IT IS generally recognized that at steady-state conditions less than 10% of cyclosporine (CyA)1,2 or FK 5063 is excreted by the kidney and that renal function has little or no effect on the blood or plasma levels of either of these two drugs. Experimental studies, however, have shown that animals with severely impaired renal function have higher blood CyA trough levels than do controls.4,5 Therefore the presence of renal ischemia and/or nephrotoxicity may have an effect as yet unidentified on the regulation of the CyA or FK 506 blood level.
To investigate this question, the effect of renal ischemia on single and multiple IV administration of FK 506 was studied in rats subjected to unilateral left nephrectomy and contralateral total renal ischemia.
MATERIALS AND METHODS
Experimental Protocol
These experiments were carried out in male Lewis rats weighing 200 to 250 g. All of the animals had been acclimatized to the Animal Research Laboratory for at least 1 week before being utilized for these studies. The animals were anesthetized with metofane administered by inhalation and when fully anesthetized, they were subjected to laparotomy and renal manipulation as described below. The animals were divided into five groups as follows.
Experiment 1 (Single Dose Administration)
Group 1 (n = 10): a left nephrectomy was performed and a single dose of FK 506 (0.3 mg/kg in 0.5 mL saline) was injected IV via the inferior vena cava (lVC).
Group 2 (n = 10): the right perirenal fascia was dissected. The right renal vessels were identified and clamped for a 60-minute period using nontraumatizing microvascular clamps. Upon reperfusion of the right kidney, a left nephrectomy was peliormed and a similar dose of FK 506 was injected IV via the IVC.
From each animal in groups 1 and 2, blood samples (0.5 mL) were withdrawn from the jugular vein at 10, 20, 30, 60, 120, and 240 minutes after saline or FK 506 administration for determination of the plasma FK 506 levels. The blood withdrawn from each rat at each time point was replaced with an equal volume of heparinized Ringers lactate.
Experiment 2 (Multiple FK 506 Administration)
Group 3 (n = 20): animals in this group were subjected to a left nephrectomy and a daily IV administration ofFK 506 (0.3 mg/kg) via the jugular vein for a period of 10 days.
Group 4 (n = 20): animals in this group were subjected to a left nephrectomy, right renal ischemia for a 60-minute period, and a daily IV administration of FK 506 (0.3 mg/kg) for 10 days.
Group 5 (n = 10): animals in this group were subjected to a left nephrectomy and right renal ischemia for a period of 60 minutes without FK 506 administration.
All animals were allowed to recover spontaneously and postoperatively were observed regularly at 12-hour intervals for a total of 10 days. From each animal, blood samples were collected (from the jugular vein) at days 0 (preoperative), 1, 2, 3, 5, 7, and 10 (postoperatively) for blood urea nitrogen (BUN), creatinine, alanine transaminase (AL T), and FK 506 levels. The blood withdrawn from each rat was replaced with an equal volume of lactated Ringers solution.
Biochemical Analyses and FK 506 Bioassay
Serum levels of BUN, creatinine, and ALT were estimated using standard biochemical methods and diagnostic kits obtained from Sigma. FK 506 plasma concentrations were measured by a monoclonal antibody (MAb)-based enzyme-linked immunosorbent assay (ELISA) (Fujisawa Pharmaceuticals Co Ltd, Osaka, Japan) as described elsewhere.6
Statistical Analyses
Data are reported as the mean and the standard error ofthe mean. BUN and creatinine data were analyzed using the two-way analysis of variance. Comparisons between the means were performed using the Student's t test. The chi-square test was used to compare the proportions of surviving animals between groups. A P value of < .05 was considered to be significant.
RESULTS
Experiment 1
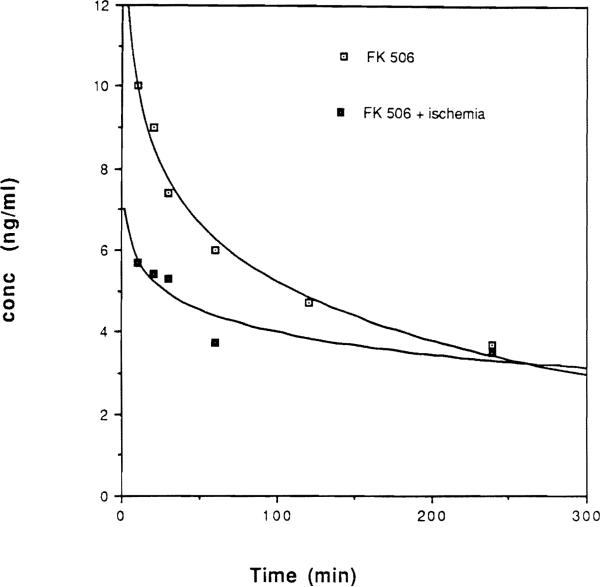
Plasma levels after a single IV dose administration of FK 506 were fit by a monoexponential curve assuming that FK 506 was distributed in a single compartment (Fig 1). The rate constant of FK 506 elimination from blood was 0.004 minute−1 and 0.0038 minute−1 for the data obtained from the nonischemic (group 1) and renal ischemic (group 2) rats, respectively. Consequently, there was no difference between the FK 506 half-lives in the nonischemic and ischemic animals. The corresponding half-life times were 173.3 minutes and 182.4 minutes, respectively.
Fig 1.
Monoexponential curve for plasma levels of FK 506 after a single IV dose administration in group 1 (renal non ischemic) and group 2 (renal ischemic) animals.
Experiment 2
Plasma FK 506 Levels
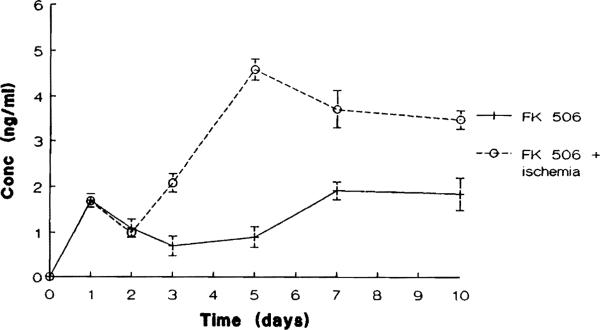
As may be seen in Fig 2, after repeated daily administration of FK 506 for 10 days, the steady-state levels of FK 506 found in the renal ischemic rats (group 4) were significantly greater than those seen in the renal nonischemic animals (group 3) (P < .02). On days 1 and 2 after operation, the plasma FK 506 levels were similar in both groups. However, the FK 506 levels increased rapidly on days 3 and 5 in the renal ischemic animals as compared with the renal nonischemic animals.
Fig 2.
Steady state levels of FK 506 found in group 3 (renal nonischemic) and group 4 (renal ischemic) after repeated daily administration of FK 506 (*P < .02).
Animal Mortality and Morbidity
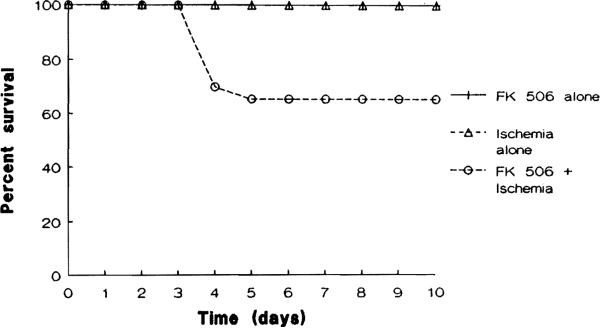
All animals in groups 3 (FK 506 alone) and 5 (ischemia alone) survived throughout the total 10-day period of the experiment. The survival of group 4 animals (FK 506 + ischemia) was reduced to 65% at 5 days postoperatively and was maintained thereafter through 10 days (P < .02) (Fig 3).
Fig 3.
Survival time course of studied animals in group 3 (FK 506 alone), group 4 (FK 506 + ischemia), and group 5 (ischemia alone) (*P < .02).
Rats subjected to both renal ischemia and repeated administration of FK 506 (group 4) appeared somewhat emaciated with a moderate degree of hair loss as compared with rats subjected to either alone. A postoperative mean weight loss of 25.3 ± 2% and severe diarrhea (five rats) were noted also in the group 4 animals. The animals that died were not autopsied. Their deaths were attributed to renal failure as a result of ischemia, FK 506 renal toxicity, or both, which were amplified by hypovolemia occurring as a result of the diarrhea and lack of oral intake they experienced.
Hepatic Injury and Renal Function
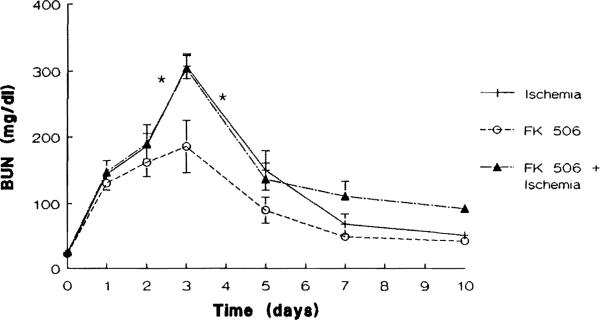
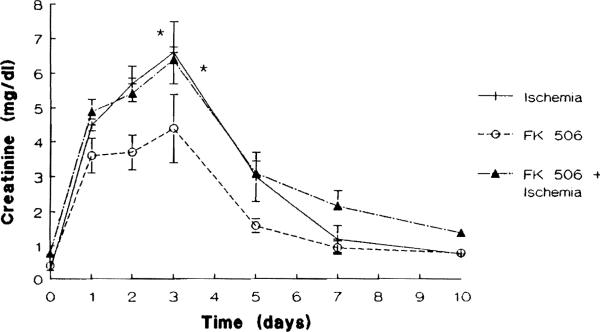
Liver injury as assessed by the serum ALT level remained normal in all groups studied throughout the duration of the study. As shown in Figures 4 and 5, renal function remained stable in the group 3 animals that received FK 506 daily for 10 days. Animals in groups 4 (FK 506 + ischemia) and those in group 5 (ischemia alone) had similar abnormal serum BUN and creatinine levels. Both groups showed a marked deterioration of renal function with a maximum elevation of BUN and creatinine levels on day 3 after operation (P < .02). From this point on renal function improved daily until day 10 when the mean BUN and creatinine levels were not significantly different from those seen in the group 3 renal nonischemic animals.
Fig 4.
Mean serum levels of BUN (mg/dL) in group 3 (FK 506 alone), group 4 (FK 506 + ischemia), and group 5 (ischemia alone) animals (*P < .02).
Fig 5.
Mean creatinine levels (mg/dL) in group 3 (FK 506 alone), group 4 (FK 506 + ischemia), and group 5 (ischemia alone) animals (*P < .02).
DISCUSSION
FK 506 is a potent immunosuppressive agent with a high efficacy and a low incidence of side effects.7–9 Similar to CyA, FK 506 is almost completely metabolized in the liver before elimination from the body and only a small percentage of the drug is excreted in the urine. This finding suggested that, in clinical transplantation, changes in FK 506 dosing regimen may not be necessary in patients with renal failure or in patients on dialysis, but may be necessary in patients with hepatic dysfunction.
In clinical transplantation, it is difficult to establish whether renal dysfunction plays a role in determining CyA or FK 506 blood levels. It has been reported that the elimination of parent CyA in the urine is not markedly different between patients with advanced renal failure and bone marrow recipients with normal renal function.10 In contrast, experimental studies have shown that animals with deteriorating renal function had significantly higher CyA levels than animals with improving renal function. 4,5
To date there is little if any studies on the renal handling of FK 506 when function is impaired. In the present study, our results demonstrated that there was no difference in the rate constant of FK 506 elimination between ischemic and nonischemic rats after a single IV dose of the drug (Fig 1). However, with repeated FK 506 administration over 10 days, renal ischemia resulted in an increase in plasma FK 506 levels as compared with those seen in nonischemic animals (Fig 2). The high plasma levels of FK 506 declined from day 5 to day 10 after operation as renal function markedly improved. This indicates that the plasma level of FK 506 is altered by the injury associated with ischemia and reperfusion and that renal impairment was responsible for changes in FK 506 distribution kinetics. The possibility of involvement of the liver in the elevation of plasma FK 506 levels may be ruled out by the normal ALT level herein observed throughout the experiment.
Experimental data have shown that the nephrotoxic effect of CyA is more pronounced in the presence of a renal ischemic insult. and that these two factors may act synergistically to produce a more severe renal functional impairment than is caused by either alone. 11-14 Our results showed that the daily IV administration of FK 506 at a dose of 0.3 mg/kg for a period of 10 days causes no impairment of renal function. Whereas a 60-minute period of ischemia resulted in deterioration of renal function, the concomitant daily administration of FK 506 did not aggravate the renal function impairment (Fig 4 and 5) despite the elevated plasma FK 506 levels seen in such cases. However, it is noteworthy that in clinical transplantation, although some reports of CyA nephrotoxicity have disclosed a close association between drug level and serum creatinine,15 with FK 506 a poor correlation exists between drug level and serum creatinine.9 It must be noted, however, that current FK 506 assays use a MAb that is not specific for FK 506 alone but also measures metabolites. While CyA-induced acute renal failure has been described, usually associated with toxic drug levels, this phenomenon was not noted with FK 506. It is therefore unlikely that changes in serum creatinine or BUN levels will be a reliable marker of FK 506 toxicity.9
Previous studies have shown that rats can tolerate well a repeated administration of FK 506 at a dose of 1 mg/kg/d with no impairment in renal or liver functions.16 In contrast, the present study shows that the repeated administration of FK 506 at a dose of 0.3 mg/kg/d in the presence of a renal ischemic injury resulted in animal morbidity and even mortality (Fig 3), which were not observed with either alone. The profound weight loss herein observed suggests that FK 506 (in high levels) may result in diminished appetite and/or augmented catabolism, which may contribute to the raised urea levels. Severe malnutrition and diarrhea were clearly major factors leading to the deaths seen in this group since the high levels of BUN and creatinine seen with renal ischemia alone were not associated with any decrease in survival. No overt evidence of a change in behavior was noted between FK 506 and control treated animals.
From the data presented it may be concluded that rats tolerate a daily IV dose of FK 506 (0.3 mg/kg) without impairment of renal function, animal morbidity or mortality. A 60-minute period of total renal ischemia results in reversible elevations of serum BUN and creatinine levels but no mortality. Renal ischemic injury results in increase of plasma FK 506 levels, but within the limits of this study the high levels of FK 506 do not further aggravate the renal function impairment caused by ischemia.
Based on these data it is possible to speculate that in clinical transplantation, although the kidney normally plays a relatively minor role in the clearance of FK 506, its importance in the presence of severe renal impairment should not be ignored. Consequently, FK 506 blood levels should be monitored in patients with severe renal dysfunction because even transiently high levels may increase the risk of drug toxicity, especially in the immediate postoperative period when the kidney may have experienced an ischemic insult. Additionally, with the return of renal function it is possible that plasma FK 506 concentrations may fall to levels that are inadequate for effective immunosuppression.
Acknowledgments
This work was supported in part by grants from NIDDK AM32556 and AM39789.
REFERENCES
- 1.Wood AJ, Maurer G, Niederberger W, et al. Transplant Proc. 1983;15:2409. [Google Scholar]
- 2.Maurer G. Transplant Proc. 1985;17:19. [PubMed] [Google Scholar]
- 3.Venkataramanan R, Jain E, Cadoff E, et al. Transplant Proc. 1990;22:52. [PMC free article] [PubMed] [Google Scholar]
- 4.Karim MS, Wood RFM. Transplant Proc. in press. [Google Scholar]
- 5.Karim MS, Wood RFM, Dawnay AB, et al. Transplantation. 1990;49:500. doi: 10.1097/00007890-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cadoff EM, Venkataramanan R, Krajack A, et al. Transplant Proc. 1990;22:50. [PMC free article] [PubMed] [Google Scholar]
- 7.Fung JJ, Todo S, Jain A, et al. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Fung JJ, Demetris AJ, et al. Transplant Proc. 1990;22:13. [PMC free article] [PubMed] [Google Scholar]
- 9.McCauley J, Fung J, Jain A, et al. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 10.Follath F, Wenk M, Vozeh S, et al. Clin Pharmacal Ther. 1983;34:638. doi: 10.1038/clpt.1983.226. [DOI] [PubMed] [Google Scholar]
- 11.Chow S, Thorner P, Baumal R, et al. Transplantation. 1986;41:152. doi: 10.1097/00007890-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Jablonski P, Harrison C, Howden B, et al. Transplantation. 1986;41:147. doi: 10.1097/00007890-198602000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gianello P, Ramboux A, Poelart D, et al. Transplantation. 1989;47:512. [PubMed] [Google Scholar]
- 14.Kanazi G, Stowe N, Steinmuller D, et al. Transplantation. 1986;41:782. doi: 10.1097/00007890-198606000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Meyers BG. Kidney Int. 1986;30:964. doi: 10.1038/ki.1986.280. [DOI] [PubMed] [Google Scholar]
- 16.Stephen ME, Woo J, Hasan NU, et al. Transplantation. 1989;47:60. doi: 10.1097/00007890-198901000-00014. [DOI] [PubMed] [Google Scholar]