal erythrocytes by trophoblasts was observed, suggesting a defect in placental circulation. Third, the fetomaternal circulation barrier in the labyrinth was structurally abnormal and disorganized. Fourth, the fetal erythrocytes were observed in the maternal blood sinuses and maternal erythrocytes were also observed in the fetal blood vessels, indicating that fetomaternal blood transfusion had occurred in DKO placentas. Therefore, further investigation of NCOA1- and NCOA3-regulated placental morphogenesis and function will shed light on understanding the molecular mechanisms and pathways responsible for Kline’s hemorrhage in pregnant women.
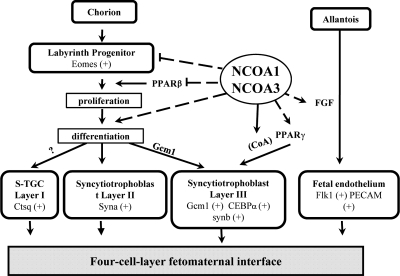
Summary
During labyrinth morphogenesis, the three layers of trophoblasts are derived from the chorion-originated progenitor cells, whereas the vascular endothelial cells originate from the allantois. Both NCOA1 and NCOA3 regulate multiple important genes involved in the labyrinth morphogenesis. Our observations suggest a working model depicted in Fig. 7. First, NCOA1 and NCOA3 control the population size of the Eomes-expressing labyrinth trophoblast progenitors by restricting PPARβ expression and hence, reducing the PPARβ-enhanced progenitor proliferation. Second, NCOA1 and NCOA3 play a role to promote the differentiation of trophoblasts from their progenitors as indicated by the decreased expression of mature trophoblast marker genes in DKO placentas. Third, NCOA1 and NCOA3 up-regulate PPARγ and CEBPα expression and may also serve as their coactivators to regulate the development and morphogenesis of layer III syncytiotrophoblasts. This notion is also supported by the coexpression of NCOA1 and NCOA3 and the severely disrupted morphology of layer III trophoblasts in DKO placentas. Finally, NCOA1 and NCOA3 may also be involved in regulation of labyrinth angiogenesis through modulating FGF expression. Given these important roles of NCOA1 and NCOA3 in the labyrinth morphogenesis, it becomes apparent why disruption of both NCOA1 and NCOA3 causes structural and functional defects during labyrinth development and results in fetomaternal blood transfusion.
Figure 7.
A working model for NCOA1 and NCOA3 functions in labyrinth morphogenesis. Arrows indicate downstream developmental events or positive regulatory effects. Dashed lines indicate regulatory events without clear molecular mechanisms at this time. The question mark indicates that the cell lineage origin of S-TGC is contentious. Eomes is a marker gene of the labyrinth progenitors. Ctsq is a marker gene of Layer I sinusoidal trophoblast giant cells (S-TGC). Syna is a marker gene of Layer II syncytiotrophoblasts. Gcm1, CEBPα, and Synb are marker genes of Layer III syncytiotrophoblasts. Flk1 and PECAM are markers of endothelia. CoA, indicates that NCOA1 and NCOA3 are coactivators of PPARγ.
Materials and Methods
Mice
Animal protocols were approved by the Animal Care and Use Committee of Baylor College of Medicine. NCOA1−/− and NCOA3−/− mice with a backcrossed FVB genetic background were described previously (73,74). NCOA1+/−;NCOA3+/− mice were generated by crossbreeding NCOA1−/− and NCOA3−/− mice. NCOA1−/−NCOA3−/− mice were generated from NCOA1+/−;NCOA3+/− and/or NCOA1−/−;NCOA3+/− breeding pairs. The day when the matting plug was observed was considered as E0.5. The embryos were dissected and examined on different pregnant days as needed. Genotypes were analyzed by PCR using allele-specific primer pairs and genomic DNA isolated from ear tips of 3-wk-old mice or yolk sacs of embryos after proteinase K digestion as described previously (11,17). The survival percentage for each genotype group was calculated by using the following formula: (Number of pups at weaning time)/(Expected number of pups) × 100. The expected number of pups was calculated according to Mendelian ratio by designating a full survival rate to mice with the following genotypes: WT, NCOA1+/−, NCOA1−/−, and NCOA3+/−. For example, NCOA3+/− breeding pairs with C57BL/6J-129SvEv strain background produced 229 WT, 479 NCOA3+/−, and 109 NCOA1−/− mice (Table 1). Their expected ratio was 1/4:1/2:1/4 if all pups were viable. The expected total number was: (229 + 479)/(1/4+1/2) = 944. The expected WT, NCOA3+/−, and NCOA3−/− numbers were 944/4 = 236, 944/2 = 472, and 944/4 = 235. Their survival percentages were 97% (229/944), 101% (472/944), and 46% (109/236), respectively. The data were statistically analyzed by Χ2 test between the observed and expected number of progenies.
Histological examination
For regular histological examination, the dissected mouse placentas were fixed overnight with 4% paraformaldehyde (PFA) at 4 C. The fixed specimens were embedded in paraffin and sectioned to a thickness of 5 μm. Sections were stained by H&E for light microscopy. For semithin and thin sections, placentas were fixed overnight with 2.5% glutaraldehyde and 2% formaldehyde at 4 C and embedded in Suprr’s low viscosity resin (EMS, Hatfield, PA). Semithin (1 μm) and thin (70–75 nm) sections were cut using an RMC MT6000-XL ultramicrotome (Tucson, AZ). Semithin sections were stained with toluidine blue for light microscopy. The thin sections mounted on 300-mesh hexagonal copper grids were stained with a saturated aqueous solution of uranyl acetate for 15 min and counterstained with Reynold’s lead citrate solution for 6 min. The stained thin sections were examined by using a Hitachi H7500 transmission electron microscope (Hitachi Scientific Instruments, Inc., San Jose, CA) at an accelerating voltage of 80 kV.
Quantitative RT-PCR (qPCR)
The mouse placentas were quickly isolated from the uterus in PBS, frozen in dry ice, and granulated into fine powder under frozen conditions. Total RNA was extracted from the tissue powder by using the TRIzol reagent (Invitrogen, Carlsbad, CA). After RNA was reversely transcribed into cDNA by using the reverse transcriptase core kit (Eurogenec, San Diego, CA), TaqMan qPCR was performed with 50 ng cDNA and the qPCR MasterMix Plus kit (Eurogenec, San Diego, CA). Relative standard curves were generated by using serial dilutions of E12.5 mouse placental RNA samples. Parallel measurements of 18S RNA or glyceraldehyde-3-phosphate dehydrogenase RNA were served as internal controls. The primers and probes for NCOA1 and NCOA3 qPCR have been described elsewhere (12,75). The qPCR primers and probes for measuring mRNA concentrations of mouse Eomes, Gcm1, Syna, Synb, Ctsq, CEBPα, PPARδ, PPARγ, RXRα, PDK1, ILK, Muc1, GCNF, GLUT1, Cx26, Flk1, PECAM, p21, p15, FGF2, FGFR2IIIb, and FGFR2IIIc were designed by using the online software of Universal ProbeLibrary Assay Design Center (Roche Applied Science, Switzerland).
IHC and X-gal staining
In addition to paraffin tissue sections, frozen tissue sections in a thickness of 6 μm were also prepared from Tissue-Tek OCT-embedded fresh mouse placentas. IHC was performed as described previously (12,75). Primary antibodies were against PECAM (CD31) (catalog no. 557355, BD biosciences, San Jose, CA), Flk1 (catalog no. 550549, BD Biosciences, San Jose, CA), NCOA1 (catalog no. 2191, Cell Signaling Technology, Danvers, MA), NCOA3 (catalog no. 2126, Cell Signaling Technology), phosphorylated-Ser (10) Histone H3 (catalog no. 06-570, Millipore Corp., Billerica, MA), and CEBPα (SC-61, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Appropriate biotinylated secondary antibodies were used. X-gal staining was performed by using frozen sections prepared from fresh placentas of embryos with a β-galactosidase sequence knocked in the NCOA3 gene locus (17).
BrdU labeling and detection
BrdU (B5002, Sigma-Aldrich Corp., St. Louis, MO) in saline (10 mg/ml) was ip injected into pregnant mice at day post coitus 12.5 (10 μl/g body mass). Injected mice were killed at 1 h after injection. Their placentas were collected, fixed in 4% PFA for 2 h, and embedded in paraffin. Sections (5 μm in thickness) were prepared for detecting BrdU signals by using the BrdU In-Situ Detection Kit (catalog no. 550803, BD Biosciences). Three samples were examined for each genotype. Images were taken from six randomly selected fields for each sample. The numbers of total trophoblasts and BrdU-positive trophoblasts were counted. Other cell types, such as endothelial cells, hematopoietic cells, and stromal cells, were distinguishable from trophoblasts based on their locations and morphologies, and these cells were excluded from the counting. The statistical analysis was performed by unpaired t test.
Western blot
Freshly frozen placentas and embryos were granulated into powder under frozen conditions. Western blot was performed as described previously (76). The primary antibodies were against NCOA1 (catalog no. 2126, Cell Signaling Technology), NCOA3 (catalog no. 2126, Cell Signaling Technology, CEBPα and β-actin (A2228, Sigma-Aldrich Corp.). Appropriate secondary antibodies conjugated with horseradish peroxidase were used to visualize the signals with the enhanced chemiluminescent reagent kit.
Histochemical analysis of AP
Placental frozen sections were fixed in 0.2% PFA for 10 min at 4 C, washed twice with PBS, and incubated at room temperature for 10 min with a Tris-HCl buffer (100 mm, pH 9.5) containing 0.375 mg/ml of nitro blue tetrazolium chloride and 0.188 mg/ml of 5-bromo-4-chloro-3-indoyl phosphate. After washing three times in PBS, the slides were mounted in Aqua-Mount (catalog no. 138000; Learner Laboratories, Pittsburgh, PA) for microscopy.
Image analysis
The light-field images were taken by an RT Color Spot camera (Diagnostic Instruments, Sterling Heights, MI). The number of cells, sinusoids, and capillaries in the placenta was counted on the images by using the ImageTool software (University of Texas Health Science Center, San Antonio, TX). The areas with different cell marker staining were measured by using the i-Solution software (IMT, Inc., Vancouver, British Columbia, Canada). An appropriate threshold above the background was set for all measurements. The sum of area with positive signal was normalized to the corresponding total tissue area.
Supplementary Material
Acknowledgments
We thank Lan Liao, Suoling Zhou, Jean Tien, and Brian York in Baylor College of Medicine for experimental assistant or manuscript preparation. We also thank Michael Mancini and Debra Townley in Integrated Microscopy Core in Baylor College of Medicine for electron microscopy.
Footnotes
This work was partially supported by National Institutes of Health grants DK058242, CA112403, CA119689, and DK059820.
Disclosure Summary: The authors have no disclosure of potential conflict of interest.
First Published Online August 4, 2010
Abbreviations: AP, Alkaline phosphatase; BrdU, bromodeoxyuridine; CEBP, CCAAT/enhancer binding protein; DKO, double knockout; E13.5, embryonic d 13.5; FGF, fibroblase growth factor; FGFR, FGF receptor; H&E, hematoxylin and eosin; IHC, immunohistochemistry; NCOA, nuclear receptor coactivator; PECAM, platelet endothelial cell adhesion molecule; PFA, paraformaldehyde; PPAR, peroxisome proliferator activated-receptor; qPCR, quantitative RT-PCR; SRC, steroid receptor coactivator; S-TGCs, sinusoidal trophoblast giant cells; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; WT, wild type.
References
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- Xu J, Wu RC, O'Malley BW 2009 Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer 9:615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Louie MC, Zou JX, Rabinovich A, Chen HW 2004 ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24:5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi P, Yu C, O'Malley BW, Xu J 2006 Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol 20:3105–3119 [DOI] [PubMed] [Google Scholar]
- Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, O'Malley BW, Xu J 2008 The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol 28:5937–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Z, Chen H, Xu J 2009 The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res 69:3819–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim HJ, Na SY, Kim TS, Choi HS, Im SY, Lee JW 1998 Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits. J Biol Chem 273:16651–16654 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S 1999 Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW 1998 Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- Nishihara E, Yoshida-Komiya H, Chan CS, Liao L, Davis RL, O'Malley BW, Xu J 2003 SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J Neurosci 23:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Xu J 2007 Loss-of-function deletion of the steroid receptor coactivator-1 gene in mice reduces estrogen effect on the vascular injury response. Arterioscler Thromb Vasc Biol 27:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Géhin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, Chambon P, Auwerx J 2002 SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111:931–941 [DOI] [PubMed] [Google Scholar]
- Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P 2002 The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol 22:5923–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW 2008 Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science 322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW 2000 The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Liao L, Tulis DA, Xu J 2002 Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation 105:2653–2659 [DOI] [PubMed] [Google Scholar]
- Liao L, Chen X, Wang S, Parlow AF, Xu J 2008 Steroid receptor coactivator 3 maintains circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. Mol Cell Biol 28:2460–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG 1997 The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677–684 [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM 1997 Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580 [DOI] [PubMed] [Google Scholar]
- Li H, Gomes PJ, Chen JD 1997 RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA 94:8479–8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Yoshida-Komiya H, Gehin M, Liao L, Tsai MJ, O'Malley BW, Chambon P, Xu J 2004 Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci USA 101:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Qi C, Krones A, Woodring P, Zhu X, Reddy JK, Evans RM, Rosenfeld MG, Hunter T 2006 Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab 3:111–122 [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC 2001 Placental development: lessons from mouse mutants. Nat Rev Genet 2:538–548 [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PPL 2002 Mouse development: patterning, morphogenesis, and organogenesis. San Diego: Academic Press [Google Scholar]
- Kaufman MH, Bard JBL 1999 The anatomical basis of mouse development. San Diego, London: Academic Press [Google Scholar]
- Enders AC 1965 A comparative study of the fine structure of the trophoblast in several hemochorial placentas. Am J Anat 116:29–67 [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D 1974 Morphogenesis of the syncytium in the mouse placenta. Ultrastructural study. Cell Tissue Res 148:381–396 [DOI] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ 2005 Ultrastructural changes in the interhaemal membrane and junctional zone of the murine chorioallantoic placenta across gestation. J Anat 207:783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC 2007 Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 304:567–578 [DOI] [PubMed] [Google Scholar]
- Los FJ, Noomen P, Vermeij-Keers C, Gaillard JL, Brandenburg H, Jahoda MG, Luider TM 1996 Chorionic villus sampling and materno-fetal transfusions: an immunological pathogenesis of vascular disruptive syndromes? Prenat Diagn 16:193–198 [DOI] [PubMed] [Google Scholar]
- Lloyd LK, Miya F, Hebertson RM, Kochenour NK, Scott JR 1980 Intrapartum fetomaternal bleeding in Rh-negative women. Obstet Gynecol 56:285–288 [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM 1999 PPAR γ is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595 [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Zhang H, Pereira FA, Yuan Y, DeMayo FJ, Ko L, Xu J 2002 Deletion of the cancer-amplified coactivator AIB3 results in defective placentation and embryonic lethality. J Biol Chem 277:45356–45360 [DOI] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C 1998 Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125:753–765 [DOI] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM 2002 Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA 99:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Sadovsky Y, Shalom-Barak T 2008 PPAR signaling in placental development and function. PPAR Res 2008:142082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom-Barak T, Nicholas JM, Wang Y, Zhang X, Ong ES, Young TH, Gendler SJ, Evans RM, Barak Y 2004 Peroxisome proliferator-activated receptor γ controls Muc1 transcription in trophoblasts. Mol Cell Biol 24:10661–10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Jia Y, Nye JS, Rao MS, Reddy JK 2000 Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J Biol Chem 275:14779–14782 [DOI] [PubMed] [Google Scholar]
- Wendling O, Chambon P, Mark M 1999 Retinoid X receptors are essential for early mouse development and placentogenesis. Proc Natl Acad Sci USA 96:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM 2000 Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA 97:10454–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J, Riethmacher-Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bösl MR, Wegner M 2000 Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol 20:2466–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Bützler C, Temme A, Traub O, Winterhager E, Willecke K 1998 Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol 140:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Enders AC 2004 Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol 2:46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Enders AC, Kunzle H, Oduor-Okelo D, Vogel P 2004 Placentation in species of phylogenetic importance: the Afrotheria. Anim Reprod Sci 82–83:35–48 [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD 1997 Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360 [DOI] [PubMed] [Google Scholar]
- Calzonetti T, Stevenson L, Rossant J 1995 A novel regulatory region is required for trophoblast-specific transcription in transgenic mice. Dev Biol 171:615–626 [DOI] [PubMed] [Google Scholar]
- Lescisin KR, Varmuza S, Rossant J 1988 Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev 2:1639–1646 [DOI] [PubMed] [Google Scholar]
- Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC 2008 Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 135:2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ 2000 Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 404:95–99 [DOI] [PubMed] [Google Scholar]
- Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, Thompson JC, Ostrowski MC, Rosol TJ, Woollett LA, Weinstein M, Cross JC, Robinson ML, Leone G 2003 Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421:942–947 [DOI] [PubMed] [Google Scholar]
- Simmons DG, Cross JC 2005 Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol 284:12–24 [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J 2005 Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132:2093–2102 [DOI] [PubMed] [Google Scholar]
- Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B 2002 Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 10:721–733 [DOI] [PubMed] [Google Scholar]
- Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K 1997 Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 138:3997–4004 [DOI] [PubMed] [Google Scholar]
- Shin BC, Suzuki T, Matsuzaki T, Tanaka S, Kuraoka A, Shibata Y, Takata K 1996 Immunolocalization of GLUT1 and connexin 26 in the rat placenta. Cell Tissue Res 285:83–89 [DOI] [PubMed] [Google Scholar]
- O'Malley BW 2007 Coregulators: from whence came these “master genes”. Mol Endocrinol 21:1009–1013 [DOI] [PubMed] [Google Scholar]
- Tien JC, Zhou S, Xu J 2009 The role of SRC-1 in murine prostate cancinogenesis is nonessential due to a possible compensation of SRC-3/AIB1 overexpression. Int J Biol Sci 5:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadra K, Anghel SI, Joye E, Tan NS, Basu-Modak S, Trono D, Wahli W, Desvergne B 2006 Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor β/δ. Mol Cell Biol 26:3266–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ 2000 Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ). Mol Cell Biol 20:5119–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Moon I, Han K 2004 Transcriptional cofactors exhibit differential preference toward peroxisome proliferator-activated receptors α and δ in uterine cells. Endocrinology 145:2886–2895 [DOI] [PubMed] [Google Scholar]
- Cho MC, Yoon HE, Kang JW, Park SW, Yang Y, Hong JT, Song EY, Paik SG, Kim SH, Yoon DY 2006 A simple method to screen ligands of peroxisome proliferator-activated receptor δ. Eur J Pharm Sci 29:355–360 [DOI] [PubMed] [Google Scholar]
- Peters JM, Gonzalez FJ 2009 Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta 1796:230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR 1996 Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol 16:4128–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Qiang L, Farmer SR 2006 Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBP β during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/ebp α gene promoter. J Biol Chem 281:7960–7967 [DOI] [PubMed] [Google Scholar]
- Louet JF, Coste A, Amazit L, Tannour-Louet M, Wu RC, Tsai SY, Tsai MJ, Auwerx J, O'Malley BW 2006 Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci USA 103:17868–17873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube DW, Schauberger CW 1982 Fetomaternal bleeding as a cause for “unexplained” fetal death. Obstet Gynecol 60:649–651 [PubMed] [Google Scholar]
- Pitkin RM 1987 Fetal death: diagnosis and management. Am J Obstet Gynecol 157:583–589 [DOI] [PubMed] [Google Scholar]
- Incerpi MH, Miller DA, Samadi R, Settlage RH, Goodwin TM 1998 Stillbirth evaluation: what tests are needed? Am J Obstet Gynecol 178:1121–1125 [DOI] [PubMed] [Google Scholar]
- Devi B, Jennison RF, Langley FA 1968 Significance of placental pathology in transplacental haemorrhage. J Clin Pathol 21:322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley AS, Killackey MA, Cederqvist LL 1983 Elevated maternal serum α-fetoprotein levels associated with breakdown in fetal-maternal-placental barrier. Am J Obstet Gynecol 146:859–861 [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J 2005 Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res 65:7993–8002 [DOI] [PubMed] [Google Scholar]
- Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J 2009 Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci USA 106:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J 2004 AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res 64:1875–1885 [DOI] [PubMed] [Google Scholar]
- Li Q, Chu MJ, Xu J 2007 Tissue- and nuclear receptor-specific function of the C-terminal LXXLL motif of coactivator NCoA6/AIB3 in mice. Mol Cell Biol 27:8073–8086 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.