Abstract
Vascular endothelial growth factor (VEGF) is one of the best characterized angiogenic factors controlling placental angiogenesis; however, how VEGF regulates placental angiogenesis has not yet completely understood. In this study, we found that all the components of assembling a functional NADPH oxidase (NOX2, p22phox, p47phox, p67phox, and Rac1) are expressed in ovine fetoplacental artery endothelial cells (oFPAECs) in vitro and ex vivo. Treatment with VEGF (10 ng/ml) rapidly and transiently activated Rac1 in oFPAECs in vitro and increased Rac1 association with p67phox in 5 min. Intracellular superoxide formation began to significantly increase after 25–30 min of VEGF stimulation, which was mediated by both VEGFR1 and VEGFR2. VEGF also stimulated oFPAE cell proliferation and migration and enhanced the formation of tube-like structures on Matrigel matrix. In oFAPEC transfected with specific Rac1 small interfering RNA (siRNA, 40 nm), VEGF-induced intracellular superoxide formation was completely abrogated in association with a 78% reduction of endogenous Rac1. In oFPAE cells transfected with the specific Rac1 siRNA, but not with transfection reagent alone or scrambled control siRNA, VEGF-induced cell proliferation, migration, and tube-like structure formation were dramatically inhibited. Pretreatment of an NADPH oxidase inhibitor apocynin also abrogates the VEGF-stimulated intracellular superoxide production and DNA synthesis in oFPAECs. Taken together, our results demonstrated that a Rac1/Nox2-based NADPH oxidase system is present in placental endothelial cells. This NADPH oxidase system appears to generate the second messenger superoxide that plays a critical role in the signaling control of the VEGF-induced placental angiogenesis.
Intracellular superoxide generation from a Rac1/Nox2-based NADPH oxidase system in placental artery endothelial cells plays a role in the signaling control of the VEGF-induced placental angiogenesis.
Vascular endothelial growth factor (VEGF) stimulates placental angiogenesis and nitric oxide (NO) production (i.e. two key mechanisms for implementing the bidirectional maternal-fetal exchanges essential for fetal growth and survival) (1,2). The biological functions of VEGF are mediated by its tyrosine kinase receptors [i.e. fms-related tyrosine kinase 1 (Flt-1/VEGFR1) and kinase insert domain receptor (KDR/VEGFR-2)] (3). Ligand binding initiates tyrosine phosphorylation of the intracellular domains of KDR and Flt-1, resulting in activation of downstream signaling pathways (4,5,6). In placental endothelial cells, we have shown that VEGF activates complex signaling pathways, including extracellular signal-regulated kinases, phosphoinositol-3-kinase/protein kinase B, and endothelial NO synthase (eNOS)/NO, etc., which are critical for endothelial cell angiogenesis and gene expression (5,6,7,8,9); however, how VEGF regulates placental angiogenesis remains incompletely understood.
NADPH oxidase derived intracellular superoxide (O2−) has been shown recently to mediate growth factor signaling (10,11,12). This oxidase was first identified in phagocytes (i.e. macrophages and neutrophils) as a multi-subunit enzyme assembled by the flavocytochrome b558 composed of the plasma membrane bound gp91phox and p22phox and several cytosolic components including p47phox, p67phox, and the small G protein Rac (13). When phagocytes are activated, membrane translocation of the cytosolic components activates the oxidase; with NADPH as the electron donor, extracellular O2− is produced in burst for host defense (14). Vascular endothelial and smooth muscle cells express most of the NADPH oxidase subunits (15); however, the vascular oxidase differs functionally from the phagocyte NADPH oxidase as it produces constitutively low levels of O2− inside the cells even in resting conditions (16).
Human placental tissues contain NADPH oxidase activity, which is greater in early compared with term pregnancy (17,18). Both trophoblastic and capillary endothelial cells in the placenta express NADPH oxidase mRNA and protein (19,20). Apart from distinct biochemical features from phagocyte NADPH oxidase, the function of placental NADPH oxidase is currently unknown (17). Placental NADPH oxidase expression is elevated in preeclampsia (PE) compared with normal pregnancy (19,20,21). Elevated NADPH oxidase is expected to increase the formation of O2− that is the major reactive oxygen species (ROS) in the placenta (20); combined with other sources of ROS, the balanced reduction-oxidation (redox) status shifts to cause oxidative stress and cell damage, which have been reasoned as one of the leading etiologies of PE (22). However, several large-scale clinical trials have failed to show any beneficial effects of antioxidants on ameliorating the symptoms of PE (23,24,25). These data have shaken the oxidative stress theory of PE pathogenesis and implicated a different image of the role that ROS plays in pregnancy.
VEGF activates Rac1 and recruits p47phox and p67phox to the membrane in human umbilical vein endothelial cells (HUVECs). This facilitates the assembling and activation of an endothelial NADPH oxidase (26). Low levels of intracellular O2− formation via NADPH oxidase functions as an important second messenger for transmitting VEGF signaling via KDR (12) to angiogenic responses and redox-sensitive gene expression (10,11,27). In this study, we hypothesized that O2− derived from endothelial NADPH oxidase plays a key role in mediating placental angiogenesis. Our data show that VEGF rapidly and transiently activates Rac1 and increases intracellular O2− formation in a time-dependent fashion in primary ovine fetoplacental artery endothelial cells (oFPAECs). Down-regulation of endogenous Rac1 inhibits the VEGF-induced O2− formation and in vitro angiogenesis. Pharmacological inhibition also confirmed a role of NADPH oxidase in VEGF-induced placental angiogenesis. Our data suggest that endothelial NADPH oxidase-derived intracellular O2− plays a key role in the VEGF-regulated placental angiogenesis in vitro.
Materials and Methods
Materials
Recombinant human VEGF165 was from R&D Systems (Minneapolis, MN). MCDB131, medium-199 (M-199), and antibiotic-antimycotic solution were from Invitrogen (Grand Island, NY). Fetal bovine serum was from Biomedia (Foster City, CA). Goat anti-p22phox polyclonal antibodies (pAb), horseradish peroxidase conjugated rabbit antigoat IgG were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-p67phox pAb, anti-p47phox pAb, and anti-gp91phox pAb, and mouse anti-Rac1 monoclonal antibody (mAb), and Rac activation assay kit and the siRNA/siAB assay kit were from Upstate Biotechnology, Inc. (UBI, Lake Placid, NY). Anti–β-actin mAb was from Ambion (Austin, TX). Anti-eNOS mAb was from Cell Signaling Technology, Inc. (Danvers, MA). Dihydroethidium and Prolong Gold antifade reagent were from Molecular Probes (Eugene, OR). Peroxidase-conjugated antirabbit and antimouse IgGs and [Methyl-3H]-thymidine (2.85 TBq/mmol, 1 mCi/ml) were from Amersham Biosciences (Arlington Heights, IL). BSA, gelatin, and all other chemicals were from Sigma (St. Louis, MO) unless indicated.
Animals and tissue sample collection
Late pregnant (D120–130; gestation ∼145 days) ewes were purchased from Nebeker Ranch (Lancaster, CA). The animal use protocol was approved by the University of California San Diego Animal Subjects Committee. Immediately after sacrifice, secondary fetoplacental artery (PA) segments were harvested, fixed in 3.7% paraformaldehyde for preparation of paraffin-embedded tissue blocks. Fresh PAs were used for isolation of endothelial cells as described below. A segment of PA (∼100 mg) per placenta were homogenized in 4 volumes per wet weight of 20 mmol/liter Tris-HCl (pH 7.6), 1% Triton X-100, and 20% glycerol with a Pro200 tissue homogenizer (3 × 20 sec bursts) on ice. The homogenates were cleared by centrifugation and protein content was measured by using a Bio-Rad protein kit using BSA as the standard.
Fluorescence microscopy
Ovine placental artery segments were fixed with 3.7% paraformaldehyde, paraffin embedded, and 6-μm sections (arterial rings) were cut and mounted onto slides. After deparaffinization and rehydration, the tissue sections were blocked in PBS + 1% BSA containing 2% goat serum for 30 min at room temperature, followed by incubation with rabbit antihuman gp91phox/NOX2 pAb (5 μg/ml, UBI) in PBS + 1% BSA overnight at 4 C. Anti-eNOS mAb (2 μg/ml) was applied to the samples for 1 h. After washing (3 × 5 min) in PBS + 0.3% Triton X-100, the samples were incubated with Alexa Fluor 488-labeled goat antimouse IgG (8 μg/ml) and rhodamine-labeled donkey antirabbit IgG (Molecular Probes, 0.8 μg/ml) for 1 h at room temperature. After washing, the samples were mounted with ProLong Gold antifade reagent containing 4′-6-diamidino-2-phenylindole (DAPI, blue). The samples were then analyzed by fluorescence microscopy. Digitalized images were captured and processed by using a high-resolution charge-coupled device (CCD) camera using the Simple PCI software. Omitting first antibody and replacing the first antibodies with rabbit/mouse IgGs were used as controls.
Cell culture and preparation of total cell extracts
oFPAECs were isolated as previously described (7,28). Cells were subcultured and used at passages 7–10. The immortalized oFPAE cell line (SV40-oF) containing a neomycin resistance gene and a simian virus 40 gene encoding large T and small t antigens were kindly provided by Dr. Zheng (29) and used at passage 19–21. Before each experiment, subconfluent (∼70–80%) cells were serum starved in M-199 containing 1% fetal bovine serum, 0.1% BSA, 25 mm HEPES overnight. After stimulation, cellular proteins were harvested in a nondenaturing lysis buffer (28) and protein content was measured as above.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed with 500 μg protein per sample. The samples were incubated with 4 μg anti-Rac1 mAb in lysis buffer (1 ml) overnight at 4 C. Protein-G agarose beads (50/50 slurry beads, 50 μl) were added and incubated at 4 C for 1 h. The beads were pelleted, washed, and boiled in Laemmli buffer for 5 min. The supernatants were subjected to immunoblotting with anti-p67phox or anti-p22phox pAb and anti-Rac1 mAb. Immunoblotting was carried out as described previously (28). The immunoreactive protein signals were visualized by using the Chemi-Glow substrate, and digital images were captured with the ChemiImager Imaging System with a high-resolution CCD camera and quantified by the ChemiImager 4400 software (Alpha Innotech Corp., Santa Clara, CA).
Rac1 GST-PAK pull-down assay
Rac1 activation was measured by a Rac activation assay kit from UBI. Briefly, equal amounts of protein samples (500 μg/assay) were precleared with glutathione agarose beads. The samples were incubated with the GST-PAK-CD fusion protein bound to glutathione-coupled Sepharose beads at 4 C for 1 h. The beads and proteins bound to the fusion protein were washed three times with lysis buffer and eluted in 2× Laemmli sample buffer. The samples were boiled for 5 min and then used for analyzing the amounts of active GTP-bound Rac1 by immunoblotting.
Rac1 small interfering RNA (siRNA)
oFPAECs were transfected with 40 nm SMARTpool siRNA directed against the Rac1 mRNA sequence (UBI) by using the siLentFect Lipid Reagent (Bio-Rad, Hercules, CA). Transient transfection of Rac1 or scrambled siRNAs (control) was carried out as described (30). The cells were used for experiments at 24 h posttransfection. Trypan blue assay confirmed that cell viability was not affected by siRNA transfection.
Measurement of intracellular O2−
Dihydroethidium (DHE) was used to detect intracellular O2− as the later oxidizes DHE to produce ethidium which in turn permanently stains nucleotide acids to produce red fluorescence and thus can be used specifically for measuring intracellular O2− (31). Cells, grown on gelatin-coated cover slides, were placed in Hank’s balanced buffer [20 mm HEPES (pH 7.5), 0.5 mm MgCl2, 137 mm NaCl, 10 mm glucose, 5.4 mm KCl, 4.2 mm NaHCO3, 3 mm Na2HPO4, 0.4 mm KH2PO4, 0.8 mm MgSO4] containing 2 mm calcium and 0.2% BSA. Calcium was supplemented freshly in the buffer prior as calcium is connected to O2− generation by stimuli (32). VEGF (10 ng/ml) and 10 μm DHE were added simultaneously from the beginning of cell stimulation. After 10 min incubation at 37 C, the cells were examined under a fluorescence microscope with a ×20 objective. Digitalized images of the cells were captured at 5-min intervals at room temperature with excitation at 518 nm and emission at 605 nm by a Hamamatsu CCD camera using the SimplePCI software (Compix Inc., Cranberry Township, PA). Relative fluorescence intensity (RFU) of the images was calculated offline. Each experiment was run in triplicate and repeated three times with different cell preparations. Images of different areas were taken from each slide and the RFU of the images were averaged as mean ± sem.
Cell proliferation assays
[3H]-thymidine incorporation was used to measure DNA synthesis. Briefly, serum-starved cells in 24-well plate were stimulated with VEGF (10 ng/ml) for 48 h. [3H]-thymidine (1 μCi per well per 0.5 ml medium) was added during the last 6 h of VEGF treatment. After washing with cold PBS, the acid-insoluble materials of the cells were precipitated with 5% (wt/vol) trichloroacetic acid. The DNA was extracted with 0.1 m NaOH (200 μl per well) and neutralized with HCl. [3H]-thymidine incorporation was quantified by liquid scintillation spectrophotometer. Cell number was measured by direct counting the cells using a hemocytometer. The cells grown in 6-well plate were treated with or without VEGF for 72 h, trypsinized, and counted.
Cell migration assays
Cells (30,000 per well) were seeded on the top of the filter membrane of BD Falcon FluoroBlok Insert (8.0 μm pores; BD Biosciences, San Jose, CA) and placed in a 24-well plate. The bottom chambers were filled with 0.8 ml of MCDB131-0.2%FCS with or without VEGF. Calcein acetoxymethyl ester (calcein-AM, 0.2 μg/ml) was added for 30 min at the end of stimulation. The plate was examined under an inverted fluorescence microscope. Digitalized images of green fluorescently labeled cells migrated through the filter membrane were captured with excitation at 494 nm and emission at 517 nm by a CCD camera using SimplePCI. The number of migrating cells was counted from four randomly chosen areas per insert. Cell migration was confirmed by the Scratch “wound” assay as described (5,6).
Tube formation assay
Tube formation on matrigel was measured as previously described (5,6). Images from five microscopic fields per well were analyzed. The branch points of the formed tubes were counted and averaged. Experiments were repeated in triplicate.
Experimental replications and data analysis
All experiments were repeated at least three times using oFPAECs from different ewes. Data are presented as mean ± sem and analyzed by one way ANOVA. When a F-test is significant, treatment responses will be compared with the corresponding controls by multiple comparisons using SigmaStat/plot software (SPSS, Chicago, IL). P < 0.05 was considered as significant.
Results
Expression of NADPH oxidase in placental artery endothelial cells
The phagocyte-like multi-component NADPH oxidases, composed of cytochrome b558 (gp91phox/NOX2 and p22phox) and cytosolic proteins (p47phox, p67phox, and Rac1), is the major source of O2− in endothelial cells (11). With fluorescence microscopic analyses, in sections of fetal placental artery rings immunoreactive NOX2 was detected in fetoplacental artery endothelial cells strongly labeled with an anti-eNOS antibody; smooth muscle cells also expressed high levels of NOX2 protein but expressed no or very low levels of eNOS (Fig. 1A). To further confirm the expression of NOX2/NADPH oxidase in placental endothelial cells, immunoblotting was performed to detect other components of the oxidase. As shown in Figures 1B, 3, and 4, all the cytosolic components were expressed in oFPAECs. NOX2, p47phox, and p67phox proteins were also detected in the SV40-transformed oFPAE cell line (SV40-oF) and in HUVECs (data not shown) known to express functional NADPH oxidases (15).
Figure 1.
NADPH oxidase expression in ovine fetoplacental artery ex vivo and fetoplacental artery endothelial cells in vitro. A, Immunofluorescence microscopic analysis of NADPH oxidase and eNOS expression in ovine fetal placental artery rings. Digital images were first captured with fluorescence microscopy under a ×40 objective. A field of the ×40 image (top merged) in the box was then examined under a ×100 oil objective to detail endothelial/smooth muscle colocalization. Incubation with antirabbit and mouse IgGs were used as experimental controls. Triangles and asterisks depict endothelial and smooth muscle cells, respectively. B, Immunoblot analysis of NOX2, p47phox, and p67phox expression in protein extracts (40 μg per lane) of ovine fetoplacental artery endothelial cells (oFPAECs), ovine placental artery homogenates (PA), and SV40-transfected oFPAE cell line (SV40-oF).
Figure 3.
Effects of VEGF on the small G protein Rac1 activation in oFPAECs. A, Cells were treated with VEGF (10 ng/ml) for up to 10 min. GTP bound Rac1 was pulled down from total cell extracts (500 μG protein per lane) by using 5 μg PAK-PDB agarose beads. The pull-down Rac1-GTP was measured by immunoblotting with an anti-Rac1 mAb (upper panel). Total protein (20 μg) from the same samples was immunoblotted with Rac1 mAb to verify sample loading. PC, positive control; NC, negative control. Blots of atypical experiment are shown. Data (mean±sem) were summarized from three independent experiments. *, P < 0.05 vs. time 0. B, Cells were treated with VEGF (10 ng/ml) for up to 30 min. Equal amounts of proteins (500 μg) were immunoprecipitated with 4 μg anti-Rac1 mAb, and immunoblotting with anti-p67phox pAb, anti-p27phox pAb, and Rac1 mAb. Blots of atypical experiment shown represent three independent experiments. Data (mean ± sem) were summarized from three independent experiments. *, P < 0.05 vs. time 0.
Figure 4.
Effects of Rac1 down-regulation by siRNA on intracellular superoxide formation in oFPAECs. A, Subconfluent (∼70%) oFPAE cells were transfected without (sham) or with 40 nm of either Rac1 or scrambled siRNAs using the Bio-Rad siLentFect lipid reagent. Rac1 protein levels were measured by immunoblotting at 24 h posttransfection. β-actin was probed as a control. ***, P < 0.001 vs. sham and scrambled siRNA controls. B, Cells transfected with Rac1 or scrambled siRNAs were treated with 40 ng/ml VEGF in Hank’s buffer containing dihydroethidium (10 μm) for 1 h at 37 C. DHE fluorescence was recorded every 10 min, and digital images were captured. Fluorescence images of a typical experiment at 30 min poststimulation are shown and data (means ± sem) of the relative fluorescence intensity from three independent experiments are summarized in the bar graph. Bars with different letters differ significantly (P < 0.05).
VEGF stimulates intracellular O2− formation in oFPAECs
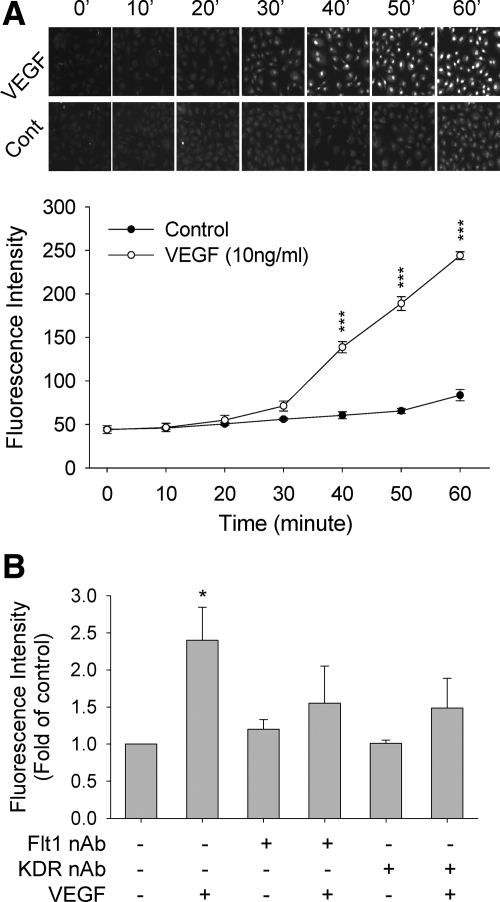
Although ROS including O2− are generally considered as toxic byproduct to induce DNA damage, apoptosis, and cell death, solid evidence also showed recently that at low levels they function as important signal mediators participating in normal cell activities (27,33). It is possible that intracellular O2− may function as second messenger for VEGF signaling toward placental angiogenesis. To this end, we first used the oxidative fluorescent dye DHE as a probe to detect O2− generation in response to VEGF stimulation in oFPAECs. DHE is cell permeable; when oxidized by O2− it is converted to ethidium that binds to DNA to form red fluorescence (31). It has been widely used as a means for measuring intracellular O2− (31). In oFPAECs, treatment with VEGF (10 ng/ml, up to 1 h) increased intracellular O2− in a time-dependent fashion. Compared with controls, the DHE fluorescence began to increase around 30 min and was significantly enhanced thereafter by VEGF (Fig. 2A). Because oFPAECs express both VEGFR1 and VEGFR2 (5), we then determined their specific role by using the neutralizing antibodies that effectively blocked their respective functions (5). Pretreatment with the neutralizing antibodies of both KDR and Flt1 blocked VEGF stimulation of intracellular O2−, suggesting that both are required (Fig. 2B).
Figure 2.
Effects of VEGF on intracellular superoxide formation in oFPAECs. A, Time course studies. Cells grown on gelatin-coated glass coverslips were treated without or with VEGF (10 ng/ml) in Hank’s buffer containing dihydroethidium (10 μm) simultaneously. DHE fluorescence was examined under an inverted fluorescence microscope. Digitalized fluorescent images of the cells were captured at 5-min intervals in dark at room temperature with excitation at 518 nm and emission at 605 nm. RFU of the images was calculated offline by using SimplePCI. One series of DHE fluorescence image in control and VEGF-treated oFPAECs at 10-min interval were recorded every 5 min were shown. B, Role of specific VEGF receptors. After 60 min pretreatment with the VEGFR1 or VEGFR2 neutralizing antibodes (20 μg/ml), the effects of VEGF (10 ng/ml) on intracellular superoxide in oFPAECs was measured. Flt1 nAb, VEGFR1 neutralizing antibody; KDR nAb, VEGFR2 neutralizing antibody. Each experiment was run in triplicate and repeated three times with different cell preparations. Images of different areas were taken from each coverslip, and the RFU of the images were averaged as mean ± sem. ***, P < 0.001; *, P < 0.05, vs. control.
VEGF rapidly and transiently activates Rac1 in oFPAECs
Current model for the assembling and activation of NADPH oxidase in nonphagocytic cells highlights a critical role of Rac1 (34,35). Rac1 is a cytosolic 21-kD small G protein, and its activation is switched on and off by GTP/GDP exchange (35). Treatment of oFPAECs with VEGF (10 ng/ml) rapidly and transiently increased the levels of active Rac1GTP in a time-dependent manner. Rac1GTP was significantly increased at 1–5 min and returned to baseline at 10 min after treatment with VEGF (Fig. 3A). One possible mechanism for assembling an active NADPH oxidase is that Rac1GTP recruits the cytosolic components to the cytochrome b558 (16). p67phox possesses an N-terminal domain comprising four tetratricopeptide repeat (TPR) motifs that specifically interact with active Rac1, which is required for NADPH oxidase activation (34). In unstimulated oFPAECs, we found that the amounts of Rac1 bound p67phox and p22phox was detectable but low. Treatment with VEGF significantly increased Rac1 bound p67phox protein at 5–15 min and returned to baseline at 30 min poststimulation. Rac1 bound p22phox appeared to be increased, though not statistically significant, at 5 min after VEGF treatment (Fig. 3B).
Rac1 down-regulation abrogates VEGF-induced intracellular O2− formation
To determine the role of Rac1 in the VEGF-induced O2− formation and angiogenic responses in vitro, we developed a method for specifically down-regulating endogenous Rac1 protein in oFPAECs. After optimizing conditions for duplex siRNA transfection, we found that transfection of 40 nm of mixed Rac1 siRNAs showed a maximal inhibition of Rac1 protein expression with minimal cytotoxicity. Transfection of specific Rac1 siRNAs, but not the scrambled control siRNA, Rac1 protein was significantly reduced by 77.6 ± 7.8%. Transfections of both scrambled and Rac1 siRNAs did not alter β-actin protein in oFPAECs (Fig. 4A). VEGF significantly stimulated intracellular O2− formation in oFPAECs transfected with control but not Rac1 siRNAs (Fig. 4B).
Rac1 down-regulation blocks the VEGF-induced angiogenic responses
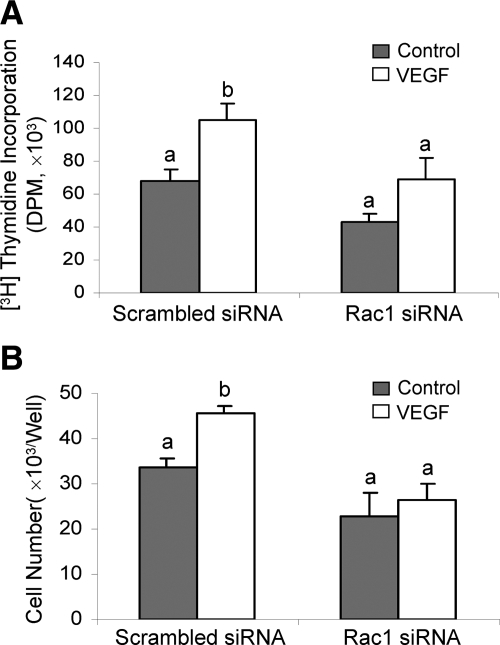
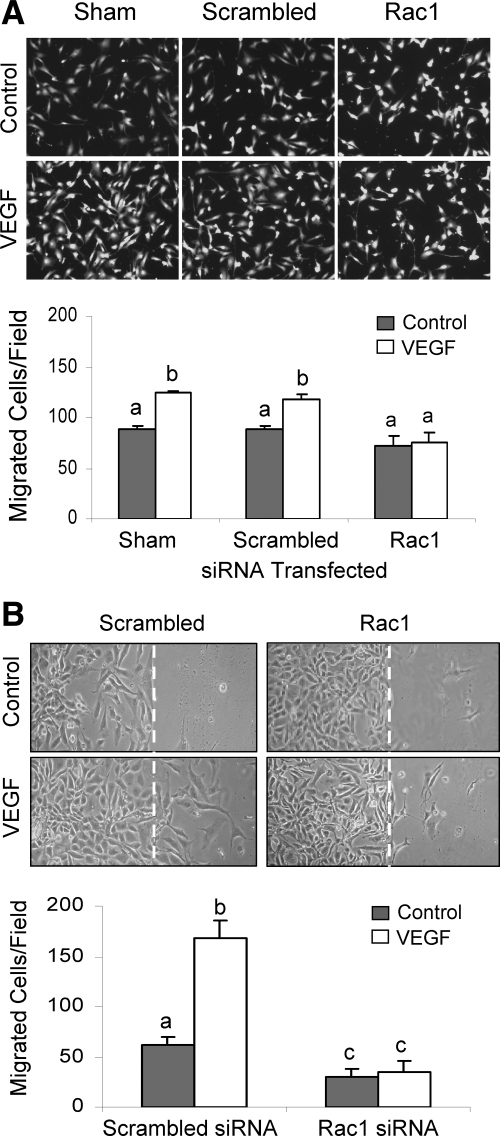
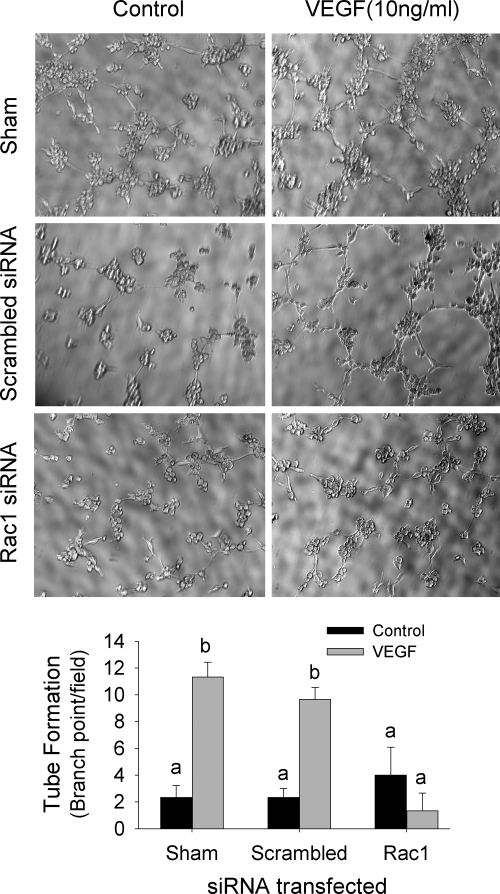
VEGF is one of the best studied endothelial cell mitogens with the ability of stimulating endothelial cell to proliferate and migrate as well as to form tube-like structure on extracellular matrix proteins (36). We have recently shown that VEGF stimulates oFAPEC cell proliferation, migration, and tube formation on Matrigel (5). In this study, we assessed the effects of Rac1-dependent O2− formation on the VEGF-induced in vitro angiogenesis using oFPAECs. In cells transfected with the scrambled siRNA, VEGF was able to significantly increase DNA synthesis at 48 h and cell number at 72 h; however, in cells transfected with Rac1 siRNAs, VEGF no longer stimulated DNA synthesis and cell number (Fig. 5). With the transwell migration assay, we observed that treatment with VEGF (10 ng/ml) for 16 h significantly stimulated cell migration in sham (transfection reagent alone) transfected cells or transfected with scrambled control siRNA; however, in cells transfected with specific Rac1 siRNAs, VEGF failed to stimulate oFPAE cell migration (Fig. 6A). The stimulatory effect of VEGF on oFPAEC migration was confirmed by a “scratch” wound assay as shown in Figure 6B. Treatment with VEGF (10 ng/ml) stimulated formation of tube-like structures on Matrigel using sham and scrambled control siRNA-transfected oFPAECs; however, VEGF did not stimulate tube-like structures on Matrigel in cells transfected with specific Rac1 siRNAs (Fig. 7).
Figure 5.
Effects of Rac1 down-regulation on VEGF-induced oFPAE cell proliferation. Subconfluent cells in 12-well plate were transfected with Rac1 or scrambled siRNAs. After 24 h of recovery, the cells were serum starved overnight. Cells were treated with VEGF (10 ng/ml) for 48 h for measuring DNA synthesis or 72 h for measuring cell number. For DNA synthesis, [3H]-tymidine (1 μCi) was added into each well 6 h before completion of VEGF treatment. [3H]-tymidine incorporation into DNA was measured by liquid scintillation counting. For cell number measurement, the cells were trypsinized after 72 h of treatment with or without VEGF for counting cell numbers. Data (means ± sem) of [3H]-tymidine (DPM) for de novo DNA synthesis (A) and cell number (B) from three independent experiments are summarized. Bars with different letters differ significantly (P < 0.05).
Figure 6.
Effects of Rac1 down-regulation on VEGF-induced oFPAE cell migration. A, Subconfluent cells were transfected without (sham) or with Rac1 or scrambled siRNAs and allowed to recover for 24 h. The cells were then trypsinized and seeded into the 24-well FluoroBlok Insert System. After treatment with or without 10 ng/ml VEGF for 16 h, cells migrated to the bottom of the filter membranes were labeled with calcein acetoxymethyl ester (calcein-AM, 0.2 μg/ml) for 30 min. Digitalized images (×100) of green fluorescently labeled cells migrated to the bottom side of the filter membrane were captured with excitation at 494 nm and emission at 517 nm. The number of cells migrated from four randomly chosen areas were counted and analyzed. B, Subconfluent (∼80%) cells were transfected with Rac1 or scrambled siRNAs and cultured until confluence. Confluent monolayers were starved overnight and scrapped with a sterilized tip. After treatment with or without VEGF (10 ng/ml) for 24 h, digitalized images of cell migration were captured. The white dashed line shows the position of “wounding” of the confluent monolayer. Photographs of a typical experiment of images of migrated cells in the transwell assay and the Scratch “wound” assay are shown. Data (means ± sem) from three independent experiments using different cell preparations are summarized in the lower bar graphs. Bars with different letters differ significantly (P < 0.05).
Figure 7.
Effects of Rac1 down-regulation on VEGF-induced formation of tube-like structures in oFPAECs. Subconfluent cells were transfected without (sham) or with Rac1 or scrambled siRNAs for 24 h and then starved overnight. After trypsinization, the cells (2000 per well) were added to 96-well plates precoated with the Matrigel. After the cells were cultured for 3 h, formation of the tube-like structures was examined under an inverted Leica microscope. Digitalized images (×10) were captured with the simplePCI software. Images shown represent one typical experiment, and bar graphs summarize data (means ± sem) from three independent experiments using different cell preparations. Bars with different letters differ significantly (P < 0.05).
Intracellular O2− mediates the VEGF-stimulated proliferation in oFPAECs
Apocynin is capable of preventing p47phox translocation to the plasma membrane thereby inhibiting the assembling of a functional NADPH oxidase (37); it has been used as a pharmacological inhibitor for NADPH oxidase (38). To further test the role of intracellular O2− formation in the VEGF-induced oFPAE cell proliferation, oFAPEC were pretreated with apocynin (0.5 mm) for 1 h, followed by incubation with VEGF for measuring intracellular O2− at 1 h or DNA synthesis at 48 h. We observed that treatment with apocynin effectively blocked VEGF-induced intracellular O2− formation (Fig. 8A). Lastly, we examined whether O2− formation is involved in endothelial cell proliferation. Consistent with the data shown in Fig. 7A, treatment with VEGF significantly stimulated DNA synthesis; however, in cells pretreated with apocynin (0.5 mm), the VEGF-stimulated DNA synthesis was completely attenuated (Fig. 8B).
Figure 8.
Effects of apocynin on VEGF-induced intracellular superoxide formation and cell proliferation in oFPAECs. A, Subconfluent cells grown on 0.2% gelatin coated coverslips were serum-starved overnight. After pretreated with 0.5 mm apocynin, the cells were treated with or without 10 ng/ml VEGF and concurrently loaded with 10 μm dihydroethidium for 1 h. Fluorescent Images were captured as described in Fig. 2. Photographs of one typical experiment shown represent similar results obtained from three independent experiments using different cell preparations. B, Serum-starved subconfluent cells were pretreated with 0.5 mm apocynin for 1 h and then treated with or without 10 ng/ml VEGF for 48 h. DNA synthesis was determined as described in Fig. 5A. Bar graph summarizes data (means ± sem) from three independent experiments using different cell preparations. Bars with different letters differ significantly (P < 0.05).
Discussion
Angiogenesis is essential for the establishment and execution of the bidirectional maternal-fetal exchange of nutrients and respiratory gases, which is arguably the most important event for pregnancy (1,2). Placental angiogenesis is a multi-factorial complex involving many cell types, of which endothelial cells play a central role in activating matrix protein degradation, proliferate, migrate and eventually form tube-like structures. VEGF and its receptors are the best studied angiogenic regulatory system in the placenta (5,6,39). Compelling data have shown that VEGF and its receptors KDR and Flt1 are increased in uterine and fetal tissues accompanied with placental angiogenesis during pregnancy. Ligand-receptor interactions result in a plethora of signaling pathways including mitogen-activated protein kinases, protein kinase B/Akt, and eNOS-NO, etc. (5,6,7,8,9). These pathways are important in mediating VEGF stimulation of placental artery endothelial cell proliferation (7,40), migration and tube formation (5,6), and NO production via eNOS that is critical for placental vasodilatation (40). However, the precise signaling mechanisms of VEGF action in the placental endothelial cells are not yet completely understood. In this study, we have shown that VEGF provokes rapid intracellular O2− formation in oFPAECs. oFPAECs express high levels of Rac1, which can be rapidly and transiently activated by VEGF. Down-regulation of endogenous Rac1 by its specific siRNAs inhibited the VEGF-induced intracellular O2− formation and VEGF-stimulated in vitro angiogenic responses, including cell proliferation, migration, and tube formation. Pharmacologic inhibition of NADPH oxidase by apocynin also attenuated VEGF-induced intracellular O2− formation and in vitro angiogenesis. Hence, our findings suggest that Rac1-dependent intracellular O2− formation is directly linked to the VEGF-induced placental artery endothelial cell proliferation, migration, and tube formation (angiogenesis in vitro), implicating a novel route for VEGF action in placental endothelial cells.
O2− is a short-lived ROS, which can be generated during any cellular oxygen metabolisms from many enzymatic sources, including mitochondrial respiration (41,42), xanthine oxidase (43), cytochrome P450 (44), lipoxygenase and cyclooxygenase (45), hemeoxygenase (46), and uncoupled endothelial nitric oxide synthase (47), etc. We have shown herein that VEGF stimulation of intracellular O2− formation is completely inhibited by apocynin in oFPAECs. In keeping with previous data showing that VEGF stimulation of O2− formation was inhibited only by inhibition of NADPH oxidase but not by inhibition of other enzymatic pathways in HUVECs (11), our data suggest that O2− formed by VEGF stimulation in oFPAECs might be exclusively derived from endothelial NADPH oxidase.
In mammals, Rac1 belongs to a family of small GTPases composed of the ubiquitously expressed Rac1 (48), hematopoetic Rac2 (48), and the predominantly neuronal Rac3 (49). They participate in the regulation of cell spreading (50,51), cell cycle progression (52), proliferation and migration (53), and transformation (54). In vascular endothelial and smooth muscle cells, as a key component for the assembling of the multicomponent NADPH oxidase, activation of Rac1 directly regulates intracellular O2− formation thereby playing a key role in the vascular redox homeostasis (34). In endothelial cells, intracellular O2− is likely derived from NADPH oxidase assembled by gp91phox/NOX2 or its homologues by Rac1 (12,55). Transient transfection of dominant negative Rac1 has demonstrated a critical role of Rac1 in the VEGF-induced O2− formation via NADPH oxidase (10,11), which is linked to VEGF-induced angiogenesis in HUVECs (11,27,35). Our current study show that a similar NOX2/NADPH oxidase is possibly important for the VEGF-induced angiogenesis in oFPAECs because: 1) Rac1 down-regulation by its specific siRNAs, presumptively disrupting the assembling of endothelial NOX2/NADPH oxidase, inhibited VEGF-induced angiogenesis in vitro; 2) pharmacological inhibition of NADPH oxidase by apocynin inhibited VEGF-induced O2− formation and cell proliferation.
With the identification of four homologues of the phagocyte NADPH oxidase gp91phox in nonphagocytic cells, these catalytic subunits of NADPH oxidase have been renamed as NADPH oxidases (NOX) (16). Apart from the gp91phox/NOX2, endothelial cells also express Nox1, Nox 3–4 (56,57). In most studies, Nox2 is found in HUVECs and functional in redox mediated angiogenesis in vitro and in vivo (11,12). However, one study claimed that Nox2 is not expressed, or if any, at very low levels in HUVECs (58). Nox4 is expressed in endothelial cells (56,57). Our current study shows that NOX2, p21phox, p47phox, p67phox, and Rac1 proteins are expressed in oFPAECs. VEGF stimulates Rac1 and Rac1GTP binding of p67phox. Together with inhibition of VEGF stimulation of intracellular O2− formation by Rac1 knockdown, our findings suggest that the Nox2/NADPH oxidase is present and functional in oFPAECs. Our study does not exclude the possibility that other NOX/NADPH oxidase(s) is also involved. In human placenta, Nox1 mRNA and protein are expressed in term trophoblastic and villous capillary endothelial cells (19,20). We have found high levels of Nox4 mRNA expression and low levels of NOX1 in oFPAECs; interestingly, Nox1 expression appears to be up-regulated by VEGF and fibroblast growth factor in oFPAECs (Barker and Chen, unpublished data). The function of these NOXs are not known in the placenta; however, these enzymes may participate in regulating angiogenesis as NADPH oxidase assembled with these NOXs also produces low levels of intracellular O2− that functions as cell signaling mediators (59).
How a specific NADPH oxidase is assembled and activated is complex because different NOXs assemble NADPH oxidase differently in different cell types, which can be further complicated by their different subcellular localizations. Nox1 and Nox4 are exclusively localized in intracellular membranes of vascular smooth muscle cells, suggesting that they produce O2− inside this cell type (60). Although NOX2/NADPH oxidase similarly assembles at plasma membrane in phagocytes (61) and nonphagocytes including endothelial cells (26), O2− is produced outside phagocytes on activation whereas is produced inside endothelial cells even without stimulation. Thus, subcellular localization cannot fully explain why NADPH oxidase produces O2− outside in phagocytes vs. inside in endothelial cells. In phagocytes, NOX2/NADPH oxidase is assembled in the phagosome possibly localized in caveolae (61). Caveolae are high abundantly in placental endothelial cells (5). Thus, it would be interesting to investigate if NOX2/NADPH oxidase is assembled and activated in endothelial caveolae by VEGF.
Angiogenesis is a multi-step process that is initiated by endothelial cell activation in response to increased local or systemic angiogenic factors. Activated endothelial cells secrete factors resulting in basement matrix protein degradation, endothelial cell proliferation and migration, and eventually formation of tube-like structures (36). As a member of the Rho family small GTPases, Rac1 plays an important role in cell migration, proliferation, transformation, and gene expression (34). We have shown that VEGF stimulates oFPAEC proliferation, migration, and tube formation [Current study, (5)]. Rac1 knockdown by its specific siRNA abrogated these VEGF-induced angiogenic responses. Thus, our data suggest that Rac1 participates into the regulation of placental endothelial cell angiogenesis. However, whether Rac1 is involved in other aspects of angiogenesis by VEGF such as basement breakdown and vascular permeability awaits further investigation.
Once formed, the short-lived O2− can be rapidly converted to the relative stable hydrogen peroxide, when produced in excess, causing oxidative stress toxic to placental endothelial and trophoblastic cells (62). Oxidative stress in the placenta has been considered as one of the potential leading etiologies of complicated pregnancies (20). This theory has provided a rationale for several clinical trials of antioxidant therapy for preeclampsia (63). Although an initial clinical trial showed that supplementation of antioxidants potentially reduced the risk for pregnant women of developing preeclampsia (64), the follow-up large scale VIP trial (23) and the studies (24,25) of vitamin C/E supplementation to pregnant women showed no benefits of reducing the risk of preeclampsia, intrauterine growth restriction, or death or other serious outcomes in their infants. In contrast, the VIP trial revealed the potential of antioxidant supplementation to increase the rate of babies born with low birthweight (23). The reason of causing these unexpected results is elusive. However, a physiological role of O2− derived from NADPH oxidase in placental angiogenesis as shown herein and other endothelial cells (11,27) might have provided one possible clue. A large body of evidence also suggests that at a certain concentration window intracellular ROS are signaling mediators essential for normal functions (65). For example, low levels of exogenous hydrogen peroxide (<10 μm) stimulates DNA synthesis, cell proliferation, migration, and tube formation in HUVECs (27). Consistent with our findings that inhibition of NADPH oxidase by apocynin and Rac1 knockdown impairs in vitro placental angiogenesis, others have shown that various antioxidants also decrease the VEGF-induced angiogenesis in vitro and in vivo (11,66). In keeping with the fact that normal pregnancy is accompanied by increased ROS formation with gestational age due to increased maternal-fetal metabolism, it is possible that antioxidant supplementation during pregnancy for preeclampsia prevention may have bought the oxidative stress occurring in preeclampsia lower than the levels seen in normal pregnancy, thereby causing harmful impacts on ROS-mediated normal cell physiology, in particular vascular morphology and/or function, in the aforementioned clinical trials.
Altogether, our current in vitro studies have established a critical role for Rac1-dependent intracellular superoxide formation via endothelial NADPH oxidase in mediating VEGF stimulation of placental angiogenesis. Cautiously, the physiological and pathophysiological significance of the conclusions drawn based on these studies will need to be verified by in vivo animal studies. In particular, the effect of antioxidant supplementation on placental angiogenesis in vivo is an intriguing area that deserves further investigation. However, our current studies provide evidence for the first time from a unique perspective for explaining the unexpected results of the antioxidant trials (23,24,25) in the prevention of preeclampsia.
Acknowledgments
We thank Dr. Robert Brace and Dr. Cecilia Cheung for assistance in animal handling and Dr. Jing Zheng for providing the SV40-transformed ovine fetoplacental artery endothelial cells.
Footnotes
Current address for S.-m.L.: School of Veterinary Medicine and Biomedical Sciences, University of Nebraska-Lincoln, Lincoln, Nebraska 68583.
This work was supported by National Institutes of Health RO1 Grants HL74947 and HL70562 and R21 HL98746 (to D.-b.C). S.-m.L. was a Lalor Foundation Postdoctoral Fellow.
Disclosure Summary: The authors have nothing to declare.
First Published Online September 15, 2010
Abbreviations: CCD, Charge-coupled device; DHE, dihydroethidium; eNOS, endothelial NO synthase; HUVEC, human umbilical vein endothelial cell; mAb, monoclonal antibody; NO, nitric oxide; NOX, NADPH oxidase; oFPAEC, ovine fetoplacental artery endothelial cells; PA, placental artery; pAb, polyclonal antibody; PE, preeclampsia; redox, reduction-oxidation; RFU, relative fluorescence intensity; ROS, reactive oxygen species; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
References
- Reynolds LP, Redmer DA 2001 Angiogenesis in the placenta. Biol Reprod 64:1033–1040 [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL 2005 Latest advances in understanding preeclampsia. Science 308:1592–1594 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J 2003 The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N 1998 Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343 [DOI] [PubMed] [Google Scholar]
- Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB 2009 Compartmentalizing VEGF-induced ERK2/1 signaling in placental artery endothelial cell caveolae: a paradoxical role of caveolin-1 in placental angiogenesis in vitro. Mol Endocrinol 23:1428–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WX, Feng L, Zheng J, Chen DB 2010 Deciphering mechanisms controlling placental artery endothelial cell migration stimulated by vascular endothelial growth factor. Endocrinology 151:3432–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Bird IM, Melsaether AN, Magness RR 1999 Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology 140:1399–1407 [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E, Liao WX, Zheng J, Chen DB 2008 Differential activation of multiple signalling pathways dictates eNOS upregulation by FGF2 but not VEGF in placental artery endothelial cells. Placenta 29:708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E, Liao WX, Wang W, Zheng J, Chen DB 2010 Activation of AP-1 transcription factors differentiates FGF2 and vascular endothelial growth factor regulation of endothelial nitric-oxide synthase expression in placental artery endothelial cells. J Biol Chem 285:17348–17358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC 2001 Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J 15:2548–2550 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW 2002 Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91:1160–1167 [DOI] [PubMed] [Google Scholar]
- Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T 2002 Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 277:3101–3108 [DOI] [PubMed] [Google Scholar]
- Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM 1993 Translocation of Rac correlates with NADPH oxidase activation: evidence for equimolar translocation of oxidase components. J Biol Chem 268:20983–20987 [PubMed] [Google Scholar]
- Babior BM 1995 The respiratory burst oxidase. Curr Opin Hematol 2:55–60 [DOI] [PubMed] [Google Scholar]
- Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT 1996 Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271:H1626–H1634 [DOI] [PubMed] [Google Scholar]
- Babior BM 2000 The NADPH oxidase of endothelial cells. IUBMB Life 50:267–269 [DOI] [PubMed] [Google Scholar]
- Manes C 2001 Human placental NAD(P)H oxidase: solubilization and properties. Placenta 22:58–63 [DOI] [PubMed] [Google Scholar]
- Raijmakers MT, Burton GJ, Jauniaux E, Seed PT, Peters WH, Steegers EA, Poston L 2006 Placental NAD(P)H oxidase mediated superoxide generation in early pregnancy. Placenta 27:158–163 [DOI] [PubMed] [Google Scholar]
- Cui XL, Brockman D, Campos B, Myatt L 2006 Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 27:422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Cui X 2004 Oxidative stress in the placenta. Histochem Cell Biol 122:369–382 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Sato I 2001 Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts: normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem Cell Biol 116:1–7 [DOI] [PubMed] [Google Scholar]
- Hubel CA 1999 Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 222:222–235 [DOI] [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH 2006 Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 367:1145–1154 [DOI] [PubMed] [Google Scholar]
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS 2006 Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med 354:1796–1806 [DOI] [PubMed] [Google Scholar]
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp Jr JM, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med 362:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Murata K, Shishido T, Hanafusa H 2002 v-Crk activates the phosphoinositide 3-kinase/AKT pathway by utilizing focal adhesion kinase and H-Ras. Mol Cell Biol 22:7015–7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid MR, Kachra Z, Spokes KC, Aird WC 2000 NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486:252–256 [DOI] [PubMed] [Google Scholar]
- Chen DB, Li SM, Qian XX, Moon C, Zheng J 2005 Tyrosine phosphorylation of caveolin 1 by oxidative stress is reversible and dependent on the c-src tyrosine kinase but not mitogen-activated protein kinase pathways in placental artery endothelial cells. Biol Reprod 73:761–772 [DOI] [PubMed] [Google Scholar]
- Song Y, Zheng J 2007 Establishment of a functional ovine fetoplacental artery endothelial cell line with a prolonged life span. Biol Reprod 76:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Song Y, Chen DB, Zheng J 2008 Protein phosphatase 3 differentially modulates vascular endothelial growth factor- and fibroblast growth factor 2-stimulated cell proliferation and signaling in ovine fetoplacental artery endothelial cells. Biol Reprod 79:704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W, Narayanan PK, Robinson JP 1994 Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55:253–258 [DOI] [PubMed] [Google Scholar]
- Goldman R, Moshonov S, Zor U 1998 Generation of reactive oxygen species in a human keratinocyte cell line: role of calcium. Arch Biochem Biophys 350:10–18 [DOI] [PubMed] [Google Scholar]
- Colavitti R, Finkel T 2005 Reactive oxygen species as mediators of cellular senescence. IUBMB Life 57:277–281 [DOI] [PubMed] [Google Scholar]
- Hordijk PL 2006 Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98:453–462 [DOI] [PubMed] [Google Scholar]
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW 1991 Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668–670 [DOI] [PubMed] [Google Scholar]
- Risau W 1997 Mechanisms of angiogenesis. Nature 386:671–674 [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL 2002 Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90:1205–1213 [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E, Grobe A, Kumar S, Noskina Y, Black SM 2005 Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species: a requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 289:L288–L289 [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA 2004 Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350:672–683 [DOI] [PubMed] [Google Scholar]
- Zheng J, Wen Y, Austin JL, Chen DB 2006 Exogenous nitric oxide stimulates cell proliferation via activation of a mitogen-activated protein kinase pathway in ovine fetoplacental artery endothelial cells. Biol Reprod 74:375–382 [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT 2000 Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol 88:1880–1889 [DOI] [PubMed] [Google Scholar]
- Corda S, Laplace C, Vicaut E, Duranteau J 2001 Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol 24:762–768 [DOI] [PubMed] [Google Scholar]
- Sanganahalli BG, Joshi PG, Joshi NB 2005 Xanthine oxidase, nitric oxide synthase and phospholipase A(2) produce reactive oxygen species via mitochondria. Brain Res 1037:200–203 [DOI] [PubMed] [Google Scholar]
- Fleming I 2001 Cytochrome p450 and vascular homeostasis. Circ Res 89:753–762 [DOI] [PubMed] [Google Scholar]
- Leopold JA, Loscalzo J 2005 Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol 25:1332–1340 [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Choi AM 2000 Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279:L1029–L1037 [DOI] [PubMed] [Google Scholar]
- Endemann DH, Schiffrin EL 2004 Nitric oxide, oxidative excess, and vascular complications of diabetes mellitus. Curr Hypertens Rep 6:85–89 [DOI] [PubMed] [Google Scholar]
- Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R 1989 rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem 264:16378–16382 [PubMed] [Google Scholar]
- Haataja L, Groffen J, Heisterkamp N 1997 Characterization of RAC3, a novel member of the Rho family. J Biol Chem 272:20384–20388 [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM 1998 Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 9:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroanne C, Vouret-Craviari V, Wang B, Pouyssegur J 2003 EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci 116:1367–1376 [DOI] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA 2001 Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev 11:48–53 [DOI] [PubMed] [Google Scholar]
- Fryer BH, Field J 2005 Rho, Rac, Pak and angiogenesis: old roles and newly identified responsibilities in endothelial cells. Cancer Lett 229:13–23 [DOI] [PubMed] [Google Scholar]
- Aznar S, Fernandez-Valeron P, Espina C, Lacal JC 2004 Rho GTPases: potential candidates for anticancer therapy. Cancer Lett 206:181–191 [DOI] [PubMed] [Google Scholar]
- Lassègue B, Clempus, RE 2003 Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297 [DOI] [PubMed] [Google Scholar]
- Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M 2004 Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109:227–233 [DOI] [PubMed] [Google Scholar]
- Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL 2005 Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7:308–317 [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Miyano, K, Takeya, R 2005 Molecular composition and regulation of the Nox family NADPH oxidases. Biochem Biophys Res Commun 338:677–686 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW 2004 Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264:85–97 [DOI] [PubMed] [Google Scholar]
- Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK 2004 Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24:677–683 [DOI] [PubMed] [Google Scholar]
- Vilhardt F, van Deurs B 2004 The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J 23:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y 2000 Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol 156:321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijmakers MT, Dechend R, Poston L 2004 Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension 44:374–380 [DOI] [PubMed] [Google Scholar]
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L 1999 Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet 354:810–816 [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM 2006 Redox signaling in hypertension. Cardiovasc Res 71:247–258 [DOI] [PubMed] [Google Scholar]
- Albini A, Morini M, D'Agostini F, Ferrari N, Campelli F, Arena G, Noonan DM, Pesce C, De Flora S 2001 Inhibition of angiogenesis-driven Kaposi’s sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res 61:8171–8178 [PubMed] [Google Scholar]