Abstract
Organophosphorus (OP) pesticides elicit acute toxicity by inhibiting acetylcholinesterase (AChE), the enzyme responsible for inactivating acetylcholine (ACh) at cholinergic synapses. A number of OP toxicants have also been reported to interact directly with muscarinic receptors, in particular the M2 muscarinic subtype. Parasympathetic innervation to the heart primarily regulates cardiac function by activating M2 receptors in the sinus node, atrial-ventricular node and conducting tissues. Thus, OP insecticides can potentially influence cardiac function in a receptor–mediated manner indirectly by inhibiting acetylcholinesterase and directly by binding to muscarinic M2 receptors. Young animals are generally more sensitive than adults to the acute toxicity of OP insecticides and age related differences in potency of direct binding to muscarinic receptors by some OP toxicants have been reported. We thus compared the effects of the common OP insecticide chlorpyrifos (CPF) on functional signs of toxicity and cardiac ChE activity and muscarinic receptor binding in neonatal and adult rats. Dosages were based on acute lethality (i.e., 0.5 and 1 × LD10: neonates, 7.5 and 15 mg/kg; adults, 68 and 136 mg/kg). Dose- and time-related changes in body weight and cholinergic signs of toxicity (involuntary movements) were noted in both age groups. With 1 × LD10, relatively similar maximal reductions in ChE activity (95%) and muscarinic receptor binding (≈ 30%) were noted, but receptor binding reductions appeared earlier in adults and were more prolonged in neonates. In vitro inhibition studies indicated that ChE in neonatal tissues was markedly more sensitive to inhibition by the active metabolite of chlorpyrifos (i.e., chlorpyrifos oxon, CPO) than enzyme in adult tissues (IC50 values: neonates, 17 nM; adults, 200 nM). Chelation of free calcium with EDTA had relatively little effect on in vitro cholinesterase inhibition, suggesting that differential A-esterase activity was not responsible for the age-related difference in cholinesterase sensitivity between age groups. Pre-incubation of neonatal and adult tissues with selective inhibitors of AChE and butyrylcholinesterase (BChE) indicated that a majority (82–90%) of ChE activity in the heart of both neonates and adults was BChE. The rapid onset (by 4 hours after dosing) of changes in muscarinic receptor binding in adult heart may be a reflection of the more potent direct binding to muscarinic receptors by chlorpyrifos oxon previously reported in adult tissues. The results suggest that ChE activity (primarily BChE) in neonatal heart may be inherently more sensitive to inhibition by some anticholinesterases and that toxicologically significant binding to muscarinic receptors may be possible with acute chlorpyrifos intoxication, potentially contributing to age-related differences in sensitivity.
Keywords: organophosphate, acetylcholinesterase, butyrlcholinesterase, cardiac, muscarinic receptors, age-related
Introduction
In 2000–2001, US pesticide use exceeded 0.5 billion kilograms, with organophosphorus insecticide use accounting for approximately 50 million kilograms of active ingredients (Kiely et al., 2004). While the indoor use of chlorpyrifos (CPF) has been eliminated in the US (U.S. EPA, 2000), it remains one of the most commonly used OP insecticides worldwide. The acute toxicity of OP insecticides including CPF is typically initiated by inhibition of acetylcholinesterase (AChE; E.C. 3.1.1.7), with consequent alteration of cholinergic neurotransmission in the peripheral and central nervous systems. Acetylcholine (ACh) is the neurotransmitter involved in cholinergic neurotransmission, released by presynaptic cholinergic terminals and activating nicotinic and/or muscarinic receptors to modify postsynaptic cell function. Normally, acetylcholinesterase rapidly and efficiently degrades ACh, thereby terminating its signaling action (Saunders and Harper, 1994; Ecobichon, 1996; Lefkowitz et al., 1996). OP toxicants alter cholinergic neurotransmission by “irreversibly” binding to AChE and blocking the enzyme’s ability to cleave Ach molecules.
Accumulation of synaptic ACh following acetylcholinesterase inhibition leads to prolonged stimulation of cholinergic receptors. It is generally thought that there are “spare” enzyme molecules, requiring extensive inhibition to affect cholinergic neurotransmission (Lotti, 1990; Norstrandt et al., 1997; Casida and Quistad, 2005). A variety of cholinergic signs are possible following exposure to anticholinesterases including salivation, lacrimation, urination and defecation (SLUD signs), bradycardia, tachycardia, nausea, vomiting, and respiratory dysfunction.(Kumawat et al., 1984; Kecik et al., 1993; Saunders and Harper, 1994; Ecobichon, 1996). Serious complications are often associated with disruption of cardiac function (Saadeh et al., 1997; Peter and Cherian 2000).
Although OP pesticides elicit toxicity through a common mechanism initiated by AChE inhibition, some discrepancies have been reported in the expression of toxicity relative to degree of acetylcholinesterase inhibition (Chaudhuri et al., 1993; Pope et al., 1995; Liu and Pope, 1998). Pope (1999) suggested that different OP pesticides likely act via additional pathways to either modulate the expression of cholinergic toxicity elicited by AChE or to modify other non-cholinergic signaling pathways. Many reports have indicated that some OP insecticides can interact with macromolecular targets in addition to AChE, including muscarinic receptors (Bakry et al., 1988; Jett et al., 1991; Ward et al., 1993, Huff et al., 1994; Huff and Abou-Donia, 1995; Ward and Mundy, 1996; van den Beukel et al., 1997, Quistad et al., 2001, 2002, Quistad and Casida, 2004). It is well known that prolonged AChE inhibition leads to a reduction in cholinergic receptor density at innervated sites, contributing to the development of tolerance to cholinergic toxicity (Costa et al., 1982a). The toxicological consequences of direct binding of some OPs to cholinergic receptors remains unclear, however.
Bomser and Casida (2001) reported that radiolabeled chlorpyrifos oxon phosphorylated muscarinic receptors in adult mouse heart in vitro. We reported a concentration-dependent inhibition of [3H]oxotremorine-M binding to muscarinic receptors in both neonatal and adult rat heart by chlorpyrifos oxon, with somewhat higher potency towards binding in adult tissues (IC50 = 7 vs 15 nM, Howard and Pope, 2002). We hypothesized that the direct binding of chlopyrifos oxon to muscarinic receptors in neonatal and adult heart may contribute to age-related differences in sensitivity to chlorpyrifos. The current study evaluated the effects of equitoxic dosages of chlorpyrifos on cholinergic toxicity, cardiac cholinesterase inhibition and muscarinic receptor binding in neonatal and adult rats.
Materials and Methods
Animals
Neonatal (7-day, males and females) and adult (90-day, male only) Sprague-Dawley rats were used throughout the experiments. Untimed pregnant females were obtained from Harlan Sprague Dawley (Indianapolis, IN) and pups culled to 10–12/dam on postnatal day 2 (date of birth was defined as postnatal day 0). All animals were kept in polycarbonate cages and acclimated for 7 days prior to any treatments. Animals were maintained on 12L:12D cycle and were given food (PMI® Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN) and tap water ad libitum. All studies were conducted with the approval from the Animal Care and Use committee of Oklahoma State University.
Chemicals
Chlorpyrifos (CPF, O, O′-diethyl-O-(3,5,6-tricholoro-2-pyridyl) phosphorothioate) and chlorpyrifos oxon (CPO, O,O′-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphate) were purchased from Chem Service (West Chester, PA). Both were ≥97% in purity. Solutions of chlorpyrifos were made in peanut oil (2 ml/kg) on the day of treatment for in vivo studies. A stock solution of chlorpyrifos oxon was prepared in dry ethanol (100%), kept at −70°C and diluted in potassium phosphate buffer on the day of assay for in vitro studies (final concentration of solvent was kept constant in all assays at 0.1%).
Acetylcholine iodide, [acetyl-3H], specific activity 82.0 mCi/mmol; oxotremorine-M acetate, [methyl-3H], specific activity 85.8–86.4 Ci/mmol; and quinuclidinyl benzilate,L-[benzilic-4,4’-3H], specific activity 37.0–49.0 Ci/mmol were purchased from Perkin Elmer (Boston, MA). All other reagents were obtained from Sigma Chemical Company (St. Louis, MO).
Animal Treatments
Neonatal and adults animals were treated with chlorpyifos by gavage. The dosages selected were based on the LD10 (i.e., either vehicle, 0.5 × LD10 or 1 × LD10: neonates = 0, 7.5 or 15 mg/kg; adults = 0, 68 or 136 mg/kg [Zheng et al., 2000]). Functional signs of toxicity were evaluated and rats were euthanized at 4, 24 and 96 hours after treatment.
Functional Signs of Toxicity
Animals were evaluated for cholinergic signs of toxicity measured as involuntary movements (IM) and SLUD (salivation, lacrimation, urination, defecation) signs by the method of Moser et al. (1988) as described previously (Liu and Pope, 1996).
Tissue Collection and Membrane Preparation
Animals were euthanized by decapitation. The hearts were quickly removed and rinsed in 10 mM Tris, pH 7.4, 1 mM EDTA (McMahon, 1989). Heart tissue was separated from blood vessels, blood clots and connective tissue, after which it was minced and frozen at −70°C until the day of assay. Thawed tissues were homogenized (1:15; w/v) in 5 mM Hepes buffer, pH 7.4 on ice for four 30-second cycles at 25,000 rpm (Polytron, Brinkmann Instruments, Westbury, NY), pausing one minute between cycles. Homogenates were centrifuged at 1,000 × g at 4°C for 10 minutes and the resulting supernatant was subsequently centrifuged at 40,000 × g at 4°C for 45 minutes. The final membranous pellet was resuspended in the original homogenate volume of buffer with 10 strokes of a Dounce-type homogenizer and used immediately for the biochemical assays.
Cholinesterase Activity
ChE activity was determined by the radiometric method of Johnson and Russell (1975) as described previously (Won et al., 2001). Tissues were simultaneously incubated at room temperature with the substrate [3H] ACh (1 mM) under conditions established to cause linear rates of hydrolysis.
In Vitro Inhibition of Cholinesterase by Chlorpyrifos oxon
Hearts from neonatal and adult rats were collected and homogenized as described above. Aliquots of homogenates were incubated with equal volumes of either vehicle (0.1% ethanol) or CPO (30 pM - 10 µM final concentration) for 30 minutes at 37°C in the presence or absence of 2 mM EDTA (to eliminate potential CPO hydrolysis by A-esterases). Following the pre-incubation period, residual cholinesterase activity was measured as described above.
Evaluation of relative AChE and BChE content in neonatal and adult rat heart
BW284C51 (BW) and iso-OMPA (tetraisopropyl pyrophosphoramide) are highly selective inhibitors of acetylcholinesterase and butyrylcholinesterase, respectively (Caselli et al., 2006). Preliminary studies with a range of concentrations of BW and iso-OMPA (1–100 µM) indicated that 10 µM concentration of both inhibitors was maximally effective at inhibiting the respective enzyme activities. Therefore this concentration of both BW and iso-OMPA was used to estimate the relative amounts of AChE and BChE. Homogenates were pre-incubated with BW or iso-OMPA for 15 min at 37°C and then residual ChE activity was measured as described above.
Muscarinic Receptor Binding
Muscarinic receptor binding was conducted essentially by the method of Silveria et al. (1990) with minor modifications. Membranes (300 – 350 µg/rxn) were incubated in the presence of the high affinity muscarinic agonist, [3H] oxotremorine-M acetate (OXO; 1 nM final concentration) for 90 minutes at 21°C. Incubation was terminated by vacuum filtration over Whatman GF/B filters (Brandel Inc., Gaithersburg, MD) saturated with cold 0.05% (w/v) polyethylenimine in 5 mM HEPES buffer, pH 7.4 prior to harvest (van den Beukel et al., 1997) to reduce non-specific binding. Filter disks were allowed to equilibrate overnight in scintillation cocktail (Fisher Scientific, Pittsburgh, PA) prior to counting. Protein content was determined by the method of Lowry et al. (1951). Assays were conducted in duplicate and specific binding was calculated as the difference in radioligand binding in the presence and absence of atropine (10 µM).
Data Analyses
Receptor binding and ChE activity data were analyzed by ANOVA and Newman-Keul’s where appropriate. Body weight was analyzed by repeated measures ANOVA. Functional signs of toxicity were analyzed using MANOVA. IC50 values were estimated by one-site competition using non-linear regression. All analyses were conducted using GraphPad Prism ® software with p<0.05 being considered statistically significant.
Results
Body Weight and Functional Signs Following Acute Chlorpyrifos
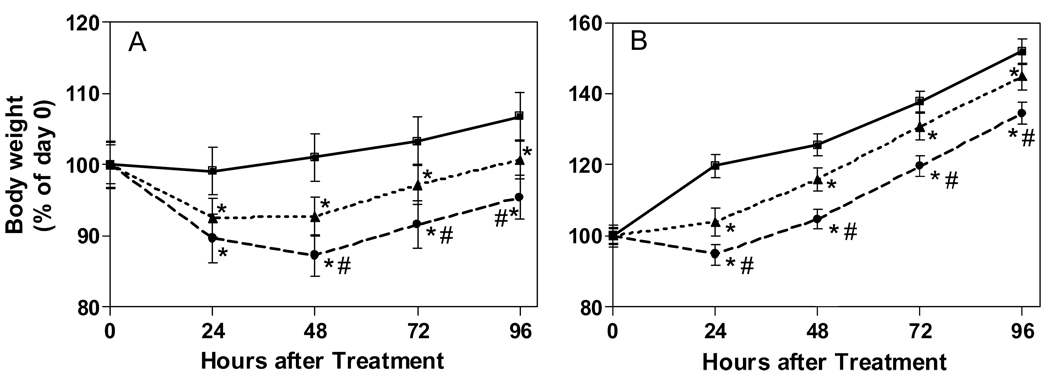
Figure 1 shows body weight changes in both age groups following chlorpyrifos exposure. There was a significant dose-related decrease in body weight in both adults and neonates occurring by 24 hours after treatment in both age groups (Figure 1A, 1B). Body weight then increased in neonates at a relatively similar rate in both controls and treated animals from 24–96 hours after treatment. In adults, body weight reductions peaked later (48 hours with 136 mg/kg). In both neonates and adults, body weights were still significantly lower in both CPF treatment groups relative to controls at the last timepoint.
Figure 1.
In vivo effects of chlorpyrifos on body weight in adult (A) and neonatal (B) rats after acute chlorpyrifos exposure. Rats were treated with vehicle (square), 0.5 × LD10 (triangle: 7.5 mg/kg in neonate; 68 mg/kg in adult) or 1 × LD10 (circle: 15 mg/kg, neonate; 136 mg/kg, adult). Values represent mean (± SEM) body weight relative to pre-treatment weight (i.e., percent of day 0). Data were analyzed using repeated measures ANOVA. An asterisk indicates statistically different from control (p< 0.05). A pound sign indicates statistical difference from the lower dose group (p< 0.05).
Tables 1 and 2 show signs of toxicity in adults and neonates at 4, 24 and 96 hours after chlorpyrifos dosing. Adults treated with CPF showed cholinergic signs (involuntary movements) as early as 24 hours after treatment in the low dose group and by 4 hours after exposure in the high dose group (Table 1). No significant differences were noted by 96 hours after dosing. In neonates, involuntary movements were only noted in the high dose group starting at 4 hours after treatment, and these dissipated by 48 hours after treatment (Table 2). No SLUD signs were noted in either adults or neonates (data not shown).
Table 1.
Involuntary movements in adult rats following acute exposure to chlorprifos.
| Hours after treatment | |||||
|---|---|---|---|---|---|
| 4 | 24 | 48 | 72 | 96 | |
| Control | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 |
| 68 mg/kg | 2 ± 0 | 3 ± 1 * | 3 ± 1 * | 2.5 ± 0.5 | 2 ± 0.5 |
| 136 mg/kg | 3 ± 0.5 *# | 3 ± 0 *# | 3 ± 0 * | 2.5 ± 0 * | 2 ± 0.5 |
Rats were treated with vehicle, 0.5 × LD10 (68 mg/kg) or 1 × LD10 (136 mg/kg). Rats were observed for involuntary movements and scored according to the protocol described in the Materials and Methods section. Data represent median ± inter-quartile range. Data were analyzed using MANOVA (JMP statistical package).
Asterisks represent values statistically different from respective controls (p< 0.05).
Pound signs represent values statistically different from the low dose group (p< 0.05).
Table 2.
Involuntary movements in neonatal rats following acute exposure to chlorpyrifos.
| Hours after treatment | |||||
|---|---|---|---|---|---|
| 4 | 24 | 48 | 72 | 96 | |
| Control | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 |
| 7.5 mg/kg | 2 ± 1 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 |
| 15 mg/kg | 2.5 ± 1 *# | 2 ± 0.25 * | 2 ± 0 | 2 ± 0 | 2 ± 0 |
Rats were treated with vehicle, 0.5 × LD10 (7.5 mg/kg) or 1 × LD10 (15 mg/kg). Rats were observed for involuntary movements and scored according to the protocol described in the Materials and Methods section. Data represent median ± inter-quartile range. Data were analyzed using MANOVA (JMP statistical package).
Asterisks represent values statistically different from respective controls (p< 0.05).
Pound signs represent values statistically different from the low dose group (p< 0.05).
Cholinesterase Activity and Muscarinic Receptor Binding Following Acute Chlorpyrifos
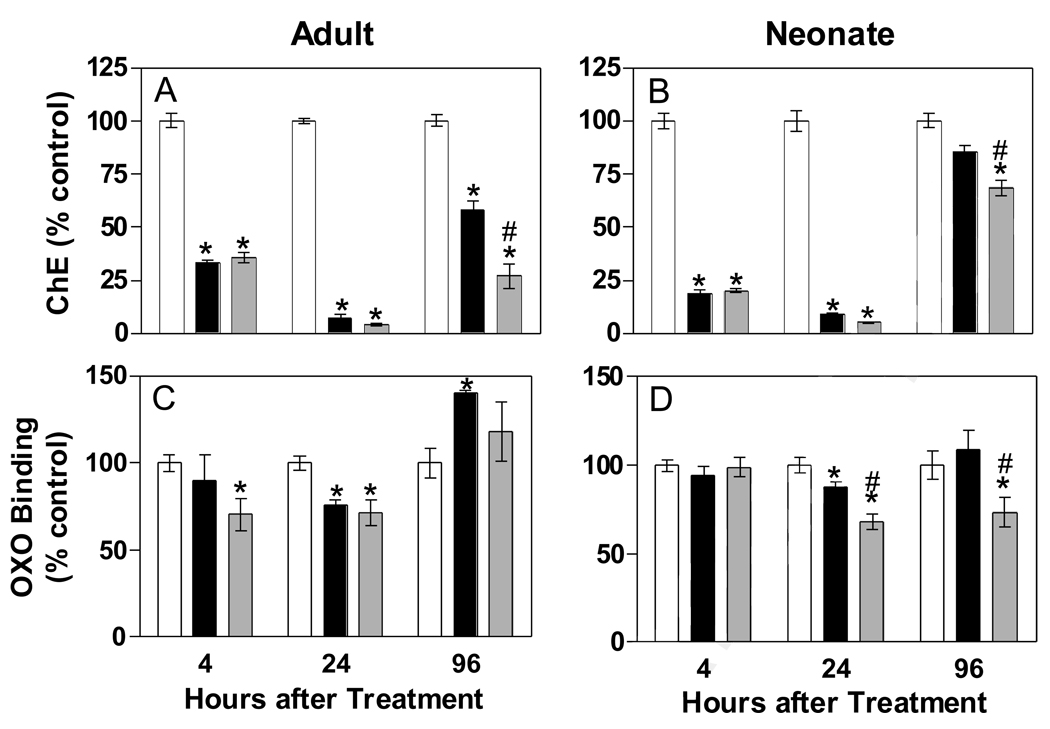
Changes in cholinesterase activity and muscarinic receptor binding following chlorpyrifos dosing are shown in Figure 2. Cholinesterase activity in the heart was significantly reduced in adults with both dosages and in neonates with the high dosage at all time points examined (Figures 2A and 2B). Heart cholinesterase activity was significantly reduced in the neonatal tissues following the lower dosage (7.5 mg/kg) at 4 and 24 hours but not at the final time-point (96 hours, Figure 2B). Peak inhibition with the higher dosage was reached at 24 hours after dosing in both age groups. Figures 2C and 2D show changes in muscarinic receptor agonist ([3H]oxotremorine-M) binding following chlorpyrifos dosing. Binding was maximally reduced (approximately 30%) in tissues from both adults and neonates, but significant reductions were noted earlier (by 4 hours after dosing) in adults. Interestingly, an increase in OXO binding was noted at 96 hours after dosing in adult tissues, but only with the lower dosage of exposure, with no differences in agonist binding between controls and the higher dosage group (Figure 2C). In contrast, agonist binding was reduced in neonatal tissues at 24 hours, and remained decreased at 96 hours in the higher dosage treatment group (Figure 2D).
Figure 2.
In vivo effects of chlorpyrifos on cholinesterase activity (A, B) and [3H]oxotremorine-M binding (C, D). Rats were treated with vehicle (open bar), 0.5 × LD10 (black bar: 7.5 mg/kg in neonate; 68 mg/kg in adult) or 1 × LD10 (shaded bar: 15 mg/kg, neonate; 136 mg/kg, adult) and sacrificed at the specified times. Data represent percent of control (mean ± SEM). Data for each time point were analyzed individually by ANOVA and Newman-Keuls post hoc test. An asterisk indicates statistically different from control (p< 0.05). A pound sign indicates statistically different from the lower dose group (p< 0.05).
In Vitro Sensitivity of Neonatal and Adult Cardiac Cholinesterase to Chlorpyrifos Oxon
The in vitro potency of chlorpyrifos oxon (CPO) against cholinesterase activity in neonatal and adult heart and its sensitivity to calcium chelation is shown in Table 3. CPO was approximately 12 times more potent at inhibiting cholinesterase activity in tissues from neonates than adults. A previous report (Mortensen et al., 1998) suggested no age-related differences in sensitivity of acetylcholinesterase from brain and other tissues to inhibition by CPO if the enzymes were immuno-isolated from other tissue components, e.g., A-esterases. We therefore evaluated whether maturational differences in A-esterase activity in heart might be responsible for the observed higher sensitivity of neonatal heart cholinesterase to inhibition by CPO. A-esterases are completely dependent on Ca2+ for activation. Homogenates of heart tissue were prepared as above except in the presence of EDTA (2 mM final concentration) to sequester Ca+2 ions. As shown in Table 3, chelation of Ca+2 had relatively little effect on inhibition of neonatal ChE activity by CPO, but did slightly reduce the IC50 value (1.6 fold) in adult tissues. There remained about a 9-fold difference in IC50 values between the two age groups under these conditions, however.
Table 3.
Effects of addition of EDTA on in vitro inhibition of cholinesterase in neonatal and adult rat heart by chlorpyrifos oxon.
| EDTA | Adult | Neonate |
|---|---|---|
| 0 mM | 200 (78–514) | 17 (12–25) |
| 2 mM | 128 (74–221) | 14 (9–24) |
Adult and neonatal hearts were minced and frozen at −70°C. Thawed samples were then homogenized in 5 mM HEPES buffer, pH 7.4 in the presence or absence of EDTA (2 mM). Aliquots of homogenates were pre-incubated with a range of chlorpyrifos oxon concentrations for 30 minutes at 37°C. Residual enzyme activity was then measured as described in the Materials and Methods section. Data represent IC50 values (nM) with 95% confidence intervals in parentheses.
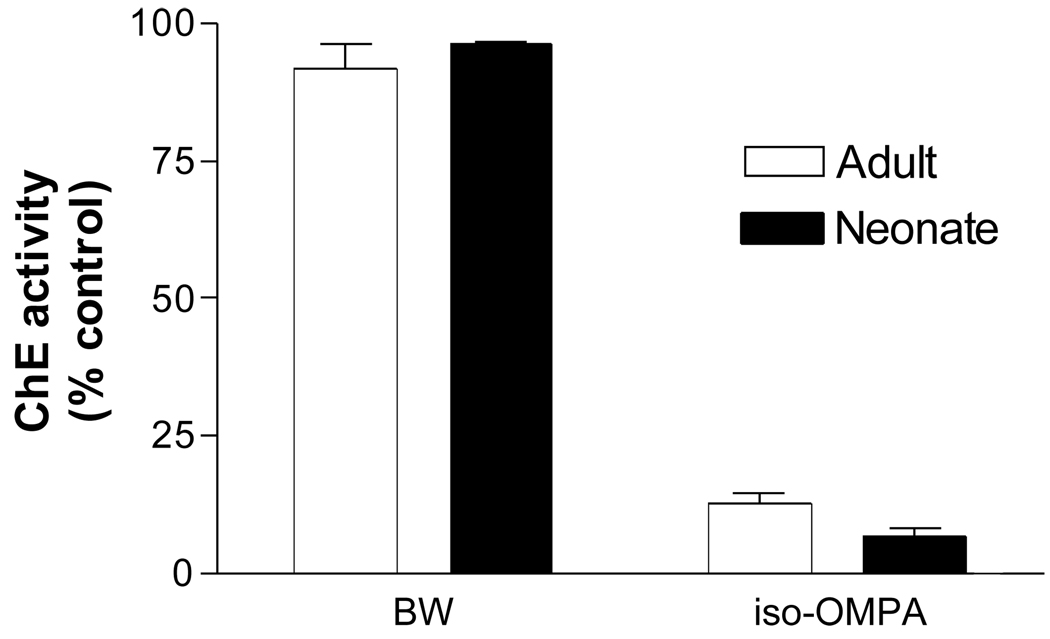
The in vitro effects of BW and iso-OMPA on ChE activity in neonatal and adult tissues are shown in Figure 3. While BW had minimal effect (4–8 % inhibition), iso-OMPA inhibited ≥ 88% of the ChE activity in tissues from both adult and neonatal heart.
Figure 3.
In vitro effects of BW and iso-OMPA on cholinesterase activities in neonatal and adult heart. Tissue homogenates from adult and neonatal rats (n=3/age group) were prepared as described in Materials and Methods and pre-incubated with either BW284C51 (BW, 10 µM, a selective inhibitor of acetylcholinesterase) or tetraisopropyl pyrophosphoramide (iso-OPMA, 10 µM, a selective BChE inhibitor) for 15 minutes at 37°C and residual ChE activity measured.
Discussion
A number of studies have evaluated differences in acute sensitivity between neonatal and adult rats to the OP insecticide chlorpyrifos. These studies focused on neurotoxicity endpoints, however, with few studies evaluating possible differences in sensitivity of the heart. As vagal cholinergic innervation is critical in the regulation of heart function, and as chlorpyrifos oxon has been reported to bind directly to the predominant form of muscarinic receptors in the heart (M2), we studied the comparative effects of acute chlorpyrifos exposure on cholinesterase activity and muscarinic receptor binding in neonatal and adult rats.
Neonatal and adult rats were challenged with vehicle, the respective LD10, or one-half of that dosage. Even though LD10 was approximately nine-fold lower in the neonates (neonates, 15 mg/kg; adults, 136 mg/kg), relatively similar dose-related decreases in body weight (Figure 1A, 1B), functional signs of toxicity (Tables 1, 2) and dose-related reduction in ChE activity in heart (Figure 2A, 2B) were observed in adult and neonatal rats treated with equitoxic dosages. There appeared to be a time-dependent difference in cholinesterase inhibition, however, with more extensive reduction in neonates (about 80%) than in adults (about 65%) at the initial time-point evaluated (4 hours after dosing), but more rapid recovery in cholinesterase activity being noted in neonates (Figure 2A, 2B). More rapid peak inhibition and faster recovery of cholinesterase activity in neonates compared to adults has also been reported in brain following chlorpyrifos exposure (Pope et al. 1991; Won et al. 2001).
We previously reported that muscarinic receptor agonist ([3H]oxotremorine-M) binding was displaced in vitro in a potent manner by chlorpyrifos oxon in membranes from both neonatal and adult rat heart (IC50, 90 minutes at 21°C: neonates, 15 nM; adults, 7 nM, Howard and Pope, 2002). Following in vivo challenge, agonist binding in adult heart was significantly reduced with the LD10 dosage by 4 hours and both dosages by 24 hours after exposure. In neonates, while cholinesterase inhibition was more extensive by 4 hours after dosing, no significant effect on muscarinic receptor agonist binding was noted at this timepoint, but significant reductions were noted at later time-points (24–96 hours after treatment, Figure 2C, 2D).
Down-regulation of muscarinic receptors following prolonged acetylcholinesterase inhibition has been reported for decades (Costa et al., 1982a,b). Schwab and coworkers (1981) found that repeated exposure to the OP insecticide disulfoton caused a significant reduction in brain [3H] QNB binding but no receptor binding changes in the heart. Zhu et al. (1991) reported that repeated exposure to subcutaneous di-isopropylfluorophosphate (DFP) in rats led to a decline in both the concentration of cardiac mAChR and mRNA, suggesting that prolonged AChE inhibition may also alter muscarinic receptor transcription in heart. Repeated oral disulfoton significantly reduced [3H] QNB binding and differentially affected mRNA levels in brain (i.e., unchanged or reduced mRNA in a region-specific manner; Yagle and Costa, 1996). Together, these studies suggested that there may be differential adaptive changes in cardiac muscarinic receptors depending on the specific toxicant and route of administration.
The observed changes in muscarinic agonist binding in response to chlorpyrifos exposure could be due to either direct or indirect actions at the receptor. It generally takes some time before down-regulation can occur. Zhu and coworkers (1991) reported a significant reduction in [3H]quinuclidinyl benzilate (QNB) binding 24 hours following acute DFP exposure in adult rats. The early (4 hours after dosing) reduction in [3H]oxotremorine-M binding noted in adult heart following the LD10 of chlorpyrifos (Figure 2C) could be an indication that chlorpyrifos oxon bound directly to the receptors and prevented subsequent agonist binding. All five muscarinic receptor subtypes (M1–M5) have been found in human heart (Wang et al., 2001) although the M2 subtype predominates (Lefkowitz et al., 1996). Chlorpyrifos oxon appears to irreversibly phosphorylate the M2 receptor in heart (Bomser and Casida, 2001; Howard and Pope, 2002). The greater in vitro potency of chlorpyrifos oxon in displacing agonist binding to cardiac muscarinic receptors in adult compared to neonatal heart (Howard and Pope, 2002) may be sufficient to differentially modulate cardiac muscarinic receptor binding between the two age groups following in vivo chlorpyrifos exposure.
A number of studies have shown that neonatal rats are more sensitive than adults to the acute toxicity of chlorpyrifos and other OP insecticides (Benke and Murphy, 1975; Mendoza, 1976; Pope et al., 1991; Atterberry et al., 1997). While neonates are more sensitive than adults to acute chlorpyrifos exposure, brain cholinesterase showed similar sensitivity to inhibition by chlorpyrifos oxon in vitro across age groups (Mortensen et al., 1998b). We evaluated the relative sensitivity of neonatal and adult heart cholinesterase activity to chlorpyrifos oxon (Table 3). Cholinesterase activity in neonatal heart was markedly more sensitive to inhibition by chlorpyrifos oxon than activity in adult tissues. By immunoprecipitating acetylcholinesterase from liver samples prior to evaluating in vitro sensitivity, Mortensen and coworkers (1998a) showed that acetylcholinesterase was markedly influenced by detoxification capacity within the specific tissues, e.g., the enzyme in liver homogenate was approximately 35–175-fold less sensitive to inhibition than following its immunological isolation. Thus, cholinesterase in neonatal heart could be more sensitive to in vitro inhibition by chlorpyrifos oxon due to lesser detoxification capacity in the tissue compared to adult tissues. A primary mechanism of chlorpyrifos oxon detoxification in liver is degradation by Ca+2-dependent A-esterases (Costa et al., 2005). Chelation of free Ca+2 had relatively little effect, however, on cholinesterase inhibition by CPO in tissues from either age group.
Our results (Figure 3) suggest that BChE in neonatal rat heart is inherently more sensitive to inhibition by CPO than the enzyme in adult heart. Li and coworkers (2000) previously reported that BChE activity was higher in concentration than AChE in the adult rat heart and suggested BChE may be a “back-up” for AChE. BChE may also serve a protective role during OP exposure by limiting inhibition of AChE. As the predominant cholinesterase in both neonatal and adult heart was BChE (Figure 3), Darvesh and colleagues (1998) reported that BChE-selective inhibitors could alter the tonic activity of cardiac ganglia. Based on our in vitro findings regarding CPO potency in 7-day versus 90-day rat heart, there could be maturational differences in BChE isoforms, thus potentially influencing the response to OP inhibitors.
These studies suggest that while relatively similar changes in cholinesterase activity in the heart and cholinergic signs of toxicity may be elicited, cardiac muscarinic receptors may be differentially affected in neonatal and adult rats by “equitoxic” dosages of chlorpyrifos. Changes in muscarinic receptor dynamics following acute exposure to chlorpyrifos may be due to both direct interaction of chlorpyrifos oxon with the receptor, and indirect receptor regulation mediated by extensive cholinesterase inhibition. Differential changes in cardiac muscarinic receptors following exposure to chlorpyrifos or other OP insecticides could contribute to age-related differences in response.
Acknowledgements
This work was partially supported by research grant R01 ES009119 from the National Institute of Environmental Health Sciences, NIH (C.N.P.), and by the Oklahoma State University Board of Reagents. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol Appl Pharmacol. 1997;147(2):411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Bakry NMS, El-Rashidy AH, Eldefrawi AT, Eldefrawi ME. Direct actions of organophosphate anticholinesterases on nicotinic and muscarinic acetylcholine receptors. J. Biochem. Toxicol. 1988;3:235–259. doi: 10.1002/jbt.2570030404. [DOI] [PubMed] [Google Scholar]
- Benke GM, Murphy SD. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol Appl Pharmacol. 1975;31(2):254–269. doi: 10.1016/0041-008x(75)90161-1. [DOI] [PubMed] [Google Scholar]
- Bomser JA, Casida JE. Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol. Lett. 2001;119(1):21–26. doi: 10.1016/s0378-4274(00)00294-0. [DOI] [PubMed] [Google Scholar]
- Caselli F, Gastaldi L, Gambi N, Fabbri E. In vitro characterization of cholinesterases in the earthworm Eisenia andrei. Comp, Biochem, Physiol, C Toxicol, Pharmacol. 2006;143:416–421. doi: 10.1016/j.cbpc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem. Biol. Interact. 2005;157–158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chaudhuri J, Chakraborti TK, Chanda S, Pope CN. Differential modulation of organophosphate-sensitive muscarinic receptors in rat brain by parathion and chlorpyrifos. J. Biol. Toxicol. 1993;8(4):207–216. doi: 10.1002/jbt.2570080406. [DOI] [PubMed] [Google Scholar]
- Costa LG, Schwab BW, Murphy SD. Tolerance to anticholinesterase compounds in mammals. Toxicology. 1982a;25:79–97. doi: 10.1016/0300-483x(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Costa LG, Schwab BW, Murphy SD. Differential alterations of cholinergic muscarinic receptors during chronic and acute tolerance to organophosphorus insecticides. Biochem. Pharmacol. 1982b;31(21):3407–3413. doi: 10.1016/0006-2952(82)90619-0. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin. Chim. Acta. 2005;352(1–2):37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Darvesh S, MacDonald SE, Losier AM, Martin E, Hopkins DA, Armour JA. Cholinesterases in cardiac ganglia and modulation of canine intrinsic cardiac neuronal activity. J. Auton. Nerv. Syst. 1998;71(2–3):75–84. doi: 10.1016/s0165-1838(98)00064-2. [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett and Doull’s Toxicology. The Basic Sciences of Poisons. 5th ed. New York: McGraw-Hill; 1996. pp. 643–689. [Google Scholar]
- Howard MD, Pope CN. In vitro effects of chlorpyrifos, parathion, methyl parathion and their oxons on cardiac muscarinic receptor binding in neonatal and adult rats. Toxicology. 2002;170(1–2):1–10. doi: 10.1016/s0300-483x(01)00498-x. [DOI] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J. Pharmacol. Exp. Ther. 1994;269(1):329–335. [PubMed] [Google Scholar]
- Huff RA, Abou-Donia MB. In vitro effect of chlorpyrifos oxon on muscarinic receptors and adenylate cyclase. Neurotoxicology. 1995;16(2):281–290. [PubMed] [Google Scholar]
- Jett DA, Abdallah EAM, El-Fakahany EE, Eldefrawi ME, Eldefrawi AT. High-affinity activation by paraoxon of a muscarinic receptor subtype in rat brain striatum. Pest. Biochem. Physiol. 1991;39:149–157. [Google Scholar]
- Johnson CD, Russell RL. A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal. Biochem. 1975;64:229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Kecik Y, Yorukoglu D, Saygin B, Sekerci S. A case of acute poisoning due to organosphosphate insecticide. Anaesthesia. 1993;48:141–143. doi: 10.1111/j.1365-2044.1993.tb06854.x. [DOI] [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube A. Pesticides Industry Sales and Usage: 2000 and 2001 Market Estimates. Washington, DC: United States Environmental Protection Agency; Office of Prevention, Pesticides, and Toxic Substances. 2004 http://www.epa.gov/oppbead1/pestsales/01pestsales/market_estimates2001.pdf.
- Kumawat DC, Bomb BS, Chhaparwal JK, Goyal V, Bedi HK. Acute organophosphorus poisoning. Indian J. Pub. Hlth. 1984;28(3):163–167. [PubMed] [Google Scholar]
- Lefkowitz RJ, Hoffman BB, Taylor P. Neurotransmission: The autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LL, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; 1996. pp. 105–139. [Google Scholar]
- Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J. Neurochem. 2000;75(3):1320–1331. doi: 10.1046/j.1471-4159.2000.751320.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Pope CN. Effects of chlorpyrifos on high-affinity choline uptake and [3H]hemicholinium-3 binding in rat brain. Fundam Appl Toxicol. 1996;34(1):84–90. doi: 10.1006/faat.1996.0178. [DOI] [PubMed] [Google Scholar]
- Liu J, Pope CN. Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion. J. Toxicol. Environ. Health, Part A. 1998;53:101–114. doi: 10.1080/009841098159123. [DOI] [PubMed] [Google Scholar]
- Lotti M. Central neurotoxicity and behavioural effects of anticholinesterase. In: Ballantyne B, Marrs TC, editors. Clinical and Experimental Toxicology of Organophosphates and Carbamates. Guildford: Wright-Butterworth; 1990. pp. 75–83. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McMahon KK. Developmental changes of the G proteins-muscarinic cholinergic receptor interactions in rat heart. J. Pharmacol. Exp. Ther. 1989;251(1):372–377. [PubMed] [Google Scholar]
- Mendoza CE. Toxicity and effects of malathion on esterases of suckling albino rats. Toxicol Appl Pharmacol. 1976;35(2):229–238. doi: 10.1016/0041-008x(76)90284-2. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Brimijoin S, Hooper MJ, Padilla S. Comparison of the in vitro sensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: what do tissue IC50 values represent? Toxicol Appl Pharmacol. 1998a;148(1):46–49. doi: 10.1006/taap.1997.8287. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Hooper MJ, Padilla S. Rat brain acetylcholinesterase activity: developmental profile and maturational sensitivity to carbamate and organophosphorus inhibitors. Toxicology. 1998b;125:13–19. doi: 10.1016/s0300-483x(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Moser VC, McCormick JP, Creason JP, MacPhail RC. Comparison of chlordimeform and carbaryl using a functional observational battery. Fundam. Appl. Toxicol. 1988;11(6):189–206. doi: 10.1016/0272-0590(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Nostrandt AC, Padilla S, Moser VC. The relationship of oral chlorpyrifos effects on behavior, cholinesterase inhibition, and muscarinic receptor density in rat. Pharmacol. Biochem. Behav. 1997;58(1):15–23. doi: 10.1016/s0091-3057(96)00458-3. [DOI] [PubMed] [Google Scholar]
- Peter JV, Cherian AM. Organic insecticides. Anaesth. Intensive Care. 2000;28:11–21. doi: 10.1177/0310057X0002800102. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chaudhuri J, Chakraborti TK. Organophosphate-sensitive cholinergic receptors: Possible role in modulation of anticholinesterase-induced toxicity. In: Quinn DM, Balasubramanian AS, Doctor BP, Taylor P, editors. Enzymes of the Cholinesterase Family. New York: Plenium Press; 1995. pp. 305–312. [Google Scholar]
- Pope CN. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health, Part B. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol. 2001;173(1):48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. 2002;135(1–2):89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Casida JE. Lysophospholipase inhibition by organophosphorus toxicants. Toxicol Appl Pharmacol. 2004;196(3):319–326. doi: 10.1016/j.taap.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Saadeh AM, Farsakh NA, Al-Ali MK. Cardiac manifestations of acute carbamate and organophosphate poisoning. Heart. 1997;77:461–464. doi: 10.1136/hrt.77.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DS, Harper C. Pesticides. In: Hayes AW, editor. Principles and Methods of Toxicology. 3rd ed. New York: Raven Press; 1994. pp. 389–415. [Google Scholar]
- Schwab BW, Hand H, Costa LG, Murphy SD. Reduced muscarinic receptor binding in tissues of rats tolerant to the insecticide disulfoton. Neurotoxicology. 1981;2(4):635–647. [PubMed] [Google Scholar]
- Silveira CLP, Eldefrawi AT, Eldefrawi ME. Putative M2 muscarinic receptors of rat heart have high affinity for organophosphorus anticholinesterase. Toxicol. Appl. Pharmacol. 1990;103:474–481. doi: 10.1016/0041-008x(90)90320-t. [DOI] [PubMed] [Google Scholar]
- U. S. EPA. Chlorpyrifos revised risk assessment and agreement with registrants. Washington, D. C.: United States Environmental Protection Agency; Office of Prevention, Pesticides and Toxic Substances (7506C) 2000 June; http://www.epa.gov/pesticides/op/chlorpyrifos/agreement.pdf.
- Van den Beukel I, Dijcks FA, Vanderheyden P, Vauquelin G, Oortgiesen M. Differential muscarinic receptor binding of acetylcholinesterase inhibitors in rat brain, human brain and Chinese hamster ovary cells expressing human receptors. J. Pharmacol. Exp. Ther. 1997;281(3):1113–1119. [PubMed] [Google Scholar]
- Wang H, Han H, Zhang L, Shi H, Schram G, Nattel S, Wang Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol. Pharmacol. 2001;59(5):1029–1036. doi: 10.1124/mol.59.5.1029. [DOI] [PubMed] [Google Scholar]
- Ward TR, Ferris DJ, Tilson HA, Mundy WR. Correlation of the anticholinesterase activity of a series of organophosphates with their ability to compete with agonist binding in muscarinic receptors. Toxicol. Appl. Pharmacol. 1993;122:300–307. doi: 10.1006/taap.1993.1199. [DOI] [PubMed] [Google Scholar]
- Ward TR, Mundy WR. Organophosphorus compounds preferentially affect second messenger systems coupled to M2/M4 receptors in rat frontal cortex. Brain Res. Bull. 1996;39(1):49–55. doi: 10.1016/0361-9230(95)02044-6. [DOI] [PubMed] [Google Scholar]
- Won YK, Liu J, Olivier K, Jr, Zheng Q, Pope CN. Age-related effects of chlorpyrifos on acetylcholine release in rat brain. NeuroToxicology. 2001;22:39–48. doi: 10.1016/s0161-813x(00)00009-7. [DOI] [PubMed] [Google Scholar]
- Yagle K, Costa LG. Effects of organophosphate exposure on muscarinic acetylcholine receptor subtype mRNA levels in the adult rat. NeuroToxicology. 1996;17(2):523–530. [PubMed] [Google Scholar]
- Zheng Q, Olivier K, Won YK, Pope CN. Comparative cholinergic neurotoxicity of oral chlorpyrifos exposures in preweanling and adult rats. Toxicol. Sci. 2000;55:124–132. doi: 10.1093/toxsci/55.1.124. [DOI] [PubMed] [Google Scholar]
- Zhu S-Z, Wang S-Z, Abdallah EAM, El-Fakahany EE. DFP-induced regulation of cardiac muscarinic receptor mRNA in vivo measured by DNA-excess solution hybridization. Life Sci. 1991;48:2579–2584. doi: 10.1016/0024-3205(91)90615-i. [DOI] [PubMed] [Google Scholar]