Abstract
The human calcium-sensing receptor (hCaR) is a family-3/C G-protein-coupled receptor that regulates Ca2+ homeostasis by controlling parathyroid hormone secretion. Here we investigated the role of Rab1, a small GTP-binding protein that specifically regulates protein transport from the endoplasmic reticulum to the Golgi, in cell surface transport of the hCaR. Cell surface expression of hCaR transiently expressed in human embryonic kidney 293 cells was strongly augmented by coexpression of Rab1 and attenuated by disruption of endogenous Rab1 function by expression of the dominant-negative Rab1N124I mutant or depletion of Rab1 with small interfering RNA. Rab1N124I expression also partially attenuated cell surface expression and signaling response to gain-of-function mutants of hCaR with truncated carboxyl-terminal sequences at positions 895 and 903. These carboxyl-tail truncations are similar to a deletion between residues S895 and V1075 found in a patient family causing autosomal dominant hypocalcemia. In addition, coexpression with wild-type Rab1 increased cell surface expression of the loss-of-function missense mutation R185Q, located on the hCaR amino-terminal extracellular ligand-binding domain (ECD), which causes familial hypocalciuric hypercalcemia. Truncated hCaR variants containing either the ECD with the first transmembrane helix or only the ECD also display Rab1-dependent cell surface expression or secretion into the culture medium, respectively. These data reveal a role for Rab1 in hCaR trafficking from the endoplasmic reticulum to the Golgi that regulates receptor cell surface expression and thereby cell signaling responsiveness to extracellular calcium.
A Rab1-dependent mechanism regulates intracellular and cell surface delivery of the human calcium-sensing receptor.
The human calcium-sensing receptor (hCaR) is the cation-sensing receptor of the family-3 (or family C) G protein-coupled receptor (GPCR) gene family. Family-3 GPCRs includes eight metabotropic glutamate receptors (mGluR1–8), two γ-aminobutyric acid receptor subunits (γ-aminobutyric acidB1 and γ-aminobutyric acidB2), three sweet and umami taste receptors (T1R1, T1R2, and T1R3), and several putative rodent pheromone receptors (1,2). The hCaR is responsible for Ca2+ homeostasis by regulating parathyroid hormone synthesis and secretion via Gq activation of phospholipase-Cβ. The activation of phospholipase-Cβ by hCaR is steeply cooperative, providing exquisite sensitivity to changes in serum Ca2+. The primary role of hCaR in regulating plasma Ca2+ concentration has been demonstrated by the identification of more than 200 mutations in the hCaR gene (www.casrdb.mcgill.ca/). Naturally occurring inactivating mutations of hCaR cause familial benign hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism, whereas activating mutations cause a form of autosomal dominant hypocalcemia (ADH). Several of these mutants exhibited altered cell surface expression resulting in concomitantly enhanced or diminished receptor activities (1,3,4,5).
Whereas the signaling of hCaR has been extensively investigated, the intracellular trafficking and its role in regulating the receptor function and pathogenesis of diseases related to Ca2+ homeostasis is less well understood. Cell surface expression of the hCaR requires intra- and interchain disulfide-linkages and N-glycosylation of the N-terminal extracellular ligand-binding domain (ECD). hCaR dimerization in the endoplasmic reticulum (ER) plays an essential role in regulating receptor signaling activity (6,7). Mutation of several highly conserved cysteine residues found within the ECDs of hCaR, mGluR1 and mGluR5, which form intrachain disulfide linkages, either abolish or dramatically reduce hCaR cell surface expression, likely by disrupting trafficking of the receptors from the ER (8,9,10). In addition, N-linked glycosylation of the hCaR ECD plays an important role in receptor trafficking to the cell surface (11). Thus, during biosynthesis, hCaR navigates a multifaceted quality control process that monitors folding and proper assembly of the receptor within the ER.
Correctly folded hCaR, as well as many GPCRs destined for Golgi and plasma membrane, exits the ER via vesicular or tubular structures. Rab GTP-binding proteins, the largest branch of the Ras-related superfamily, are involved in almost every step of this vesicle-mediated protein transport including targeting, tethering, and fusion of transport vesicles with the appropriate acceptor membrane (12). To date, Rab proteins have been documented mostly in internalization and degradation of GPCRs (13). Rab1, Rab2, and Rab6 are shown to be involved in both anterograde and retrograde transport of angiotensin II type 1 receptor (AT1R), α1A-adrenergic receptor (AR) and α1B-AR (14,15). Rab1 is involved in vesicular transport and assembly of the fusion complex for delivery to Golgi membranes. Vesicular transport from the ER of many cell surface proteins involves their concentration in ER-derived coat protein complex II (COPII)-coated vesicles. After the assembly of COPII-coated vesicles from the ER, Rab1 regulates the anterograde vesicular trafficking of the cargo proteins from the ER to the cis-Golgi network and possibly to intra-Golgi transport (16,17). Rab1 has been shown to play an important role in ER-Golgi anterograde transport of transmembrane proteins such as cystic fibrosis transmembrane conductance regulator (CFTR) and vesicular stomatitis glycoprotein (18,19). Cystic fibrosis transmembrane conductance regulator transport is dependent on Rab1 in HeLa (a human epithelial cervical cancer line) and human embryonic kidney 293 (HEK293) cells but independent in baby hamster kidney (BHK) and Chinese hamster ovary (CHO) cells and thus is regulated in a cell-specific manner (19). Of the GPCRs, the AT1R, and β2-AR, α1A-AR use conventional Rab1-dependent transport pathway in HEK293 cells, whereas, α2B-AR undergoes Rab1-independent export in these cells (20).
The molecular mechanisms underlying the intracellular trafficking remain largely unknown for hCaR and for any other family-3 GPCR. As an initial approach to elucidate the molecular mechanisms of hCaR trafficking to the cell surface, we investigated the role of Rab1 in the anterograde transport of hCaR.
Materials and Methods
Materials
The coding sequences for the hCaR were inserted into the in pCR3.1 expression plasmid. The hCaR K644X (previously named TM1 mutant) containing the ECD and first-transmembrane helix, the wild-type hCaR, and G903X (previously referred to as T903) carboxyl-tail truncation mutant with rhodopsin epitope tag MNGTEGPNFYVPFSNKTGVV after the N terminus signal peptide between residues Y20 and G21 of the hCaR have been previously described (21,22). The plasmid construct, myc epitope-tagged wild-type Rab1 in pcDNA3.1, was a gift from Dr. Victor Rebois (National Institute of Neurological Disorders and Stroke, National Institutes of Health) (23). The monoclonal antibody against hCaR, ADD, was purchased from Thermo Scientific (Worcester, MA) and antibodies against the myc epitope and α-actin were purchased from Sigma Corp. (Ronkonkoma, NY). A monoclonal antibody (B6–30N) recognizing the N terminus of rhodopsin was kindly provided by Dr. Paul Hargrave, Department of Ophthalmology (University of Florida, Gainesville, FL) (24).
Mutagenesis and other hCaR mutant constructs
In the G613X and S895X constructs, truncation of the seven-transmembrane domains or carboxyl-terminal tail of the hCaR was achieved by introducing stop codons at amino acid position 613 or 895 of the hCaR and rhodopsin epitope-tagged hCaR using QuickChange mutagenesis kit from Stratagene Inc. (La Jolla, CA) as described previously (6). R185Q and E297K single missense mutations and the dominant-negative myc epitope-tagged Rab1N124I were created similarly. Briefly, pairs of complementary primers of 35 bases were designed with either the stop codons or amino acid changes placed in the middle of the primers. These primers were used for site-directed mutagenesis and verified by automated DNA sequencing using a Taq DyeDeoxy terminator cycle sequencing kit and ABI prism-377 DNA sequencer (Applied Biosystems Inc., Minneapolis, MN).
Cell culture and transient transfection
HEK293, HeLa, and COS-7 (African green monkey Simian virus-40 transformed kidney fibroblast) cells were cultured using DMEM supplemented with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 mg/ml streptomycin. These cells were transfected with the plasmid DNA constructs using lipofectamine transfection (Invitrogen) as described previously (11). Six-well plates containing 90% confluent cells in each well were transfected with a total of 2 μg/well plasmid DNA or 1 μg each of plasmid DNA when two different cDNAs were added in cotransfection experiments. For 80-cm2 flask transfection, the DNA concentration was adjusted to a total of 8 μg. Membrane protein extraction for Western blots, intact cell ELISA or phosphoinositide (PI) hydrolysis assays were performed 48 h after transfection.
Western blot analysis with detergent solubilized cell extracts
Whole-cell extracts were prepared as described previously (6). Confluent cells in six-well plates were rinsed with ice-cold PBS, scraped, and solubilized on ice in solution B containing 20 mm Tris-HCL (pH 6.8), 150 mm NaCl, 10 mm EDTA, 1 mm EGTA, 1% Triton X-100 with freshly added protease inhibitor mix (Complete protease inhibitor cocktail; Roche Applied Science, Indianapolis, IN) and 10 mm iodoacetamide. Detergent-solubilized proteins were first denatured under reducing conditions at room temperature for 10 min in the presence of 0.05% sodium dodecyl sulfate and 50 mm 2-mercaptoethanol. An equal amount of protein (20 or 30 μg) was loaded in each lane and subjected to SDS-PAGE using either 6 or 4–12% gradient Tris-glycine gels. The proteins were electrophoretically transfered to nitrocellulose membranes, blocked with 5% milk, and incubated first with a primary antibody and then with secondary antibody conjugated to horseradish peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The immunoreactive protein bands were detected using an enhanced chemiluminescence system containing substrate for horseradish peroxidase (Pierce Laboratories, Rockford, IL). For cleavage with Endoglycosidase H (EndoH; Roche Applied Science), 30 μg of total protein was incubated with 0.5 mU of EndoH for 1.5 h at room temperature before SDS-PAGE.
RNA interference (RNAi) assay
Double-stranded small interfering RNAs (siRNAs), chemically modified custom-synthesized oligonucleotides (Stealth RNAi) targeting Rab1 were obtained from Invitrogen Life Technologies (Carlsbad, CA). We also used scrambled control RNAi duplexes with similar GC content as Rab1 siRNA designed to minimize sequence homology to any known vertebrate transcripts. siRNA transfections were carried out using confluent cells in six-well plates. Ten microliters of lipofectamine reagent (Invitrogen, Carlsbad, CA) were diluted with 10 μl of serum-free DMEM along with double-stranded siRNA diluted with 80 μl of DMEM to a final concentration of 500 nm and incubated for 30 min at room temperature. Each transfection mixture was then added slowly to the culture dish containing 800 μl of antibiotic-free DMEM with no FCS. Cells were incubated for 5 h in this transfection mix, and an additional 3 ml of DMEM containing 10% FCS was added to each dish. Experimental tests of siRNA-treated cells were conducted 48 h after transfection.
Intact cell ELISA
Cells were detached from 80-cm2 culture flasks with 1 mm EDTA in PBS containing 0.5% BSA. To measure the cell surface expression constructs with a rhodopsin epitope tag at the N terminus, cells were washed with PBS and incubated for 1 h on ice with 1 mg/ml monoclonal antirhodopsin antibody, B6–30N. After three washes with PBS, the cells were incubated with 5 μg/ml peroxidase-conjugated goat antimouse IgG in DMEM containing 10% FBS for 1 h on ice. After three subsequent washes with PBS, peroxidase substrate [2.5 mm each of H2O2 and O-phenylenediamine in 0.1 m phosphate-citrate buffer (pH 5.0)] was added to each sample and the color reaction was stopped by centrifugation and removal of the cells. The reaction product was measured by reading absorbance at 580 nm using an ultrospec 3000 spectrophotometer (Amersham Pharmacia Biotech, Piscataway, NJ). The cell surface expression of receptors was normalized to control values after subtracting the A580 value determined for equal number of nonexpressing cells.
PI hydrolysis assay
PI hydrolysis was measured as previously described (11). Briefly, cells plated in 24-well culture plates were incubated in medium containing 3.0 μCi/ml of 3H-myoinositol (Perkin Elmer, Wellesley, MA) in complete DMEM for 16 h. To deplete Ca2+ and 3H-myoinositol present in the culture medium, after removal of the medium, attached cells were incubated with PI solution [120 mm NaCl, 5 mm KCl, 5.6 mm glucose, 0.1 mm MgCl2, 20 mm LiCl in 25 mm PIPES buffer (pH 7.2)] for 30 min. After removal of the PI solution, cells were treated for 30 min with two different concentrations of Ca2+ (0.5 and either 10 or 20 mm Ca2+) in PI solution. The PI-hydrolysis reactions were terminated by the addition of 1 ml of HCl-methanol [1:1000 (vol/vol)] per well. Total inositol phosphates were separated by chromatography on Dowex-1-X8 columns (Bio-Rad, Hercules, CA) and samples were counted in a LS 6500 multi-purpose scintillation spectrometer (Beckman Coulter, Brea, CA).
Statistical analysis
All data are reported as mean ± sem. Paired Student’s t test or one-way ANOVA test was used to assess significant differences where appropriate. Significance was set at P < 0.05.
Results
Regulation of expression and signaling of hCaR by Rab1
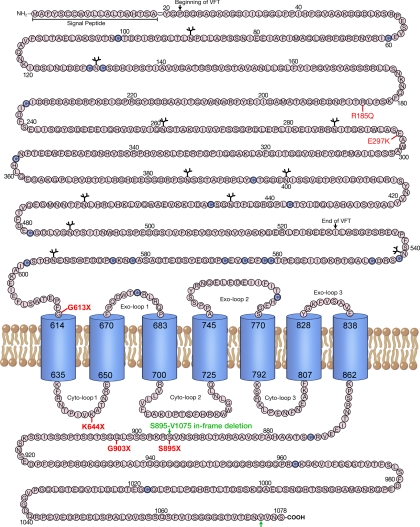
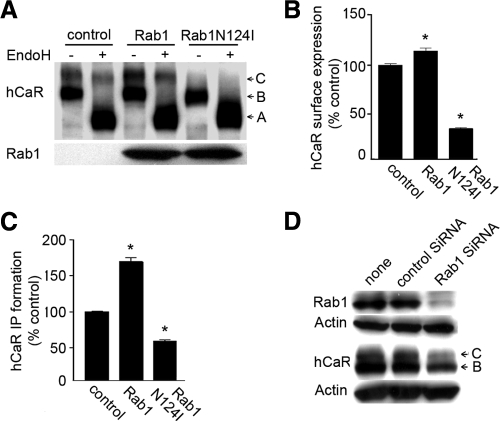
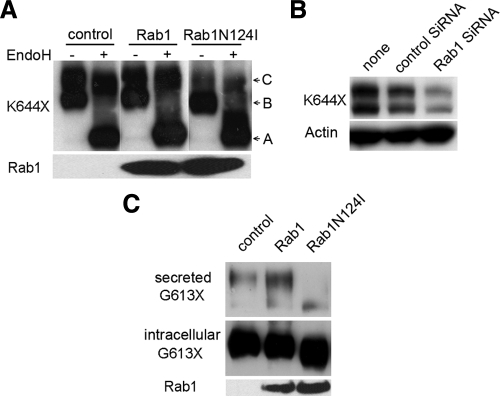
A schematic depiction of the hCaR structure showing the overall topology of this receptor along with the locations of the several truncated and loss-of-function missense mutant receptors tested in this study is presented in Fig. 1. We sought to determine the involvement of Rab1 in the regulation of trafficking of the hCaR to the cell surface. To examine this and the sequences of hCaR important for cell-surface trafficking, we used the hCaR constructs of Fig. 1 together with the mutant Rab1N124I, a dominant-negative Rab1 mutant locked in the GTP-bound form, which competes with endogenous Rab1 and interferes with anterograde vesicular transport from the ER to Golgi, leading to the accumulation of immature proteins in the ER (17). We monitored the trafficking of the wild-type hCaR in the presence of RabN124I by Western blot detection and EndoH digestion (Fig. 2 A). As seen on the Western blot (control lane), the core glycosylated high-mannose form of hCaR (band B) migrates with an apparent molecular mass of 130 kDa and is sensitive to EndoH digestion characteristic of ER retained proteins that have not yet trafficked from the ER to the Golgi (4,11), whereas hCaR modified with complex carbohydrates migrates as a 150-kDa protein (band C), which is resistant to EndoH digestion, confirming them as hCaR forms trafficked from the ER to the Golgi. Cells transiently expressing hCaR alone or coexpressed with wild-type Rab1 both contain a distinct pool of band C protein resistant to EndoH digestion. In several experiments the apparent abundance of this mature EndoH-resistant form of hCaR was increased when coexpressed with Rab1. Cells expressing hCaR coexpressed with Rab1N124I exhibited diminished expression of the 150-kDa band C form. Densitometric analysis of thye EndoH-resistant band C ratios for hCaR revealed a greater than 60% reduction in Rab1N124I cotransfected cells compared with hCaR expressed alone (Fig. 2A). Similar results were obtained for Rab1 and Rab1N124I coexpression of hCaR in both HeLa and COS-7 cells as well as the HEK293 cells (data not shown).
Figure 1.
Schematic diagram of the hCaR. The amino acid sequence of the extracellular and intracellular sequence of the hCaR is presented. Predicted extracellular and intracellular sequences are shown as the single-letter code inside circles with the signal peptide, beginning and end of VFT motif within the N terminal ECD, cysteines (purple circles), and N-linked glycosylation sites (ψ) highlighted. Predicted transmembrane helices are represented as barrels. The C-terminal and ECD truncations S895X, G903X, K644 × and G613 × and missense mutations R185Q and E297K are shown in red. The two brown arrows show the location of the in-frame deletion from S895 to V1075 in the hCaR C-tail known for gain-of-function receptor activity causing ADH.
Figure 2.
Cell surface transport of hCaR is dependent on Rab1. A, Western blot analysis of protein extracts from transiently transfected HEK293 cells expressing wild-type hCaR alone (control) and together with Rab1 or Rab1N124I. Thirty micrograms of proteins from whole-cell lysates were loaded per lane and subjected to SDS-PAGE on 6% Tris-glycine gels as described in Materials and Methods. The hCaR protein bands were detected by anti-hCaR monoclonal antibody ADD. The position of the 150- (band C) and 130-kDa (band B) representing differentially N-glycosylated hCaR forms are indicated, and the EndoH digested nonglycosylated band migration is shown as band A. The samples were either left untreated (−) or treated (+) with EndoH. Separately, samples not digested with EndoH were subjected to SDS-PAGE on 4–12% gradient gels, and the expression of wild-type Rab1 and Rab1N124I was detected by anti-myc monoclonal antibody 9E10. B, To measure hCaR expression at the cell surface, an amino-terminal rhodosin epitope tagged wild-type hCaR was expressed alone (control) or together with Rab1 or Rab1N124I in HEK293 cells. hCaR expression at the cell surface was quantified by intact cell ELISA assay after incubation with a anti-rhodopsin monoclonal antibody B6–30N. Values presented are normalized to the A580 measured for the hCaR control and are presented as the mean ± sem of triplicate measurements from two experiments. *, P < 0.05 vs. control. C, hCaR stimulation of PI hydrolysis in HEK293 cells transiently expressing hCaR (control) together with either Rab1 or Rab1N124I was assessed in the presence of 10 mm Ca2+. The values shown have been normalized to that for the hCaR alone (control) and are the means ± sem (n = 4) obtained from a single experiment with two independent experiments providing similar results. *, P < 0.05 vs. control. D, HEK293 cells were transiently transfected with control low G-C siRNA or Rab1 siRNA together with myc-tagged Rab1 and hCaR as described in Materials and Methods. Thirty micrograms of proteins from whole-cell lysates were separated by SDS-PAGE on 4–12% gradient gels, and Western blots were probed with anti-myc monoclonal 9E10, anti-hCaR monoclonal ADD, and anti α-actin as a loading control.
To quantify cell surface receptor expression of hCaR, we used two different approaches: intact cell ELISA for intact cells to measure surface antigen abundance and extracellular Ca2+-stimulated PI hydrolysis to compare changes in cell surface abundance by the signaling efficacy of the receptor. For intact cell ELISA, an amino-terminal rhodopsin epitope-tagged hCaR construct was used for detection with anti-rhodopsin monoclonal antibody B6–30N. The cell surface expression of the hCaR measured by ELISA was reduced by 70% in cells expressing RabN124I compared with cells expressing hCaR alone (Fig. 2B). In contrast, cells expressing Rab1 together with hCaR displayed increased cell surface expression of the receptor by 20% compared with hCaR alone. The functional assessment of cell surface expressed hCaR by Ca2+-stimulated PI hydrolysis displayed a greater enhancement of receptor activity in Rab1 expressing cells (70% increase over the hCaR alone) but a lesser inhibition in cells coexpressing RabN124I (45% decrease of the hCaR alone) (Fig. 2C). Taken together, these results demonstrate that wild-type Rab1 augments whereas Rab1N124I reduces cell surface expression and thus inversely influence the functional activity of wild-type hCaR.
To confirm these results, we used an alternative approach using a siRNA-mediated knockdown of endogenous Rab1 expression to test Rab1-mediated regulation of hCaR trafficking. The success of the Rab1 siRNA was assessed by coexpression of myc epitope-tagged Rab1 together with Rab1-specific siRNA in HEK293 cells measuring the expression level of Rab1 by Western blot (Fig. 2D). Rab1-specific siRNA significantly knocked down the expression of transiently expressed Rab1 protein (Fig. 2D, upper panel). Introduction of Rab1 siRNA with hCaR into HEK293 cells reduced the intensity of both hCaR band B and C levels by 2.5-fold compared with the control (Fig. 2D, lower panel).
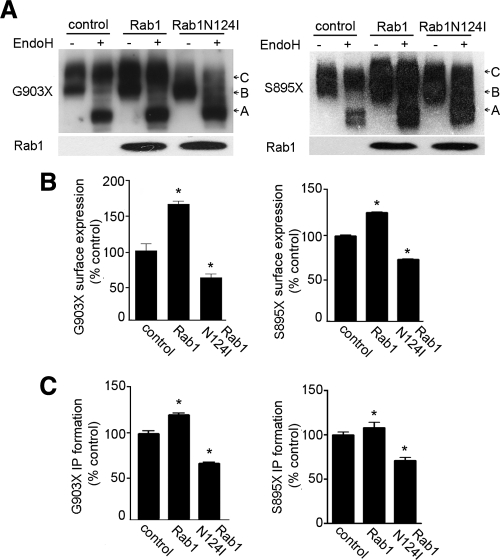
Rab1N124I reduces cell surface expression and signaling activities of carboxyl-tail truncated G903X and S895X hCaR
Because sequence motifs in the carboxyl-tail of GPCRs such as rhodopsin, luteinizing hormone receptor and V2 vasopressin receptor have been demonstrated to participate in cell-surface trafficking (14), we examined a carboxyl-tail-truncated hCaR construct G903X we and others have previously demonstrated was highly expressed at the cell surface and showed increased signaling activity (22). We coexpressed G903X with Rab1 or Rab1N124I in HEK293 cells and compared the expression levels by Western blot analysis. Figurer 3A (left panel) shows that cells expressing G903X receptor alone (control) or with Rab1 contain two bands similar to the full-length hCaR with a slower migrating band C resistant to EndoH digestion. Conversely, cells expressing G903X receptor with Rab1N124I contain mostly immature band B sensitive to EndoH digestion. EndoH-resistant band C ratios for G903X receptor exhibited a 2-fold reduction in Rab1N124I-expressing cells determined by densitometric analyses. In addition to the apparent reduction in abundance, band C presented a ladder-like heterogeneous migration pattern in Rab1N124I-expressing cells. Therefore, we used intact cell ELISA to quantify the reduction in cell surface expression. The cell surface receptor level of G903X receptor was reduced 45% in cells coexpressing Rab1N124I compared with cells expressing G903X alone, whereas coexpression of wild-type Rab1 increased cell surface expression of G903X more than 70% compared with control (Fig. 3B, left panel). The functional assessment of cell surface G903X expression revealed a 20% increase of Ca2+-stimulated PI hydrolysis in cells coexpressing Rab1 and 35% inhibition in cells coexpressing RabN124I (Fig. 3C, left panel).
Figure 3.
Truncation of hCaR carboxyl-tail does not prevent Rab1-mediated cell surface transport. A, HEK293 cells were transfected with hCaR truncation mutants G903X and S895X alone (control) or together with Rab1 or Rab1N124I. Thirty micrograms of proteins from whole-cell lysates were separated by SDS-PAGE on 6% gels, and receptor protein expression was detected by anti-hCaR monoclonal antibody ADD. The samples were either untreated (−) or treated (+) with EndoH before SDS-PAGE. Separately, the undigested samples were separated by SDS-PAGE on 4–12% gradient gels, and the expression of wild-type Rab1 and Rab1N124I was detected by anti-myc monoclonal antibody 9E10. B, N-terminal rhodopsin epitope-tagged G903X and S895X hCaR constructs were expressed alone (control) or with Rab1 or Rab1N124I in HEK293 cells. Cell surface expression levels were quantified by intact cell ELISA assay after incubation with an antirhodopsin monoclonal antibody B6–30N. Values presented are normalized to the A580 measured for the G903X or S895X hCaR controls and are the mean ± sem of triplicate measurements. *, P < 0.05 vs. control. C, hCaR stimulation of PI hydrolysis in HEK293 cells transiently expressing G903X and S895X mutant receptors alone (control) or together with either Rab1 or Rab1N124I was assessed in the presence of 10 mm Ca2+. The values shown have been normalized to the control value and are the means ± sem of three determinations. Two independent experiments showed similar results. *, P < 0.05.
Because coexpression of RabN124I with G903X reduced cell surface receptor level, we tested the influence of Rab1N124I on the expression of another gain-of-function mutant S895X. The S895X truncation of hCaR is similar to a naturally occurring gain-of-function deletion mutation that contains a carboxyl-tail deletion between residues S895 and V1075 in the hCaR in a patient family with ADH (see Fig. 1). As shown in Fig. 3, A and B (right panels), both Western blot analysis and intact cell surface ELISA confirmed reduced abundance of EndoH-resistant protein and cell surface expression for S895X in cells coexpressing Rab1N124I. Figure 3C (right panel) shows that the signaling analysis of the influence of Rab1 on the S895X expression extracellular Ca2+ was able to induce PI hydrolysis more robustly in control and Rab1 expressing cells than in cells expressing Rab1N124I. Overall, these experiments demonstrate that Rab1 influences the trafficking and receptor density at the cell surface for both G903X and S895X truncations of hCaR.
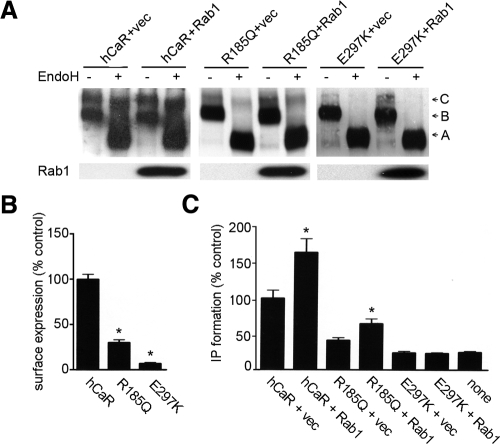
Rab1 augments cell surface expression and functional activity of R185Q loss-of-function hCaR mutant
The majority of loss-of-function missense mutations are located in the ECD of the hCaR. We tested whether two such ECD mutants, R185Q and E297K, could be rescued by coexpressing the mutant receptors with the wild-type Rab1 in HEK293 cells. Previous studies of R185Q revealed reduced cell surface level and highly reduced delayed functional activity for this mutant in response to high calcium concentrations, whereas the E297K mutant failed to reach the cell surface with no measurable signaling activity (4,5). When expressed alone, both R185Q and E297K mutant receptors displayed dominant expression of the high-mannose band B (130 kDa) susceptible to EndoH digestion with a reduced or absent expression of the EndoH-resistant band C (Fig. 4A). Quantitative ELISA demonstrated highly reduced levels of cell surface-localized receptor for both mutant receptors as compared with the wild-type hCaR (Fig. 4B). Cells expressing R185Q or E297K together with wild-type Rab1 showed an apparent increase of band B and R185Q mutant receptor displayed an increase of complex carbohydrate-modified, 150-kDa band C forms (Fig. 4A). These data suggest a partial cell surface rescue of R185Q mutant receptor in the presence of exogenously expressed Rab1. The functional rescue of the R185Q receptor was investigated by assessing its signaling activity. Figure 4C presents the analysis of PI signaling response of HEK293 cells transiently expressing the R185Q or E297K receptors alone or together with Rab1. As seen, 20 mm Ca2+ stimulated PI hydrolysis to a greater extent in cells transiently expressing Rab1 together with R185Q. We detected no Ca2+-stimulated PI hydrolysis in cells expressing E297K mutant receptor with or without Rab1 compared with the control cells.
Figure 4.
Rab1 rescues cell surface expression of loss-of-function missense mutant R185Q hCaR. A, HEK293 cells were transfected with rhodopsin-epitope-tagged wild-type hCaR, R185Q, or E297K mutant hCaR cotransfected with vector (+vec) plasmid DNA as control or with wild-type Rab1. Whole-cell lysates were either untreated (−) or treated with EndoH, and 30 μg of proteins were separated by SDS-PAGE on 6% gels, and hCaR expression was detected by the anti-hCaR antibody ADD. Separately, samples not digested with EndoH were subjected to SDS-PAGE on 4–12% gradient gels, and the expression of Rab1 was detected by the anti-myc monoclonal antibody 9E10. B, HEK293 cells were transfected with rhodopsin-epitope-tagged wild-type, R185Q, or E297K mutant hCaR, and cell surface expression was quantified by an intact cell ELISA assay after incubation with an antirhodopsin B6–30N monoclonal antibody. Values presented are normalized to the A580 measured for the wild-type hCaR and are the mean ± sem from three experiments. *, P < 0.05 vs. wild type. C, HEK293 cells were transfected with wild-type hCaR, R185Q, and E297K mutant receptors with or without Rab1. hCaR-stimulated PI hydrolysis was determined for cells stimulated with 20 mm Ca2+. Values presented are normalized to the wild-type hCaR alone and are the means of six determinations from two separate experiments. *, P < 0.05.
Lastly, we examined whether the N-terminal ECD of hCaR exhibits Rab1-dependent cell surface transport. For this purpose, we analyzed two truncated hCaR mutants; K644X containing only the ECD and the first transmembrane helix and G613X containing the hCaR ECD terminating at the G613 predicted to initiate the first transmembrane helix. Previous studies indicated that the K644X-truncated receptor is expressed at the cell surface in HEK293 cells (6). Figure 5A shows that expression of K644X in HEK293 cells results in an EndoH-resistant, 97-kDa band C and EndoH-digested, 90-kDa band B detected on Western blot. Transient expression the K644X with Rab1N124I significantly reduced the level of mature complex carbohydrate-modified EndoH-resistant, 97-kDa band C intensity (Fig. 5A). Moreover, Rab1 siRNA cotransfected with K644X drastically reduced expression of both the mature 97- and 90-kDa proteins (Fig. 5B). These results suggest that the trafficking and cell surface transport of the hCaR ECD linked only to the first transmembrane portion of the hCaR is also Rab1 dependent. Therefore, we examined the trafficking of a construct of the hCaR comprised solely of the ECD. In HEK293 cells transfected with the G613X-truncated construct, the hCaR ECD was secreted into the culture medium as a soluble 95-kDa protein detected by Western blot (Fig. 5C). We tested the Rab1 dependence of G613X trafficking by coexpression with wild-type Rab1 or the Rab1N124I mutant. The results shown in Fig. 5C illustrate that in all conditions a minor fraction of the translated G613X protein was secreted into the culture medium. However, when coexpressed with Rab1N124I, this secreted protein band intensity was diminished and increased when coexpressed with wild-type Rab1. Interestingly, both the 90-kDa intracellular and the secreted 95-kDa G613X protein bands showed faster mobility in cells coexpressing RabN124I compared with control or Rab1-expressing cells, possibly reflecting differential N-glycosylation patterns.
Figure 5.
Rab1-dependent trafficking of a cell surface bound and a secreted amino-terminal hCaR ECD structure. A, HEK293 cells were transfected with the ECD containing truncated K644X hCaR alone or together with Rab1 or Rab1N124I. thirty micrograms of proteins from cell lysates were separated by SDS-PAGE on 6% gels, and hCaR expression was detected by the anti-hCaR monoclonal antibody ADD. The samples were either left untreated (−) or treated (+) with EndoH. Separately, untreated cell lysates were separated by SDS-PAGE on 6–12% gradient gels, and the expression of Rab1 or Rab1N124I was detected by anti-myc antibody 9E10. B, HEK293 cells were transiently transfected with K644X alone, with negative control siRNA or Rab1 siRNA, and 20 μg of proteins from whole-cell lysates was separated by 4–12% gradient SDS-PAGE. Expression of K644X receptor was detected with anti-hCaR monoclonal antibody ADD. The blots were probed for α-actin expression as a control for equal loading (bottom panel). C, HEK293 cells were transfected with truncated hCaR G613X alone or together with Rab1 or Rab1N124I. One milliliter of conditioned cell culture medium was collected and concentrated to 100 μl using a Millipore ultrafiltration membrane (CM 30; Bedford, MA) and an Amicon concentrator (Beverly, MA). Ten microliters of the concentrate was loaded per lane for detection of the secreted proteins. Thirty micrograms of proteins from whole-cell lysates were analyzed for cell-retained hCaR. All samples were separated by SDS-PAGE on 6% gels run under reducing conditions, and hCaR expression was detected with the monoclonal antibody ADD. The upper blot shows the secreted G613X receptor and the middle blot shows the intracellular G613X. Separately, 30 μg of whole-cell lysate was separated by SDS-PAGE on 4–12% gradient gels, and Rab1 and Rab1N124I expression was detected with anti-myc antibody 9E10.
Discussion
GPCR export trafficking between the ER to Golgi and to plasma membrane is a highly regulated process. Naturally occurring inactivating mutations and truncations in GPCRs such as those found in nephrogenic diabetes insipidus for V2 vasopressin receptor and retinitis pigmentosa for rhodopsin, often lead to ER retention and which is implicated as the pathogenesis of these disease phenotypes (25,26). Several inactivating mutants of hCaR identified in patients with familial benign hypocalciuric hypercalcemia/neonatal severe hyperparathyroidism remain trapped in the intracellular space and are not trafficked to the plasma membrane. Similarly, a few activating mutants found in patients with ADH have shown higher cell surface expression of the receptor (1,4,5). Little is known about the ER export mechanisms that dictate the level of the hCaR expression at the plasma membrane. We addressed this issue by investigating the ability of the small GTP-binding protein Rab1, known to play an essential role in vesicular transport from ER to Golgi, to regulate hCaR transport to the cell surface. Transient expression of the dominant negative Rab1N124I mutant and Rab1 siRNA inhibited cell surface expression of the full-length and carboxyl-tail-truncated hCaR variants. Thus, the hCaR transport in HEK293 cells is Rab1 dependent similar to AT1R, β2-AR, and α-1AAR and unlike the α2B-AR, which uses a nonconventional Rab1-independent pathway in HEK293 cells (20). Our data demonstrate that hCaR transport to the cell surface is dependent on Rab1 also in HeLa and COS-7 cells, and thus, this is not unique to the HEK293 cell type.
Interestingly, wild-type Rab1 transiently coexpressed with hCaR in most experiments augmented the total and also cell surface expression of the receptor. This suggests that endogenous Rab1 is limiting for hCaR transport from the ER to the cell surface in our transient expression paradigm. Transient expression of wild-type Rab1 similarly increased the cell surface level of AT1R in cardiac myocytes (27) and the AT1R, angiotensin II type 2 receptor, α-1AAR and α-1B AR but not β1-AR and β2-AR in HEK293 cells (28,29). Our data further show that Rab1 can rescue cell surface expression and functional response of the loss-of-function missense mutation R185Q but not the E297K, both mutated within the large ECD of the hCaR. Both R185Q and E297K mutant receptors are not present in significant amount at the cell surface and remain mostly as high-mannose forms in the ER. Quality control mechanisms in the ER may serve as a check point that regulates proteosomal degradation of these mutant receptors (5). Addition of exogenous Rab1 resulted in an increase in R185Q and E297K total protein expression accompanied by a significant increase in R185Q receptor signaling, whereas E297K, despite an increase in protein expression, displayed no functional activity. The increase in the functional response of R185Q mutant receptor correlated well with the increase of the 150-kDa complex carbohydrate modified forms, demonstrating cell surface transport of this mutant receptor. Thus, the retention of different missense hCaR mutants in early secretory compartments may be regulated differentially. Previously we have identified an essential role for the Sar1 GTP binding protein in trafficking of the hCaR, presumably in transit through the ER (30). The current results suggest that Rab1 may constitute a second mechanism to control the regulated exit from the ER to the Golgi that enables supply of hCaR to the cell surface.
With the exception of an in-frame deletion, S895-V1075, activating mutations of hCaR that cause ADH are all missense mutations. This large in-frame hCaR deletion mutant missing 181 amino acids displays increased activity and a significantly left-shifted saturation curve for Ca2+ signaling (31). These properties correlated well with a higher cell surface expression of this mutant receptor. Experimental introduction of other truncations and point mutations within the hCaR C-tail have also been found to produce similar phenotypes (22,32,33). For example, truncation at residue 888, 892, and 903 result in both enhanced signaling activities and greater level of cell surface expression of these C-tail truncated mutants (22,33). The mechanisms through which these truncation and deletion of the carboxyl tail of the receptor induce higher expression levels are unknown. The present data show that RabN124I can partially reduce cell surface expression of two highly expressed C-tail truncated receptors, G903X and S895X. Concomitantly, RabN124I decreased the maximum signaling response of the G903X and S895X mutant receptors.
The majority of loss-of-function missense mutations including R185Q and E297K found in the hCaR are located in the large amino-terminal ECD (1,4). The ECD of the hCaR and other family-3 GPCRs show structural homology to bacterial periplasmic binding proteins resembling a venus flytrap (VFT)-like module. Along with family-3 GPCRs, N-terminal extracellular domains in several other receptor families including ionotropic glutamate (N-methyl-d-aspartate) receptors, natriuretic peptide receptors (atrial natriuretic peptide, brain natriuretic peptide, C-type natriuretic peptide) also possess periplasmic binding protein-like VFT structures (34). The crystal structure of mGluR1 suggests that the VFT-like ligand binding module oscillates between open and closed conformations with the ligand binding stabilizing a closed conformation (35). Recent studies on hCaR ECD variants with defects in N-glycosylation and disulfide-linked dimer formation suggests that a correctly folded ECD structure is necessary to traverse ER quality control checkpoints via Sar1-dependent COPII-coated vesicles (30). We suggest that the ECD behaves like a soluble secreted cargo and may contain primary structural determinants for ER-Golgi forward transport because our present data confirm that transport to the cell surface of the autonomously folded hCaR ECD is also regulated by Rab1. Together these results identify a Rab1-dependent mechanism regulating the intracellular trafficking and membrane delivery of both the full-length transmembrane hCaR and the secreted hCaR ECD. A more detailed knowledge of the mechanisms regulating hCaR and other family-3 GPCR intracellular trafficking will be essential to understand cellular processes controlling cell surface expression and activities of these therapeutically important receptors.
Acknowledgments
We are thankful to Drs. James Battey and Victor Rebois for critical reading of this manuscript.
Footnotes
This work was supported by the Intramural Research Program of the National Institute on Deafness and Other Communication Disorders.
Present address for K.A.A.: Department of Molecular Genetics and Microbiology, Duke University Medical Center, CARL 261, Box 3509, Durham, North Carolina 27710.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
Abbreviations: ADH, Autosomal dominant hypocalcemia; AR, adrenergic receptor; AT1R, angiotensin II type 1 receptor; COPII, coat protein complex II; C-tail, carboxyl-terminal-tail; ECD, extracellular ligand-binding domain; EndoH, Endoglycosidase H; ER, endoplasmic reticulum; FCS, fetal calf serum; GPCR, G protein-coupled receptor; hCaR, human calcium-sensing receptor; HEK293, human embryonic kidney 293; mGluR, metabotropic glutamate receptor; N-linked, asparagines-linked; PI, phosphoinositide hydrolysis; RNAi, RNA interference; siRNA, small interfering RNA; 7TMD, seven-transmembrane domains; VFT, venus flytrap motif.
References
- Brown EM, MacLeod RJ 2001 Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–297 [DOI] [PubMed] [Google Scholar]
- Bräuner-Osborne H, Wellendorph P, Jensen AA 2007 Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8:169–184 [DOI] [PubMed] [Google Scholar]
- Pearce SH, Bai M, Quinn SJ, Kifor O, Brown EM, Thakker RV 1996 Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J Clin Invest 98:1860–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM 1996 Expression and characterization of inactivating and activating mutations in the human Ca2+-sensing receptor. J Biol Chem 271:19537–19545 [DOI] [PubMed] [Google Scholar]
- White E, McKenna J, Cavanaugh A, Breitwieser GE 2009 Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol 23:1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM 1999 Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca2+ receptor critical for dimerization: implications for function of monomeric Ca2+ receptor. J Biol Chem 274:27642–27650 [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN 2004 Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CaSR mutants retained intracellularly. Hum Mol Genet 15:2200–2209 [DOI] [PubMed] [Google Scholar]
- Fan GF, Ray K, Zhao XM, Goldsmith PK, Spiegel AM 1998 Mutagenesis analysis of the cysteines in the extracellular domain of the human Ca2+ receptor: effects on cell surface expression, dimerization and signal transduction. FEBS Lett 436:353–356 [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC 2000 Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J Biol Chem 275:34245–34251 [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennericks S, Takeuchi Y, Goldberg MP, O'Malley KL 2001 Covalent and non-covalent interactions mediate metabotropic glutamate receptor mGluR5 dimerization. Mol Pharmacol 59:46–53 [PubMed] [Google Scholar]
- Ray K, Clapp P, Goldsmith PK, Spiegel AM 1998 Identification of the sites of N-glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 273:34558–34567 [DOI] [PubMed] [Google Scholar]
- Martinez O, Goud B 1998 Rab proteins. Biochim Biophys Acta 1404:101–112 [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SS 2003 Regulation of G protein coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74:225–235 [DOI] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G 2007 Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 1768:853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G 2007 Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal 19:2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE 1992 GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol 119:749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Nuoffer C, Meinkoth JL, McCaffery M, Feramisco JR, Balch WE, Farquhar MG 1994 A Rab1 mutant affecting guanine nucleotide exchange promotes cis-assembly of the Golgi apparatus. J Cell Biol 125:557–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson HW, Balch WE 1993 Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J Biol Chem 268:4216–4226 [PubMed] [Google Scholar]
- Yoo JS, Moyer BD, Bannykh S, Yoo HM, Riordan JR, Balch WE 2002 Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J Biol Chem 277:11401–11409 [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y 2003 Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G-protein-coupled receptor. J Biol Chem 278:47062–47069 [DOI] [PubMed] [Google Scholar]
- Ray K, Tisdale J, Dodd RH, Dauban P, Ruat M, Northup JK 2005 Calindol, a positive allosteric modulator of the human Ca2+ receptor, activates an extracellular ligand-binding domain-deleted rhodopsin-like seven-transmembrane structure in the absence of Ca2+. J Biol Chem 280:37013–37020 [DOI] [PubMed] [Google Scholar]
- Ray K, Fan GF, Goldsmith PK, Spiegel AM 1997 the carboxyl terminus of the human calcium receptor: requirements for cell surface expression and signal transduction. J Biol Chem 272:31355–31361 [DOI] [PubMed] [Google Scholar]
- Dupré DJ, Baragli A, Rebois RV, Ethier N, Hébert TE 2007 Signalling complexes associated with adenylnyl cyclase II are assembled during their biosynthesis. Cell Signal 19:481–489 [DOI] [PubMed] [Google Scholar]
- Adamus G, Zam ZS, Arendt A, Palczewski K, McDowell JH, Hargrave PA 1991 Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res 31:17–31 [DOI] [PubMed] [Google Scholar]
- Kosmaoglou M, Schwarz N, Bett JS, Cheetham ME 2008 Molecular chaperones and photoreceptor function. Prog Retin Eye Res 27:434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben JH, Sze M, Knoers NV, Deen PM 2006 Rescue of vasopressin V2 receptor mutants by chemical chaperones: specificity and mechanism. Mol Biol Cell 17:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G 2004 Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum to Golgi transport in cardiac myocytes. J Biol Chem 279:41077–41084 [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Fugetta EK, Wu G 2006 Differential regulation of cell surface targeting and function of β- and α-adrenergic receptors by Rab1 in cardiac myocytes. Mol Pharmacol 69:1571–1578 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang G, Dupré DJ, Feng Y, Robitaille M, Lazartigues E, Feng YH, Hébert TE, Wu G 2009 Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther 330:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Chowdhury S, Northup JK, Ray K 2010 Sar1-dependent trafficking of the human calcium receptor to the cell surface. Biochem Biophys Res Comm 396:874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt A, Garabédian M, Bai M, Sinding C, Zhang Z, Lagarde JP, Boulesteix J, Rigaud M, Brown EM, Kottler ML 2000 A large homozygous or heterozygous in-frame deletion within the calcium-sensing receptor’s carboxylterminal cytoplasmic tail that causes autosomal dominant hypocalcemia. J Clin Endocrinol Metab 85:1695–1702 [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM 1998 Protein kinase C phosphorylation of threonine at position 888 in Ca2+-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem 273:21267–21275 [DOI] [PubMed] [Google Scholar]
- Gama L, Breitwieser GE 1998 A carboxyl terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium-sensing receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem 273:29712–29718 [DOI] [PubMed] [Google Scholar]
- Felder CB, Graul RC, Lee AY, Merkle HP, Sadee W 1999 The venus flytrap periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS PharmSci 1:E2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K 2000 Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]