Abstract
Women with Cushing’s syndrome (CS) and polycystic ovarian syndrome (PCOS) may present with similar symptoms. Subjects with mild CS lack clinical stigmata of classical CS and often have normal laboratory tests measuring hypercortisolism. Thus, distinguishing mild CS from PCOS may be difficult. We hypothesized that either total testosterone (TT) or bioavailable testosterone (BT) levels or the calculation of the free androgen index (FAI) would be low in patients with mild CS and elevated in patients with PCOS, and could help differentiate the two conditions. TT, BT, and FAI were measured in a group of 20 patients of reproductive age with mild CS and 20 PCOS patients matched for age and BMI. We used receiver operator characteristic (ROC) curves to assess the sensitivity and specificity of these measurements for the diagnosis of CS. TT (p<0.0001), BT (p=0.02), and FAI (p=0.003) were significantly elevated in PCOS patients compared to mild CS patients. Sex hormone-binding globulin was similar in both groups. The optimal cut-point for TT was 1.39 nmol/L, yielding a sensitivity of 95 % and a specificity of 70%. The cut-point for BT was 0.24 nmol/L, resulting in a sensitivity of 75 % and a specificity of 80%. The cut-point for FAI was 5.7, with a sensitivity of 88 % and a specificity of 60 %. We conclude that TT levels may be useful to discriminate between mild CS and PCOS. In patients with signs and symptoms consistent with CS and PCOS, a TT level of <1.39nmol/L warrants a workup for CS.
Keywords: Cushing’s syndrome, PCOS, episodic, periodic, androgens, testosterone, ROC
Introduction
Cushing’s syndrome (CS) is a relatively rare disorder with potential catastrophic consequences when diagnosis is delayed. The incidence of the disorder ranges from 0.7 to 2.4 per million population per year, however, recent data suggest that CS is more common than previously thought [1]. Most of the affected adults are aged 25–50, and the female/male ratio is 3:1 to 8:1 [2]. Most published articles on CS have examined patients with sustained and severe hypercortisolism. However, because of increased recognition of CS, a greater number of mild cases of CS are being detected [3]. These patients may have episodic hypercortisolism, so that tests used to diagnose CS may be normal on some occasions and elevated on other occasions [4,5]. Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder of reproductive-aged women, affecting approximately 6–8% [6]. The diagnosis of PCOS requires exclusion of other causes of androgen excess and ovulatory dysfunction disorders, one of those being CS [6]. There are three principal features of PCOS; hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology on ultrasound [6].
The presentations of CS and PCOS are often quite similar. Both diseases can present with weight gain, hirsutism, acne, and irregular periods in women. However, CS usually includes symptoms such as easy bruising, sleep disturbances, decreased libido, a buffalo hump, and stretch marks, which are not present in PCOS. Women with mild and/or periodic CS, however, can be difficult to diagnose as they may lack classic manifestations of the disease and the diagnosis may require multiple hormonal measurements [4, 5, 7]. The treatment of CS is primarily the surgical removal of a pituitary, adrenal, or ectopic tumor. The treatment for PCOS is primarily medical and includes oral contraceptives, biguanides (metformin), thiazolidinediones (rosiglitazone or pioglitazone) or androgen antagonists (spironolactone or flutamide) [8]. Thus, distinguishing the two conditions is important.
We hypothesized that either total testosterone (TT) or bioavailable testosterone (BT) levels or the calculated free androgen index (FAI) would be lower in women with mild CS than in women with PCOS and could be used to help differentiate the two conditions. Therefore, we measured TT and BT, and calculated the FAI in a group of 20 Cushing’s syndrome patients of reproductive age and 20 PCOS patients matched for age and BMI.
Materials and Methods
Patients
Twenty consecutive women diagnosed with mild CS of pituitary origin, < 45 years of age, were compared to 20 women diagnosed with PCOS according to 1990 NIH criteria [9]. Subjects were matched for age (±5 years) and BMI (±4kg/m2). The CS group included 17 Caucasian, 1 Hispanic, 1 Black, and 1 woman of Pacific Island origin. The age range was 21–44 years (median 36 years) and BMI was 37.7±1.7kg/m2 (mean±SEM). The PCOS group included 14 Caucasian, 3 Hispanic, 2 Black, and one woman of Iranian origin. The age range of the PCOS group was 16–39 years (median 31.5 years) and BMI was 37.7±1.6kg/m2 (mean ± SEM). None of the women included in the study were on biguanides, thiazolidinediones, birth control pills, spironolactone, or other treatments for hyperandrogenism.
Diagnosis of mild Cushing’s Syndrome
Diagnosis of mild CS was made if elevated values of at least two of the following tests were found: 1) 24 h urinary free cortisol (UFC), 2) 24 h urinary 17-hydroxycorticosteroids (17-OHS), 3) 2300±0030h salivary cortisol level, or 4) 2300±0100h serum cortisol level [4, 10]. Urine creatinine was measured in 24 h urine samples to insure completeness of collection. The type of CS was then determined by assessing nonsuppressed adrenocortico-tropic hormone (ACTH) level (excluding adrenal adenomas) followed by biochemical testing including dexamethasone suppression, imaging, and if needed, petrosal sinus sampling [11]. The diagnosis of Cushing’s disease was confirmed by pathologic examination at the time of surgery and/or postoperative resolution of hypercortisolism with clinical remission. Although some patients had mild dysthymic or anxiety-related symptoms, no patient had major depression, alcoholism, or other causes of pseudo-Cushing’s syndrome [12]. Two patients with CS were taking antidepressants at the time of diagnosis.
Diagnosis of polycystic ovarian syndrome (PCOS)
Only women with PCOS according to the NIH 1990 criteria were included. Diagnosis included the presence of all of the following: 1) hirsutism and/or hyperandrogenemia, 2) oligo/anovulation, and 3) exclusion of other disorders such as congenital adrenal hyperplasia, androgen secreting tumors, hyperprolactinemia, CS, and thyroid disorders [9]. Oligo-ovulation was defined by a history of eight menstrual cycles per year or less. In women with 26–35 d cycles, ovulatory dysfunction was also defined as a luteal phase progesterone measurement with less than 4 ng/ml. Hirsutism was defined as a modified Ferriman–Gallwey score of 6 or above [13]. The study was approved by the IRBs at The Charles Drew University of Medicine and Science and Cedars-Sinai Medical Center in Los Angeles, CA, USA.
Biochemical testing
As per clinical praxis, blood sampling for TT, BT, and sex hormone-binding globulin (SHBG) was performed in follicular phase in women with PCOS and during any time-point in the mild CS group. All androgen measurements for this study were performed in duplicate at Esoterix Laboratories (Calabasas Hills, CA) and batch processed. Plasma cortisol levels were measured by direct RIA in diluted serum [14]. A positive test was defined as a night-time plasma cortisol greater than 207 nmol/L [15]. Patients taking oral estrogens or birth control pills were excluded from this analysis, as these agents are known to increase total cortisol levels [16, 17]. Salivary cortisol was measured by enzyme immunoassay (EIA) [18] by ACL Laboratories (Milwaukee, WI, USA) [19]. A positive test was defined as a salivary cortisol greater than 4.3nmol/L [18].
Twenty-four-hour UFC was assayed by HPLC Tandem Mass Spectrometry. The 95 % tolerance interval (normal range) for women was determined by measurement in 20 healthy adult females and found to be 28–94 nmol/d in females. A positive test was, therefore, defined as a 24h UFC greater than 94nmol/d. Twenty four-hour urine for 17-OHS was measured as Porter–Silber chromogens following enzymatic hydrolysis and purification by solvent extraction and column chromatography on silica gel [20] and expressed as μmol/d. A positive test was defined as a 24 h 17-OHS greater than 16.6 μmol/d, as defined by the normal range of the laboratory for women. Similar results were obtained when a 24 h 17-OHS/Cr was determined.
Total testosterone was measured by liquid chromatography coupled with mass spectrometry after nonpolar solvent extraction [21, 22]. Testosterone was calculated from a linear plot generated by purified testosterone standards ranging from 0.087 to 173nmol/L. The sensitivity was 0.10nmol/L. The reference range for women of reproductive age as supplied by the laboratory was 0.35–1.9nmol/L, and the intra-assay coefficient of variation (CV) less than 8.1%.
Bioavailable testosterone was determined by separation of the SHBG-bound steroid from albumin-bound and free steroid with ammonium sulfate as described [23, 24]. Serum was incubated with 3 H-testosterone. Sex hormone-binding globulin was precipitated by the addition of ammonium sulfate solution. Samples were centrifuged, and aliquots of the supernatant containing the “non-SHBG bound ” steroid were taken for scintillation counting. The sensitivity of the test was 3 % bioavailable with an intra-assay CV of 1.3–5.8 %. The bioavailable steroid concentration was derived from the product of the total serum testosterone by mass spectrometry and the percent non-SHBG bound steroid determined from the separation procedure. The normal range of BT for women of reproductive age as supplied by the laboratory was 0.038–0.50nmol/L.
The binding capacity of SHBG was directly measured in serum using a displacement technique previously described [23, 24]. Quadruplicate aliquots of each sample were incubated for 1 h with 3 H-testosterone in borate buffer. Two aliquots from each sample were incubated in the presence of purified testosterone standard. Ammonium sulfate precipitation was used to separate free and protein bound steroid. Sensitivity was 0.1mg/dl with an intra-assay CV of 6.7–9.2 % and a normal female range of 35–105nmol/L as supplied by the laboratory.
Free androgen index (unitless) was calculated according to the formula (100×TT/SHBG) as described [25]. Total testosterone levels were measured in all 40 patients, BT was not measured in 4 of the patients with CS, and SHBG/FAI was not measured in 4 of the patients with CS.
Statistical analyses
The receiver operating characteristic (ROC) curves were generated using the binomial model as suggested by Metz [26]. The area under the curve (AUC) was determined from this model. Area under the curve represents accuracy, with a value of 0.90–1.0=excellent, 0.80–0.90=good and 0.70–0.80=fair [26]. The determination of the optimal cut-point for the various laboratory criteria considered was based on the minimization of average cost of evaluation as suggested by Metz [26] and Zhou et al. [27]. A 10 % prevalence for CS in the population to be screened was used to perform these calculations. Differences in groups were evaluated by Student’s t-test and a p value of <0.05 was considered significant.
Results
Table 1 depicts the abnormal biochemical values in the 20 patients diagnosed with CS. All subjects with CS reported that their symptoms were more severe at certain times suggesting episodic hypercortisolism. In this group, 16 of 20 patients had at least one UFC in the normal range, supporting the diagnosis of episodic hypercortisolism. All patients had a maximal UFC less than three times the upper limit of the normal range for UFC, supporting the diagnosis of mild CS [10]. The clinical presentation of the mild CS patients and PCOS patients was similar. Fourteen of 19 (74 %) patients with mild CS had oligomennorhea. One subject had had a hysterectomy. All patients with PCOS had oligomennorhea. Twelve of 20 (60 %) patients with CS had hirsutism, while 12 of 20 (60 %) patients with PCOS had hirsutism. Data in Table 2 reveal that the age and BMI of CS patients and PCOS patients do not differ.
Table 1.
Abnormal biochemical test conducted in 20 patients with Cushing’s syndrome.
| Patient | Abnormal bio-chemical test | Test | ||
|---|---|---|---|---|
| 1 | Salivary F X3 | Night F | 17OHS X5 | |
| 2 | Salivary F X1 | UFC X1 | ||
| 3 | Night F | UFC X2 | ||
| 4 | Salivary F X2 | UFC X3 | ||
| 5 | Salivary F X3 | Night F | UFC X1 | 17OHS X2 |
| 6 | Salivary F X3 | Night F | 17OHS X1 | |
| 7 | Salivary F X2 | Night F | 17OHS X1 | |
| 8 | Salivary F X1 | 17OHS X2 | ||
| 9 | Salivary F X1 | 17OHS X3 | ||
| 10 | Night F | UFC X1 | 17OHS X1 | |
| 11 | Salivary F X2 | UFC X1 | ||
| 12 | Salivary F X3 | UFC X1 | 17 OHS X1 | |
| 13 | Salivary F X4 | Night F | UFC X1 | 17 OHS X1 |
| 14 | UFC X3 | 17 OHS X4 | ||
| 15 | Salivary F X2 | UFC X4 | 17 OHS X4 | |
| 16 | Salivary F X2 | Night F | UFC X4 | 17 OHS X1 |
| 17 | Salivary F X1 | UFC X1 | 17 OHS X3 | |
| 18 | UFC X1 | 17 OHS X3 | ||
| 19 | Salivary F X4 | 17 OHS X1 | ||
| 20 | UFC X4 | 17 OHS X2 |
F: cortisol; UFC: urinary free cortisol; 17OHS: 17-hydroxycorticosteroids; Salivary F: salivary cortisol. Salivary F was collected at 2300 ± 0030 h (abnormal value of cortisol >4.3mmol/l). Night F plasma cortisol was collected between 2300±0100h (abnormal value-cortisol ≥ 207nmol/L). UFC and 17OHS were collected from 0800 to 0800h the following day (abnormal value of UFC >94nmol/day, 17OHS >16.6 μmol/day)
Table 2.
Demographic characteristics and androgen levels in women with Cushing’s syndrome and PCOS.
| Test | Cushing’s syndrome | PCOS | p-Value |
|---|---|---|---|
| age (years) | 34.0±6.7 | 29.8±7.6 | 0.08 |
| BMI (kg/m2) | 37.7±1.7 | 37.7±1.6 | 0.99 |
| total testosterone (nmol/L) | 0.75±0.08 | 1.75±0.17 | <0.0001 |
| bioavailable testosterone (nmol/L) | 0.29±0.08 | 0.56±0.08 | 0.02 |
| SHBG (nmol/L) | 28.4±4.1 | 28.0±4.6 | 0.95 |
| FAI (testosterone/SHBG) | 3.43±0.66 | 9.30±1.60 | 0.003 |
All values are mean±SEM, except age, which is mean±SD
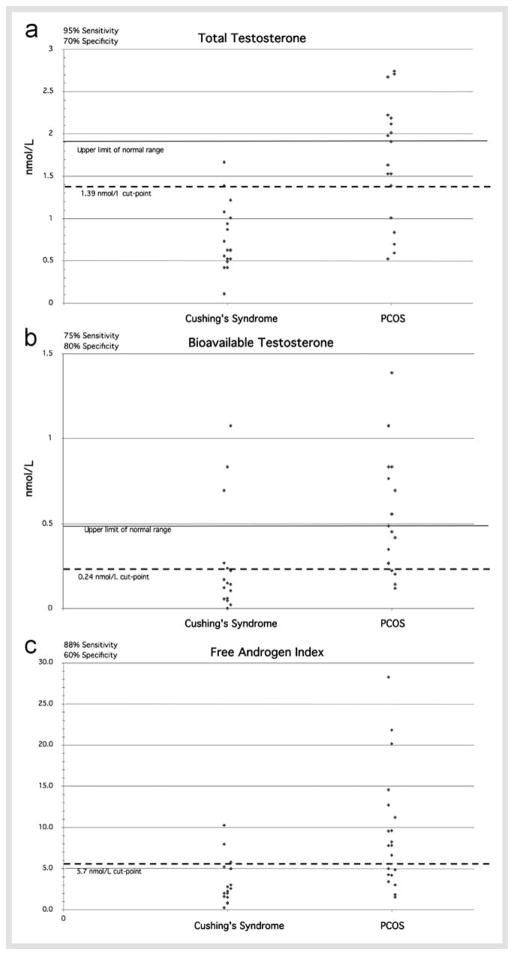
As shown in Table 2 and  Fig. 1, TT (
Fig. 1, TT ( Fig. 1A, p<0.0001), BT (
Fig. 1A, p<0.0001), BT ( Fig. 1B, p=0.02), and FAI (
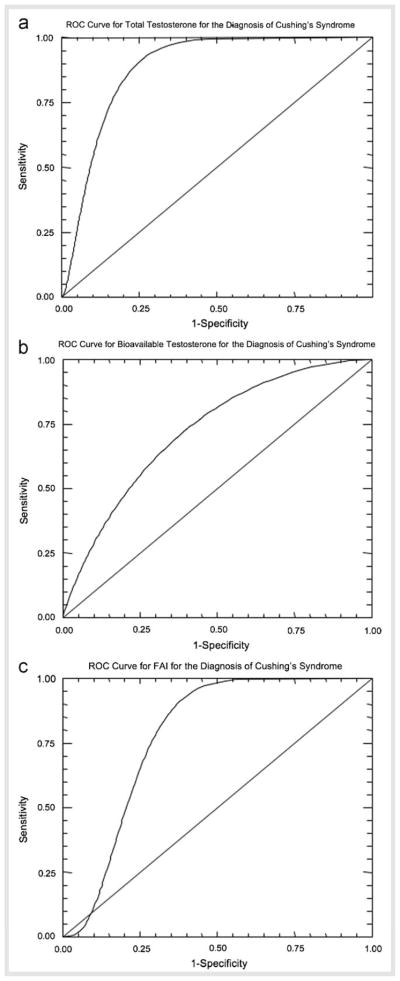
Fig. 1B, p=0.02), and FAI ( Fig. 1C, p=0.003) were significantly elevated in PCOS patients as compared to CS patients. Sex hormone-binding globulin was similar in both groups (Table 1). The ROC curves shown in
Fig. 1C, p=0.003) were significantly elevated in PCOS patients as compared to CS patients. Sex hormone-binding globulin was similar in both groups (Table 1). The ROC curves shown in  Fig. 2 for TT (
Fig. 2 for TT ( Fig. 2A), BT (
Fig. 2A), BT ( Fig. 2B), and FAI (
Fig. 2B), and FAI ( Fig. 2C) were used to determine a cut-point for each value that gave the optimum sensitivity and sensitivity for the diagnosis of CS (Table 3). Total testosterone gave the highest sensitivity, positive predictive value (PPV) and negative predictive value (NPV) while BT gave the highest specificity for the diagnosis of CS. Determinations AUC demonstrate that use of a TT level gave “good” diagnostic accuracy, while BT and FAI gave “fair ” diagnostic accuracy.
Fig. 2C) were used to determine a cut-point for each value that gave the optimum sensitivity and sensitivity for the diagnosis of CS (Table 3). Total testosterone gave the highest sensitivity, positive predictive value (PPV) and negative predictive value (NPV) while BT gave the highest specificity for the diagnosis of CS. Determinations AUC demonstrate that use of a TT level gave “good” diagnostic accuracy, while BT and FAI gave “fair ” diagnostic accuracy.
Fig. 1.
Total testosterone (A), bioavailable testosterone (B), and free androgen index (FAI) (C) in PCOS and CS patients. The upper limit of the normal range (except for FAI), mean (short thick bar), optimal cut-point and resultant sensitivity and specificity for the diagnosis of CS is depicted.
Fig. 2.
Receiver operating characteristic (ROC) curves for total testosterone (A), bioavailable testosterone (B), and FAI (C) for the diagnosis of CS. The diagonal line represents sensitivity and specificity that would be expected for a random test.
Table 3.
Cut-points for the optimal diagnosis of Cushing’s syndrome, the resultant sensitivity and specificity, and the area under the curve (AUC).
| Test | Cut-point | Sensitivity | Specificity | AUC | NPV | PPV |
|---|---|---|---|---|---|---|
| total testosterone (nmol/L) | 1.39 | 95% | 70% | 0.87 | 0.93 | 0.76 |
| bioavailable testosterone (nmol/L) | 0.24 | 75% | 80% | 0.77 | 0.80 | 0.75 |
| FAI | 5.7 | 88% | 60% | 0.79 | 0.80 | 0.69 |
Positive predictive value (PPV) and negative predictive value (NPV) are also depicted for each study
Discussion
This is the first study comparing TT, BT, and FAI levels between PCOS and mild CS in order to evaluate if cutoff values could help differentiate the two conditions. The PCOS group had significantly higher levels of these hormones as compared to the mild CS group. We were able to define cut-points for TT, BT, and FAI, which could be used to help distinguish the two syndromes. In the mild CS group, TT<1.39nmol/L, had the highest AUC (proportional to diagnostic accuracy), PPV, NPV, as well as specificity (95 and 75 %), as compared to BT and FAI. The present study suggests that women with TT<1.39nmol/L, when analyzed by mass spectrometry, should be evaluated for CS especially if they have clinical stigmata of the condition.
Identification of mild CS is important as surgery may provide a cure for these patients. Recent studies by our group have shown that a single overnight dexamethasone suppression test or urine, serum or salivary cortisol measurement may be normal in some CS patients [10].
Prior studies have found that women with sustained CS have low androgen levels. Kaltsas et al. [28] examined the occurrence of PCOS and polycystic ovaries in 13 women with sustained CS. Androstenedione, DHEAS, and testosterone levels were within normal limits in women with CS, but lower as compared to the PCOS group. Patients with CS and higher cortisol secretion developed hypogonadotrophic hypogonadism. Lado-Abeal et al. [29] demonstrated that hypercortisolism, not hyperandrogenism, had an inhibitory effect on hypothalamic control resulting in menstrual abnormalities in women with CS. Glucocorticoids may also exert a direct inhibitory effect on ovarian steroid output, further reducing testosterone production [30, 31]. In the current study, we have examined testosterone production (made primarily by the ovaries in women) and not adrenal androgens, including DHEAS and androstenedione. As adrenal androgens, but not ovarian testosterone production is stimulated by ACTH [32], adrenal androgens might be elevated in both CS and PCOS and would not be useful to distinguish between the two conditions. Cunnigham and Mckenna [33] found that 4 of 15 women with Cushing’s disease had elevated serum DHEAS levels, 8 of 15 had normal levels, and 23 of 15 had marginally low DHEAS levels. They also found suppressed serum DHEA levels in 3 of 12 women with Cushing’s disease (normal values in the other 9 patients) and normal serum testosterone levels in all their female patients, but an elevated FAI in most of their female patients.
There are several limitations to our study. The prevalence of CS is low and thus it is difficult to conduct larger studies on this population. Only subjects identified with mild CS of pituitary origin were included and therefore the cut-points of testosterone levels cannot be applied to patients with severe CS or adrenal or ectopic CS. The hormonal analysis of PCOS subjects was performed in the follicular phase; while analysis in CS subjects was performed throughout the menstrual cycle since cycle-dependent analysis has not been praxis when evaluating these patients. Free and total testosterone levels, measured in precise assays, validated in women, demonstrate a mild degree of fluctuation with phase of cycle with a small peak a few days prior to ovulation and with slightly lower levels in the early follicular phase [34]. Thus, the androgen levels of the CS patients who may have had their blood drawn during the pre-ovulatory phase could have been lower than if the blood had been drawn in follicular phase. In these cases, the differences in testosterone levels between PCOS and mild CS patients would have been greater.
There is variability between androgen assays in different laboratories. Most commercial assays lack the accuracy and precision of assays when measuring testosterone in women, as recently reviewed in an Endocrine Society position statement [35]. The use of extraction and chromatography followed by mass spectroscopy was recommended, as was done in the present study. Thus, the cut-points mentioned here using mass spectroscopy analysis and performed at Esoterix Laboratories may be different in testosterone assays performed at other laboratories.
The findings in our study do not lead to the conclusion that testosterone measurement should replace assessment of cortisol production or dexamethasone suppression in those patients in whom CS is being considered. We did not measure cortisol production or postdexamethasone cortisol levels in our PCOS subjects. Rather, testosterone measurements should be used as an additional piece of information available to the clinician confronted with a patient who has signs and symptoms consistent with either PCOS or CS. In fact, a prior study has demonstrated that PCOS patients had elevated UFC and midnight cortisol, but not post-dexamethasone cortisol levels compared to obese subjects [36]. Additionally, as discussed above and as shown in Table 1, patients with mild or episodic CS can have some normal urine UFC or 17-OHS determinations, night-time plasma, or salivary cortisol determinations [4, 7], or postdexamethasone cortisol levels [10] interspersed with high values. Thus, there is likely to be an overlap when determining cortisol production or dexamethasone suppression in patients with either PCOS or mild CS.
In conclusion, the presenting phenotypes of women with mild CS and PCOS may be similar. This study demonstrates that analysis of serum testosterone, using a well-validated assay may be helpful to distinguish patients with symptoms of androgen excess who should be evaluated for CS.
Acknowledgments
Dr. Friedman was supported in part by the following grants: U54 RR14616, S06 GM068510, U54 HD41748, and R25 RR019488. We thank Ricardo Azziz, M.D. (Cedars-Sinai Medical Center) for allowing us to use samples from PCOS patients under his care.
Footnotes
Some of this work was presented in abstract form at the 88th Endocrine Society meeting in 2005.
References
- 1.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 2.Boscaro M, Barzon L, Fallo F, Sonino N. Cushing’s syndrome. Lancet. 2001;357:783–791. doi: 10.1016/S0140-6736(00)04172-6. [DOI] [PubMed] [Google Scholar]
- 3.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 4.Friedman TC, Zuckerbraun E, Lee ML, Kabil MS, Shahinian H. Dynamic pituitary MRI has high sensitivity and specificity for the diagnosis of mild Cushing’s syndrome and should be part of the initial workup. Horm Metab Res. 2007;39:451–456. doi: 10.1055/s-2007-980192. [DOI] [PubMed] [Google Scholar]
- 5.Velez DA, Mayberg MR, Ludlam WH. Cyclic Cushing syndrome: definitions and treatment implications. Neurosurg Focus. 2007;23:E4. doi: 10.3171/foc.2007.23.3.5. discussion E4a. [DOI] [PubMed] [Google Scholar]
- 6.Trivax B, Azziz R. Diagnosis of polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:168–177. doi: 10.1097/GRF.0b013e31802f351b. [DOI] [PubMed] [Google Scholar]
- 7.Friedman TC, Zuckerbraun E, Daigle K, Shahinian H. The changing face of Cushing’s syndrome: mild and periodic cases makes the diagnosis more difficult. Endocrine Soc (Abstract) 2004;86:P3–408. [Google Scholar]
- 8.Dronavalli S, Ehrmann DA. Pharmacologic therapy of polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:244–254. doi: 10.1097/GRF.0b013e31802f35a0. [DOI] [PubMed] [Google Scholar]
- 9.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovarian syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 10.Friedman TC. An update on the overnight dexamethasone suppression test for the diagnosis of Cushing’s syndrome: limitations in patients with mild and/or episodic hypercortisolism. Exp Clin Endocrinol Diabetes. 2006;114:356–360. doi: 10.1055/s-2006-924281. [DOI] [PubMed] [Google Scholar]
- 11.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 12.Friedman TC. Pseudo-Cushing syndrome. In: Margioris AN, Chrousos GP, editors. Contemporary Endocrinology: Adrenal Disorders. Totowa, NJ: Humana Press; 2001. pp. 203–218. [Google Scholar]
- 13.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 14.Foster LB, Dunn RT. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin Chem. 1974;20:365–368. [PubMed] [Google Scholar]
- 15.Papanicolaou DA, Yanovski JA, Cutler G, Jr, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing’s syndrome from pseudo-Cushing states. J Clin Endocrinol Metab. 1998;83:1163–1167. doi: 10.1210/jcem.83.4.4733. [DOI] [PubMed] [Google Scholar]
- 16.Burke CW. Biologically active cortisol in plasma of oestrogen-treated and normal subjects. Br Med J. 1969;2:798–800. doi: 10.1136/bmj.2.5660.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doe RP, Zinneman HH, Flink HB, Ulstrom RA. Significance of the concentration of non protein bound plasma cortisol in normal subjects, Cushing’s syndrome, pregnancy and during estrogen therapy. J Clin Endocrinol Metab. 1960;20:1484–1492. doi: 10.1210/jcem-20-11-1484. [DOI] [PubMed] [Google Scholar]
- 18.Ra H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–204. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- 19.Ra H, Ra JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:2681–2686. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- 20.Silber RH, Porter CC. The determination of 17,21-dihydroxy-20-keto-steroids in urine and plasma. J Biol Chem. 1954;210:923–932. [PubMed] [Google Scholar]
- 21.Furuyama S, Mayes DM, Nugent CA. A radioimmunoassay for plasma testosterone. Steroids. 1970;16:415–428. doi: 10.1016/s0039-128x(70)80124-6. [DOI] [PubMed] [Google Scholar]
- 22.Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffer J, Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2006;91:1683–1690. doi: 10.1210/jc.2005-2596. [DOI] [PubMed] [Google Scholar]
- 23.Mayes D, Nugent CA. Determination of plasma testosterone by the use of competitive protein binding. J Clin Endocrinol Metab. 1968;28:1169–1176. doi: 10.1210/jcem-28-8-1169. [DOI] [PubMed] [Google Scholar]
- 24.Nankin HR, Pinto R, Fan DF, Troen P. Daytime titers of testosterone, LH, estrone, estradiol, and testosterone-binding protein: acute effects of LH and LH-releasing hormone in men. J Clin Endocrinol Metab. 1975;41:271–281. doi: 10.1210/jcem-41-2-271. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor P, Luttrell BM, Williams D. The free androgen index is not valid for adult males. J Steroid Biochem Mol Biol. 1993;45:325–326. doi: 10.1016/0960-0760(93)90350-6. [DOI] [PubMed] [Google Scholar]
- 26.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Obuchowski N, MacClish D. Statistical Methods in Diagnostic Medicine. New York: 2002. [Google Scholar]
- 28.Kaltsas GA, Korbonits M, Isidori AM, Webb JA, Trainer PJ, Monson JP, Besser GM, Grossman AB. How common are polycystic ovaries and the polycystic ovarian syndrome in women with Cushing’s syndrome? Clin Endocrinol (Oxf) 2000;53:493–500. doi: 10.1046/j.1365-2265.2000.01117.x. [DOI] [PubMed] [Google Scholar]
- 29.Lado-Abeal J, Rodriguez-Arnao J, Newell-Price JD, Perry LA, Grossman AB, Besser GM, Trainer PJ. Menstrual abnormalities in women with Cushing’s disease are correlated with hypercortisolemia rather than raised circulating androgen levels. J Clin Endocrinol Metab. 1998;83:3083–3088. doi: 10.1210/jcem.83.9.5084. [DOI] [PubMed] [Google Scholar]
- 30.Karpas AE, Rodriguez-Rigau LJ, Smith KD, Steinberger E. Effect of acute and chronic androgen suppression by glucocorticoids on gonadotropin levels in hirsute women. J Clin Endocrinol Metab. 1984;59:780–784. doi: 10.1210/jcem-59-4-780. [DOI] [PubMed] [Google Scholar]
- 31.Odagiri E, Yamanaka Y, Ishiwatari N, Jibiki K, Demura R, Demura H, Suda T, Shizume K. Studies on pituitary-gonadal function in patients with Cushing’s syndrome. Endocrinol Jpn. 1988;35:421–427. doi: 10.1507/endocrj1954.35.421. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DC. The adrenal androgen-stimulating hormone does not exist. Lancet. 1980;2:454–456. doi: 10.1016/s0140-6736(80)91889-9. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham SK, MacKenna TJ. Dissociation of adrenal androgen and cortisol secretion in Cushing’s syndrome. Clin Endocrinol (Oxf) 1994;41:795–800. doi: 10.1111/j.1365-2265.1994.tb02795.x. [DOI] [PubMed] [Google Scholar]
- 34.Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1998;83:1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 35.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 36.Putignano P, Bertolini M, Losa M, Cavagnini F. Screening for Cushing’s syndrome in obese women with and without polycystic ovary syndrome. J Endocrinol Invest. 2003;26:539–544. doi: 10.1007/BF03345217. [DOI] [PubMed] [Google Scholar]