Abstract
Rats selectively bred for low aerobic running capacity exhibit the metabolic syndrome, including hyperinsulinemia, insulin resistance, visceral obesity, and dyslipidemia. They also exhibit features of nonalcoholic steatohepatitis, including chicken-wire fibrosis, inflammation, and oxidative stress. Hyperinsulinemia in these rats is associated with impaired hepatic insulin clearance. The current studies aimed to determine whether these metabolic abnormalities could be reversed by caloric restriction (CR). CR by 30% over a period of 2–3 months improved insulin clearance in parallel to inducing the protein content and activation of the carcinoembryonic antigen-related cell adhesion molecule 1, a main player in hepatic insulin extraction. It also reduced glucose and insulin intolerance and serum and tissue (liver and muscle) triglyceride levels. Additionally, CR reversed inflammation, oxidative stress, and fibrosis in liver. The data support a significant role of CR in the normalization of insulin and lipid metabolism in liver.
Caloric restriction exerts its beneficial metabolic effect by promoting insulin extraction and reducing lipogenesis in liver.
Low aerobic capacity constitutes a strong risk factor of mortality from metabolic diseases (1). We have shown that rats selectively bred for low aerobic running capacity [low-capacity runners (LCR)] via selection based on treadmill running distance to exhaustion exhibit features of the metabolic syndrome, including visceral obesity, insulin resistance, and high blood pressure, compared with age-matched high-capacity runners (HCR) (2). They also exhibit elevated hepatic and serum triglyceride levels with a progressive form of hepatic steatosis (3), characterized by features of nonalcoholic steatohepatitis (NASH), including chicken-wire fibrosis and inflammation (4,5).
Hyperinsulinemia in LCR is associated with impaired hepatic insulin clearance, which appears to correlate with reduced mRNA of the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) (2). CEACAM1, a substrate of the insulin receptor kinase in liver, but not muscle or adipose tissue, promotes receptor-mediated insulin endocytosis and degradation, a process that underlies insulin clearance (6). Consistent with the liver being the main site of endogenous insulin clearance, L-SACC1 mice with liver-specific inactivation of CEACAM1 and mice with global Ceacam1 null mutation (Cc1−/−) develop hyperinsulinemia resulting from impaired insulin clearance. The hyperinsulinemic state of these mice causes whole-body insulin resistance, liver steatosis, and redistribution of triglyceride to the adipose tissue and, subsequently, visceral obesity (7,8,9,10). The similarity in the phenotype of L-SACC1 and Cc1−/− mice emphasizes the role of hepatic insulin and fat metabolism in regulating the metabolic response in extrahepatic tissues and assigns a major role for CEACAM1 in this process.
Exercise and caloric restriction (CR) reduce comorbidities of metabolic diseases. The recently completed 10-yr follow-up of the diabetes prevention program achieved its most favorable results in the cohort of intensive lifestyle modification that maintained significant weight loss through a program of regular exercise and CR (11,12). The mechanisms of the beneficial effect of regular exercise and CR are not well delineated, in part due to the limitation imparted by the paucity of human tissues for mechanistic analyses. To circumvent this problem, we subjected LCR, a replicate model of the human disease, to high-intensity interval endurance training for 8 wk (13). This program effectively reduced cardiovascular risk, such as endothelial dysfunction and hypertension, fat mass, and glucose intolerance (13). However, it did not reduce the hepatic protein content of fatty acid synthase (FAS), a key enzyme in de novo fatty acid synthesis (13). This suggests that endurance training does not suffice to reverse all metabolic abnormalities in LCR. Thus, here, we examined whether CR regulates hepatic lipid metabolism in LCR.
Materials and Methods
Caloric restriction
Three-month-old male LCR rats (n = 20) from generation 20 were randomized to either CR or ad libitum (AL) feeding of regular rat chow (Lab Diet no. 5001) for up to 90 d (14). Initially, after monitoring food intake for 2 wk, rats were placed on a 10% CR program for 2 wk before restricting their diet by 30% of each animal’s individual initial caloric consumption throughout the remainder of the study. Food was dispensed to CR animals daily at 1700 h. Age-matched HCR were fed AL throughout the studies. Total food intake was defined as the average daily food intake over the 3-month period of the study.
Endurance training
Liver and serum samples were obtained from our previously used set of rats, which at 3 months of age had been either left sedentary (S) or subjected to an aerobic interval endurance training for 8 wk (T) (13). This high-intensity interval training consisted of running for 1 h/d, 5 d/wk until VO2max (oxygen consumption) reached stability. The animals were euthanized 48 h after exercise (13). The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Intraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT) and blood glucose measurements
IPGTT and ITT were measured after an overnight fast (1700–0800 h the next day for IPGTT, 1700–1100 h the next day for ITT). Rats were anesthetized, injected ip with 0.50 U insulin/kg body weight (for ITT) or 2 g glucose/kg body weight (for IPGTT), and their whole venous blood from catheterized tail vein was drawn at 0–2 h after injection to determine blood glucose levels. Automated “Accu-chek Aviva” test strips (Roche, Indianapolis, IN) were used for all glucose measurements. Tests were repeated at 6 and 9 wk of feeding to account for changes in body weight.
Metabolic analysis
After an overnight fast, rats were anesthetized with sodium pentobarbital. Whole venous blood was drawn from the tail vein to measure serum insulin and C-peptide by RIAs (Linco Research, St. Charles, MO), serum free fatty acids (FFA) by NEFA C kit (Wako, Richmond, VA) and serum triglycerides by Triglycerides reagent (Pointe Scientific, Inc., Canton, MI). Liver tissue triglycerides were measured from livers harvested at end of treatments, homogenized in sucrose lysis buffer, and separated by chloroform-methanol and aqueous sulfuric acid; the organic layer was dried, reconstituted by chloroform, dried and measured by Triglycerides reagent (Pointe Scientific, Inc.), and normalized to protein content of lysates. Visceral adipose tissue was excised, weighed, and visceral adiposity expressed as percentage of total body weight.
Western blot analyses
Livers were removed at euthanization from age-matched rats after an overnight fast. We analyzed 20 μg of total lysates with 4–12% SDS-PAGE before transferring proteins to nitrocellulose membranes. Proteins were immunoblotted (Ib) with polyclonal antibodies against CEACAM1 (α-CC1), FAS (α-FAS) (10), and CD36 (α-CD36; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), followed by reIb with monoclonal antibody against tubulin (Sigma, St. Louis, MO) or with polyclonal antibody against α-actin (Santa Cruz Biotechnology, Inc.) to normalize for total protein content. After protein detection by enhanced chemiluminescence, protein bands were scanned and their density measured using Image J software and calculated as percentage of the amount of proteins loaded.
Ex vivo phosphorylation
Tissue lysates were treated with 10−6 m insulin for 5 min before immunoprecipitation (Ip) with monoclonal antibodies against α-phosphotyrosine (α-pY) (Upstate Biotechnology, Inc., Lake Placid, NY) or rat CEACAM1 (HA4 c19) (15), or with a polyclonal antibody against α-phospho-CEACAM1 (α-pCC1) (Bethyl Laboratories, Inc., Montgomery, TX). The α-pCC1 affinity purified polyclonal antibody was raised in rabbit against a CEACAM1 phosphorylated peptide sequence flanking the unique Tyrosine 488 phosphorylation site in mice: 482 KVDDVA (pY) TVLNF 493 (NCBI Accession no. AAK52601) (15). After analysis on 7% SDS-PAGE, CEACAM1 immunopellets were immunoprobed sequentially with α-pCC1 and α-CC1 polyclonal antibodies (10). The bands were scanned and their density measured using ImageJ software (National Institutes of Health, Bethesda, MD), normalized per the amount of proteins loaded (PhosphoCEACAM1/CEACAM1), and the ratio calculated as ±sem.
Thiobarbituric acid reactive substances (TBARS) assay
Lipid peroxidation was measured as described (16) with modifications. Briefly, liver tissue was homogenized in 1.15% KCl before centrifugation at 10,000 rpm at 4 C for 10 min. The supernatant (100 μl) was added to 8.1% sodium dodecyl sulfate, 20% acetic acid, and 0.8% thiobarbituric acid, followed by heating at 95 C for 60 min; n-butanol-pyridine (15:1) was added to the cooled mix before centrifugation at 4000 × g for 10 min, and the upper layer was removed to measure its absorbance at A532nm.
Histological analysis
Histological examination was established using hematoxylin-eosin (H&E) of formalin-fixed paraffin-embedded liver. For analysis of fibrosis, deparaffinized and rehydrated slides were also incubated in 0.1% solution of Sirius red (Direct Red 80; Sigma) and mounted with resin, following manufacturer’s instructions.
Statistical analysis
Data were analyzed with SPSS software using one-way ANOVA with Bonferroni’s multiple comparison test or Student’s t test. P values of less than 0.05 were statistically significant. Bar and line graphs were produced by GraphPad Prism 4 software (GraphPad Software, Inc., La Jolla, CA).
Results
CR reduced body mass and visceral obesity in LCR
During the initial feeding period, AL-fed HCR (AL-HCR) consumed approximately 14.6 g/d of chow (5.3% of their body weight) in food daily. This daily food consumption increased to approximately 19.3 g/d, or approximately 6.3% of body weight on average over the course of the study (Table 1). In comparison, AL-LCR consumed initially approximately 17.5 g/d of chow (4.3% of their body weight) daily. Their average food consumption increased to 21.5 g/d, or approximately 4.9% of body weight. In concordance with other reports (3), the data indicate that HCR consumed more food per body weight than LCR. Nonetheless, 6-month-old LCR males exhibited a 1.5-fold higher body weight than their age-matched HCR counterparts (Table 1), as previously observed (2). This is partly attributed to elevated visceral obesity (by ∼2-fold). Energy balance was not analyzed in these studies, but other reports have shown reduction in ambulatory activity and oxygen consumption in LCR by comparison with HCR rats (3). Nonetheless, LCR had increased food intake over the course of the study. Subjecting them to a 30% CR program reduced their body weight progressively to reach that of AL-HCR after about 50–60 d (CR-LCR vs. AL-HCR) (Fig. 1 and Table 1). Visceral obesity was also reduced by 50% by CR to become comparable with that in AL-HCR (Table 1).
Table 1.
Effect of caloric restriction on serum and tissue biochemistry of 6-month-old LCR rats
| LCR
|
HCR AL | ||
|---|---|---|---|
| AL | CR | ||
| Initial body weight (g) | 402. ± 12.6 | 412. ± 9.61 | 274. ± 8.41a,c |
| Body eeight at sacrifice (g) | 471. ± 8.29 | 335. ± 6.99b | 340. ± 9.81a |
| Initial daily food consumption (g) | 17.5 ± 0.64 | 17.5 ± 0.64 | 14.6 ± 0.65a,c |
| Total daily food consumption (g) | 21.5 ± 0.52 | 12.3 ± 0.45b | 19.3 ± 0.55c |
| %Visceral fat/body weight | 1.81 ± 0.11 | 0.81 ± 0.12b | 1.11 ± 0.11a |
| Hepatic triglyceride (μg/mg protein) | 154. ± 20.5 | 67.8 ± 14.4b | 96.7 ± 13.8a |
| Fasting blood glucose (mg/dl) | 121. ± 6.14 | 106. ± 3.38b | 103. ± 4.54a |
| Random blood glucose (mg/dl) | 100. ± 3.10 | 86.8 ± 4.70b | 87.3 ± 3.26a |
| Serum insulin (pm) | 794. ± 156. | 115 ± 12.2b | 201. ± 31.8a,c |
| Serum C-peptide (pm) | 2655 ± 259.0 | 940 ± 133b | 1397 ± 145.0a,c |
| Serum C-peptide/insulin molar ratio | 4.66 ± 0.84 | 8.23 ± 0.62b | 7.81 ± 0.63a |
| Serum FFA (mEq/liter) | 1.46 ± 0.11 | 0.70 ± 0.20b | 0.79 ± 0.07a |
| Serum triglyceride (mg/dl) | 53.1 ± 7.32 | 18.7 ± 3.05b | 31.9 ± 3.96a,c |
Male LCR (n > 8 per group; 3 months of age) were fed AL or CR for 3 months prior to blood drawing from tail vein and tissue extraction at the time of killing for biochemical analysis. Age-matched (6-month-old) AL-HCR were also examined. Values are expressed as mean ± sem.
P < 0.05 AL-HCR vs. AL-LCR.
P < 0.05 CR-LCR vs. AL-LCR.
P < 0.05 AL-HCR vs. CR-LCR.
Figure 1.
CR reduces body mass in LCR. LCR rats were randomized to either CR or AL feeding (n = 10 in each feeding group). Total body weight was measured over the course of the study and compared with that of AL-HCR (n = 10). Values are mean ± sem. AL-HCR and AL-LCR were significantly different (P < 0.001) at all feeding time points.
CR improved hepatic insulin clearance and overall insulin sensitivity in LCR
By comparison with AL-HCR, AL-LCR exhibited hyperinsulinemia with impaired insulin clearance, as assessed by an approximately 2-fold lower steady-state C-peptide-to-insulin molar ratio (Table 1). CR reduced serum insulin level in LCR by approximately 7-fold. This could be due to its cumulative reducing effect on insulin secretion, as suggested by the 3-fold decrease in C-peptide levels (CR- vs. AL-LCR) (Table 1), and inducing effect on insulin clearance, as assessed by the 2-fold increase in steady-state C-peptide/insulin ratio (CR-LCR vs. AL-LCR) (Table 1). Consistent with a role for CEACAM1 in hepatic insulin clearance (9), Western blot analysis using a rat specific α-CC1 antibody for Ib followed by reprobing (reIb) with α-actin antibody revealed a more than 50% reduction in CEACAM1 level in AL-LCR by comparison with AL-HCR livers (Fig. 2A). Accordingly, ex vivo phosphorylation of liver lysates in the presence of insulin (+) or buffer (−) followed by Ip with α-CC1 and Ib with α-pCC1 antibodies revealed failure of insulin to induce CEACAM1 phosphorylation in AL-LCR (Fig. 2B). This was confirmed by Ip with α-pCC1 and sequential Ib with α-pY and α-CC1 antibodies to account for protein loading. A nonspecific unidentified band (band X) was detected in some experiments. CR elevated hepatic CEACAM1 protein levels to the level of AL-HCR (Fig. 2A). It also restored insulin-stimulated CEACAM1 phosphorylation in liver lysates derived from AL-LCR (Fig. 2B).
Figure 2.
The effect of CR on proteins implicated in lipid and insulin metabolism in liver of LCR. A, Western blot analysis was performed in liver lysates by Ib with α-FAS, α-CEACAM1 (α-CC1), or α-CD36 antibodies followed by reIb with α-actin or α-tubulin to account for protein loading, as described in Materials and Methods. Two representative animals of at least three rats per each group are included. The mean of band density was measured and plotted as ±sem in the graphs on the right. *, P < 0.05 vs. AL-LCR. B, Liver lysates were treated with (+) or without (−) insulin (Ins), Ip with α-CC1 (top gel) or α-pCC1 (bottom gel) antibody before Ib with an antibody against α-pCC1 (top gel) or α-pY (bottom gel) followed by reIb with α-CC1 antibody to normalize per the total amount of CEACAM1 in the immunopellet. A nonspecific unidentified band (band X) was detected in some experiments. Gels are representative of three separate experiments performed on lysates from different mice per group per experiment. Separations between blots indicate removal of empty lanes on the same gel. The mean of pCC1 band density normalized per total CC1 band density was plotted in the graph on the right as a ratio of pCC1 to CC1 in the presence or absence of insulin. Units are arbitrary. *, P < 0.05 (−) vs. (+) insulin.
In addition to hyperinsulinemia, AL-LCR exhibited other features of insulin resistance by comparison with AL-HCR. These include glucose and insulin intolerance (Fig. 3, A and B, respectively) and fed hyperglycemia (Table 1), in agreement with other reports (17). CR reversed insulin tolerance (Fig. 3B), indicating restoration of whole-body insulin sensitivity. Consistent with increased insulin sensitivity, insulin excursion in response to glucose was reduced in CR-LCR compared with AL-LCR (data not shown). Although blood glucose level eventually returned to the level of HCR controls, the initial excursion in response to a glucose challenge was not reduced by CR (Fig. 3A). Because CR decreased fasting blood glucose to HCR levels (Table 1), and hence, most likely restored glucose homeostasis in liver, it is possible that persistently elevated initial glucose excursion in CR-LCR is attributed to unaffected glucose disposal in skeletal muscle.
Figure 3.
CR improves insulin and glucose tolerance in LCR. Rats (n = 10 from each feeding group per line) were anesthetized and subjected to ip glucose (A) and insulin (B) tolerance tests (IPGTT and ITT, respectively). Values are mean ± sem; *, P < 0.05 vs. AL-LCR, as analyzed by Student’s independent t test.
Of note, CR exerts a similar effect on body weight and insulin clearance in 9-month-old males from generation 18 (data not shown).
CR improved lipid metabolism in LCR
Consistent with elevation in key hepatic lipogenic enzymes, such as FAS, under conditions of chronic hyperinsulinemia (18,19,20), Western blot analysis revealed a higher FAS protein level in AL-LCR than HCR livers (Fig. 2A). This could contribute to the 2-fold higher hepatic triglyceride content in AL-LCR, which may in part underlie the approximately 2.5-fold higher level of serum triglyceride in these rats (Table 1). Increased visceral obesity in AL-LCR (by 2-fold) could, at least in part, result from redistribution of triglyceride into white adipose tissue. Elevation in visceral obesity might result in excessive lipolysis and hepatic fatty acid transport, as suggested by the 2-fold higher fasting serum FFA levels (Table 1) and hepatic CD36 protein content (Fig. 2A) in AL-LCR when compared with AL-HCR.
CR in LCR lowered hepatic FAS protein (Fig. 2A) and triglyceride content (Table 1) and serum triglyceride level (Table 1). It also reduced visceral adiposity and, consequently, serum FFA levels, rendering them all comparable with AL-HCR levels (Table 1). Consistent with the role of CD36 in fatty acid transport in liver, hepatic CD36 protein content was also reduced to the level of AL-HCR (Fig. 2A).
CR reduced NASH pathogenesis in LCR
LCR exhibit features of NASH, a more progressive form of steatosis (4,5). These include inflammation, steatosis, and fibrosis. Accordingly, H&E staining of liver sections revealed scattered mononuclear inflammatory cell infiltration in AL-LCR (Fig. 4A, panel 1, black arrows) and altered hepatocellular architecture with microsteatosis (Fig. 4A, panel 1, brown arrows) and macrosteatosis (Fig. 4A, panel 1, green arrows). CR markedly reduced inflammation, as indicated by absence of inflammatory cell islands and normal hepatic architecture in CR-LCR (Fig. 4A, panel 2), as in AL-HCR (Fig. 4A, panel 3). Early fibrosis was also apparent in AL-LCR with a NASH-like “chicken-wire” pattern of Sirius red stain (Fig. 4B, panel 1). These chicken-wire fibrogenic changes were not detected in either CR-LCR (Fig. 4B, panel 2) or AL-HCR (Fig. 4B, panel 3).
Figure 4.
CR reduces features of NASH in LCR. A, Liver sections (magnification, ×40) from more than five rats of each of feeding group were analyzed by H&E staining, as described in Materials and Methods. Small (brown arrow) and large (green arrow) lipid-containing vacuolated cells were detected throughout the section from AL-LCR (panel 1). Inflammatory cells foci were also noted in this animal (panel 1, black arrows). B, Sirius red staining revealed fibrotic changes in AL-LCR (panel 1) but not in other animals. C, TBARS assay was performed in liver lysates from six animals in each feeding group. Bars shown are mean ± sem; *, P < 0.05 vs. AL-LCR, as analyzed by Student’s independent t test.
In agreement with increased oxidative stress in LCR (3), lipid peroxidation (assessed by TBARS concentration) was elevated in AL-LCR liver compared with AL-HCR (Fig. 4C). CR reduced markedly TBARS concentration in LCR, rendering it comparable with that in AL-HCR (Fig. 4C). This suggests that CR limited the initiation of oxidative changes in LCR liver.
Metabolic effect of endurance training in LCR liver
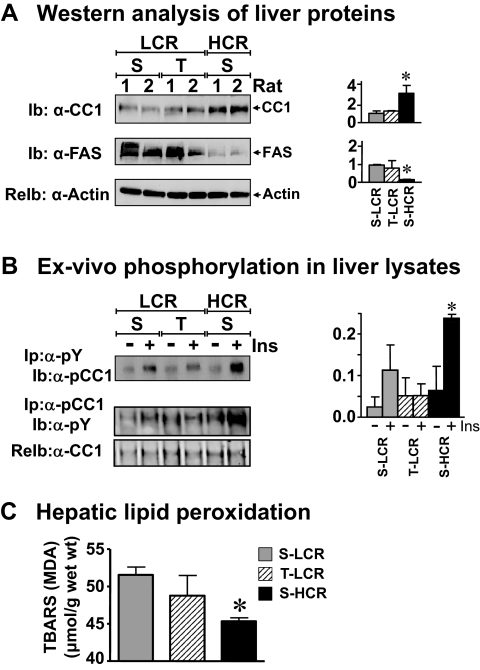
In previous studies, we have shown that 8 wk of high-intensity endurance training improved most of the cardiovascular abnormalities associated with the metabolic syndrome in LCR, including glucose tolerance and insulin receptor phosphorylation and signaling in insulin target tissues (skeletal muscle, adipose tissue, and liver) (13). In addition to reducing body weight (13), it also reduced visceral adiposity to the level of HCR (Table 2). However, this training program did not fully reverse hepatic and serum triglyceride levels, because these parameters remained higher in T-LCR than AL-HCR (Table 2). Sustained elevation in hepatic triglyceride level was due, at least in part, to persistent elevation in synthesis, as suggested by high levels of hepatic FAS protein content in T-LCR (Fig. 5A). This appears to stem from failure of high-intensity training to reverse hyperinsulinemia (Table 2), in association with persistent impairment of insulin clearance (C-peptide/insulin ratio; Table 2) and elevated insulin secretion, as suggested by C-peptide levels (Table 2). Consistent with sustained impairment of insulin clearance in T-LCR, high-intensity training did not induce hepatic CEACAM1 protein content (Fig. 5A) or insulin-stimulated phosphorylation (Fig. 5B) sufficiently enough to reach the level in S-HCR, as shown by more than 50% lower phosphorylated CEACAM1 in the α-phosphotyrosyl (α-pY) and α-pCC1 immunopellets. Moreover, endurance training did not reduce lipid peroxidation, as indicated by persistently high hepatic TBARS concentration in T-LCR by comparison with S-HCR (Fig. 5C).
Table 2.
Effect of endurance training on serum and tissue biochemistry of 6 month-old LCR rats
| LCR
|
HCR S | ||
|---|---|---|---|
| S | T | ||
| %Visceral fat/body weight | 5.1 ± 0.3 | 1.9 ± 0.3b | 1.7 ± 0.2a |
| Hepatic triglyceride (μg/mg protein) | 62.0 ± 6.01 | 58.7 ± 6.85 | 41.9 ± 3.59a,c |
| Serum insulin (pm) | 493. ± 98.9 | 658 ± 190 | 141. ± 24.2a,c |
| Serum C-peptide (pm) | 2385 ± 550.8 | 3433 ± 795.6 | 968.5 ± 67.60a,c |
| Serum C-peptide/insulin molar ratio | 4.02 ± 0.57 | 4.60 ± 0.67 | 8.12 ± 1.15a,c |
| Serum FFA (mEq/liter) | 1.44 ± 0.17 | 0.52 ± 0.08b | 0.86 ± 0.16a |
| Serum triglyceride (mg/dl) | 40.3 ± 3.13 | 34.2 ± 4.17b | 27.2 ± 2.50a,c |
Male LCR (n > 8 per group; 4 months of age) were left S or T for 2 months prior to blood drawing from tail vein and tissue extraction at the time of killing for biochemical analysis. Age-matched (6-month-old) sedentary HCR were also examined. Values are expressed as mean ± sem. Of note, this set of rats was generated, fed, and trained under different housing and feeding conditions than the set in Table 1, which may account for some of the differences in body composition and hepatic triglyceride content.
P < 0.05 AL-HCR vs. AL-LCR.
P < 0.05 CR-LCR vs. AL-LCR.
P < 0.05 AL-HCR vs. CR-LCR.
Figure 5.
The effect of endurance training in LCR liver. A, Liver lysates from rats, which had been subjected to T or left S (13), were analyzed by Ib with α-CC1 and α-FAS antibodies, followed by reimmunoprobing (reIb) with α-actin to normalize per protein loading. Two representative animals of at least three rats per each group are included. The mean of band density was measured and plotted as ±sem in the graphs on the right; *, P < 0.05 vs. AL-LCR. B, Liver lysates were treated with (+) or without (−) insulin (Ins), Ip with α-pY antibody, and analyzed by Ib with an antibody against α-pCC1. Gels are representative of three separate experiments performed on lysates from different mice per group per experiment. The mean of pCC1 band density was measured and plotted on the right as described in the legend to Fig. 2; *, P < 0.05 (−) vs. (+) insulin. C, TBARS assay was performed in liver lysates from six animals in each group. Bars shown are mean ± sem; *, P < 0.05 vs. S-LCR, as analyzed by Student’s independent t test.
Discussion
We have previously shown that LCR exhibit the metabolic syndrome, including hyperinsulinemia, hypertriglyceridemia, visceral obesity, and body weight gain. Moreover, hyperinsulinemia in LCR stems, at least in part, from compromised hepatic insulin clearance (2). The current study demonstrated that CR improved insulin action and the altered lipid metabolic state in the liver of LCR, in association with enhancement of hepatic insulin extraction. In contrast, endurance training did not significantly affect hepatic lipid or insulin metabolism.
Whether insulin resistance precedes or follows obesity in type 2 diabetes is unresolved (21). However, LCR develop hyperinsulinemia and hypertriglyceridemia at 5 wk of age, before visceral obesity and body weight increase (2), demonstrating a key role for altered insulin and triglyceride homeostasis in the deranged metabolic state in this model. The marked reduction of hepatic CEACAM1 in LCR and the similarity in the phenotype to that in Ceacam1 mutants (L-SACC1 and Cc1−/− mice) (7,8,9) provide circumstantial evidence that altered CEACAM1-dependent insulin clearance pathways are implicated in the pathogenesis of visceral obesity, hyperinsulinemia, and dyslipidemia (liver steatosis and hypertriglyceridemia) in LCR.
The cause-effect relationship between enhancement of insulin clearance and the reversal of insulin resistance in LCR by CR has not been determined. However, it is intriguing that CR was more effective in regulating hepatic insulin and lipid metabolism than endurance training in parallel to its superior effect on restoring hepatic CEACAM1 protein content. Consistent with the positive effect of chronically elevated levels of insulin on lipogenesis (22,23), endurance training did not fully reduce hepatic FAS protein content or hepatic and serum triglyceride levels despite reducing body weight and visceral obesity (13). Although this agrees with the reported failure of exercise to reduce hyperinsulinemia and hepatic FAS mRNA levels in obese Zucker rats (24), it remains possible that a reversal effect of exercise on hyperinsulinemia was diminished in the 48 h after the last bout of exercise before killing. Nevertheless, we have reported (13) that this training program improved insulin signaling in LCR liver in addition to reducing fasting blood level and the 2-h after glucose challenge. Enhanced mitochondrial biogenesis in skeletal muscle and reduced visceral obesity by training could contribute to improved glucose tolerance in T-LCR (13). Because the HCR controls were not included in these earlier studies, it is difficult, however, to assess whether training fully restored glucose homeostasis in LCR by comparison with HCR. Persistent hyperinsulinemia argues that the effect of training on glucose homeostasis was partial. This possibility remains to be tested by hyperinsulinemic clamp analysis.
The current data provide evidence that in contrast to endurance training, CR modulates hepatic insulin extraction, which is in turn tightly linked to lipid metabolism. Activation of CEACAM1 by acute rise in insulin constitutes a common regulatory pathway of insulin and lipid metabolism in liver. CEACAM1 mediates a negative effect of insulin on hepatic FAS enzymatic activity (10). Thus, it is likely that under conditions of CR, CEACAM1 is elevated to reduce insulin levels in LCR and, consequently, hepatic FAS content. Together with inhibiting FAS enzymatic activity and reducing triglyceride synthesis and output, this elevates malonyl-coenzyme A levels to reduce β-oxidation and, ultimately, gluconeogenesis. In this manner, elevation of CEACAM1 levels by CR could contribute to reduced fasting blood levels by CR. The mechanism of the up-regulatory effect of CR on CEACAM1 levels is not yet delineated. Nonetheless, our observations of a significant lowering effect of CR on hepatic steatosis are supported by human studies indicating that CR alone, without exercise, reduced liver triglyceride in a small cohort of obese individuals (25). Although these studies did not subject a group of obese humans to an exercise program alone, they serve to emphasize the beneficial therapeutic role of CR on lipid metabolism in liver.
As recently reported (3), LCR exhibit features of NASH, including steatohepatitis, fibrosis, and elevation in oxidative stress. In agreement with other reports showing improvement of hepatic histology and reversal of hepatic steatosis and insulin resistance with a more than 9% loss of body weight by dietary restriction in humans (26), CR reversed most, if not all, NASH-characteristic features of LCR. Given the cumulative effect of increased fat deposition in liver and macrophage infiltration from white adipose tissue on the hepatic inflammatory milieu (27,28), it is not all that surprising that LCR developed steatohepatitis. With Ceacam1 mutants (L-SACC1 and Cc1−/−) (16) exhibiting hepatic fibrosis and progressive steatosis in response to a high-fat diet, and NASH features subsiding by CR in parallel to restoring CEACAM1 levels in LCR, it is possible that activating CEACAM1-dependent pathways contribute to the reversal of NASH phenotype by CR in LCR.
In summary, CR appears to exert a beneficial effect on overall metabolism mainly by promoting insulin clearance and reducing lipogenesis in liver. Further analyses are required to identify the underlying pathways, but the current studies point to a possible role for CEACAM1-dependent insulin clearance pathways in this process.
Acknowledgments
We thank trainees in the Najjar (Dr. Jill Schroeder-Gloeckler, Dr. Qusai Y. Al-Share, Dr. Garrett Heinrich, Dr. Anthony M. DeAngelis, Benjamin R. Hart, and Sumona Ghosh) and the Shapiro laboratory (Dr. Amjad Shidyak, Dr. Jihad El-Kareh, and Dr. Sleiman Smaili) for their technical assistance and scientific discussions. We also thank the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan.
Footnotes
This work was supported by the National Institutes of Health Grants DK054254 and DK083850 and by the United States Department of Agriculture Grant 38903-02315 (to S.M.N.). The work was also supported by the National Center for Research Resource (a component of the National Institutes of Health) Award R24RR017718 (to S.L.B. and L.G.K.), by institutional funds (R.E.B.), and by the Norwegian Council of Cardiovascular Disease and the Norwegian Research Council (U.W.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
Abbreviations: AL, Ad libitum; AL-HCR, AL-fed HCR; CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1; CR, caloric restriction; FAS, fatty acid synthase; FFA, free fatty acids; HCR, high-capacity runners; H&E, hematoxylin-eosin; Ib, immunoblotting; Ip, immunoprecipitation; IPGTT, ip glucose tolerance test; ITT, insulin tolerance test; LCR, low-capacity runners; NASH, nonalcoholic steatohepatitis; α-pCC1, α-phospho-CEACAM1; α-pY, α-phosphotyrosine; S, sedentary; T, aerobic interval endurance training for 8 wk; TBARS, thiobarbituric acid reactive substances.
References
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE 2002 Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346:793–801 [DOI] [PubMed] [Google Scholar]
- Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL 2005 Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307:418–420 [DOI] [PubMed] [Google Scholar]
- Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA 2009 Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587:1805–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I 2004 Mechanisms of non-alcoholic steatohepatitis. Alcohol 34:67–79 [DOI] [PubMed] [Google Scholar]
- Abdeen MB, Chowdhury NA, Hayden MR, Ibdah JA 2006 Nonalcoholic steatohepatitis and the cardiometabolic syndrome. J Cardiometab Syndr 1:36–40 [DOI] [PubMed] [Google Scholar]
- Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern-Buell CL, Accili D, Gorden P 1995 Receptor-mediated internalization of insulin. Potential role of pp120/HA4, a substrate of the insulin receptor kinase. J Biol Chem 270:24073–24077 [DOI] [PubMed] [Google Scholar]
- Poy MN, Yang Y, Rezaei K, Fernström MA, Lee AD, Kido Y, Erickson SK, Najjar SM 2002 CEACAM1 regulates insulin clearance in liver. Nat Genet 30:270–276 [DOI] [PubMed] [Google Scholar]
- Park SY, Cho YR, Kim HJ, Hong EG, Higashimori T, Lee SJ, Goldberg IJ, Shulman GI, Najjar SM, Kim JK 2006 Mechanism of glucose intolerance in mice with dominant negative mutation of CEACAM1. Am J Physiol Endocrinol Metab 291:E517–E524 [DOI] [PubMed] [Google Scholar]
- DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, Hong EG, Jung DY, Assmann A, Kulkarni RN, Kim JK, Najjar SM 2008 CEACAM1: a link between insulin and lipid metabolism. Diabetes 57:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SM, Yang Y, Fernström MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM 2005 Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab 2:43–53 [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program 2002 Description of lifestyle intervention. Diabetes Care 25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Temprosa M, Haffner S, Orchard TJ, Ratner RE, Fowler SE, Mather K, Marcovina S, Saudek C, Matulik MJ, Price D 2009 Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the diabetes prevention program randomized trial by the diabetes prevention program research group. Diabetes Care 32:726–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U 2009 Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO 2005 Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310:314–317 [DOI] [PubMed] [Google Scholar]
- Najjar SM, Philippe N, Suzuki Y, Ignacio GA, Formisano P, Accili D, Taylor SI 1995 Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 34:9341–9349 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Heinrich G, Fedorova L, Al-Share QY, Ledford KJ, Fernstrom MA, McInerney MF, Erickson SK, Gatto-Weis C, Najjar SM 2008 Development of nonalcoholic steatohepatitis in insulin-resistant liver-specific S503A carcinoembryonic antigen-related cell adhesion molecule 1 mutant mice. Gastroenterology 135:2084–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EM, Whaley-Connell AT, Thyfault JP, Britton SL, Koch LG, Wei Y, Ibdah JA, Sowers JR 2009 Low aerobic capacity and high-fat diet contribute to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol 30:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B 1995 In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism 44:228–233 [DOI] [PubMed] [Google Scholar]
- McCormick KL, Susa JB, Widness JA, Singer DB, Adamsons K, Schwartz R 1979 Chronic hyperinsulinemia in the fetal rhesus monkey: effects on hepatic enzymes active in lipogenesis and carbohydrate metabolism. Diabetes 28:1064–1068 [DOI] [PubMed] [Google Scholar]
- Deng X, Cagen LM, Wilcox HG, Park EA, Raghow R, Elam MB 2002 Regulation of the rat SREBP-1c promoter in primary rat hepatocytes. Biochem Biophys Res Commun 290:256–262 [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Frontini MG, Berenson GS 2003 Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa heart study. Metabolism 52:443–450; discussion 451–443 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 2008 Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7:95–96 [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Accili D 2008 The double life of Irs. Cell Metab 8:7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig RG, Hollander JM, Ney D, Boileau R, Jeffery E, Ji LL 2002 Training down-regulates fatty acid synthase and body fat in obese Zucker rats. Med Sci Sports Exerc 34:1106–1114 [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E 2008 Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity 16:1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA 2009 Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 49:80–86 [DOI] [PubMed] [Google Scholar]
- Bigorgne AE, Bouchet-Delbos L, Naveau S, Dagher I, Prévot S, Durand-Gasselin I, Couderc J, Valet P, Emilie D, Perlemuter G 2008 Obesity-induced lymphocyte hyperresponsiveness to chemokines: a new mechanism of Fatty liver inflammation in obese mice. Gastroenterology 134:1459–1469 [DOI] [PubMed] [Google Scholar]
- Sheth SG, Gordon FD, Chopra S 1997 Nonalcoholic steatohepatitis. Ann Intern Med 126:137–145 [DOI] [PubMed] [Google Scholar]