Abstract
Transforming growth factor-β canceled the hepatocyte proliferation caused by transforming growth factor-α when the two substances were mixed and administered through a disconnected central portal vein branch after creation of an Eck fistula. In contrast, transforming growth factor-β had no antidotal action on the stimulatory effects of insulin or full test doses of insulinlike factor-2, hepatocyte growth factor, epidermal growth factor or triiodothymanine. A minor antidotal effect on hepatic stimulatory substance activity could be detected, but only with hepatic stimulatory substance was given in doses smaller than those known to cause maximum stimulatory response. These results suggest a highly specific pharmacological and physiological interaction between transforming growth factor-α and transforming growth factor-β in the modulation of liver growth control.
The Eck fistula (portacaval shunt) in dogs is a useful tool in the identification and study of hepatic growth-modulating substances, of which some of the most potent have little or no effect on hepatocytes in culture (1). This operation is followed by tripling of hepatocyte proliferation, atrophy of hepatocytes within 4 days to about half their original volume and establishment of a new stable state after 4 days (2). Growth stimulating substances, when infused into the tied-off central portal vein after this operation, augment further the heightened proliferation of the Eck fistula liver and prevent liver atrophy after creation of the fistula (1, 3–7). These effects have been termed hepatotrophic. In contrast, transforming growth factor-β (TGF-β), a known inhibitor of hepatocyte proliferation in vitro (8, 9), has the opposite effect in that it suppresses hepatocyte proliferation after creation of the Eck fistula and further reduces the size of the atrophied hepatocytes (1). The striking TGF-β effect is easily overridden with insulin (1).
In this study, we examined further the interaction of TGF-β with the five substances that were the most potently hepatotrophic in the Eck fistula model (1): insulin, insulinlike growth factor-2 (IGF-2), TGF-α, hepatocyte growth factor (HGF) and hepatic stimulatory substance (HSS). In addition, the interaction of TGF-β was tested with other molecules: epidermal growth factor (EGF), which is highly stimulatory in vitro (10) but not in vivo; and triiodothymanine (T3), which causes feeble stimulation only in the in vivo system (1, 11). The results revealed a strong, although not absolute, specificity of the TGF-α interactions with TGF-β.
MATERIALS AND METHODS
The Experimental Model
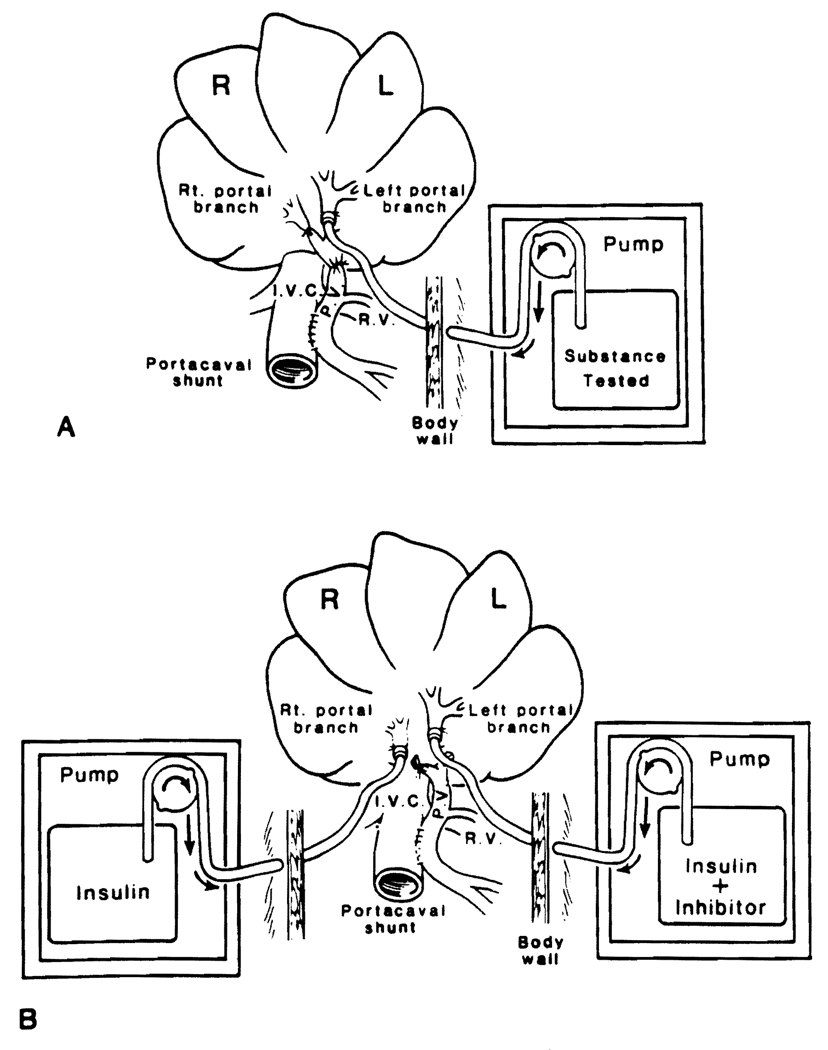
Details of the experimental model have been described (1, 3, 4). In experiments testing a single drug, the left portal vein was used for infusion (Fig. 1A) and the right portal vein was ligated. For double infusions, catheters were placed in both main portal branches and connected to separate infusion pumps (Fig. 1B). The dogs were active after surgery and appeared clinically well. They were fed sugar water ad libitum on the day of surgery and a regular diet thereafter.
FIG. 1.
Eck fistula model for postoperative infusion into the (A) left portal vein and (B) the left and right portal vein.
The pathological endpoints were the same as those previously described (1–7). Four days after creation of the portacaval shunt, 0.2 mCi/kg of intravenous [3H]thymidine was given (specific activity = 80 to 90 Ci/mmol; Du Pont–New England Nuclear, Boston, MA). Two hours later, while dogs were under sodium phenobarbital anesthesia, specimens were taken from the left and right lobes of the liver and fixed in 10% normal buffered formalin. At autopsy, after each dog was killed with an overdose of anesthesia, the patency of the anastomosis and the correct position of the catheter tip were confirmed. The liver tissue was processed and stained with hematoxylin and eosin.
Autoradiography was carried out with Kodak NTB2 liquid emulsion (Eastman Kodak Co., Rochester, NY) with an exposure time of at least 30 days. The number of replicating hepatocytes as an index of hepatocyte regeneration was determined by counting the number of [3H]thymidine-labeled nuclei per 1,000 hepatocytes. The sizes of individual hepatocytes (index of hypertrophy or atrophy) were determined by tracing the outlines of at least 500 midzonal liver cells projected onto standard-thickness paper, cutting out the individual silhouettes and weighing them. This method has been shown to be accurate in determining hepatocyte size and has been validated by planimetry and studies of unicellular organisms, the sizes of which have been determined directly (12).
In control experiments (data not shown), we established that the vehicle used for the test substances is inert. Each animal served as its own control because the cell replication and cell sizes in the directly infused liver lobes could be compared with these parameters in the contralateral lobes that were not infused or infused with different solutions. In addition, we used as fresh controls animals not subjected to surgery and animals with Eck fistula only. Animals from the group of Eck fistula controls were killed on days 1 to 4 after surgery, enabling us to note the pace of change and the difference in changes of different liver lobes (Table 1).
TABLE 1.
Hepatocyte size and autoradiographic labeling
| Group | No. of dogs |
Days after PCS |
TGF-β dose (ng/kg/day) |
No. of labeled hepatocytes per 1,000 hepatocytesa |
Cell size unitsa |
||
|---|---|---|---|---|---|---|---|
| Left lobe | Right lobe | Left lobe | Right lobe | ||||
| 1 | 2 | No surgery | – | 1.55 ± 0.18 | 1.55 ± 0.08 | 0.161 ± 0.002 | 0.160 ± 0.003 |
| 2 | 3 | 1 | – | 1.43 ± 0.43 | 1.36 ± 0.4 | 0.192 ± 0.02 | 0.192 ± 0.023 |
| 3 | 3 | 2 | – | 6.33 ± 0.82a | 6.26 ± 0.64b | 0.134 ± 0.008b | 0.134 ± 0.009b |
| 4 | 3 | 3 | – | 5.03 ± 0.27b | 4.96 ± 0.3b | 0.128 ± 0.009b | 0.130 ± 0.008b |
| 5 | 4 | 4 | – | 4.4 ± 0.3b | 4.2 ± 0.3b | 0.095 ± 0.01b | 0.096 ± 0.015b |
| 6 | 3 | 4 | 5 | 2.4 ± 0.5c, d | 4.6 ± 0.8 | 0.064 ± 0.02c, d | 0.103 ± 0.01 |
| 7 | 2 | 4 | 50 | 2.1 ± 0.05c, d | 4.9 ± 0.7 | 0.071 ± 0.006c, d | 0.106 ± 0.004 |
PCS = portacaval shunt.
Group 1: dogs that did not undergo surgery; groups 2 to 5: dogs 1 to 4 days after PCS; groups 6 and 7: dogs with PCS and 4-day continuous infusion of TGF-β into the left branch of the portal vein.
Data expressed as mean ± S.D.
p < 0.001 vs. groups 1 and 2.
p < 0.01 vs. own right lobe.
p < 0.05 vs. left lobe of group 5.
Growth Stimulators Tested
The hormones we used to stimulate growth were semisynthetic human insulin (E.R. Squibb & Sons, Inc., Princeton, NJ) and T3 (Sigma Chemical Co., St. Louis, MO). Growth stimulating factors used were HSS produced in our laboratory (13); recombinant HGF (also called hematopoietin [14]), generously donated by Dr. George Michalopoulos of the University of Pittsburgh; IGF-2 and EGF (Collaborative Research, Inc., Bedford, MA); and TGF-α, (Peninsula Laboratories, Inc., Belmont, CA).
Growth Factor Inhibitors Tested
TGF-β (Collaborative Research, Inc., Bedford, MA) was infused alone (Fig. 1A) or mixed with selected growth stimulating factors (Fig. 1B) and infused into one of the branches of the portal vein.
Statistical Analysis
Data are reported as mean ± S.D. Student’s one-tailed t test was used to determine the significance of differences. A p value less than 0.05 was considered significant.
RESULTS
Controls
The day-to-day changes in the liver after creation of Eck fistula were the same as those reported previously (1–4) with rapid atrophy that was equal in the right and left lobes. We saw a nearly threefold increase in cell mitosis (Table 1).
TGF-β Infusion
At a TGF-β dose of 5 ng/kg/day, the normal proliferative response of 1.5 to 4.5 labeled hepatocytes/1,000 hepatocytes was cut to about 2.2 labeled hepatocytes/1,000 hepatocytes in the TGF-β-infused left liver lobes; in the uninfused right lobes we found a full proliferative effect. In addition, the hepatocytes were significantly more atrophied in the left lobes than in the uninfused right lobes (Table 1).
TGF-β Plus Growth Stimulators
As previously reported (1), a strong hepatotrophic response was evident in the lobes infused only with insulin, IGF-2, HSS, HGF or TGF-α. Modest responses to T3 and EGF were seen (Table 2). TGF-β at a dose 10 times greater than that shown to be antihepatotrophic in the control experiments documented in Table 1 caused no reduction in these responses except for those induced by TGF-α (Table 2).
TABLE 2.
Hepatocyte size and autoradiographic labeling after continuous infusion of growth stimulating factor into the right portal vein branch and infusion of growth stimulating factor plus TGF-β (50 ng/kg/day) into the left portal infusion of branch
| No. of labeled hepatocytes per 1,000 hepatocytesa |
Cell size unitsa |
||||||
|---|---|---|---|---|---|---|---|
| Group | No. of subjects |
Stimulating substances |
Dose (ng/kg/day) |
Left lobe | Right lobe (no inhibitor) |
Left lobe | Right lobe (no inhibitor) |
| 1 | 3 | Insulin | 0.16 | 15.0 ± 0.8 | 14.9 ± 0.4 | 0.187 ± 0.018 | 0.165 ± 0.01 |
| 2 | 1 | IGF-2 | 50 | 11.0 | 10.3 | 0.148 | 0.1477 |
| 3 | 2 | HSS | 50 | 13.8 ± 0.2 | 13.3 ± 0.15 | 0.142 ± 0.006 | 0.143 ± 0.004 |
| 4 | 1 | HGF | 50 | 11.3 | 11.2 | 0.164 | 0.163 |
| 5 | 2 | T3 | 100 | 7.4 ± 0.65 | 7.3 ± 0.8 | 0.126 ± 0.016 | 0.127 ± 0.013 |
| 6 | 2 | EGF | 150 | 5.15 ± 0.2 | 5.05 ± 0.05 | 0.119 ± 0.001 | 0.120 ± 0.001 |
| 7 | 2 | TGF-α | 50 | 3.4 ± 0.25b | 12.6 ± 0.5 | 0.082 ± 0.006b | 0.159 ± 0.002 |
Data expressed as mean ± S.D.
p < 0.001.
TGF-β completely eliminated the TGF-α actvity. In addition, it suppressed the normally occurring proliferative response characteristic of Eck fistula and aggravated hepatocyte atrophy (Table 2).
Dose-response Relationship of HSS vs that of TGF-β
Although 50 ng/kg/day HSS still had its full hepatotrophic effect after mixing with 50 ng/kg/day TGF-β, smaller HSS doses of 25 and 10 ng were partly or completely inactivated after mixing with the fixed large dose of TGF-β (Table 3).
TABLE 3.
Hepatocyte size and autoradiographic labeling after continuous infusion of HSS into the right portal vein branch and HSS plus TGF-β (50 ng/kg/day) into the left portal vein branch
| No. of labeled hepatocytes per 1,000 hepatocytesa |
Cell size units |
|||||
|---|---|---|---|---|---|---|
| Group | No. of subjects |
HSS (ng/kg/day) |
Left lobe | Right lobe (no inhibitor) |
Left lobe | Right lobe (no inhibitor) |
| 1 | 1 | 10 | 2.3 | 7.5 | 0.093 | 0.141 |
| 2 | 1 | 25 | 5.8 | 9.2 | 0.104 | 0.153 |
| 3 | 2 | 50 | 13.0 ± 0.2 | 13.3 ± 0.15 | 0.142 ± 0.006 | 0.143 ± 0.004 |
Data expressed as mean ± S.D.
DISCUSSION
TGF-β, a 25,000-Da homodimeric peptide, is known to be a powerful in vitro inhibitor of various cell types (15, 16), including hepatocytes stimulated with EGF and TGF-α (9). During the liver regeneration that reaches its peak 24 hr after partial hepatectomy in rats, the tissue TGF-β level increases significantly, but not until after this peak of regeneration is reached. Thereafter the TGF-β concentration rises four to eight times by 72 hr after hepatectomy and then recedes to the control level by 96 hr. Such findings have prompted speculation that TGF-β modulates liver regeneration by counteracting the growth stimulation of TGF-α, EGF and other regeneration-promoting factors (17).
In contrast to the classic “burst” of liver regeneration after partial hepatectomy, the heightened cell renewal after creation of the Eck fistula builds to a new steady state within 4 days after surgery and remains constant thereafter. In this investigation it was demonstrated that 5 ng/kg/day TGF-β prevented the increase in hepatocyte proliferation invariably seen in the Eck fistula liver. It also worsened the characteristic hepatocyte atrophy.
In subsequent experiments, 50 ng/kg/day TGF-β was infused to simulate the high concentrations of endogenous TGF-β seen during regeneration after hepatectomy. These high concentrations are considered the result of the development of resistance to TGF-β (18). In the 4 days after creation of the Eck fistula, the larger dose of TGF-β (50 ng/kg/day) abrogated the stimulation usually caused by TGF-α Because the reactivity to TGF-α alone was fully retained in the contralateral lobes, it appeared that TGF-β was inactivated almost completely with one pass through the liver. Under the same conditions of testing, all of the other potent hepatotrophic factors that were examined (insulin, IGF-2, HGF, HSS, T3 and EGF) were unaffected by mixing and coadministration with TGF-β.
These data indicate that growth-stimulating factors of hepatocytes can be divided into two groups: those resistant to modulation by TGF-β and those whose proliferation-inducing qualities are dramatically reduced or even canceled by TGF-β. However, it should also be emphasized that the foregoing classification of hepatic stimulatory factors according to their reaction to coadministration with TGF-β depends in part on dose selection. This was demonstrated when HSS (which is usually unaffected by TGF-β) was given in reduced quantities (10 or 25 ng/kg/day) that by themselves evoked minimum proliferative responses. Under these circumstances, the minor HSS-induced response could be eliminated with TGF-β.
Further discussion of these findings does not seem warranted. However, we do emphasize the seemingly high degree of specificity of the TGF-β/TGF-α interaction. Even EGF, which ostensibly has the same binding sites as TGF-α (19), was not affected by TGF-β. This pharmacological specificity is consonant with a critical role of the balance of these growth factors in the control of cell proliferation, as has been proposed by others (20, 21).
Acknowledgment
We thank John Prelich for lending us his experience and technical assistance.
This work was aided by grant no. DK 29961 from the National Institutes of Health.
REFERENCES
- 1.Francavilla A, Starzl TE, Porter K, Scotti-Foglieni C, Michalopoulos GK, Carrieri G, Trejo J, et al. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck fistula. Hepatology. 1991;14:665–670. doi: 10.1016/0270-9139(91)90055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:688–767. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Jones AF, Terblanche J, Usui S, Porter KA, Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Porter KA, Scotti-Foglieni CL, Venkataramanan R, Makowka L, Rossaro L, Francavilla A, et al. The hepatotrophic influence of cyclosporine. Surgery. 1990;107:533–539. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Porter KA, Mazzaferro V, Todo S, Fung J, Francavilla A. Hepatotrophic effects of FK 506 in dogs. Transplantation. 1991;51:67–70. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Schrieber SL, Albers MW, Porter KA, Foglieni CS, Francavilla A. Hepatotrophic properties in dogs of human FKBP, the binding protein for FK 506 and rapamycin. Transplantation. 1991;52:751–753. doi: 10.1097/00007890-199110000-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun L, Mead JE, Panzica M, Mikumo R, Bell GI, Fausto N. Transforming growth factor B mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Cell Biol. 1988;85:1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type B transforming growth factor. Cancer Res. 1986;46:2330–2334. [PubMed] [Google Scholar]
- 10.Richman RA, Claus TH, Pilkis SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult hepatocyte. Proc Natl Acad Sci USA. 1976;73:3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Short JA, Brown RF, Husakova A, Gilbertson J, Zemel R, Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972;247:1757–1766. [PubMed] [Google Scholar]
- 12.Starzl TE, Francavilla A, Halgrimson CG, Francavilla FR, Porter KA, Brown TH, Putnam CW. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 13.Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Rose J, Van Thiel DH, et al. Extraction and partial purification of hepatic stimulatory substance in rats, mice and dogs. Cancer Res. 1987;47:5600–5605. [PMC free article] [PubMed] [Google Scholar]
- 14.Michalopoulos B, Houck KA, Dolan ML, Lvetteke NC. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- 15.Roberts AB, Sporn MH. Transforming growth factors. Cancer Surv. 1985;4:683–705. [PubMed] [Google Scholar]
- 16.Goustin AS, Leof EB, Shipley GD, Moses HL. Growth factors and cancer. Cancer Res. 1986;46:1015–1029. [PubMed] [Google Scholar]
- 17.Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an endocrine mechanism. Proc Natl Acad Sci USA. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houck KA, Michalopoulos GK. Altered responses of regenerating hepatocytes to norepinephrine and transforming growth factor type beta. J Cell Physiol. 1989;141:503–509. doi: 10.1002/jcp.1041410308. [DOI] [PubMed] [Google Scholar]
- 19.Gruppuso PA, Mead E, Fausto N. Transforming growth factor receptors in liver regeneration following partial hepatectomy in the rat. Cancer Res. 1990;50:464–469. [PubMed] [Google Scholar]
- 20.Fausto N. Hepatology: a textbook of liver disease. 2nd ed. Philadelphia: W.B. Saunders Co; 1990. pp. 49–64. [Google Scholar]
- 21.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176–187. [PubMed] [Google Scholar]