Abstract
Study Objectivies:
Several studies have investigated slow wave sleep EEG parameters, including slow-wave activity (SWA) in relation to somnambulism, but results have been both inconsistent and contradictory. The first goal of the present study was to conduct a quantitative analysis of sleepwalkers' sleep EEG by studying fluctuations in spectral power for delta (1-4 Hz) and slow delta (0.5-1 Hz) before the onset of somnambulistic episodes. A secondary aim was to detect slow-wave oscillations to examine changes in their amplitude and density prior to behavioral episodes.
Participants:
Twenty-two adult sleepwalkers were investigated polysomnographically following 25 h of sleep deprivation.
Results:
Analysis of patients' sleep EEG over the 200 sec prior to the episodes' onset revealed that the episodes were not preceded by a gradual increase in spectral power for either delta or slow delta over frontal, central, or parietal leads. However, time course comparisons revealed significant changes in the density of slow-wave oscillations as well as in very slow oscillations with significant increases occurring during the final 20 sec immediately preceding episode onset.
Conclusions:
The specificity of these sleep EEG parameters for the occurrence and diagnosis of NREM parasomnias remains to be determined.
Citation:
Jaar O; Pilon M; Carrier J; Montplaisir J; Zadra A. Analysis of slow-wave activity and slow-wave oscillations prior to somnambulism. SLEEP 2010;33(11):1511-1516.
Keywords: Sleepwalking, somnambulism, parasomnias, sleep EEG, slow-wave activity, slow-wave oscillations, sleep deprivation
SOMNAMBULISM (SLEEPWALKING) IS CONSIDERED TO BE A “DISORDER OF AROUSAL”1 AND IS CHARACTERIZED BY BEHAVIORAL MANIFESTATIONS, misperception and relative unresponsiveness to the environment, impaired judgment, and variable retrograde amnesia.2,3 Somnambulistic episodes of varying degrees of complexity and duration generally arise from incomplete awakenings out of slow-wave sleep (SWS; stages 3 and 4) and sometimes from stage 2 sleep.4–6 Sleepwalking is a common parasomnia, affecting up to 4% of adults,7,8 and represents a leading cause of sleep-related violence and self-injury.4,9–12
Although the occurrence of behavioral episodes in predisposed individuals can be facilitated by the use of sleep deprivation13,14 or experimentally induced via the presentation of auditory stimuli during SWS,15 the mechanisms which give rise to naturally occurring somnambulism remain unclear.16,17 The sleep of somnambulistic patients is characterized by non-rapid eye movement (NREM) sleep instability, including an inability to maintain consolidated periods of SWS.6,18,19 Considerable attention has thus focused on the potential role of sleep EEG parameters during periods of SWS immediately preceding episode onset.
One of the first sleep EEG variables investigated in relation to sleepwalking was hypersynchronous delta activity (HSD), usually described as continuous high voltage (> 150 μV) delta waves occurring during SWS or immediately prior to an episode.4,20,21 Although early reports suggested HSD was associated with episode onset,21 subsequent studies revealed that HSD occurs frequently in patients' NREM sleep that is not accompanied by sleepwalking episodes15,20,22,23 and that regardless of how it is measured, HSD has low specificity for the diagnosis of NREM parasomnias.24–26
Another sleep parameter investigated in relation to sleepwalking is EEG slow wave activity (SWA; spectral power between 0.5 and 4.5 Hz), a quantitative measure of NREM sleep dynamics and an indicator of sleep depth or sleep intensity. As SWS progresses within sleep cycles, hypersynchronization of thalamo-cortical and cortical neuron discharges begin to appear, generating high-amplitude delta waves which characterize SWS EEG recordings.27–30 Several investigations6,18,19,31 of SWA in adult sleepwalkers and controls report that sleepwalkers show significantly lower overall SWA, with the greatest difference between the two groups occurring during the 1st NREM cycle. One study6 of patients with sleepwalking and/or sleep terrors found that the absolute values of SWA averaged during the 2 min immediately preceding an episode were significantly greater than the SWA averaged during 2 min in the same stage 10 min before an episode.
In a study of sleepwalkers and controls, Guilleminault et al.31 separately investigated spectral power in delta (2.25-4 Hz) and in slow delta (0.75-2 Hz) bandwidths during the 32 sec that preceded the onset of episodes. Their results suggest a rise in the spectral power of slow delta during the 4 to 16 sec (12 sec total) preceding episode onset as compared to values from the 4 sec window representing 28 to 32 sec preceding an episode. However, the statistical analyses solely focused on the 4 to 16 sec period, ignoring the windows from 16 to 28 sec immediately preceding the episodes. In addition, analyses of SWA beyond 32 sec prior to an episode may be valuable in detecting more gradual changes in EEG signals.
Finally, the detection of slow-wave oscillations (SWO), which are high voltage (> 75 μV) waves with a peak frequency of approximately 0.7-0.8 Hz,32,33 could also help clarify mechanisms underlying the onset of somnambulistic episodes as they reflect fundamental processes underlying neural activity during SWS.33–35 SWO are characterized by hyperpolarization (down state), during which cortical neurons are silent, and depolarization (up state), during which the cortical neurons fire intensively. SWO generally originate in prefrontal-orbitofrontal regions and travel over the scalp in an anteroposterior direction as entire cortical networks alternate between hyperpolarizing and depolarizing states, in a similar fashion as the K-complex.30,32,33,36 To this date, SWO has not been investigated in relation to NREM parasomnias.
To help elucidate the potential role of the more promising sleep EEG parameters identified above in precipitating somnambulism, we used the polysomnographic (PSG) data of somnambulistic patients collected following 25 h of sleep deprivation with recovery sleep being initiated in the morning. This procedure is known to significantly increase SWS during recovery sleep37 and also increases the frequency of somnambulistic episodes recorded in the laboratory.13 The primary aim of the present study was thus to conduct a spectral analysis of sleepwalkers' sleep EEG by focusing on fluctuations in the spectral power of delta (1-4 Hz) and slow delta (0.5-1 Hz) bandwidths before the onset of somnambulistic episodes recorded from recovery SWS. We hypothesized that episodes would be immediately preceded by an increase in spectral power for slow delta. A secondary aim was to detect slow oscillations to examine changes in amplitude and density prior to behavioral episodes of somnambulism.
MATERIALS AND METHODS
Detailed information on the participants, sleep deprivation protocol, and materials used has already been published.13 The relevant information is thus presented succinctly.
Subjects
Subjects were 22 adult sleepwalkers (11 men, 11 women, mean age: 28.6 years, SD: 7.4) referred to the Sleep Disorders Clinic of the Hôpital du Sacré-Coeur by their physician for suspected somnambulism. All patients reported a clinical history (including over the previous 6 months) of somnambulism or somnambulism and sleep terrors that was not of a traumatic, neurological, or medication-induced origin, and received a final diagnosis of sleepwalking according to the International Classification of Sleep Disorders.3 None of the patients presented a history of neurological or psychiatric disorders, drug addiction or abuse, or a concomitant sleep disorder. The 22 patients included in the present study were selected on the basis of having experienced a somnambulistic episode during their first period of recovery SWS following their 25 h of sleep deprivation. Thus, a total of 22 episodes (one from each of the 22 participants) was investigated.
The study was performed in accordance with the ethical standards put forth in the 1964 Declaration of Helsinki, was approved by the hospital's ethical and scientific committee, and informed consent was obtained from each patient.
Procedures
Participants were investigated in the laboratory for one baseline night and during recovery sleep following 25 h of sleep deprivation. Recovery sleep began one hour after their previous wake time (following 25 h of wakefulness) and patients were allowed to sleep as long as they wished but were prohibited from consuming alcohol, caffeine, or other stimulating substances the day prior to and during all laboratory procedures.
PSG recordings were obtained by means of a 32-channel Grass polygraph (sensitivity at 7 μV, bandpass at 0.3-100 Hz; Grass Instruments, Quincy, MA) and digitized at a sampling rate of 256 Hz. EEG recordings and electrode placement were performed according to the international 10-20 system (Fp1, Fp2, F3, F4, F7, F8, C3, C4, P3, P4, O1, O2, T3, T4, T5, T6, Fz, Cz, Pz) with a linked-ear reference. Twenty-sec epochs of PSG were used to score sleep stages according to standard criteria.38 All patients were continuously videotaped during both sleep assessments.
Scoring of Somnambulistic Episodes
Behavioral movements identified on patients' PSG recordings were independently investigated for final classification as a somnambulistic episode by two of the authors (MP, AZ) by examining the accompanying time-synchronized video recordings. As per our established scoring protocols,13–15 behavioral episodes characterized by clumsy, stereotyped or repetitive movements, by confusion, agitation or disorientation during the event, and episodes accompanied by somniloquy received particular attention. Somniloquy alone was not considered as a partial manifestation of somnambulism. The Kappa inter-rater reliability test indicated a high degree of concordance for the presence or absence of somnambulistic episodes (κ = 0.896).
Spectral Analysis of SWA
Spectral analyses were computed with a commercial software package (Stellate Systems, Montreal, Canada) on Cz, Fz, Pz using a fast Fourier transform (cosine tapering) on 4-s artifact-free sections yielding a spectral resolution of 0.25 Hz. Epochs containing artifacts were considered as missing data to preserve sleep continuity. Spectral power in SWA (0.5-4 Hz), delta (1-4 Hz), and slow delta (0.5-1 Hz) were calculated. The 200-sec period immediately preceding each sleepwalking episode was investigated. This arbitrary time duration was selected to investigate fluctuations in spectral power over a longer period of time in comparison to previous studies.
Given the presence of considerable inter-individual variations, each 4- sec value of spectral power for SWA, delta, and slow delta was divided by that participant's mean spectral power (SWA, delta, or slow delta, respectively) over the 200 sec. This procedure produces a ratio representing variations relative to the individual's mean and not the absolute power of SWA, delta or slow delta and allows data pooling from all 22 patients. The result is presented as a series of 50 values (200 sec/4) with negative and positive deviations from the standardized mean, which is set to 1, once the ratio transformation is applied.
Detection of SWO
A proprietary in-house software package specifically designed to automatically detect EEG signals on the basis of their voltage and frequency was used to determine both the presence and amplitude of SWO. The amplitude variable refers to the mean peak-to-peak amplitude of individual SWO in a given 4-sec window, whereas the density variable refers to the mean number of SWO detected in that 4-sec window. Such windows were used to facilitate comparison with results from the spectral analysis.
SWO were automatically detected on central derivations Fz, Cz, and Pz. Data were initially bandpass filtered between 0.1 and 4.0 Hz using a linear phase FIR filter (−3 dB at 0.1 and 4.0 Hz). SWO detections were performed on artifact-free sleep EEG using the following criteria: (1) Negative peak below 40 μV; (2) Peak-to-peak amplitude > 75 μV; (3) Duration of negative deflection > 125 ms and < 1500 ms; and (4) Duration of positive deflection < 1000 ms. These criteria are illustrated in Figure 1. SWO detected by the software were also divided into either SWO or very slow oscillations (vSWO) according to established criteria34,35,39 (Fig. 1). It should be noted that SWO include vSWO, as the latter are essentially SWO with lower frequencies and higher amplitudes.
Figure 1.
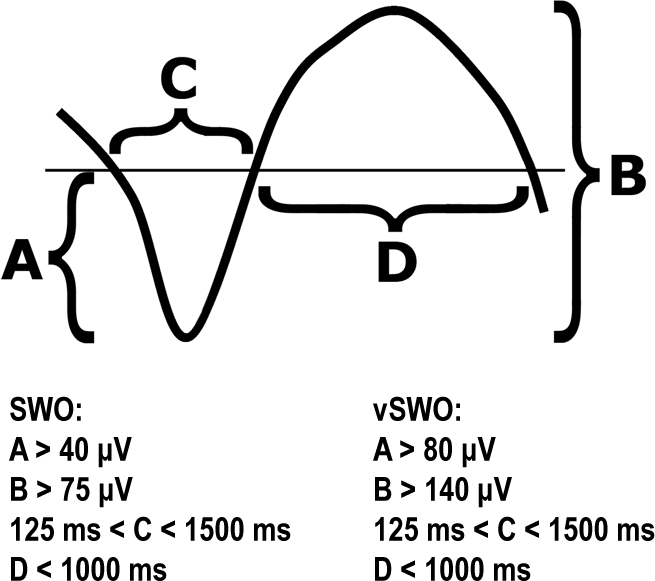
Criteria used for the automated detection of SWO. Negative is downward.
Statistical Analysis
Linear regressions were used to assess variations in SWA and in amplitude and density of SWO over the entire 200 sec as well as over the last 32 sec of sleep EEG prior to episode onset. The 32-sec window was selected for consistency with the study by Guilleminault et al.31 The value of interest extracted from these analyses was not the statistical significance of the linear regressions per se, but rather their slopes, whether positive, neutral (i.e., horizontal) or negative. These analyses were performed using Prism v4 (GraphPad Software, La Jolla, CA, USA).
A second set of analyses has been performed to compare the average SWA during the 20 sec (5 consecutively valid 4-sec mini-epochs) prior to episode onset with the SWA values for the preceding 180 sec. Similar comparative analyses were conducted on measures of SWO amplitude and density. We opted for a 20-sec window as Guilleminault et al.31 reported increases in the spectral power of low delta over the 4 to 16 sec preceding episodes, while a study of HSD26 revealed no significant differences in the proportion of episodes immediately preceded by HSD during 10-sec versus 30-sec windows either at baseline or following sleep deprivation. The use of five 4-sec mini-epochs also allowed us to compensate in cases where a mini-epoch was excluded due to artifacts or when the average signal amplitude could not be determined due to the absence of SWO in a given mini-epoch. Wilcoxon signed-rank tests were used to assess statistical differences with SPSS v15 (SPSS, Chicago, IL, USA). Given the number of comparisons computed, the level for statistical significance was set at α = 0.01.
RESULTS
Results from the linear regressions on SWA, spectral power in delta and in slow delta over the 200 sec and 32 sec prior to the onset of the somnambulistic episodes are presented in Table 1. The consecutive measures of SWA over the full 200 sec followed a significant, but neither positive nor negative, linear regression (i.e., a horizontal slope) on Fz with a small effect size. All other regressions, including for the spectral power of delta and slow delta for the 200 sec period, were not statistically significant on any of the three derivations. Results for the final 32 sec prior to the episodes indicate that SWA showed positive linear regressions with much greater effect sizes on all three derivations. Analysis of the spectral power for delta and slow delta yielded similar results with slightly stronger slopes on the Cz and Pz derivations, reflecting an increase in SWA prior to somnambulistic episodes.
Table 1.
Linear regressions of SWA, spectral power in delta and in slow delta for the 200 sec and 32 sec prior to episode onset
| SWA |
Delta |
Slow delta |
||||
|---|---|---|---|---|---|---|
| 200 sec | a | r2 | a | r2 | a | r2 |
| Fz | 0.004 ± 0.002 | 0.14* | −0.000 ± 0.009 | 0.00 | 0.025 ± 0.017 | 0.22 |
| Cz | 0.003 ± 0.002 | 0.07 | −0.001 ± 0.015 | 0.00 | 0.025 ± 0.018 | 0.20 |
| Pz | 0.003 ± 0.002 | 0.06 | 0.014 ± 0.019 | 0.06 | 0.024 ± 0.025 | 0.11 |
| 32 sec | a | r2 | a | r2 | a | r2 |
| Fz | 0.096 ± 0.032 | 0.60° | 0.099 ± 0.030 | 0.64° | 0.068 ± 0.040 | 0.32 |
| Cz | 0.110 ± 0.023 | 0.79* | 0.102 ± 0.023 | 0.77* | 0.131 ± 0.031 | 0.75* |
| Pz | 0.096 ± 0.020 | 0.79* | 0.085 ± 0.020 | 0.75* | 0.120 ± 0.028 | 0.76* |
P < 0.05,
P < 0.01,
P < 0.001; a = slope of the linear regression
Table 2 presents the results of the Wilcoxon signed-rank tests for comparisons of SWA, spectral power in delta and in slow delta over the 20 sec prior to episode onset with values obtained from the preceding 180 sec; a positive W indicates higher spectral power in the last 20-sec window than in the 180-sec baseline recording. As can be seen in Table 2, SWA during the final 20 sec prior to episode onset was significantly higher on Fz. When tested independently, analyses of both delta and slow delta revealed that a significant rise in spectral power occurred exclusively in the delta bandwidth recorded on Fz. A similar rise in spectral power with a trend towards significance (given our conservative α level) was found on Cz (P = 0.050).
Table 2.
Wilcoxon signed-rank tests of SWA, spectral power in delta and in slow delta for the 20 sec prior to episode onset and the 180 sec baseline (medians are reported)
| SWA |
Delta |
Slow delta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 180 s | 20 s | W | 180 s | 20 s | W | 180 s | 20 s | W | |
| Fz | 0.99 | 1.09 | 137* | 0.98 | 1.12 | 171* | 1.02 | 0.96 | −5 |
| Cz | 0.99 | 1.10 | 113 | 0.97 | 1.11 | 121° | 1.00 | 1.13 | 85 |
| Pz | 0.99 | 1.05 | 87 | 1.00 | 1.07 | 39 | 0.98 | 1.17 | 73 |
P < 0.05,
P < 0.01,
P < 0.001
Results from the linear regressions on the amplitude and density of SWO and vSWO over the 200 sec and 32 sec prior to the onset of the somnambulistic episodes are presented in Table 3. First, the amplitudes of both SWO and vSWO do not appear to fluctuate in either direction during the 200 sec prior to somnambulistic episodes. It should be noted that a particularly small r2 does not imply regressions with horizontal slopes, but rather the absence of any noteworthy linear regressions. Second, the density of SWO shows a significant and positive increase on all three derivations but the associated effect size is relatively small. Comparable patterns and effect sizes were obtained for the density of vSWO, with the exception on Pz.
Table 3.
Linear regressions of amplitude and density of SWO and vSWO for the 200 sec and 32 sec prior to episode onset
| SWO |
vSWO |
|||
|---|---|---|---|---|
| 200 sec | a | r2 | a | r2 |
| Amplitude | ||||
| Fz | 0.001 ± 0.001 | 0.04 | −0.001 ± 0.001 | 0.22 |
| Cz | 0.000 ± 0.001 | 0.00 | −0.001 ± 0.001 | 0.20 |
| Pz | 0.000 ± 0.001 | 0.00 | 0.000 ± 0.001 | 0.11 |
| Density | ||||
| Fz | 0.005 ± 0.002 | 0.17* | 0.008 ± 0.004 | 0.10° |
| Cz | 0.005 ± 0.002 | 0.14* | 0.011 ± 0.004 | 0.14* |
| Pz | 0.004 ± 0.002 | 0.09° | 0.011 ± 0.006 | 0.06 |
| 32 sec | a | r2 | a | r2 |
| Amplitude | ||||
| Fz | 0.025 ± 0.009 | 0.56° | 0.026 ± 0.010 | 0.51° |
| Cz | 0.021 ± 0.008 | 0.51° | 0.015 ± 0.008 | 0.38 |
| Pz | 0.014 ± 0.006 | 0.48 | 0.017 ± 0.013 | 0.21 |
| Density | ||||
| Fz | 0.055 ± 0.026 | 0.42 | 0.133 ± 0.057 | 0.47 |
| Cz | 0.089 ± 0.025 | 0.68° | 0.147 ± 0.059 | 0.51° |
| Pz | 0.096 ± 0.028 | 0.66 | 0.180 ± 0.090 | 0.40 |
P < 0.05,
P < 0.01,
P < 0.001; a = slope of the linear regression
Data restricted to the 32 sec prior to the episodes show trends toward significant increases in the amplitude of SWO on Fz (P = 0.045) and Cz (P = 0.032), and of vSWO on Fz (P = 0.048). Overall, the results obtained on all three derivations were comparable: positive yet relatively small slopes over the final 32 sec. Density values for SWO and vSWO resulted in a trend towards significant positive linear regressions on Cz (P = 0.012, P = 0.047, respectively). Similar patterns were observed on Fz and Pz. However, it should be noted that the increase in the density of vSWO follows stronger slopes in comparison to what was found for SWO.
The differences in amplitude and density of SWO between the final 20 sec prior to episode onset and the preceding 180 sec are presented in Table 4. There were no significant differences in the amplitudes of SWO and vSWO between these two time measures on any of the derivations. By contrast, significant differences were found in the density of SWO, with significant increases occurring during the final 20 sec on both Fz and Cz. Smaller but significant time course differences in the density of vSWO were confined to Fz.
Table 4.
Wilcoxon signed-rank tests of amplitude and density of SWO and vSWO for the 20 sec prior to episode onset and the 180 sec baseline (medians are reported)
| Amplitude | SWO |
vSWO |
||||
|---|---|---|---|---|---|---|
| 180 s | 20 s | W | 180 s | 20 s | W | |
| Fz | 1.00 | 1.04 | 103 | 1.00 | 0.99 | −1 |
| Cz | 1.00 | 1.00 | 25 | 1.01 | 0.96 | −39 |
| Pz | 1.00 | 1.02 | 35 | 1.00 | 0.97 | −38 |
| Density | ||||||
| Fz | 0.99 | 1.23 | 205** | 0.97 | 1.27 | 169* |
| Cz | 0.98 | 1.18 | 201** | 0.96 | 1.20 | 127° |
| Pz | 0.99 | 1.12 | 137° | 1.02 | 1.02 | −7 |
P < 0.05,
P < 0.01,
P < 0.001
DISCUSSION
The main goal of the present study was to conduct a quantitative analysis of sleepwalkers' sleep EEG by focusing on fluctuations in the spectral power of delta and slow delta bandwidths before the onset of somnambulistic episodes. The systematic analysis of our patients' sleep EEG over the 200 sec prior to the onset of 22 somnambulistic episodes revealed that these behavioral manifestations are not preceded by a progressive increase in spectral power of either delta or slow delta over frontal, central, or parietal leads. The detection of SWO also failed to show gradual increases in the amplitude of SWO and vSWO during the 200 sec preceding the episodes. Small but statistically significant SWO and vSWO density increases were noted on all three derivations, but these were due to more time-limited increases occurring approximately 20 to 30 seconds prior to the onset of somnambulistic behaviors.
Analysis of the sleep EEG signals from a more restrictive period of 32 sec before the episodes revealed significant spectral power increases in both delta and slow delta bandwidths, with substantial effect sizes. These results indicate that somnambulistic episodes are immediately preceded by relatively short-duration increases in SWA, particularly over central and parietal derivations.
With regards to measures of SWO and vSWO amplitude over the last 32 sec, the small positive slopes observed were outweighed by findings on oscillation density. A moderate increase in SWO density along with a more pronounced increase in vSWO density was found over the central lead. However, no significant differences were observed between amplitude values collected over the 20 sec immediately prior to the episode as compared to the preceding 3 min. This relatively short-duration increase in both SWO and vSWO density was evidenced by significant increases on frontal and central derivations.
Thus, the aforementioned results suggest that somnambulistic episodes recorded from SWS are not preceded by a long-duration and progressive increase in SWA, but rather by a relatively sudden SWO and vSWO density increase during the 20 sec prior to episode onset.
While Guilleminault et al.31 reported significant pre-episode increases in a bandwidth referred to as “low delta” (0.75-2 Hz), our spectral analyses reveal significant increases in the spectral power of delta (1-4 Hz) and slow delta (0.5-1 Hz). Whereas the findings of both studies point to somnambulistic behaviors being preceded by an increase in SWA, the investigation of SWO carried out in the present study allows a better understanding of the observed fluctuations in SWA. While spectral analysis presents total spectral power for successive time-defined windows, we also identified the actual number of SWO in those same windows as well as the amplitude of each SWO. The ensuing results reveal that the observed increases were associated with SWO density as opposed to SWO amplitude.
If SWO are related to sleep depth or intensity,28 it is possible that a sudden increase in SWO and vSWO is associated with somnambulistic behaviors in predisposed individuals. Thus, while precipitating factors or external stimuli (e.g., sudden noise) might induce an arousal or an episode in sleepwalkers,15 naturally occurring episodes may be due to abnormally heightened sleep depth, reflected by a sudden increase in SWO. This idea is consistent with the finding that patients with frequent respiratory related arousals but without NREM parasomnias often experience such arousals following the presence of HSD during their SWS.25 The occurrence of an abnormally heightened sleep depth may also be facilitated by sleep deprivation, a condition known to significantly increase the frequency of somnambulistic events in sleepwalkers.13,14 Finally, since neural SWO between hyperpolarized and depolarized phases protect the sleeping brain from disruptive stimulation,40 it is also possible that increases in sleepwalkers' SWO reflect the brain's attempt to maintain sleep in the face of internal or external processes or stimuli that may give rise to an arousal or actual behavioral episode. This cortical arousal reaction to brain activation during SWS may arise from the selective activation of thalamocingulate circuits and the persisting inhibition of other thalamocortical arousal systems.41
Since SWO in humans originate in prefrontal-orbitofrontal regions,34,35,39 it is not surprising to note the greatest and clearest changes in SWO and vSWO density on the frontal derivation followed by the central lead. These results are consistent with the topographical data for HSD-related variables which indicate a robust fronto-central gradient in the expression of these forms of delta activity.26 These findings are also consistent with the observation that the rebound in SWA induced by sleep deprivation (i.e., sleep pressure) is more pronounced over the brain's frontal regions42–44 and that delta wave counts and delta amplitude are higher in frontal regions.45 It has been suggested that frontal brain areas may represent a region particularly vulnerable to the effects of sleep loss or sleep fragmentation.46,47 These patterns may thus reflect the higher recovery need of the frontal brain area.
Given that sleepwalkers are known to experience an unusually high number of arousals and brief microarousals out of SWS,6,19,20 it would be interesting to investigate fluctuations in SWA and SWO prior to abrupt arousals without somnambulistic behaviors. Results from one study6 of adults with sleep terrors and/or sleepwalking suggest that parasomnia episodes out of SWS are preceded by greater SWA than are patients' normal arousals out of SWS. Further investigations along these lines could help clarify if sleepwalkers' fragmentation of NREM sleep embodies a milder manifestation of their presumed arousal dysfunction.
The results of the present study are limited due to methodological considerations. First, since the data were collected during daytime recovery sleep following 25 h of sleep deprivation, differing patterns could emerge during either normal sleep or recovery sleep recorded at nighttime during the usual circadian phase for sleep propensity. Second, no comparison group was included. Although the study of SWA prior to somnambulism is impossible in control subjects as they do not experience such episodes, overall SWA dynamics could be investigated by pairing patients with controls based on the onset of their first SWS period. One question of interest would be whether SWS in controls show comparable time course increases in SWA. Third, given that large inter-individual differences exist in SWA, some patients may show relatively little SWA during SWS paired with only a slight increase in SWA prior to an episode, while others may have abundant SWA combined with notable increases in activity prior to an episode's onset. For example, one of our patients showed 94 SWO on the parietal derivation during the 200 sec of sleep EEG investigated while another only had seven. However, four of the latter seven SWO occurred during the final 12 sec prior to the episode. Even though the ratio transformations used in the present study ensured that each of the recorded events had an equal weight in the analyses, this variability adds to the complexity of proposing neural mechanisms potentially implicated in the occurrence of somnambulistic behaviors.
In summary, the findings provide partial support for the hypothesis that somnambulistic episodes are preceded by increases in SWA. Whereas some investigations found no evidence that somnambulism is immediately preceded by a build-up in HSD or by any HSD-related variable,24,26 the present findings indicate that the occurrence of such episodes is preceded by increases in SWA, and specifically in SWO density over the 20 sec immediately preceding their onset. However, since SWO and vSWO have yet to be similarly investigated in healthy controls and in other sleep-disorders populations, the specificity of these sleep EEG parameters for the occurrence or even diagnosis of NREM parasomnias remains to be determined.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by the Canadian Institutes of Health Research (grant # MOP 49515 to A. Zadra and J. Montplaisir).
REFERENCES
- 1.Broughton RJ. Sleep disorders: disorders of arousal? Science. 1968;159:1070–8. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- 2.Broughton R. NREM arousal parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 693–706. [Google Scholar]
- 3.American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 4.Kavey NB, Whyte J, Resor SR, Jr, Gidro-Frank S. Somnambulism in adults. Neurology. 1990;40:749–52. doi: 10.1212/wnl.40.5.749. [DOI] [PubMed] [Google Scholar]
- 5.Zucconi M, Oldani A, Ferini-Strambi L, Smirne S. Arousal fluctuations in non-rapid eye movement parasomnias: the role of cyclic alternating pattern as a measure of sleep instability. Clin Neurophysiol. 1995;12:147–54. [PubMed] [Google Scholar]
- 6.Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–39. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 7.Hublin C, Kaprio J, Partinen M, Heikkila K, Koskenvuo M. Prevalence and genetics of sleepwalking: a population-based twin study. Neurology. 1997;48:177–81. doi: 10.1212/wnl.48.1.177. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM, Guilleminault C, Priest RG. Night terrors, sleepwalking, and confusional arousals in the general population: their frequency and relationship to other sleep and mental disorders. J Clin Psychiatry. 1999;60:268–76. doi: 10.4088/jcp.v60n0413. [DOI] [PubMed] [Google Scholar]
- 9.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, et al. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry. 1989;146:1166–73. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 10.Moldofsky H, Gilbert R, Lue FA, MacLean AW. Forensic sleep medicine: Violence, sleep, nocturnal wandering: Sleep-related violence. Sleep. 1995;18:731–9. doi: 10.1093/sleep/18.9.731. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright R. Sleepwalking violence: a sleep disorder, a legal dilemma, and a psychological challenge. Am J Psychiatry. 2004;161:1149–58. doi: 10.1176/appi.ajp.161.7.1149. [DOI] [PubMed] [Google Scholar]
- 12.Mahowald MW, Schenck CH. Violent parasomnias: forensic medicine issues. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 960–9. [Google Scholar]
- 13.Zadra A, Pilon M, Montplaisir J. Polysomnographic diagnosis of sleepwalking: effects of sleep deprivation. Ann Neurol. 2008;63:513–9. doi: 10.1002/ana.21339. [DOI] [PubMed] [Google Scholar]
- 14.Joncas S, Zadra A, Paquet J, Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58:936–40. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 15.Pilon M, Montplaisir J, Zadra A. Precipitating factors of somnambulism: Impact of sleep deprivation and forced arousals. Neurology. 2008;70:2284–90. doi: 10.1212/01.wnl.0000304082.49839.86. [DOI] [PubMed] [Google Scholar]
- 16.Pressman MR. Factors that predispose, prime and precipitate NREM parasomnias in adults: Clinical and forensic implications. Sleep Med Rev. 2007;11:5–30. doi: 10.1016/j.smrv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Pressman MR. Disorders of arousal from sleep and violent behavior: the role of physical contact and proximity. Sleep. 2007;30:1039–47. doi: 10.1093/sleep/30.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilleminault C, Kirisoglu C, da Rosa A, Lopes C, Chan A. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7:163–70. doi: 10.1016/j.sleep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Gaudreau H, Joncas S, Zadra A, Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000;23:755–60. [PubMed] [Google Scholar]
- 20.Blatt I, Peled R, Gadoth N, Lavie P. The value of sleep recording in evaluating somnambulism in young adults. Electroencephalogr Clin Neurophysiol. 1991;78:407–12. doi: 10.1016/0013-4694(91)90058-c. [DOI] [PubMed] [Google Scholar]
- 21.Kales A, Jacobson A, Paulson MJ, Kales JD, Walter RD. Somnambulism: psychophysiological correlates. I. All-night EEG studies. Arch Gen Psychiatry. 1966;14:586–94. doi: 10.1001/archpsyc.1966.01730120026004. [DOI] [PubMed] [Google Scholar]
- 22.Broughton R, Billings R, Cartwright R, et al. Homicidal somnambulism: a case report. Sleep. 1994;17:253–64. [PubMed] [Google Scholar]
- 23.Jacobson A, Kales A. Somnambulism: all-night EEG and related studies. Res Publ Assoc Res Nerv Ment Dis. 1967;45:424–55. [PubMed] [Google Scholar]
- 24.Schenck CH, Pareja JA, Patterson AL, Mahowald MW. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol. 1998;15:159–66. doi: 10.1097/00004691-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Pressman MR. Hypersynchronous delta sleep EEG activity and sudden arousals from slow-wave sleep in adults without a history of parasomnias: Clinical and forensic implications. Sleep. 2004;27:706–10. doi: 10.1093/sleep/27.4.706. [DOI] [PubMed] [Google Scholar]
- 26.Pilon M, Zadra A, Joncas S, Montplaisir J. Hypersynchronous delta waves and somnambulism: Brain topography and effect of sleep deprivation. Sleep. 2006;29:77–84. doi: 10.1093/sleep/29.1.77. [DOI] [PubMed] [Google Scholar]
- 27.Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 28.Borbely AA, Acherman P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Philadelphia: WB Saunders; 2000. pp. 377–90. [Google Scholar]
- 29.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:878–99. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 30.Steriade M. Brain electrical activity and sensory processing during waking and sleep states. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. pp. 101–19. [Google Scholar]
- 31.Guilleminault C, Poyares D, Aftab FA, Palombini L. Sleep and wakefulness in somnambulism: A spectral analysis study. J Psychosom Res. 2001;51:411–6. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 32.Amzica F, Steriade M. The K-complex: its slow (< 1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–9. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- 33.Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–22. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 34.Molle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neuroscience. 2002;22:10941–7. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang-Vu TT, Schabus M, Desseilles M, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105:15160–5. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res. 1998;7(Suppl 1):30–5. doi: 10.1046/j.1365-2869.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 37.Gaudreau H, Morettini J, Lavoie HB, Carrier J. Effects of a 25-h sleep deprivation on daytime sleep in the middle-aged. Neurobiol Aging. 2001;22:461–8. doi: 10.1016/s0197-4580(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Bethesda, MD: Neurological Information Network; 1968. [Google Scholar]
- 39.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–70. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–5. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 42.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 43.Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 44.Münch M, Knoblauch V, Blatter K, et al. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 45.Sekimoto M, Kato M, Kajimura N, Watanabe T, Takahashi K, Okuma T. Asymmetric interhemispheric delta waves during all-night sleep in humans. Clin Neurophysiol. 2000;111:924–8. doi: 10.1016/s1388-2457(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 46.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 47.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]


