Abstract
In the rat, tail skin vasomotion is a primary heat loss mechanism that can be monitored by changes in tail skin temperature (TSKIN). Previous studies showed that ovariectomy and estrogen replacement modify TSKIN in the rat. Based on these findings, the ovariectomized (OVX) rat has been used as a model to study the mechanisms and treatment of menopausal hot flushes. It is not known, however, if TSKIN changes across the estrous cycle in intact rats. Here, we describe an improved method for monitoring TSKIN in freely moving rats using a SubCue Mini datalogger mounted on the ventral surface of the tail. This method is noninvasive, cost-effective, and does not require restraints or tethering. We observed a distinct pattern of TSKIN across the estrous cycle characterized by low TSKIN on proestrous night. To determine whether this pattern was secondary to secretion of ovarian steroids, we monitored the thermoregulatory effects of 17β-estradiol (E2) and E2 plus progesterone, administered via SILASTIC capsules to OVX rats. E2 treatment of OVX rats significantly reduced TSKIN in the dark phase from 2 to 21 d after hormone treatment. The TSKIN of E2-treated OVX animals was not significantly different from OVX rats receiving E2 plus progesterone. These data provide evidence that the reduction in TSKIN on proestrous night was secondary to elevated levels of ovarian estrogens. This study provides the first description of TSKIN changes with the estrous cycle and supports the role of estrogens in normal thermoregulation in the rat.
An improved method for long-term monitoring of tail skin temperature in unrestrained rats revealed tail skin vasoconstriction on proestrous night.
Hot flushes are a common symptom of menopause characterized by a coordinated but inappropriate activation of heat loss effectors, including peripheral vasodilatation, sweating, and behavioral mechanisms (1,2,3,4). They occur as a result of ovarian failure and are effectively treated by estrogen replacement therapy (2,3,4). In the rat, vasodilatation of the tail is a primary heat dissipation mechanism (5), and this effector can be monitored by measuring tail skin temperature (TSKIN) (6). TSKIN is increased by ovariectomy and reduced by administration of exogenous estrogens in ovariectomized (OVX) animals (7,8,9,10,11,12,13). Based on these findings, the OVX rat has been used as a model to study the mechanisms and treatment of menopausal flushes. However, the methods of recording TSKIN in these studies have incorporated either restraints (7,10,14), tethering devices that interfere with long-term monitoring (8,13), or expensive and invasive surgically implanted telemetry devices (9,12,15,16).
In 2002, Gordon et al. (17) described a method to monitor TSKIN by mounting a telemetry device in a protective covering on the surface of the tail. In the present study, we adapted this method using a SubCue Mini datalogger rather than the telemetry device. We reasoned that this would provide a noninvasive method to monitor TSKIN over prolonged periods (days to weeks) that does not require an expensive telemetry system. If the effects of estrogens on TSKIN in OVX rats are physiologically relevant, we predicted that there would be changes in TSKIN during the estrous cycle, a naturally occurring event in which ovarian hormone levels are fluctuating. Therefore, in our first experiment, we monitored TSKIN in intact cycling female rats. In a second experiment, we administered 17β-estradiol (E2) or E2 plus progesterone (E2P) to OVX rats in a protocol previously used to explore the positive and negative effects of estrogens on LH secretion (18,19,20). This experiment allowed us to examine the acute and chronic actions of gonadal steroids on thermoregulation, including the circadian and ultradian rhythms of core temperature (TCORE) and TSKIN.
Materials and Methods
Female Sprague Dawley rats (∼10 wk old, 200–250 g; Harlan Sprague Dawley, Inc., Houston, TX) were housed in the Animal Care Facility at the University of Arizona on a 12-h light, 12-h dark cycle (lights on at 0700 h) in a temperature- and humidity-controlled environment. The range of ambient temperatures was 21.1–22.5 C. Rats were fed (ad libitum) a low phytoestrogen diet (Harland Teklan 2014 rat chow) to minimize thermoregulatory effects of phytoestrogens (10). Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Arizona and followed National Institutes of Health guidelines.
TSKIN recording
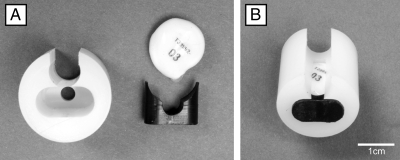
SubCue Mini dataloggers (Canadian Analytical Technologies, Inc., Alberta, Canada) were calibrated using the manufacturer’s specifications. Additionally, each datalogger was validated by comparing its temperature recordings with that of a National Institute of Standards and Technology certified device in our laboratory (TC4000 thermocouple recorder; Madgetech, Inc., Contoocok, NH). A SubCue Mini datalogger was inserted into a protective covering and attached 4.0 cm from the base of the tail (on the ventral surface) using double-sided tape while rats were briefly anesthetized with isofluorane (<5 min). The protective covering consisted of two parts: a main covering (nylon 6-6) and a cap (polyacetal plastic) manufactured by the University Research Instrumentation Center at the University of Arizona (Fig. 1). The main coverings were manufactured in three groove widths, 7.5, 8.0, and 8.5 mm, which allowed placement on tails of different diameters.
Figure 1.
A, Protective covering (left) and SubCue Mini datalogger (top right) and cap (bottom right) used for measuring TSKIN. A pocket in the nylon covering houses the datalogger. B, The datalogger is inserted, and the cap is secured at the end of the pocket to protect the datalogger. The rat’s tail is nestled into the groove of the protective covering so that the datalogger comes in close contact with the ventral surface of the tail.
Experiment 1
Estrous cycles were evaluated by daily vaginal smears in eight rats (21). Rats were housed in pairs or triplets in an animal room shared by numerous investigators. After regular 4- or 5-d cycles were detected, a SubCue Mini datalogger was mounted on the ventral surface of the tail as described above. The datalogger was programmed to record TSKIN every 5 min and replaced every 7 d for 21 d.
Experiment 2
Twenty-six rats were bilaterally OVX under general anesthesia and implanted (ip) with a PhysioTel transmitter (TA10TA-F40; Data Sciences International, St. Paul, MN) to record TCORE by telemetry. TSKIN was recorded as described above. Animals were housed individually in an isolated room of the facility with minimal contact with laboratory personnel.
After a 7-d recovery period, on experimental d 0 and 2 (between 0800 and 0830 h), rats were implanted sc with two SILASTIC (Dow Corning Corp., Midland, MI) capsules (OD, 3.18 mm; ID, 1.57 mm) under isoflurane anesthesia. The capsules were 30 mm long with 5-mm wood sticks inserted to the end of the tubing (effective capsule length, 20 mm). The experimental groups consisted of: 1) OVX, capsules with sesame oil, n = 5 or saline, n = 5; 2) OVX + E2, n = 8, capsules with E2 (360 μg/ml in sesame oil; Sigma-Aldrich, St. Louis, MO) on d 0, then sesame oil on d 2; and 3) OVX + E2P, n = 8, capsules with E2 (360 μg/ml in sesame oil) on d 0, then progesterone (50 mg/ml in sesame oil; Sigma-Aldrich) on d 2. Temperature recordings were obtained at 5-min intervals until 21 d after the first capsule implant, and TSKIN dataloggers were replaced every 7 d between 0800 and 0900 h. On d 22, between 0800 and 1200 h, animals were killed by an overdose of sodium pentobarbital and a terminal blood sample was collected via cardiac puncture. Serum was stored at −20 C until hormone assays were performed.
Statistical analysis
Statistical tests were performed using SigmaPlot (Systat Software, San Jose, CA) software. Mean TSKIN was calculated using data from a 6-h block of time in the light and dark phases. Averages from each rat were used to generate group averages. Statistical comparisons for experiment 1 were made using two-way ANOVA (estrous cycle vs. light-dark cycle) and Tukey’s post hoc test with α = 0.05.
Statistical comparisons for experiment 2 were made by two-way ANOVA (time vs. hormone treatment) with repeated measures followed by Tukey’s post hoc test with α = 0.05. Values for d −3 to −1 were combined for baseline data (designated −1). The heat loss index, an indirect measure of skin vasodilatation, was calculated using the formula (5):
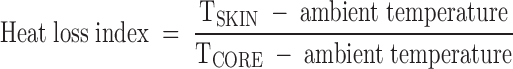 |
Because there were no significant differences between OVX animals receiving saline or sesame oil capsules, data for these animals were pooled (n = 10). Data from d 3 to 7 were used to evaluate ultradian rhythms using Fourier analysis and circadian physiology software (22).
Hormone assays
Assays were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Serum E2 concentrations were measured using a RIA (Diagnostic Systems Laboratories, Inc. ultrasensitive E2 rat-RIA) with a sensitivity of 1.5 pg/ml and intraassay coefficient of variation of 5.8. Progesterone levels were determined using RIA (sensitive progesterone-RIA) with a sensitivity of 0.05 ng/ml and an intraassay coefficient of variation of 4.7.
Results
Experiment 1, the estrous cycle is characterized by reduced TSKIN and TSKIN variability on proestrous night
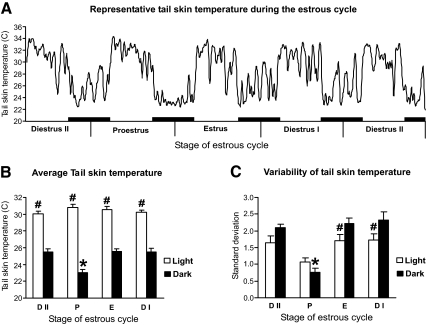
A prominent circadian rhythm of TSKIN was seen during all phases of the estrous cycle. Spontaneous large-amplitude fluctuations were observed, as well as fluctuations associated with vaginal smearing or other animal handling. In addition, there was a distinct infradian pattern, characterized by low TSKIN with decreased fluctuations during the dark phase of proestrous evening/estrous morning (Fig. 2). For simplicity, this period will be referred to as proestrous night. Analysis revealed the average TSKIN (Fig. 2B) and the average TSKIN variability (Fig. 2C) on proestrous night to be significantly lower than all other nights of the cycle.
Figure 2.
TSKIN during the rat estrous cycle. A, Recording of an intact rat shows circadian rhythms and decreased TSKIN on proestrous night. The black bars represent the dark phase. The graph is generated using a moving average of five data points. B, In addition to the circadian rhythms throughout the cycle, the average TSKIN is significantly reduced during proestrous night, compared with the dark phase of other stages of the cycle. C, The average TSKIN variability (measured by the sd) is also significantly reduced on proestrous night. The averages (±sem) in B and C were calculated from a 6-h period in the light (0900–1500 h) or dark (2100–0300 h) phases (n = 6 cycles). D II, Diestrous day II; P, proestrus; E, estrus; D I, diestrous day I. *, Significantly different from dark phases of other stages of the cycle; #, significantly different from dark phase within each stage of the cycle.
Experiment 2, treatment of OVX rats with E2 reduces TSKIN during the dark phase of the light-dark cycle
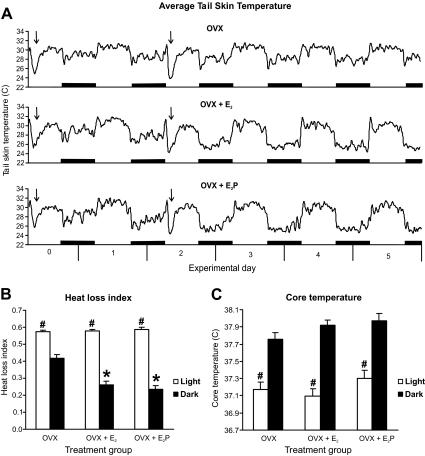
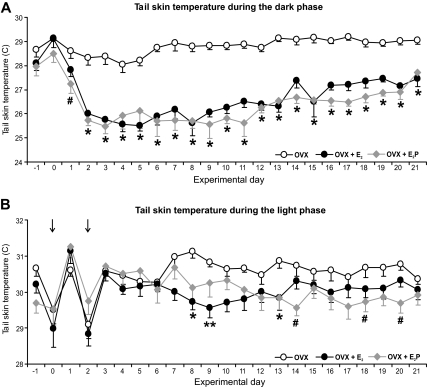
All treatment groups displayed circadian rhythms of TSKIN (Fig. 3). Additionally, E2 treatment of OVX rats resulted in decreased TSKIN during the dark phase (Figs. 3 and 4). The heat loss index was also significantly reduced in both hormone-treated groups, compared with OVX (Fig. 3B). The reduction of TSKIN in the E2P-treated rats was first significant on the dark phase of d 1 (≈35 h after capsule implantation), with a maximal effect achieved by the dark phase of d 2 in both groups (Fig. 4A). The effects of hormone-treatment on the heat loss index were similar to the effects on TSKIN over the 21 d of recording (data not shown). In contrast, ovarian steroid treatment did not significantly affect TSKIN during the light phase until experimental d 8 (Fig. 4B). Thereafter, E2 produced a mild suppression of TSKIN during the light phase that was only significantly different from OVX rats on a few occasions. There were no significant differences between OVX + E2 and OVX + E2P groups in any parameter. The average TSKIN variability was similar across treatment groups for both the light and dark phases (data not shown).
Figure 3.
Effects of E2 or E2P on TSKIN in OVX rats. A, Average TSKIN recordings in OVX (top), OVX + E2 (middle), and OVX +E2P (bottom) from experimental d 0 through experimental d 5. Circadian rhythms in T SKIN were detected in all groups. In the dark phase of experimental d 2–5, TSKIN was lower in both OVX + E2 and OVX + E2P rats compared with OVX rats. The black bars represent the dark phase, and arrows mark the time of capsule implantation. The graph is generated using a moving average of five data points. B, Compared with the OVX group, the average heat loss index was significantly reduced in OVX + E2 and OVX + E2P animals during the dark phase. C, Circadian rhythms of TCORE were present in all groups, but there was no significant effect of hormone treatment. The averages (±sem) in B and C are calculated from a 6-h period in the light (1000–1600 h) or dark (2200–0400 h) on experimental d 3. *, Significantly different from the dark phase of OVX rats; #, significantly different from the dark phase within each treatment group.
Figure 4.
Time course of the effects of E2 or E2P on TSKIN in OVX rats. A, In the dark phase, E2 treatment of OVX rats significantly reduced TSKIN from experimental d 2 through the end of the experiment. In E2P-treated rats, this effect was first significant on experimental d 1. B, In the light phase, E2 or E2P-treated rats exhibited lower TSKIN staring on experimental d 7, but this change was only significant on a few occasions. The acute drop in TSKIN during the light phase of d 0 and 2 (arrows) can be attributed to capsule implantation. There were no significant differences between OVX + E2 and OVX + E2P groups in any parameter. The averages (±sem) are calculated from a 6-h period in the light (1000–1600 h) or dark (2200–0400 h). *, Significantly different in OVX + E2 and OVX + E2P rats compared with OVX; **, significantly different in OVX + E2 rats compared with OVX; #, significantly different in OVX + E2P rats compared with OVX.
TCORE also displayed prominent circadian rhythms, but there were no differences between groups in average TCORE (Fig. 3C). Fourier analysis of both TCORE and TSKIN rhythms revealed no significant ultradian rhythms across treatment group. Body weights were not significantly different among treatment groups on the day of ovariectomy (224.9 ± 3.7 g, n = 26). On d 22, OVX rats (306.4 ± 5.5 g, n = 10) weighed significantly more than OVX + E2 and OVX + E2P rats (245.7 ± 3.7 and 248.1 ± 3.4 g, respectively; n = 8/group).
Pilot studies of an identical treatment regimen showed that on experimental d 3, OVX + E2 rats (n = 4) had serum E2 levels of 14.2 ± 0.7 pg/ml, similar to levels reported in intact animals on diestrous d 2 (23,24). On experimental d 3, serum progesterone of OVX + E2P rats (n = 4) was 10.4 ± 0.5 ng/ml, similar to levels reported for intact animals on diestrous d 1 (24). On d 22, serum E2 levels of the OVX + E2 (7.9 ± 0.5 pg/ml) and OVX + E2P groups (8.4 ± 0.4 pg/ml) were still higher than OVX rats (3.46 ± 0.29 pg/ml). On d 22, serum progesterone in OVX + E2P animals (6.8 + 2.0 ng/ml) was not significantly different from the OVX (5.0 + 0.9 ng/ml) or OVX + E2 (6.3 + 1.9 ng/ml) groups. A similar decline in hormone release from SILASTIC capsules after a 3-wk implantation period has been described (25).
Discussion
In the present study, TSKIN was monitored using a SubCue Mini datalogger mounted on the surface of the tail. This method was noninvasive, cost-effective, and did not require restraints or tethering. Moreover, there was no visible damage to the tail after prolonged use. The datalogger recorded circadian rhythms and a wide range of TSKIN from 23 to 32 C. We also observed spontaneous fluctuations in TSKIN characteristic of normal thermoregulation (5), as well as large fluctuations secondary to animal handling, such as vaginal smearing. Of note, the effects of E2 on TSKIN in OVX rats during the dark phase were similar in magnitude to that recorded by surgically implanted telemetry devices (9,12) and to temperatures recorded in the light phase using thermocouples (13). Simultaneous recording of TSKIN and TCORE could be accomplished by a datalogger mounted on the tail’s surface and a second datalogger implanted in the peritoneal cavity. This technology is readily accessible to most laboratories.
Here, we provide the first description of changes in TSKIN during the estrous cycle of the rat. Accompanying a noticeable circadian rhythm was a distinct infradian rhythm characterized by low TSKIN and low TSKIN variability during proestrous night. In our second experiment, we observed that E2 produced a pronounced decrease in TSKIN during the dark phase 2–21 d after capsule implantation, consistent with previous reports (9,12,15). The TSKIN of animals receiving E2 was not significantly different from those receiving E2P. These data suggest that the lowering of TSKIN during proestrous night is a result of increased secretion of estrogens.
On the night of proestrus, the TSKIN approached ambient temperatures and exhibited decreased fluctuations with low variability. Moreover, the TSKIN and heat loss index during the dark phase was low in E2-treated OVX rats. These parameters are characteristic of tail skin vasoconstriction, a thermoregulatory mechanism activated in subneutral ambient temperatures to reduce heat loss to the environment (5). Thus, rats with elevated levels of E2 (endogenous or exogenous) exhibited the vasomotor responses that would be expected in animals exposed to a subneutral environment. We have previously shown that E2 treatment of OVX rats shifted the thermoneutral zone to higher ambient temperatures (13). The present results are consistent with this shift in the thermoneutral zone, because the E2-treated OVX rats exhibited more vasoconstriction than OVX rats in the relatively cool ambient temperatures of the animal facility.
In the present study, ovarian steroids were administered in SILASTIC capsules. Similar protocols showed E2 capsules to initially produce negative feedback effects followed by positive feedback effects observed as daily LH surges beginning in the afternoon of d 2 (19,20). The addition of progesterone capsules on the morning of d 2 amplifies the LH surge that afternoon, and then abolishes the surge on the following days (18,19,20). Interestingly, there was no difference in the TSKIN between the OVX + E2 and OVX + E2P-treated rats. These data are consistent with a previous study showing that two different concentrations of progesterone (without estrogen pretreatment) had no effect on TSKIN in OVX rats (26). Moreover, implantation of an identical dose of progesterone was sufficient to amplify the LH surge in previous studies (19,20). Although these findings provide evidence that the vasoconstriction on proestrous night is not secondary to increased progesterone secretion, the level of progesterone achieved by our capsules was lower that reported in proestrous animals (24). Therefore, further experiments, including a dose-response curve, would be necessary to exclude progesterone as a contributing factor to the changes in tail TSKIN on the night of proestrus.
By d 22, the body weights of the OVX animals were significantly increased, compared with both hormone-replaced groups. However, changes in body weight do not account for the E2-induced vasoconstriction during the dark phase. First, TSKIN changes were observed as early as the dark phase of d 2, before significant differences in body weight can be detected (Dacks, P. A., and N. E. Rance, unpublished data). Second, OVX rats continued to gain weight over the course of the experiment but maintained stable TSKIN. Finally, in intact cycling rats, the vasoconstriction observed on proestrous night occurs every 4–5 d, at a time frame which could not be explained by significant weight changes. Thus, it is unlikely that the lower body weight of E2-treated rats is a major factor producing tail vasoconstriction during the dark phase. The lower TSKIN in E2-treated rats also cannot be explained by changes in TCORE, because there were no differences in TCORE between the OVX and hormone-treated groups.
In summary, we describe a simple, cost-effective method for recording TSKIN in unrestrained animals that allowed the first characterization of changes in TSKIN over the rat estrous cycle. A distinctive pattern of TSKIN was identified, characterized by vasoconstriction of tail skin blood vessels during proestrous night. A similar reduction in TSKIN was observed in OVX rats treated with E2 capsules, with no additional effect of progesterone treatment. The TSKIN recording technique could greatly facilitate future studies of the effects of estrogens on the thermoregulatory axis. Understanding the basic biology of estrogens’ effects on thermoregulation will be necessary for the development of physiological models of the menopausal flush (27,28).
Acknowledgments
We thank Dr. Christopher Gordon for his expert assistance in setting up the method for recording TSKIN in our laboratory; and Dr. Patricia Hoyer, Dr. Janet Funk, Dr. Nathaniel McMullen, Sally Krajewski, Melinda Smith, Marina Cholanian, and David L. Williams for critically reading the manuscript.
Footnotes
This work was supported by the National Institutes of Health (NIH) National Institute on Aging Grant R01 AG-032315 and the Arizona Biomedical Research Commission. Hormone assays were performed by the Ligand Assay and Analysis Core at the University of Virginia Center for Research in Reproduction supported by The National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934. P.A.D. was supported by a NIH National Institutes on Aging Predoctoral Training Fellowship 1F31-AG030881.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
Abbreviations: E2, 17β-Estradiol; E2P, E2 plus progesterone; OVX, ovariectomized; TCORE, core temperature; TSKIN, skin temperature.
References
- Molnar GW 1975 Body temperatures during menopausal hot flashes. J Appl Physiol 38:499–503 [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA 1984 Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas 6:31–43 [DOI] [PubMed] [Google Scholar]
- Freedman RR 2001 Physiology of hot flashes. Am J Human Biol 13:453–464 [DOI] [PubMed] [Google Scholar]
- Sturdee DW 2008 The menopausal hot flush—anything new? Maturitas 60:42–49 [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP 2002 Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92:2667–2679 [DOI] [PubMed] [Google Scholar]
- Gordon CJ 1990 Thermal biology of the laboratory rat. Physiol Behav 47:963–991 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tamura M, Hayashi M, Katsuura Y, Tanabe H, Ohta T, Komoriya K 2000 Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol 278:R863–R869 [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen XM, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K 2001 Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Reg Integr Comp Physiol 280:R1341–R1347 [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Weekers AHJ, Kloosterboer HJ 2001 Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol 419:47–54 [DOI] [PubMed] [Google Scholar]
- Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A 2004 Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas 48:463–471 [DOI] [PubMed] [Google Scholar]
- Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC 2004 Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res 1028:191–202 [DOI] [PubMed] [Google Scholar]
- Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O'Byrne K 2006 The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol 191:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Rance NE 2010 Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology 151:1187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opas EE, Scafonas A, Nantermet PV, Wilkening RR, Birzin ET, Wilkinson H, Colwell LF, Schaeffer JM, Towler DA, Rodan GA, Schmidt A 2009 Control of rat tail skin temperature regulation by estrogen receptor-β selective ligand. Maturitas 64:46–51 [DOI] [PubMed] [Google Scholar]
- Deecher DC, Alfinito PD, Leventhal L, Cosmi S, Johnston GH, Merchenthaler I, Winneker R 2007 Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endocrinology 148:1376–1383 [DOI] [PubMed] [Google Scholar]
- Cosmi S, Pawlyk AC, Alfinito PD, Roman J, Zhou T, Deecher DC 2009 Simultaneous telemetric monitoring of tail-skin and core body temperature in a rat model of thermoregulatory dysfunction. J Neurosci Meth 178:270–275 [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Puckett E, Padnos B 2002 Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J Pharmacol Toxicol Methods 47:107–114 [DOI] [PubMed] [Google Scholar]
- Rance N, Wise PM, Barraclough CA 1981 Negative feedback effects of progesterone correlated with changes in hypothalamic norepinephrine and dopamine turnover rates, median eminence luteinizing hormone-releasing hormone, and peripheral plasma gonadotropins. Endocrinology 108:2194–2199 [DOI] [PubMed] [Google Scholar]
- Wise PM, Rance N, Barraclough CA 1981 Effects of estradiol and progesterone on catecholamine turnover rates in discrete hypothalamic regions in ovariectomized rats. Endocrinology 108:2186–2193 [DOI] [PubMed] [Google Scholar]
- Petersen SL, McCrone S, Keller M, Shores S 1995 Effects of estrogen and progesterone on luteininzing hormone-releasing homone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology 136:3604–3610 [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL 2007 The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B 80:84–97 [DOI] [PubMed] [Google Scholar]
- Refinetti R 2006 Circadian physiology. 2nd ed. Boca Raton, FL: CRC Press/Taylor and Francis Group [Google Scholar]
- Ström JO, Theodorsson A, Theodorsson E 2008 Substantial discrepancies in 17β-oestradiol concentrations obtained with three different commercial direct radioimmunoassay kits in rat sera. Scand J Clin Lab Invest 68:806–813 [DOI] [PubMed] [Google Scholar]
- Freeman ME 2006 Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM, eds. Knobil and Neill’s physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 2327–2388 [Google Scholar]
- Ström JO, Theodorsson E, Theodorsson A 2008 Order of magnitude differences between methods for maintaining physiological 17β-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest 68:814–822 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tamura M, Hayashi M, Itano Y, Tanabe H, Katsuura Y, Ohta T, Komoriya K 2000 Effects of progesterone on the elevation of tail skin temperature in ovariectomized rats. Jpn J Physiol 50:651–656 [DOI] [PubMed] [Google Scholar]
- Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF 2002 Hot flushes. Lancet 360:1851–1861 [DOI] [PubMed] [Google Scholar]
- Miller HG, Li RM 2004 Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc 79:777–781 [DOI] [PubMed] [Google Scholar]