Abstract
Hedgehog (Hh) signaling and laminin-111, a basement membrane protein, are required for early muscle development. Hh signaling specifies different populations of muscle fibers and laminin-111 is critical for early muscle morphogenesis. However, additional requirements for Hh signaling and laminin during later phases of muscle development are not known. Furthermore, interactions between Hh signaling and laminin in this context are unknown. We used laminin gamma1 mutant zebrafish and cyclopamine to block Hh signal transduction separately and in combination to investigate their functions and interactions. We found that both Hh signaling and laminin are required for normal myosin chain expression. In addition, Hh signaling and laminin act synergistically during fast-twitch fiber elongation: fast muscle cells do not elongate in embryos deficient for both Hh signaling and laminin. Finally, we present evidence which suggests that Hh signaling is indirectly required via slow fiber specification for recovery of fast fiber elongation in laminin gamma1 mutant embryos.
INTRODUCTION
Vertebrate skeletal muscle is derived from transient, segmentally reiterated structures called somites. Muscle specification requires Myogenic Regulatory Factors (MRFs) of the Myod family: Myod, Myf5, and Mrf4. Although mice that harbor mutations in one MRF are viable, no skeletal muscle forms in mice that lack function of all three MRFs (Kassar-Duchossoy et al., 2004; Kaul et al., 2000; Rudnicki et al., 1992; Rudnicki et al., 1993). Recent analysis of Myod, Myf5, Mrf4, and myogenin function in the zebrafish model has shown that the requirement for specific MRFs is different in distinct cell lineages, but there is some redundancy (Hinits et al., 2009). These results highlight the complexity of muscle specification as well as the robustness of skeletal muscle development.
Musculoskeletal differentiation involves multiple morphogenetic processes. Some of these processes, in a rough approximation of temporal progression, are: (1) elongation of initially short muscle precursor cells, (2) fusion to generate multinucleate myotubes, (3) assembly of contractile myofibrils, (4) secretion and assembly of the extracellular basement membrane, (5) attachment to the basement membrane that surrounds individual muscle cells, as well as the basement membrane at tendons and myotendinous junctions (MTJs), and (6) dynamic maintenance of muscle structure.
Interestingly, both the cellular and molecular mechanisms underlying elongation of the first muscle fibers in chick and the first fast-twitch muscle fibers in zebrafish are similar. In chick, cells delaminate from the overlying dermomyotome and migrate into the forming myotome at which point they elongate prior to fusion (Gros et al., 2004). Time-lapse analysis of elongating myocytes shows that they exhibit extensive protrusive activity. Filopodia form in all directions, but the elongation in the anterior-posterior axis is driven by planar cell polarity signaling and polarized lamellipodia (Gros et al., 2009). Similarly, time-lapse analysis and mathematical modeling of zebrafish myocyte elongation shows that a two-step process of protrusion extension and filling mediates elongation in the anterior-posterior axis, and that the extracellular matrix protein laminin is required for normal orientation (Snow et al., 2008).
As major constituents of the basement membrane, hetereotrimeric laminins are critical for early muscle development. Adhesion to laminin via Integrin alpha6beta1 is required for efficient elongation of muscle precursor cells in mice (Bajanca et al., 2006). In zebrafish, laminin beta1 (lam beta1) and laminin gamma1 (lam gamma1) are required for efficient muscle cell elongation. However, muscle cells eventually recover and elongate in lam beta1 and lam gamma1 mutant embryos (it is not known if elongation recovers in the mouse) (Snow et al., 2008). The eventual recovery of fast muscle cell elongation in lam beta1 and gamma1 mutant zebrafish embryos implies the existence of a latent secondary mechanism for fast muscle morphogenesis. An understanding of this mechanism will shed light on developmental robustness and lead to a more comprehensive understanding of muscle morphogenesis.
Multiple reciprocal interactions occur during musculoskeletal development and may contribute to developmental robustness. These interactions are observed at large (muscle-tendon) and small (transcriptional compensation) size scales. On a large scale, muscle and tendon development are spatiotemporally correlated and the formation of tendon progenitor cells is dependent upon the presence of the early myotome in amniotes (Brent et al., 2005; Brent et al., 2003; Brent and Tabin, 2002). One example of transcriptional compensation is observed in Congenital Muscular Dystrophy 1A (MDC1A), caused by mutations in laminin alpha2. In MDC1A, there is a partial increase in laminin alpha4 synthesis and a decrease in amounts of two laminin receptors, Integrin alpha7beta1 and the dystroglycan complex (DGC) (Bentzinger et al., 2005; Kortesmaa et al., 2000; Moll et al., 2001; Patton et al., 1997; Ringelmann et al., 1999; Vachon et al., 1997). Unfortunately, increased laminin alpha4 does not fully compensate for the lack of laminin alpha2 because laminin alpha4 doesn’t self-polymerize or bind to the DGC or Integrin alpha7beta1 with high affinity (Kortesmaa et al., 2000; Talts et al., 2000). It is possible that such interactions at multiple size scales may contribute to the phenotypic complexity of many congenital myopathies that vary in severity and age of onset (Babovic-Vuksanovic et al., 2005; Biancheri et al., 2006; Pace et al., 2008; Uro-Coste et al., 2009). Thus, elucidating how interactions between different morphogenetic processes contribute to phenotypic complexity and developmental robustness is critical to further our understanding of musculoskeletal development and functional physiology.
We aimed to elucidate mechanisms underlying the recovery of muscle cell elongation in lam gamma1 mutant embryos. We asked whether Hh signaling was required for elongation recovery in lam gamma1 mutant embryos. Hh signaling is indirectly required for efficient fast-twitch muscle elongation during zebrafish muscle morphogenesis. Hh signaling specifies slow-twitch muscle fibers (Barresi et al., 2000; Blagden et al., 1997; Du and Dienhart, 2001; Henry and Amacher, 2004; Roy et al., 2001), which are necessary and sufficient for efficient fast-twitch muscle cell elongation (Henry and Amacher, 2004). We find that laminin and Hh signaling play distinct and cooperative roles during different aspects of muscle development. Muscle differentiation is slightly abnormal in embryos deficient for either/both Hh signaling and laminin. Laminin is required for timely MF20 and F310 expression and Hh signaling is required for maintenance of the normal anterior-posterior gradient of MF20 and F310 expression. Significantly, we show that Hh signaling and laminin act synergistically in fast-twitch muscle cell elongation. We suggest that Hh signaling is indirectly (through slow muscle specification) required for the recovery of fast-twitch muscle cell elongation in lam gamma1 mutant embryos. Taken together, these results show a novel interaction between Hh signaling and laminin during early muscle development.
RESULTS
Segments Form in Embryos Deficient for both Hh Signaling and Laminin
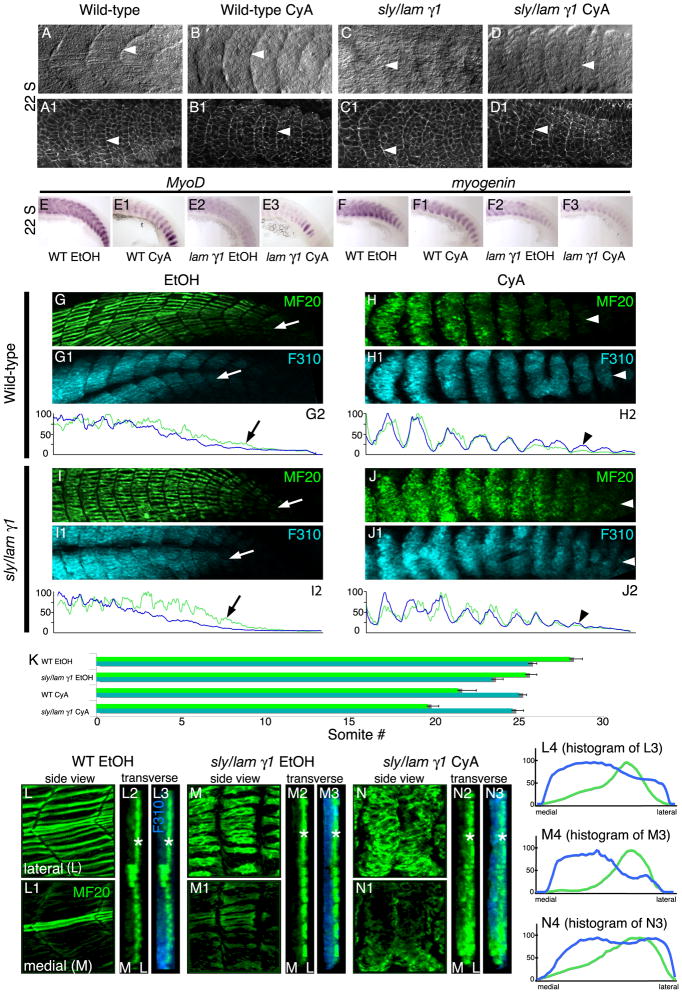
Most of the musculature is derived from somites, reiterated segments that form along the anterior-posterior axis. Neither Hh signaling nor laminin are required for initial somite formation (Barresi et al., 2000; Parsons et al., 2002; van Eeden et al., 1996; Wiellette et al., 2004). However, it is not known whether somites form in the absence of both laminin and Hh signaling. Because axial skeletal muscle is derived from somites, it is necessary to determine whether somites form prior to elucidating potential disruptions of muscle morphogenesis. Somites are visible with DIC imaging in wild-type embryos at the 22-somite stage (Fig. 1A, white arrowhead). Staining for β-catenin to outline cells also allows visualization of somites (Fig. 1A1, white arrowhead). Hh signaling was disrupted with cyclopamine (CyA). CyA blocks the action of Smoothened, a membrane receptor essential for Hh signal transduction (Chen et al., 2002; Ingham and McMahon, 2001). Somites form in CyA-treated embryos (Fig. 1B, B1, white arrowheads) and in lam gamma1 mutant embryos (Fig. 1C, C1, white arrowheads). Somites also form in CyA-treated lam gamma1 mutant embryos (Fig. 1D, white arrowhead). β-catenin staining shows that, although cells are slightly more disorganized than in wild-type embryos, segments still form (Fig. 1D1, white arrowhead). The expression of segmentally reiterated somite patterning genes such as her1, deltaD, and papc in the presomitic mesoderm is also normal in CyA-treated lam gamma1 mutant embryos (data not shown). Taken together, these data indicate that somites form in embryos deficient for both laminin and Hh signaling and that failure of somitogenesis is not responsible for subsequent defects in morphogenesis.
Fig. 1.
Somite formation, muscle specification, and muscle differentiation proceed in embryos deficient for both Hh signaling and laminin gamma1. A-J: Side views, anterior left, dorsal top. A-F3: 22-somite stage embryos. Lettered panels A-D are DIC images. Numbered panels A1-D1 are stained for β-catenin (white) to outline cells. White arrowheads point to somite boundaries. Note that somite boundaries form in the absence of both laminin and Hh signaling (D, D1). E-F3: In Situ Hybridizations showing MyoD and myogenin expression in wild-type and lam gamma1 mutant embryos treated with EtOH or CyA. Segmental expression of MyoD and myogenin persists. G-J: Laminin and Hh signaling are required for proper anterior-posterior myosin distribution. Lettered panels show MF20 (light meromyosin) in green. Panels numbered 1 show F310 (myosin light chain) in blue. Panels numbered 2 are histograms that correlate with the images above. The posterior expression domains are indicated by white arrows for EtOH-treated embryos and white arrowheads for CyA-treated embryos. G-G2: EtOH-treated wild-type embryos. MF20 is expressed more posteriorly than F310. H-H2: CyA-treated wild-type embryos. MF20 is not expressed more posteriorly than F310. I-I2: Similar to EtOH-treated wild-type embryos, MF20 is expressed in younger somites than F310 in EtOH-treated lam gamma1 mutant embryos. J-J2: The same shift in myosin chain expression as seen in CyA-treated wild-type embryos is observed in CyA-treated lam gamma1 mutant embryos. K: Graph showing the average posterior limits of MF20 and F310 expression (error bars reflect standard error). L-N3: The lateral-medial pattern of MF20 expression is preserved in CyA-treated embryos. Lettered panels (lateral focal planes) and panels numbered 1 (medial focal planes) are side views, anterior left, dorsal top. Panels numbered 2 and 3 are 3-D reconstructed, transverse views, medial left, lateral right. MF20 expression (white *) is strongest laterally in all embryos (note the lateral peak of MF20 expression in histograms numbered 4).
Muscle is Specified in Embryos Deficient for both Hh Signaling and Laminin
The expression of MRFs by muscle precursor cells is the first overt indication of muscle specification. During zebrafish development, MyoD-mediated expression of myogenin plays a significant role in development of slow-twitch fibers, medial fast-twitch fibers, and lateral fast-twitch fibers (Hinits et al., 2009). We find that both MyoD and myogenin are segmentally expressed in 22-somite stage CyA-treated lam gamma1 mutant embryos (Fig. 1E3, F3). With regards to expression levels, we find that expression levels of MyoD and myogenin are varible in CyA-treated embryos, lam gamma1 mutant embryos, and CyA-treated lam gamma1 mutant embryos. Thus, the only conclusion we can draw from these data is that the muscle differentiation program initiates in CyA-treated lam gamma1 mutant embryos.
Both Hh Signaling and Laminin are Required for the Normal Anterior-Posterior Distribution of Myosin Chains
The development of contractile muscle fibers requires assembly of sarcomeres, the fundamental units of contraction. Myosins are major components of sarcomeres and convert chemical energy to mechanical force during muscle contraction. The importance of myosins in muscle physiology is highlighted by the subset of congenital myopathies called “myosin myopathies” (Oldfors, 2007). We assessed myosin expression in CyA-treated lam gamma1 mutant embryos with two different antibodies. MF20 recognizes light meromyosin and is a pan-muscle myosin marker (Bader et al., 1982). F310 is an anti-myosin light chain antibody that labels fast-twitch muscle myosins in zebrafish (Hamade et al., 2006). Initiation of MF20 and F310 expression during zebrafish development is heterochronic (Maves et al., 2007). MF20 labeling initiates in younger (more posterior) somites and is typically observed 5 somites more posterior than F310 labeling at 24 hours post fertilization (hpf) (Maves et al., 2007). The difference between MF20 and F310 labeling is observed both in micrographs and when normalized fluorescence intensity is plotted (see methods). In wild-type embryos, relatively high MF20 expression is maintained more posteriorly than high F310 expression (Fig. 1G-G2, the histograms correlate with the images above them). The average posterior limits of expression for both MF20 and F310 also reflect this pattern (Fig. 1K, see methods).
The relatively earlier initiation of MF20 expression is preserved in lam gamma1 mutant embryos (Fig. 1I-I2, note the higher levels of MF20 expression relative to F310 in the posterior). There is a small delay in both MF20 and F310 labeling in lam gamma1 mutant embryos compared to wild-type embryos (Fig. 1K). This delay correlates with the delay in muscle morphogenesis in lam gamma1 mutant embryos.
Hh signaling is required for slow-twitch muscle differentiation (Barresi et al., 2000; Blagden et al., 1997; Du et al., 1997). The pan-muscle marker MF20 is highly expressed in slow-twitch fibers, which differentiate prior to fast-twitch muscle fibers (Devoto et al., 1996). Thus, it is not surprising that CyA treatment shifts the relative posterior limits of MF20 and F310 expression domains. In CyA-treated wild-type embryos, F310 labeling is found in younger (more posterior) somites than MF20 labeling (Fig. 1H-H2). The relative expression levels of MF20 and F310 are more closely correlated throughout the anterior-posterior axis than in wild-type embryos (Fig. 1H2).
F310 and MF20 are expressed in CyA-treated lam gamma1 mutant embryos. Similar to CyA-treated embryos, F310 is expressed in younger somites than MF20 (Fig. 1J-J2). These data show that fast-twitch muscle differentiation occurs in embryos lacking laminin and/or Hh signaling. Thus, subsequent disruptions of morphogenesis cannot be attributed to a failure of myosin expression. However, laminin is required for efficient expression of both myosin chains and Hh signaling is required for timely expression of MF20.
Slow-Twitch Fibers are not Necessary for the Lateral-Medial Gradient of MF20 Expression
MF20 labeling at 24 hpf is strongest in the superficial slow-twitch fibers that are developmentally older than medial fast-twitch fibers (Fig. 1L). This lateral-medial gradient of MF20 is clear in 3-dimensionally (3-D) projected transverse views (Fig. 1L2, L3, MF20 green, F310 blue). Graphs of fluorescence intensity of the 3-D transverse views also illustrate this gradient (Fig. 1L4). The medial-lateral gradient of MF20 is preserved in lam gamma1 mutant embryos, in which slow-twitch muscle fibers differentiate (Wiellette et al., 2004) (Fig. 1M). Surprisingly, the gradient is also preserved in CyA-treated wild-type (data not shown) and CyA-treated lam gamma1 mutant embryos (Fig. 1N). Thus, despite the delay in MF20 expression in CyA-treated embryos, these data indicate that neither slow-twitch fibers nor Hh signaling are required for the lateral-medial initiation of MF20 expression. Taken together with data showing that a medial-lateral degradation of Fn proceeds in Hh signaling-deficient embryos (Snow and Henry, 2009), these data indicate that Hh signaling and slow-twitch muscle fibers are not required for dynamic morphogenetic changes that occur in the medial-lateral axis.
Hh Signaling and Laminin Function Synergistically during Fast-Twitch Muscle Morphogenesis
Slow-twitch muscle fibers in zebrafish are a unique population of cells. Multiple lines of evidence suggest that these cells impact morphogenesis of both the adjacent fast-twitch fibers and the MTJ. Slow-twitch fiber migration is both necessary and sufficient to trigger fast-twitch fiber elongation. Slow-twitch fiber migration also correlates with changes in the MTJ microenvironment. Finally, it has been hypothesized that slow-twitch fibers play a significant role in the recovery of segmentation, via MTJ formation, in Notch pathway mutants with disrupted initial somite formation (van Eeden et al., 1998).
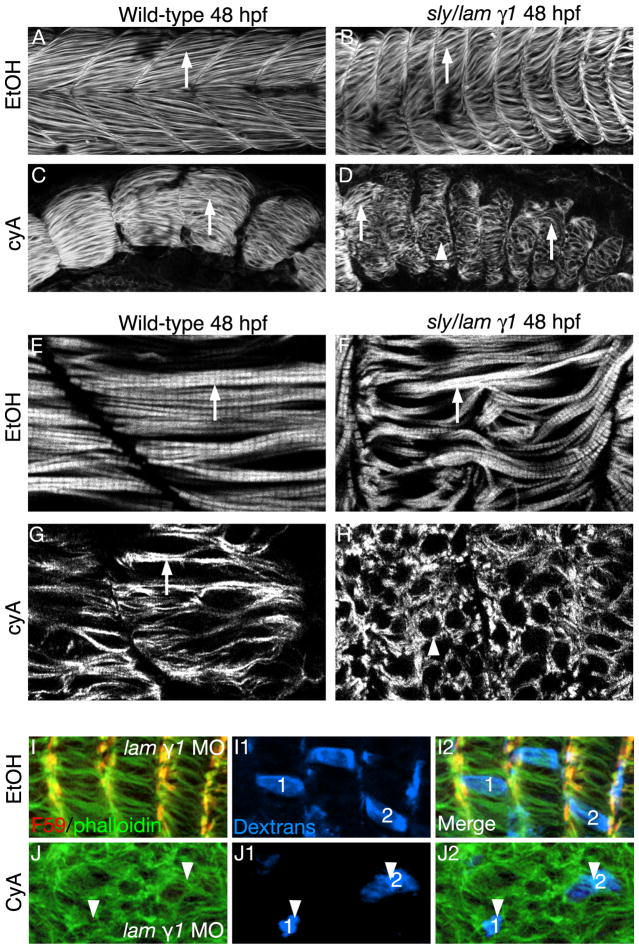
We asked whether slow-twitch fibers play a role in the recovery of fast-twitch fiber elongation in lam gamma1 mutant embryos. Although fast muscle cell elongation is delayed in embryos deficient for either Hh signaling or lam gamma1, elongated fibers form by 26 hpf and are clearly visible by 48 hpf (Fig. 2B, C, F, G). In contrast, fiber elongation is disrupted in CyA-treated lam gamma1 mutant embryos. Although some cells, elongate (Fig. 2D, arrows), most cells are short and rounded (Fig. 2D, H, arrowheads). The rounded cells observed in CyA-treated lam gamma1 mutant embryos could either indicate: (1) disruption in fiber elongation, or (2) misoriented long fibers. We obtained a “scatter label” of dextran filled cells by injecting dextrans into the yolk close to the blastula at the 512-1k cell stage of embryos that had been injected with lam gamma1 morpholinos at the one cell stage. Projections of dextran filled cells show that round cells are indeed short (Fig. 2J1, J2, arrowheads). Thus, Hh signaling is necessary for the normal recovery of fast-twitch fiber elongation in lam gamma1 mutant embryos.
Fig. 2.
Laminin and Hh signaling act synergistically in fast-twitch muscle cell elongation. Side views, anterior left, dorsal top, 48 hpf embryos stained with phalloidin to visualize actin. White arrows point to elongated fast-twitch muscle fibers. White arrowheads points to short, round fast-twitch muscle cells. Panels E-H are higher magnification images from a different experiment than panels A-C. A-C, E-G: Fast-twitch fibers are long in wild-type, lam gamma1 mutant embryos, and CyA-treated wild-type embryos at 48 hpf. D, H: Fast-twitch cells are shorter and round in CyA-treated lam gamma1 mutant embryos. I, J: Three-dimensional projections of dextran-filled cells show highlight the short fast cells in CyA-treated laminin gamma1 mutant embryos.
Hh Signaling Likely Promotes Delayed Fast-Twitch Fiber Elongation in laminin gamma1 Mutant Embryos via Slow-Twitch Fiber Specification
The above data show that Hh signaling is required for delayed fast fiber elongation in lam gamma1 mutant embryos, but do not determine whether there is a direct or indirect requirement for Hh. One possibility is that Hh is indirectly required for delayed elongation. In this scenario, Hh signaling is required for slow-twitch fiber specification, which is in turn required for delayed fast-twitch fiber elongation. Alternatively, given that cell autonomous perception of Hh signaling promotes differentiation of fast-twitch cells from the dermomyotome (Feng et al., 2006), it is also possible that Hh signaling is directly required for delayed fast-twitch fiber elongation. We endeavored to distinguish between these two possibilities by treating with CyA at the 10-somite stage (as opposed to shield stage). It has been shown that commitment of slow-twitch muscle precursors occurs 4-6 hours prior to somite formation. Thus, slow-twitch muscle precursor cells in the anterior presomitic mesoderm are committed and do not require Hh signaling (Hirsinger et al., 2004). For example, slow-twitch muscle fibers are observed through somite 16 when embryos are treated at the 10-somite stage with CyA (Hirsinger et al., 2004). Late CyA treatment therefore allows one to determine when, and in which cells, perception of Hh signaling is required.
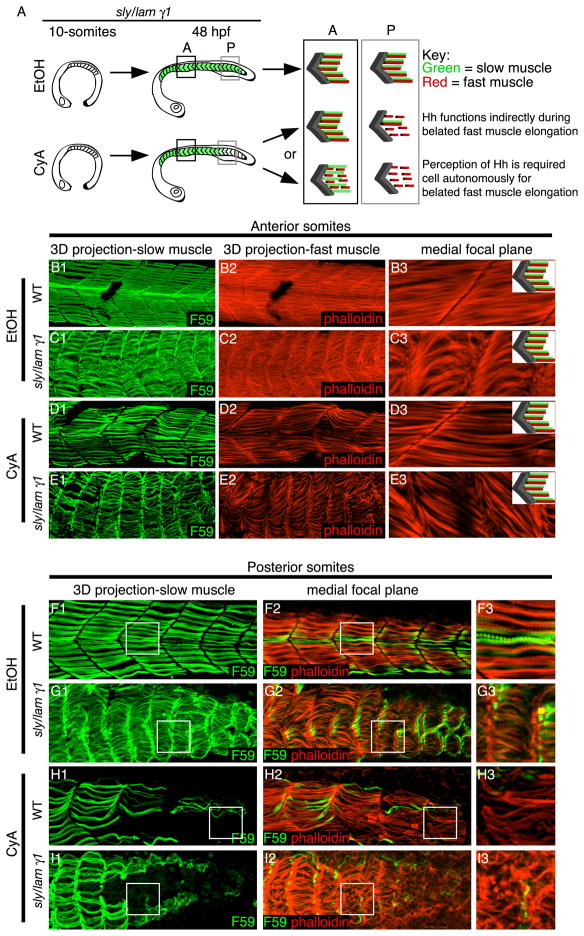
Fast-twitch muscle elongation in wild-type embryos does not begin until about 18 hpf (Roy et al., 2001). We treated lam gamma1 mutant embryos with CyA at the 10-somite stage so that Hh signaling would be inhibited long before fast fibers belatedly elongate in lam gamma1 mutant embryos. If cell autonomous perception of Hh signaling is required (direct requirement) for belated fast muscle cell elongation in lam gamma1 mutant embryos, then fast cells in CyA-treated lam gamma1 mutants would not elongate even when slow-twitch fibers are present (Fig. 3A). In contrast, if slow-twitch fibers mediate recovery (indirect requirement) in lam gamma1 mutant embryos, then the disruption of fast muscle cell elongation should correlate with progressive loss of slow-twitch muscle fibers in the posterior of embryos treated at 10-somites with CyA (Fig. 3A).
Fig. 3.
Hh signaling is required indirectly for the recovery of fast-twitch fibers in laminin mutant embryos. Panels B-I are side views, anterior left, dorsal top, 48 hpf embryos treated with EtOH or CyA at the 10-somite stage and stained with F59 (green) to visualize slow muscle fibers and phalloidin (red) to visualize actin. A: Cartoon describing expected results if Hh signaling is directly or indirectly required. B-E: Anterior somites are shown. Panels numbered 1 are 3-D projections. Panels numbered 3 are higher magnification views of individual focal planes. Note the presence of slow-twitch fibers and elongated fast fibers in all conditions. F-I: Posterior somites are shown. The boxes in panels numbered 1 and 2 correspond with the higher magnification medial focal plane shown in panels numbered 3. Slow-twitch fibers are present in EtOH-treated embryos (F, G) but are reduced and/or absent in posterior somites in CyA-treated embryos (H, I). Note that defects in fast fiber elongation correlate with the lack of slow fibers in the posterior of CyA-treated lam gamma1 mutant embryos (I).
Elongated fast-twitch fibers form medial to slow-twitch fibers in the anterior of late CyA-treated lam gamma1 mutant embryos (Fig. 3E). This result suggests that perception of Hh signaling is not cell autonomously required for recovery of fast cell elongation in lam gamma1 mutant embryos. The correlation of short fast cells with the loss of slow-twitch fibers in the posterior of CyA-treated lam gamma1 mutant embryos supports the above data (Fig. 3I). Thus, results from this experiment strongly suggest that slow-twitch muscle fibers play a significant role in mediating the recovery of fast-twitch muscle cell elongation in lam gamma1 mutant embryos.
Our data suggests that lam gamma1 and Hh signaling play synergistic roles during muscle morphogenesis. Initial fast-twitch muscle cell elongation is delayed in embryos deficient for either lam gamma1 or Hh signaling, but recovers by 26 hpf. The eventual recovery of fast muscle cell elongation in lam gamma1 mutant embryos implies the existence of a latent secondary mechanism underlying muscle fiber morphogenesis. Here, we show that fast-twitch muscle is specified in embryos deficient for both lam gamma1 and Hh signaling, but muscle morphogenesis is disrupted. We also suggest that Hh signaling is indirectly required, via slow-twitch muscle specification, to mediate recovery of fast muscle cell elongation in lam gamma1 mutant embryos. Thus, these results shed new light on an alternate mechanism of muscle morphogenesis. In addition, these results support the growing body of evidence indicating that slow-twitch muscle cells in zebrafish are an interesting population of cells that may have unique instructive properties.
EXPERIMENTAL PROCEDURES
Zebrafish Husbandry and Mutant Alleles
Zebrafish embryos were obtained from natural spawnings of adult fish kept at 28.5°C on a 16 h light/8 h dark cycle and were staged according to (Kimmel et al., 1995) The allele of sly/lam gamma1 used (sly ti263a) has previously been described (Parsons et al., 2002). Sly/lam gamma1 embryos were a generous gift from the Max Planck Institute for Developmental Biology Zebrafish genomics unit.
Morpholino Injections
Morpholino-modified antisense oligonucleotides (MOs) were synthesized by Gene-Tools, LCC. The lam gamma1 MO is as previously described: 5’-tgtgccttttgctattgcgacctc-3’ (Parsons et al., 2002). Morpholinos were dissolved to a stock concentration of 50 ng/nl in sterile water. Three nanoliters of MOs were injected into embryos at the 1–2 cell stage using an ASI pressure injector (ASI Systems).
CyA Treatment
Embryos were treated with CyA (Toronto Research Chemicals) to disrupt Hh signaling as previously described (Barresi et al., 2001). In brief, embryos were incubated in 100 μM CyA (with 1% EtOH) beginning at shield stage until fixation. Wild-types were treated with 1% EtOH. In each experiment, CyA-treated embryos had U-shaped somites and were partially or fully cyclopic. For late-CyA treatments, embryos were not treated until the 10-somite stage.
In Situ Hybridization and Immunocytochemistry
Whole mount in situ hybridization was performed as previously described (Jowett, 1999). The β-catenin antibody was obtained from Sigma (C7207, diluted 1:500), and the MF20 and F310 antibodies were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (diluted 1:10). The secondary antibodies were Alexa-conjugated (Invitrogen). Alexa Fluor 488, and 546 phalloidin were obtained from Molecular Probes (diluted 1:20). For antibody staining, embryos were fixed in 4% paraformaldehyde (PFA) for 4 hours at room temperature and incubated in block (5% BSA, 1% DMSO, 1% Triton X-100, 0.2% saponin in PBS) for 1 hour. Staining was conducted in PBDT (1% BSA, 1% DMSO, 1% Triton X-100 in PBS). For visualization of actin using phalloidin staining, embryos were fixed as above, followed by permeabilization in 2% Triton X-100/PBS for 1.5 hours and incubation in Alexa-Fluor 488 or 543 conjugated phalloidin (Invitrogen) for 1 hour. Embryos were then rinsed overnight prior to proceeding with antibody staining. We obtained a “scatter” label of cells that were filled with fluoro-ruby dextrans (Molecular Probes) by microinjecting dextrans into the yolk cell just below the blastoderm at the 512-1k cell stage.
Analysis of MF20 and F310 Expression
It has previously been shown that MF20 expression is initiated in younger (more posterior) somites than F310 expression (Maves et al., 2007). We analyzed MF20 and F310 expression in two different ways. The posterior limit of MF20/F310 expression shown in the graph (Fig. 2E) was qualitative and involved an individual judging the most posterior somite expressing myosin. The trends shown in the graph were consistent between two different individuals. The histograms presented in Fig. 2 panels numbered 2 are quantitative. For each panel, in order to control for varied exposure times, the maximum intensity for each myosin chain was determined and the values were all normalized to this intensity. Thus, what is shown is a “percentage of maximum intensity.”
Imaging
Images were acquired using a Zeiss ApoTome running on a Zeiss Axio Imager Z1. Embryos were mounted in 80% glycerol/20% PBS with 1.5 coverslips and visualized using the Plan-Apochromat 10X/0.45 and Plan-Apochromat 20X/0.8 objectives. Image quality was optimized by averaging 4-5 frames. Images were processed in Adobe Photoshop (only linear modifications to brightness/contrast were made) and collated in Adobe Illustrator.
Acknowledgments
We thank Ian McNulty, Robert Jones, and Emma Oster for contributing to the initial experiments. We thank members of the Henry lab, especially Michelle Goody, for critical reading of the manuscript. This work was supported by NIH grant RO1 HD052934.
References
- Babovic-Vuksanovic D, et al. Selective antibody immune deficiency in a patient with Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2005;28:181–6. doi: 10.1007/s10545-005-5515-3. [DOI] [PubMed] [Google Scholar]
- Bader D, et al. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–70. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajanca F, et al. Integrin alpha6beta1-laminin interactions regulate early myotome formation in the mouse embryo. Development. 2006;133:1635–44. doi: 10.1242/dev.02336. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, et al. Distinct mechanisms regulate slow-muscle development. Curr Biol. 2001;11:1432–8. doi: 10.1016/s0960-9822(01)00428-6. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, et al. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–99. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, et al. Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-alpha2-deficient mice. Faseb J. 2005;19:934–42. doi: 10.1096/fj.04-3376com. [DOI] [PubMed] [Google Scholar]
- Biancheri R, et al. POMGnT1 mutations in congenital muscular dystrophy: genotype-phenotype correlation and expanded clinical spectrum. Arch Neurol. 2006;63:1491–5. doi: 10.1001/archneur.63.10.1491. [DOI] [PubMed] [Google Scholar]
- Blagden CS, et al. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–75. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, et al. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132:515–28. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- Brent AE, et al. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12:548–57. doi: 10.1016/s0959-437x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Chen JK, et al. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto SH, et al. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–80. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Du SJ, et al. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J Cell Biol. 1997;139:145–56. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Dienhart M. Gli2 mediation of hedgehog signals in slow muscle induction in zebrafish. Differentiation. 2001;67:84–91. doi: 10.1046/j.1432-0436.2001.067003084.x. [DOI] [PubMed] [Google Scholar]
- Feng X, et al. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol. 2006;300:736–46. doi: 10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Gros J, et al. A two-step mechanism for myotome formation in chick. Dev Cell. 2004;6:875–82. doi: 10.1016/j.devcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Gros J, et al. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–93. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- Hamade A, et al. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev Biol. 2006;289:127–40. doi: 10.1016/j.ydbio.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Henry CA, Amacher SL. Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev Cell. 2004;7:917–23. doi: 10.1016/j.devcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Hinits Y, et al. Differential requirements for myogenic regulatory factors distinguish medial and lateral somitic, cranial and fin muscle fibre populations. Development. 2009;136:403–14. doi: 10.1242/dev.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsinger E, et al. Hedgehog signaling is required for commitment but not initial induction of slow muscle precursors. Dev Biol. 2004;275:143–57. doi: 10.1016/j.ydbio.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jowett T. Analysis of protein and gene expression. Methods Cell Biol. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, et al. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature. 2004;431:466–71. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- Kaul A, et al. Myf-5 revisited: loss of early myotome formation does not lead to a rib phenotype in homozygous Myf-5 mutant mice. Cell. 2000;102:17–9. doi: 10.1016/s0092-8674(00)00006-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kortesmaa J, et al. Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification,and interactions with integrins. J Biol Chem. 2000;275:14853–9. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- Maves L, et al. Pbx homeodomain proteins direct Myod activity to promote fast-muscle differentiation. Development. 2007;134:3371–82. doi: 10.1242/dev.003905. [DOI] [PubMed] [Google Scholar]
- Moll J, et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–7. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- Oldfors A. Hereditary myosin myopathies. Neuromuscul Disord. 2007;17:355–67. doi: 10.1016/j.nmd.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Pace RA, et al. Collagen VI glycine mutations: perturbed assembly and a spectrum of clinical severity. Ann Neurol. 2008;64:294–303. doi: 10.1002/ana.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–46. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- Patton BL, et al. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–21. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelmann B, et al. Expression of laminin alpha1, alpha2, alpha4, and alpha5 chains, fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ dy/dy mice. Exp Cell Res. 1999;246:165–82. doi: 10.1006/excr.1998.4244. [DOI] [PubMed] [Google Scholar]
- Roy S, et al. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15:1563–76. doi: 10.1101/gad.195801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, et al. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–90. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–9. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Snow CJ, et al. Time-lapse analysis and mathematical characterization elucidate novel mechanisms underlying muscle morphogenesis. PLoS Genet. 2008;4:e1000219. doi: 10.1371/journal.pgen.1000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow CJ, Henry CA. Dynamic formation of microenvironments at the myotendinous junction correlates with muscle fiber morphogenesis in zebrafish. Gene Expr Patterns. 2009:37–42. doi: 10.1016/j.gep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, et al. Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–9. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- Uro-Coste E, et al. Striking phenotypic variability in two familial cases of myosin storage myopathy with a MYH7 Leu1793pro mutation. Neuromuscul Disord. 2009;19:163–6. doi: 10.1016/j.nmd.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Vachon PH, et al. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–81. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden FJ, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–64. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, et al. Zebrafish segmentation and pair-rule patterning. Dev Genet. 1998;23:65–76. doi: 10.1002/(SICI)1520-6408(1998)23:1<65::AID-DVG7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Wiellette E, et al. Combined haploid and insertional mutation screen in the zebrafish. Genesis. 2004;40:231–40. doi: 10.1002/gene.20090. [DOI] [PubMed] [Google Scholar]