Abstract
Study Objectives:
Single motor unit recordings of the genioglossus (GG) muscle indicate that GG motor units have a variety of discharge patterns, including units that have higher discharge rates during inspiration (inspiratory phasic and inspiratory tonic), or expiration (expiratory phasic and expiratory tonic), or do not modify their rate with respiration (tonic). Previous studies have shown that an increase in GG muscle activity is a consequence of increased activity in inspiratory units. However, there are differences between studies as to whether this increase is primarily due to recruitment of new motor units (motor unit recruitment) or to increased discharge rate of already active units (rate coding). Sleep-wake state studies in humans have suggested the former, while hypercapnia experiments in rats have suggested the latter. In this study, we investigated the effect of hypercapnia on GG motor unit activity in humans during wakefulness.
Setting:
Sleep research laboratory.
Participants:
Sixteen healthy men.
Measurements and Results:
Each participant was administered at least 6 trials with PetCO2 being elevated 8.4 (SD = 1.96) mm Hg over 2 min following a 30-s baseline. Subjects were instrumented for GG EMG and respiratory measurements with 4 fine wire electrodes inserted subcutaneously into the muscle. One hundred forty-one motor units were identified during the baseline: 47% were inspiratory modulated, 29% expiratory modulated, and 24% showed no respiratory related modulation. Sixty-two new units were recruited during hypercapnia. The distribution of recruited units was significantly different from the baseline distribution, with 84% being inspiratory modulated (P < 0.001). Neither units active during baseline, nor new units recruited during hypercapnia, increased their discharge rate as PetCO2 increased (P > 0.05 for all comparisons).
Conclusions:
Increased GG muscle activity in humans occurs because of recruitment of previously inactive inspiratory modulated units.
Citation:
Nicholas CL; Bei B; Worsnop C; Malhotra A; Jordan AS; Saboisky JP; Chan JKM; Duckworth E; White DP; Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. SLEEP 2010;33(11):1529-1538.
Keywords: Single motor unit, upper airway muscles, motor control, obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA (OSA) IS AN IMPORTANT DISEASE BECAUSE OF ITS HIGH PREVALENCE AND CONSIDERABLE CONSEQUENCES.1,2 UNDERLYING mechanisms of OSA are variable, but generally involve an interaction between anatomical susceptibility of the upper airway to collapse with state-dependent effects on the motor control of upper airway dilator muscles.3 We and others have observed stable periods of breathing during sleep, even in patients with severe OSA.4,5 Recent data suggest that high levels of genioglossal activity (and likely of other phasic upper airway dilator muscles) are present when adequate pharyngeal patency is maintained in OSA patients during sleep.4,6 As such, understanding the mechanisms underlying pharyngeal dilator muscle recruitment appears crucial to the eventual goal of designing new therapies for OSA.7–9
Upper airway muscles in general, and the genioglossus (GG) in particular, have been extensively investigated by our group and others.10–14 The GG is thought to be representative of the activity of other phasic dilator muscles and has been shown to have important influences on pharyngeal mechanics.15–17 Traditional studies have been performed using multi-unit EMG recording techniques,18,19 which have led to important conclusions regarding the robust responsiveness of the GG to mechanoreceptive, e.g., negative intrapharyngeal pressure,20–22 and chemoreceptive, e.g., CO2,23,24 stimuli during wakefulness, but less so during sleep.25 More recent single motor unit (SMU) recordings have revealed considerable complexity to the behavior of the GG,26 with as many as 6 different patterns of individual motor unit behavior. These more recent SMU studies have provided considerable insight into the possible brainstem pathways mediating hypoglossal output.27–29 Recording SMU activity provides a means by which the activity of cells within the hypoglossal motor nucleus can be assessed in humans.
In humans, multi-unit EMG activity in upper airway muscles is reduced at sleep onset, including a transient reduction in GG activity.30 More recent studies27 have shown that in the GG, this effect is almost entirely due to reduced activity in inspiratory modulated motor units (motor units that either only fire during inspiration [inspiratory phasic] or fire at higher rates during inspiration [inspiratory tonic]), rather than tonic motor units that do not modulate their rate in phase with ventilation.27 Further, upon a return to wakefulness, GG activity increases as a consequence of increased activity of inspiratory modulated units.29 Of interest is the observation that the change in the activity of inspiratory modulated units at sleep-wake transitions is due to the loss or recruitment of motor units, rather than changes in their firing rate from one breath to the next (noting that, by definition, inspiratory modulated units do change their firing rate within a breath).
In rats, hypercapnia increases the activity of inspiratory phasic GG motor units,31 however the increase in activity is primarily due to increases in the discharge rate of most of the already active motor units, rather than through recruitment of previously inactive units. This finding raises the question of whether the difference in the results of these studies was due to a species difference, or to a difference in respiratory drive (hypercapnia versus changes in sleep-wake state). Recently Richardson and Bailey32 have shown that while hypercapnia in awake humans caused an increase in overall GG activity, it did not elevate the breath-by-breath discharge rate of individual tonically active motor units (inspiratory phasic units were not studied). Thus, leading to the inference that motor unit recruitment had occurred, although motor unit recruitment was not directly identified.
In the current study we sought to define further the mechanisms underlying genioglossal muscle activity patterns in response to chemical stimuli by studying individual motor unit behavior in response to hypercapnia in humans during wakefulness. This aim allowed us to test several hypotheses: first, that GG recruitment in humans occurs primarily in inspiratory phasic and inspiratory tonic motor units; second, that the increase in GG activity occurs via the recruitment of previously inactive units, rather than an increase of the discharge rate of already active units (rate coding); and third, that hypercapnia increases the proportion of a breath during which inspiratory phasic motor units are active.
METHODS
Subjects
The subjects were 16 males with a mean age of 28.6 years (± 12.4 years), a mean height of 177.9 cm (± 6.2 cm), and a mean weight of 76.4 kg (± 13.0 kg). Women were excluded from the study, as elevated CO2 may affect the fetus, and pregnancy testing was not available within the University of Melbourne laboratory. All subjects were healthy and were without sleep or respiratory complaints as determined by a questionnaire. The protocol conformed to the Declaration of Helsinki and had prior approval of the local Human Subjects Ethics Committee. Informed consent was obtained from each subject.
Design
During each experimental session, subjects were administered 6 CO2 trials with an elevation in end tidal CO2 (PetCO2) of 8.4 (SD = 1.96) mm Hg above their baseline level. Each trial was 3 minutes in duration and consisted of a 30-second baseline, 1 minute over which PetCO2 was elevated, 1 minute during which PetCO2 was maintained at the elevated level, and 30 seconds during which the manipulation was terminated and the subject allowed to return towards eucapnia. Following a trial, a new trial was not commenced until the subject had returned to stable breathing. Individual trials were restricted to 3 min, because as trials increased in length, they were more likely to be disturbed by body movements and swallows.
For data analysis purposes, the 3-min trials were converted into 6 epochs, in which each epoch consisted of the average of 3 breaths. The 6 epochs were the last 3 breaths from the baseline, the last 3 breaths from the first, second, third, and fourth 30-sec periods of hypercapnia; and the last 3 breaths of the recovery period. Four types of variables were analyzed. First, respiratory activity (airflow, airway resistance) was analyzed to document the effect of the manipulation. Second, overall GG muscle activity on each electrode was quantified by the rate of spike activity exceeding a threshold, irrespective of the motor units from which the spikes were obtained. Third, the distribution of motor units with different discharge patterns was assessed during baseline and as a function of PetCO2. Finally, the discharge properties of motor units with different discharge patterns were quantified as a function of PetCO2.
General Laboratory Procedures
Experimental sessions were conducted during the late morning or early afternoon. Subjects were requested to refrain from alcohol before the experimental session and from food and caffeine for 4 h before the session. On arriving at the laboratory a local anesthetic cream was applied under the subject's chin (Lidocaine-Prilocaine, Fogera, Melville, NY). Instrumentation of the subject commenced 30 min later.
During data collection subjects lay supine on a bed in a bedroom. The supine position was used as it is an easy position for subjects to sustain and standardize without moving and because it maximizes GG activity.33 Subjects initially breathed room air for 5 min to become accustomed to breathing through the face mask. They were then switched into the experimental breathing circuit with the inspired air containing 40% oxygen and 60% nitrogen and were given a further 5-min acclimatization period. The experimental trials then commenced. Subjects were instructed to remain still and relaxed, to relax facial muscles as much as possible, to try and avoid swallowing, and to remain awake during trials. To initiate a trial an experimenter entered the bedroom and informed the subject a trial was about to commence. The trial then commenced when the subject was no longer moving and their PetCO2 had stabilized. At the completion of a trial, the experimenter entered the bedroom and informed the subject, who was then free to adjust their body position, swallow, etc., in preparation for the next trial. All data were stored for subsequent off-line analysis.
Measurements and Recordings
Ventilation and airway mechanics
The respiratory circuit, which was based on a circuit developed by Banzett et al.,34 allowed PetCO2 levels to be manipulated. Airflow was collected via a full face mask (Fisher & Paykel, 431 anesthetics mask) connected to a 2-way breathing valve (Hans Rudolph, 2600 medium). Respiratory airflow was measured by a calibrated pneumotachometer (Fleisch #2), a differential pressure transducer (DP–45, Validyne Corp., Northridge, CA) and a Morgan carrier demodulator (CD15). The pneumotachometer was placed between the mask and the 2-way valve. The inspiratory line was connected to 2 air tanks (one 40% oxygen, 60% nitrogen, and the other 40% oxygen, 5% carbon dioxide and 55% nitrogen) via a mixer (Siemens 965), a 3-L air bladder (Ferraxis), and humidifier (Fisher & Paykel, HC150), allowing the CO2 content and volume of inspired air to be manipulated. When tidal volume exceeded the air available in the inspiratory line, the remaining inspired air was obtained from the expiratory line and thus the expiratory line operated as a re-breathing circuit, preventing large breaths from perturbing PetCO2. PetCO2 was monitored from the mask using an Ametek CO2 (CD-3A) analyzer and was displayed on an oscilloscope (Tetronix, TDS 1002), so that an experimenter could monitor and control PetCO2 levels. A hyperoxic gas mixture was used to eliminate any effects on respiratory activity due to changes in O2 levels. Oxygen levels were monitored continuously using an S-3A/I Ametek Gas Analyzer. To document the effect of increased ventilation on airway pressure, pressures were monitored in the mask with an open catheter attached to a pressure transducer (Validyne Corp) and in the airway at the level of the epiglottis using a pressure-tipped catheter (MPC-500, Millar, Houston, TX) inserted via a nostril.
Respiratory variables were recorded as DC signals with a 15-Hz low-pass filter using Grass amplifiers (Grass Telefactor, Rhode Island, USA) and were digitized at 100 Hz using a 1401 interface and Spike 2 acquisition software (Cambridge Electronic Design, Cambridge, UK).
Muscle activity
The procedures used to record GG EMG activity followed those of Eastwood et al.13 GG activity was recorded using 4 monopolar intramuscular wire electrodes referenced to a common surface electrode positioned over the bony mandible. A large flexible ground strap was placed on the left shoulder and electrodes were inserted 2.4 cm from the surface of the skin, using a percutaneous approach.13 Previous studies using ultrasound to identify the location of the GG suggest that at this depth the electrode consistently enters the GG.13,26,27 Location was also confirmed by standard maneuvers. Insertion was via a 25-gauge hypodermic needle, with 1 stainless steel, Teflon-coated 50 μm wire electrode (A-M Systems Inc. Seattle, Washington) in each of the needle insertions. The electrodes had 0.5 mm of the tip exposed. Anterior and posterior positions were used for each subject, with electrodes placed ∼3 mm each side of the midline and ∼10 mm and ∼20 mm from the inferior margin of the mandible. For the GG EMGs, the filters were set at 0.03–3 kHz, and the signal digitized and recorded at 10 kHz.
Data Reduction
Respiratory variables
Respiratory variables were analyzed on a breath-by-breath basis using software developed within the laboratory. All analyzed data were visually checked and edited where necessary to confirm that the program's breath detection algorithm was performing appropriately. The respiratory variables analyzed were minute ventilation, tidal volume, cycle duration, PetCO2, peak inspiratory flow, epiglottal pressure at peak flow, and airway resistance at peak flow. Data were averaged over trials within subjects and then over subjects.
Overall GG activity
The GG EMGs were analyzed using Spike 2 software. The level of activity recorded on each electrode was quantified independent of individual motor units by determining the spike density. Spike density was defined as the number of spikes per second that exceeded a threshold voltage. The threshold was defined as twice the voltage amplitude of the background noise. Background noise primarily reflected activity of distant motor units and, at times, electrical interference. Spike density rather than integration was used because under the recording conditions appropriate to identify single motor units, the rectified and integrated output tends to be discontinuous, unless long integration intervals are used. The spike density was determined independently for inspiration and expiration over the 6 epochs. Values were averaged over electrodes and trials within subjects and then averaged over subjects.
Identification of single motor units
The GG EMG recordings for each trial were initially inspected: electrodes without motor unit activity, electrodes with too much activity for individual motor units to be sorted, and electrodes that had technical deficiencies were discarded. Single motor units on the remaining electrodes were identified using Spike 2 analysis software (Cambridge Electronic Design, Cambridge, UK). Motor units were initially identified using a spike-triggered threshold voltage and sorted by the software into templates based on the spikes detailed amplitude and shape signatures. Subsidiary software was then used to inspect and edit the initial software classification (software courtesy of Prince of Wales Medical Research Institute, Sydney, Australia). Motor units were discarded at this stage if the spikes belonging to a motor unit were not clearly identifiable. Finally, each sorted motor unit was inspected for sudden changes in frequency (defined as a change > 40% from one inter-spike interval to the next) that indicated inappropriate sorting. If these events exceeded 5% of inter-spike intervals, the unit was discarded. As a result of these procedures, which have been described and illustrated in greater detail in an earlier publication,27 one could have every confidence that units included in subsequent analyses were correctly sorted. The discharge properties of the motor units were expressed as instantaneous frequency plots.
Five different discharge patterns were identified from the instantaneous frequency plots according to procedures reported previously.26 A sixth pattern identified by Saboisky et al.,26 tonic other, was not identified in these data. The 5 discharge patterns were:
Inspiratory Phasic.
These were units that showed peak activity during inspiration and had a minimum firing frequency < 2.0 Hz during expiration (i.e. were silent for > 500 ms).
Inspiratory Tonic.
Units that showed peak activity during inspiration and maintained a discharge rate > 2.0 Hz throughout the respiratory cycle.
Expiratory Phasic.
These were units that showed peak activity during expiration and had a minimum firing frequency of < 2.0 Hz during inspiration (i.e. were silent > 500 ms).
Expiratory Tonic.
Units that showed peak activity during expiration and maintained a discharge rate > 2.0 Hz throughout the respiratory cycle.
Tonic.
Units that fired throughout the respiratory cycle, but had no obvious respiratory or other modulation.
In order to determine a unit's degree of respiratory modulation, instantaneous frequency values for the motor unit were cross-correlated with tidal volume, derived from an integrated flow signal, at the time of each spike, over a breath. The value was calculated for each breath and averaged over the three breaths within an epoch. The greater the maximum cross-correlation value, the stronger the respiratory modulation, while the phase of the maximum value indicated whether the unit was inspiratory or expiratory. The statistic is analogous to the η2 statistic proposed by Orem and Dick,35 but modified to accommodate data with small numbers of breaths,26,27 noting that the η2 statistic requires a relatively large number of breaths for calculations as it uses between, rather than within, breath variance. In accord with values published in earlier papers,26,27 a cross-correlation value ≥ 0.49 was accepted as defining the presence of an inspiratory or expiratory phasic component. Units with cross-correlation values < 0.49 were assigned to the tonic category. The mean (± SD) cross-correlation values for units with different discharge patterns during baseline were: inspiratory phasic, 0.76 (± 0.12); inspiratory tonic, 0.71 (± 0.11); expiratory tonic, 0.65 (± 0.10); and tonic, 0.40 (± 0.08).
In allocating motor units to different discharge patterns, motor units that were active during the baseline were initially classified according to their baseline pattern and then given a second classification based on their predominant pattern following CO2 administration. A motor unit was considered to be active during the baseline if it had ≥ 5 spikes on ≥ 2 of the last 3 baseline breaths. Motor units recruited during a trial were classified according to their predominant pattern once they commenced activity. A recruited tonic unit was classified as having a phasic component when the maximum cross-correlation value averaged over all breaths was ≥ 0.49.
In some instances the same unit was recorded on a particular electrode for more than one trial. Such events were identified on the basis of the morphology of the spike. How these units were entered into statistical analyses has been described in the Statistical Analyses section. On other occasions the same motor unit was recorded simultaneously on 2 electrodes. Such events were identified by the identical timing of the spikes on the 2 electrodes. On such occasions only one electrode was sorted. Finally, some units identified during baseline could not be followed through to the end of the elevated CO2 phase, due to an increase in overall activity because of recruitment, or swallows and movement. These units were included in the baseline distribution data, but not in other analyses.
Discharge Properties of Motor Units
The instantaneous frequency plots were quantified to characterize the discharge pattern of the motor units (software developed by Prince of Wales Medical Research Institute, Sydney, Australia). A number of measures were used:
The mean frequency during either inspiration or expiration, depending on the phase of the unit.
The peak frequency during inspiration or expiration, depending on the phase of the unit (200 ms average).
The tonic frequency, which was defined as the last 200 ms before the onset of the inspiratory or expiratory phasic component. This measure only applied to inspiratory and expiratory tonic units.
The time, with respect to the onset of inspiration, of the first action potential for each breath for inspiratory phasic units and the first action potential to show a rate increase (reduced action potential interval) in association with inspiration for inspiratory tonic units. A negative value indicated activation prior to the onset of airflow (pre-activation). This measure only applied to inspiratory phasic and inspiratory tonic motor units.
The proportion of the breath during which the unit was active. This measure only applied to inspiratory phasic and expiratory phasic units.
Statistical Analyses
Analysis of the respiratory variables consisted of a 6-level (6 values over trials) one-way repeated-measures ANOVA conducted on each variable. Spike density (# of spikes/sec, irrespective of single motor unit affiliation) was analyzed by a 2 (inspiration versus expiration) × 6 (values over a trial) repeated-measures ANOVA. The distribution of motor units with different discharge patterns during the baseline period was compared to the distribution of motor units recruited during hypercapnia, and with the distribution of all units active at the end of trials, using χ2 analysis. In analyses of the discharge characteristics of motor units, breaths on which the unit did not fire were omitted from the analysis. This rarely occurred for motor units active during baseline and never involved more than an isolated breath; however, missing values were more frequent in units recruited subsequent to the onset of hypercapnia. Statistical analysis for each variable consisted of a one-way ANOVA with repeated measures. Where the same motor unit was recorded on more than one trial, values were averaged over the trials and the averaged value entered into analyses. The statistical analyses reported were based on motor units, although analyses based on subjects produced the same results. Where the assumption of sphericity was violated, indicating lack of homogeneity of variance for a repeated measures variable, an adjustment of df (Huynh-Feldt correction) was used to test statistical significance.36
RESULTS
Thirteen of the 16 participants were presented 6 CO2 trials on a single recording session, 2 were administered 12 trials over 2 recording sessions, and one was administered 15 trials over 3 recording sessions, yielding a total of 117 trials. As 4 electrodes were inserted for each recording session, there were potentially 468 electrodes available for the analysis. Of these, 121 (26%) electrodes provided decomposable motor units, with 203 motor units identified in 14 of the 16 subjects. For the 14 subjects, the mean number of motor units was 14.5 (SD = 11.0), with a range from 1 to 36. Data from other electrodes did not result in the identification of motor units due to (a) technical problems such as electrodes becoming dislodged (131 electrodes, 28%), (b) absence of single motor activity (80 electrodes, 17%), or (c) simultaneous recordings of too much motor unit activity for individual units to be identified and sorted (136 electrodes, 29%).
Respiratory Variables
Baseline values and the values for the last 3 hypercapnic breaths are shown in Table 1, although statistical analyses were conducted over the 6 values. PetCO2 began at a low eucapnic level, rose by ∼8 mm Hg over a trial and remained above the baseline level at the end of the recovery period. As would be expected and as shown in Table 1, hypercapnia produced a progressive and significant increase in minute ventilation. This was a consequence of an increase in tidal volume, not a change in cycle duration. Peak inspiratory flow also increased, producing a progressively negative change in epiglottic pressure, but not a change in airway resistance. Thus, as anticipated, the experimental manipulation resulted in an increase in PetCO2, ventilation and negative airway pressure.
Table 1.
Respiratory activity during baseline and the final three breaths of hypercapnia
| Baseline | Final 3 Breaths | F5,75 | P | |
|---|---|---|---|---|
| Breath duration (s) | 4.5 (1.9) | 4.5 (2.4) | 0.19 | 0.82 |
| Tidal volume (mL) | 673 (298) | 888 (429) | 22.3 | < 0.001 |
| Minute ventilation (L) | 9.3 (2.5) | 12.5 (4.0) | 26.2 | < 0.001 |
| PetCO2 (mm Hg) | 34.3 (4.7) | 42.6 (4.2) | 60.3 | < 0.001 |
| Peak inspiratory flow (L/min) | 29.3 (7.3) | 38.2 (12.5) | 21.1 | < 0.001 |
| Epiglottal Pres Peak Flow (cm H2O) | −2.9 (0.8) | −3.9 (1.2) | 12.5 | < 0.001 |
| Resistance Peak Flow (cm H2O)/L/s) | 4.3 (3.1) | 4.4 (2.7) | 0.58 | 0.34 |
The statistical analysis reported is a one way ANOVA over the 6 time points in the analysis. However, only values for baseline and the last 3 hypercapnic breaths have been reported in the table. All comparisons required Huynh-Feldt correction of df. SD in parenthesis.
Spike Density During a Trial
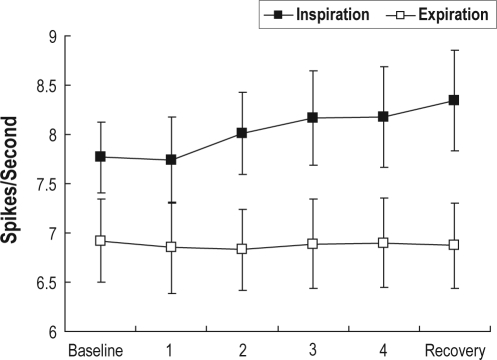
Spike density during inspiration and expiration over a trial is illustrated in Figure 1. As would be anticipated given that, with respect to respiration, GG is known to be an inspiratory phasic muscle, spike density was significantly higher during inspiration than expiration (F1,15 = 17.3, P < 0.001); and while there was no effect of time within a trial (F5,75 = 0.70, P > 0.05), there was a significant interaction effect (F5,75 = 5.84, P < 0.01), with spike density increasing over a trial during inspiration, but not during expiration.
Figure 1.
Total spikes/second, irrespective of motor unit affiliation, during inspiration and expiration as a function of consecutive 30-s blocks over a trial. Error bars indicate standard errors.
Distribution of Motor Units as a Function of Their Discharge Pattern
The group percentage distribution of the different motor unit discharge patterns that were present during baseline, were recruited during hypercapnia, and were active by the end of the trials, are shown in Figure 2. Chi-square analysis indicated there was a statistically significant difference between the distribution of motor units at baseline compared to the distribution of units recruited during a trial χ2(3) = 61.5, P < 0.001. There was also a significant difference between the distributions at baseline and at the end of a trial χ2(3) = 16.4, P < 0.001. (Expiratory phasic units were not included in these analyses as they had an expected cell frequency < 5.)
Figure 2.
Percentage distribution of motor units with different discharge patterns during baseline that were recruited during hypercapnia and were present at the end of the trial during recovery. IP = inspiratory phasic; IT = inspiratory tonic; EP = expiratory phasic; ET = expiratory tonic; TT = tonic.
There were several features of the changes in distribution of motor units with different discharge patterns. The first was that during baseline, with the exception of expiratory phasic units, there was a relatively even distribution of the different patterns. Second, in marked contrast to units active during the baseline, motor units recruited during hypercapnia were overwhelmingly inspiratory phasic (84%). Thus, by the end of the CO2 stimulation, inspiratory phasic units had more than doubled in number to almost 50% of the active units (the recruitment of an inspiratory phasic unit is illustrated in Figure 3). A third feature of the data, which is illustrated in Table 2, was that the discharge pattern of 23% of the units active during baseline changed during hypercapnia, while a further 9% ceased discharge.
Figure 3.
The top 2 traces show instantaneous frequency plots for a recruited inspiratory phasic unit (top trace) and a tonic unit (second trace) as a function of time in the trial. This segment is taken from a period during the final 30 sec of a CO2 trial. Also shown is the raw EMG, rate of flow, and CO2 recordings.
Table 2.
Discharge pattern of motor units active during baseline but which changed their discharge pattern during hypercapnia
| Baseline Distribution |
Distribution of Changed Units During Hypercapnia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Units | Units that Changed | IP | IT | EP | ET | TT | Stopped | |
| IP | 39 | 6 | - | 6 | - | - | - | - |
| IT | 27 | 8 | 4 | - | - | 1 | 1 | 2 |
| EP | 2 | 2 | - | 1 | - | 1 | - | - |
| ET | 39 | 13 | - | - | - | - | 5 | 8 |
| TT | 34 | 17 | - | - | - | 14 | - | 3 |
| 141 | 46 | 4 | 7 | - | 16 | 6 | 13 | |
Tonic units tended to acquire a phasic expiratory component. The cross correlation value, which is a measure of the strength of the phasic component (see methods), significantly increased during hypercapnia (baseline = 0.39 and hypercapnia = 0.49; F5,125 = 4.25, P < 0.001), However, the phasic expiratory component of expiratory tonic units remained constant from baseline to hypercapnia with a value of 0.65. This difference was also reflected in the distribution of the different discharge patterns. Approximately half the tonic units that continued to fire converted into expiratory tonic units (14 of 31, see Table 2, an example is shown in Figure 4), while of the 31 expiratory tonic units that continued to fire, converted to a tonic pattern. This difference was statistically significant χ2 (1) = 5.06, P < 0.05.
Figure 4.
The top tracing shows an instantaneous frequency plot for a tonic unit that acquired an expiratory phasic component late in the trial. The left panel is taken from early in the trial and the right panel from late in the trial. The average maximum cross correlation values were 0.38 and 0.70 respectively; 60 sec intervened between the 2 panels. Also shown are the raw EMG, rate of flow, and CO2 recordings.
Another aspect of the behavior of these units was that expiratory tonic units exhibited tendencies to both recruit (6 units, see Figure 2) and cease firing (8 units, see Table 2) in response to hypercapnia. Finally, the majority of the 33 units that continued to fire, but changed classification, involved changes between inspiratory phasic and inspiratory tonic patterns (10 units, see Table 2), or between expiratory tonic and tonic (19 units, see Table 2).
Discharge Properties of Motor Units Active during Baseline
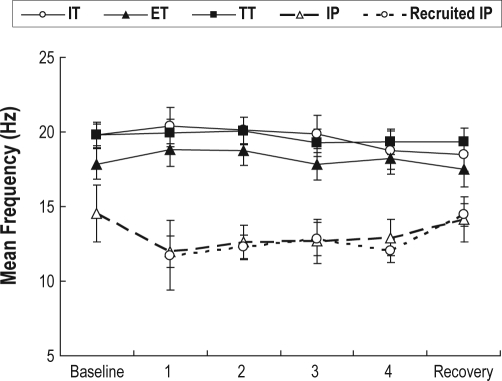
The discharge properties of inspiratory tonic and inspiratory phasic units that were active during baseline and were present during hypercapnia are shown in Table 3 with mean values illustrated in Figure 5. Neither inspiratory phasic nor inspiratory tonic motor units changed their discharge rates following CO2 administration, nor was there a significant change in pre-activation, or the proportion of a breath that inspiratory phasic units were active (see Table 3). This pattern is illustrated for 2 inspiratory phasic units in Figure 6. A subsequent analysis (not reported in Table 3) indicated that compared to inspiratory phasic units, inspiratory tonic units had higher peak discharge (F1,29 = 17.59, P < 0.001) and mean discharge rates (F1,29 = 15.98, P < 0.001) and had an earlier onset (F1, 29 = 4.22, P < 0.05). Thus, for units active during the baseline, inspiratory tonic units had higher discharge rates than inspiratory phasic units, but neither was significantly affected by the hypercapnic manipulation.
Table 3.
Discharge characteristics of inspiratory modulated motor units as a function of hypercapnia
| Time in CO2 trial |
||||||||
|---|---|---|---|---|---|---|---|---|
| Inspiratory tonic | Pre CO2 | 1 | 2 | 3 | 4 | Post CO2 | F*5, 75 | P |
| Mean freq (Hz) | 19.72 (3.03) | 20.20 (5.13) | 20.47 (3.38) | 19.86 (4.93) | 18.77 (5.17) | 18.52 (4.96) | 1.46 | 0.23 |
| Peak freq (Hz) | 22.37 (3.21) | 22.00 (4.36) | 23.18 (4.11) | 22.51 (4.61) | 21.79 (5.90) | 21.39 (5.48) | 1.04 | 0.39 |
| Tonic freq (Hz) | 13.69 (3.53) | 12.48 (4.77) | 13.47 (5.60) | 12.90 (5.81) | 13.64 (7.12) | 13.27 (5.57) | 0.22 | 0.89 |
| Time of onset (ms)† | −0.06 (0.17) | −0.07 (0.16) | −0.11 (0.24) | −0.08 (0.33) | −0.005 (0.26) | −0.08 (0.22) | 0.49 | 0.75 |
| Inspiratory phasic | F*5, 70 | |||||||
| Mean freq (Hz) | 14.54 (10.32) | 11.97 (4.69) | 12.61 (5.07) | 12.69 (6.02) | 12.91 (5.25) | 14.13 (7.39) | 0.62 | 0.52 |
| Peak freq (Hz) | 14.90 (7.76) | 13.98 (5.16) | 15.07 (5.79) | 15.30 (6.59) | 15.54 (5.65) | 16.07 (6.52) | 0.49 | 0.62 |
| Pre-activation (s) | −0.91 (2.04) | −0.70 (1.36) | −0.44 (0.97) | −0.53 (1.04) | −0.35 (1.09) | −0.09 (0.84) | 2.24 | 0.15 |
| % Duration‡ | 34.41 (16.39) | 31.49 (9.34) | 33.52 (7.97) | 35.21 (8.16) | 37.80 (6.73) | 32.91 (7.72) | 1.08 | 0.36 |
All comparisons required Huynh-Feldt correction of df;
Time of onset of phasic component compared to onset of ventilation;
Percentage of a breath a unit is active; SD in parenthesis.
Figure 5.
Mean frequency (Hz) for inspiratory phasic (IT), recruited IP, inspiratory tonic (IT), expiratory tonic (ET), and tonic (TT) motor units as a function of consecutive 30-s blocks. CO2 was added to the inspiratory line during blocks 2 through 5. Error bars indicate standard errors.
Figure 6.
The top 2 tracings show 2 inspiratory phasic motor units recorded on a single electrode, neither of which shows any evidence of rate coding in response to hypercapnia. The left vertical line indicates the onset of CO2 administration and the right line the offset. Also shown are the raw GGEMG, flow, and CO2 recordings.
The mean discharge properties for expiratory tonic and tonic motor units that were active during the baseline are illustrated in Figure 5. Statistical analysis of expiratory tonic units indicated that neither the mean (F5,75 = 1.87, P > 0.05), peak (F5,75 = 1.37, P > 0.05), or tonic frequency (F5,75 = 1.41, P > 0.05) showed any effect of hypercapnia. Similarly, mean values for tonic units remained unchanged (F5,100 = 0.80, P > 0.05).
Discharge Properties of Motor Units Recruited Subsequent to the Onset of Hypercapnia
The vast majority of recruited motor units showed an inspiratory phasic discharge pattern, with too few of the other patterns to be included in these analyses. Of the 52 inspiratory phasic units recruited, 6 began firing by the end of the first 30 sec after the onset of the hypercapnia manipulation, 24 by the end of the second 30-sec period, 11 by the end of the third, 9 by the end of the fourth, and 2 after CO2 was turned off. Recruited units were far more intermittent in their activities than units active during baseline, firing on only 63% of breaths after their initial onset. However, the discharge rate for recruited inspiratory phasic units was, on breaths that the unit fired, equivalent to units active during the baseline. This is illustrated in Figure 5 where the mean discharge rates of the 2 types of units are virtually identical. Statistically the 2 groups of units were compared at the fourth 30-sec period of hypercapnia, when almost all of the recruited units had become active. Neither mean rates (F1,44 = 0.02, P > 0.05) nor peak rates (F1,44 = 0.08, P > 0.05) showed differences. Further, as with baseline active units, recruited units did not increase their discharge rates as hypercapnia progressed (Figure 5), rather they effectively achieved their maximum firing rates on the first breath they were active (Figure 3 illustrates this effect for a single motor unit).
DISCUSSION
The hypercapnic manipulation had the expected effects on the general pattern of respiratory and total GG activity, establishing conditions appropriate to testing the hypotheses. Thus, there was an increase in ventilation and inspiratory negative pressure, while airway resistance remained constant, and spike density, a measure of multiunit activity, increased during inspiration, but not expiration. Further the proportional distribution of the different motor unit patterns during the baseline (Figure 2) was approximately the same as in previous studies conducted during daytime relaxed wakefulness,26 pre-sleep relaxed wakefulness,14,27 and following arousal from sleep,29 although there were somewhat more expiratory tonic units and fewer inspiratory tonic units in the current data set. Given these findings, the results suggest that in humans the increased output of the GG muscle under conditions of hypercapnia is primarily due to the activity of inspiratory phasic units, and specifically the recruitment of previously inactive units and not to an increase in the discharge rate of already active units, nor to an increase in the proportion of a breath the unit was active. This conclusion is consistent with a recent report, which also demonstrated an absence of rate coding in tonically active GG motor units in response to hypercapnia.32 In this paper motor unit recruitment was inferred from the absence of rate coding in tonically active units and the presence of increased GG activity, an inference that is directly confirmed by our current data.32
The most usual pattern of recruitment of motor units in skeletal muscles consists of a mixed pattern of rate coding and unit recruitment37,38; however, muscles differ as to the balance between these two processes.39 For example, hand muscles emphasize rate coding, while shoulder muscles emphasize recruitment, a distinction that may reflect muscles with fine motor control versus large muscles that require strong contractions.40 Within respiratory muscles the diaphragm is more dependent on rate coding,41 while the scalene and parasternal intercostal muscles tend to favor recruitment.42 In the current study hypercapnia was associated with motor unit recruitment, with essentially no evidence of rate coding. This observation is consistent with previous studies in humans in which changes in respiratory drive at sleep onset14,27 and arousal from sleep29 were associated with units ceasing activity and unit recruitment respectively and with only minor changes in the discharge rate of individual motor units.
The discharge pattern of recruited units also reflected an absence of rate coding. The peak and mean discharge rates of newly recruited inspiratory phasic units did not increase over time once the unit was recruited; rather, these units reached their characteristic rates on the first active breath. Further, although new units tended to fire intermittently, they had the same discharge rate as originally active units on the breaths that they did fire (see Figures 3 and 5). Similarly, an earlier study indicated that at sleep onset units maintained their firing rate until the final breath before stopping.27 The current results and those of Richardson and Bailey32 are in contrast to an earlier study in anesthetized rats, in which hypercapnia resulted in marked increases in the discharge rate of inspiratory modulated motor units.31 The results are also difficult to reconcile with studies that suggest muscles providing fine motor control emphasize rate coding.40 To speculate, it may indicate that respiratory related activity of GG units does not need to respond in a precise manner (their role being to open the airway in a uni-dimensional manner) and that the intrinsic muscles that help shape and modify the tongue in tasks such as vocalization may have different rate coding /recruitment patterns.
On the assumption that the size principle38 applies to GG, then it would be anticipated that newly recruited units would be larger and therefore have a higher probability of being recorded once they became active. Thus the recording environment may have been more sensitive to recruited units. However, while this might affect the relative distribution of recorded units, it would not alter the basic finding that recruitment is a more important process than rate coding. Further, if recruited units were larger, as predicted by the size principle, their contribution to overall GG muscle activity would also be correspondingly larger.
In the current study hypercapnia was associated with a marked increase in respiratory drive, as indicated by a progressive increase in the total number of inspiratory action potentials. Nevertheless, the manipulation of CO2, although it produced substantial changes in respiratory activity, did not elevate PetCO2 to particularly high levels. This result was primarily because baseline levels were low, most likely as a consequence of an influence of the respiratory circuit and because of the repetition of trials. Increases in motor unit discharge rates may have been observed at higher PetCO2 levels. Indeed, previous studies of SMUs in the human GG have investigated sleep and relaxed wakefulness, states also associated with low respiratory drive.14,27,29 Thus, the observation that rate coding is not an important mechanism in GG is, at this time, limited to low to medium levels of respiratory drive.
While the change in inspiratory phasic activity was the most marked effect of hypercapnia, it was not the only change. There was a slight, although statistically significant tendency for tonic units to acquire an expiratory phasic component and to be reclassified as expiratory tonic units. As illustrated in Figure 4, the magnitude of the phasic component in these units was relatively small and, although the magnitude of the expiratory phasic component was significantly increased when assessed by the correlation technique, it did not reach significance in statistical analyses of the peak discharge rate of expiratory phasic units. One interpretation of this change is that it represents more active expiratory dilatation of the airway during hypercapnia induced ventilatory stimulation.
In a previous paper,29 we argued that the tonic component of inspiratory tonic motor units is controlled separately to the tonic component of expiratory tonic and tonic units. This conclusion was based on the observation that the tonic component associated with inspiratory units responds differently to changes in sleep-wake state than the tonic component associated with expiratory tonic and tonic units. The observation in the current data, that there were changes in discharge pattern between expiratory and tonic units and between inspiratory phasic and inspiratory tonic units, but few changes between the two composite classifications, is consistent with the view that these two tonic components are derived from independent inputs to the hypoglossal motor nucleus.
A major concern with EMG measurements generally, and with single motor unit recordings in particular, is that the apparent recruitment and cessation of motor unit activity may be an artifact of electrode movement. However, we consider this an unlikely explanation of the data reported in this paper. We have previously presented evidence that GG intramuscular electrodes are stable in the absence of body movements and swallows.27,29 Further, electrode movement would be expected to produce a random pattern of recruitment and cessation of activity reflecting the underlying distribution of motor units, rather than the specific pattern of inspiratory phasic unit recruitment seen in the data.
While our underlying purpose in studying GG was a concern with OSA, the generalizability of the current findings are limited by the restriction of data collection to normal male subjects during wakefulness. However, we are aware of only one other study that has investigated SMU GG activity in humans in response to hypercapnia; it also studied subjects during wakefulness, and presumably the investigators, like us, considered wakefulness an appropriate place to start. Critically, the nature of motor control in GG revealed by the current data is quite consistent with that indicated by studies of sleep-wake transitions.27,29 While it is clear that future studies will be required to extend these findings to sleeping subjects as well as women, the aged, and OSA patients, the developing understanding of motor control in GG through both wakefulness and sleep studies is likely to assist in understanding the role of GG in OSA.
A further consideration in conducting such studies during wakefulness is the possibility that the response of the GG to hypercapnia may have been modified by behavioral factors. We have no direct evidence that such an influence was, or was not, present in this study. However, we consider it unlikely that any such effect would have been marked, as the pattern of change in respiratory and total muscle measures suggest primarily involuntary influences that were consistent with known responses to chemical stimulation.
In conclusion, the activity of the GG increases in response to moderate levels of hypercapnia due to the recruitment of inspiratory phasic motor units, rather than an increase in the discharge rate of active units. This response to hypercapnia differs from that observed in rats, suggesting a species difference in the motor control of the GG. We speculate that demands on the upper airway by respiration, vocalization, and other activities, has produced a particular pattern of motor control in the human GG.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Nicholas has received research support from Ventus Medical. Dr. Worsnop has participated in speaking engagements for GlaxoSmithKline, AstraZeneca, Pfizer, Boehringer Ingelheim, Schering Plough, Elsevier, and Nycomed. Dr. Malhotra has received research support and/or consulted for Philips, Pfizer, Cephalon, Sepracor, Itamar, NMT, SGS, Apnex, Novartis, Ethicon, and Medtronic. Dr. White is Chief Medical Officer for Philips Respironics and consults for Itamar Medical. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The project was supported by grants NHMRC-454458 and NIH-1R01HL085188-01A2
REFERENCES
- 1.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–23. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 6.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–7. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnoea. Respir Res. 2001;2:286–94. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–95. [PubMed] [Google Scholar]
- 9.Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–8. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–7. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 11.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 12.Fregosi RF. Influence of tongue muscle contraction and dynamic airway pressure on velopharyngeal volume in the rat. J Appl Physiol. 2008;104:682–93. doi: 10.1152/japplphysiol.01043.2007. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–58. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- 14.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 15.Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol. 2006;101:609–17. doi: 10.1152/japplphysiol.00204.2006. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi I, Perry A, Rhymer J, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–9. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 18.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–70. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 19.Sauerland EK, Mitchell SP. Electromyographic activity of the human Genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc. 1970;35:69–73. [PubMed] [Google Scholar]
- 20.Mathew OP. Upper airway negative-pressure effects on respiratory activity of upper airway muscles. J Appl Physiol. 1984;56:500–5. doi: 10.1152/jappl.1984.56.2.500. [DOI] [PubMed] [Google Scholar]
- 21.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52:438–44. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- 22.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–5. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 24.Lo YL, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–7. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillar G, Malhotra A, Fogel RB, et al. Upper airway muscle responsiveness to rising PCO(2) during NREM sleep. J Appl Physiol. 2000;89:1275–82. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- 26.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson V, Malhotra A, Nicholas CL, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–33. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97:933–6. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson V, Malhotra A, Nicholas CL, et al. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep. 2010;33:379–87. doi: 10.1093/sleep/33.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 31.John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med. 2005;172:1331–7. doi: 10.1164/rccm.200505-790OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol. 2010;103:1315–21. doi: 10.1152/jn.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banzett RB, Garcia RT, Moosavi SH. Simple contrivance “clamps” end-tidal PCO(2) and PO(2) despite rapid changes in ventilation. J Appl Physiol. 2000;88:1597–600. doi: 10.1152/jappl.2000.88.5.1597. [DOI] [PubMed] [Google Scholar]
- 35.Orem J, Dick T. Consistency and signal strength of respiratory neuronal activity. J Neurophysiol. 1983;50:1098–107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- 36.Noruis MJ. SPSS Inc. SPSS advanced statistics user's guide. Chicago, Ill: SPSS; 1990. [Google Scholar]
- 37.Burke RE. Motor units: anatomy, physiology, and functional organization. In: Brookhart JM, Mountcastle VB, Magoun HW, editors. Handbook of physiology. Bethesda, MD: American Physiological Society; 1981. pp. 345–422. [Google Scholar]
- 38.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–7. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 39.Binder MD, Heckman CJ, Powers RK. The physiological control of motor neuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of physiology. New York: American Physiological Society; 1996. pp. 423–507. [Google Scholar]
- 40.Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- 41.Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol. 1999;518(Pt 3):907–20. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med. 1999;160:1598–603. doi: 10.1164/ajrccm.160.5.9904023. [DOI] [PubMed] [Google Scholar]