Abstract
In adult male rats, androgens are necessary for the maintenance of the motoneurons and their target muscles of the sexually dimorphic, steroid-sensitive spinal nucleus of the bulbocavernosus (SNB) neuromuscular system, regulating motoneuron and muscle morphology, function, and expression of trophic factors. Castration of males results in somal, dendritic, and muscle atrophy as well as increases in brain-derived neurotrophic factor (BDNF) in the target musculature. Because BDNF can have either facilitative or inhibitory effects in other systems, we examined SNB neuromuscular morphology after BDNF blockade using a fusion protein (tyrosine kinase receptor type B IgG). Blockade of BDNF in gonadally intact males resulted in hypertrophy of SNB motoneuron dendrites and target musculature, suggesting that normal levels of BDNF are inhibitory in SNB neuromuscular system. BDNF blockade in castrated males prevented SNB motoneuron atrophy and attenuated target muscle weight loss. This is the first demonstration that the highly androgen-sensitive SNB motoneuron dendrites and target muscles can be maintained in the absence of gonadal hormones and, furthermore, that blocking BDNF can have trophic effects on skeletal muscle. These results suggest that whereas BDNF is involved in the signaling cascade mediating the androgenic support of SNB neuromuscular morphology, its action can be inhibitory. Furthermore, the elevations in BDNF after castration may be responsible for the castration-induced atrophy in SNB motoneurons and target muscles, and the trophic effects of androgens may be mediated in part through a suppression of BDNF. These results may have relevance to therapeutic approaches to the treatment of neurodegenerative disease or myopathies.
Using a highly androgen-sensitive neuromuscular system, we demonstrate that brain-derived neurotrophic factor blockade can prevent or reverse castration-induced atrophy in spinal motoneurons and their target musculature.
The dendritic arbors of spinal motoneurons are extensive, spanning several spinal segments and displaying elaborate branching patterns. Not only are the dendritic arbors the major site for synaptic contact, but they are also highly plastic and their shape is dynamically regulated by a combination of molecular and activity dependent cues (1,2,3). The morphology of these dendritic arbors determines the type and amount of synaptic inputs spinal motoneurons receive (4,5,6). As a consequence, changes in dendritic morphology have profound implications for the functional properties of motoneurons and the behaviors they mediate (7,8,9,10). Thus, understanding the mechanisms involved in the maintenance and plasticity of motoneuron dendrites is of central importance.
The lumbar spinal cord of the rat contains a sexually dimorphic nucleus, the spinal nucleus of the bulbocavernosus [SNB; also known as the dorsomedial nucleus (11)]. In male rats, the SNB consists of approximately 200 motoneurons that innervate the perineal muscle complex consisting of the bulbocavernosus (BC) and levator ani (LA; collectively, BC/LA) as well as the external anal sphincter (11,12,13). The BC/LA muscles attach to the penis and are essential for successful copulation and insemination (14,15). The SNB neuromuscular system is highly sensitive to gonadal hormones and many features of the SNB are regulated by androgens both during development and in adulthood. For example, androgens masculinize SNB motoneuron number (16) and soma size (17,18,19) and are critical to perineal muscle retention (20) and neuromuscular synapse elimination (21,22) during development. In adulthood, androgens regulate a variety of characteristics of the SNB neuromuscular system. For example, castration of males causes a reduction in the penile reflexes mediated by SNB-innervated musculature (23,24), and testosterone replacement prevents (23) or reverses (15,23) this reduction. In addition, testosterone regulates SNB soma size (25,26,27), dendritic length (27,28), the number and size of gap junction plaques (29,30,31), and the expression of a variety of proteins [e.g. ciliary neurotrophic factor-α (32), N-cadherin (33), calcitonin gene related peptide (34), androgen receptors (35)], and mRNA [e.g. β-actin (36), β-tubulin (37), gap junction protein connexin 32 (36), calcitonin gene-related peptide (34)]. The hormonal effects on SNB motoneuron somata and dendrites, as well as target muscle weight, are specific to androgens; blockade of estrogen synthesis or treatment of castrated males with estradiol are ineffective (26,27). Peripherally, testosterone maintains several characteristics of SNB-innervated musculature including muscle weight (38), muscle fiber diameter (39), neuromuscular junction size (40,41), and acetylcholine receptor number (40,42).
Trophic factors also play an important role in the maintenance of neuronal morphology in adulthood. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of neurotrophic factors and binds with high affinity to the tyrosine kinase receptor type B (trkB). BDNF is expressed throughout the body, including in the central (43) and peripheral nervous system (44,45) and skeletal muscles (46,47,48). Initially, it was believed that BDNF is produced in limited quantities in target tissues and retrogradely transported back to the central nervous system in which it exerts a variety of effects (49). Indeed, BDNF is retrogradely transported from skeletal muscle to motoneurons (50,51). However, BDNF is also produced centrally and released in an autocrine and/or paracrine manner within neuronal populations (2,52,53), including spinal motoneurons (54). The actions of BDNF in central nervous system have traditionally been thought of as facilitative, promoting growth or survival of neurons (55,56,57). For example, BDNF supports neuron survival (58,59,60), somal area (61), axonal outgrowth (52,62,63,64), and dendritic branching (1,65,66,67) in vitro and in vivo. More recently, however, antipodal actions of BDNF on neuron survival and morphology have been described in other systems. For example, BDNF can have deleterious effects on motoneurons in vitro by rendering them vulnerable to excitotoxic insult (68,69,70) and on dendritic morphology in vitro (1,71) and in vivo (52).
BDNF mRNA and protein are expressed in SNB motoneurons (46,72), and BDNF protein is also present in the BC target musculature (46,73). We have recently demonstrated that androgens differentially regulate BDNF protein in SNB motoneurons and BC muscle (46). For example, in adult male rats, castration decreases BDNF protein in SNB motoneurons but significantly increases BDNF protein in the BC musculature. Castration of adult male rats results in atrophy of SNB dendritic arbors (27,28,74), and previous reports suggest that this regulation of SNB dendritic morphology occurs via the BC target musculature (75). Given the increase in BDNF levels in the BC musculature after castration (46) and the inhibitory effects of BDNF on dendritic morphology demonstrated in other systems (1,52,71), we hypothesized that BDNF blockade, using a fusion protein for BDNF, would protect SNB neuromuscular morphology after castration in adult male rats.
Materials and Methods
Animals
Adult male Sprague Dawley rats (∼100 d old; Harlan, Indianapolis, IN) were maintained on a 12-h light, 12-h dark cycle, with unlimited access to food and water. To assess the role of BDNF blockade on the maintenance of adult SNB neuromuscular morphology, we used a fusion protein for BDNF (trkB IgG; R & D Systems, Minneapolis, MN). trkB IgG is constructed from the extracellular domain of the trkB receptor linked to the Fc tail of human IgG. The trkB receptor is the primary receptor for BDNF but does show low-affinity binding to other neurotrophins (nerve growth factor, neurotrophin-3). trkB IgG binds to BDNF with the same affinity and specificity as the endogenous trkB receptor (59,76) and thus effectively acts as a competitive antagonist to trkB receptors. Recombinant human IgG1Fc (IgG; R & D Systems) was used as a control for trkB IgG treatment.
To assess the effects of BDNF blockade in normal adults, two groups of gonadally intact animals were anesthetized with isoflurane. Alzet mini-osmotic pumps (model 2004; Durect Corp., Cupertino, CA) filled with either trkB IgG (n = 6) or IgG (n = 6) were sc implanted in the interscapular region. trkB IgG or IgG was dissolved in PBS containing 0.1% BSA (0.42 μg/μl) to achieve a dosage of 2.5 μg/d for 3 wk. This dosage of trkB IgG has been previously shown to inhibit androgenic rescue of SNB motoneurons during development (59). A group of age-matched, normal males was also included (n = 6). To assess the effects of BDNF blockade after castration, a group of males was anesthetized with isoflurane, gonadectomized, and immediately implanted sc, in the interscapular region, with trkB IgG-filled mini-osmotic pumps (n = 7); a second group of castrated males was left untreated (n = 6). All procedures were carried out in accordance with the Indiana University Animal Care and Use Guidelines.
Histochemistry
Horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP; List Biological, Campbell, CA) was used to retrogradely label SNB motoneurons innervating the BC muscle. Previous studies have demonstrated that BHRP labeling permits sensitive detection and quantitative analysis of SNB somal and dendritic morphologies (18,28,77). SNB motoneuron morphology was examined after 3 wk of treatment. Animals were anesthetized with isoflurane, and the left BC muscle was exposed and injected with BHRP (0.5 μl, 0.2% solution). Forty-eight hours after BHRP injection, a period that ensures optimal labeling of SNB motoneurons (18,28), animals were overdosed with urethane (0.25 g per 100 g body weight) and perfused intracardially with saline followed by cold 1% paraformaldehyde/1.25% glutaraldehyde. Lumbar spinal segments were removed, postfixed in the same solution for 5 h, and then transferred to sucrose phosphate buffer [10% (wt/vol), pH 7.4] overnight for cryoprotection. Spinal cord segments were then embedded in gelatin and frozen sectioned transversely at 40 μm; all sections were collected into four alternate series. For visualization of BHRP, the tissue was immediately reacted using a modified tetramethyl benzidine protocol (78), mounted on gelatin-coated slides, and counterstained with thionin. BC/LA muscles were removed at perfusion and weighed to evaluate potential treatment effects on gross muscle weight. Residual volume of trkB IgG or IgG solutions was measured to confirm proper functioning of mini-osmotic pumps.
Motoneuron somata
The number of BHRP-filled motoneurons was assessed in all sections through the entire rostrocaudal extent of the SNB for all animals. Counts of labeled motoneurons in the SNB were made under bright-field illumination, in which somata and nuclei could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed. Estimates of the total number of labeled SNB motoneurons were based on unbiased counts obtained using the optical dissector method as previously described (27). Counts were made at ×500 under bright-field illumination, and motoneuron somata could be easily visualized in multiple focal planes. BHRP-labeled motoneurons were counted as their somata first appeared in focus while focusing through the z-axis and labeled somata in the first focal plane (i.e. tops) were not counted. For each animal, counts were derived from sections spaced at 160-μm intervals uniformly distributed through the entire rostrocaudal extent of the SNB. Within each section, all labeled somata within the SNB were counted. Estimates of the total number of labeled SNB motoneurons were then obtained by correcting for the percentage of the tissue sampled. All counts were performed blind to experimental condition.
The cross-sectional soma area of BHRP-labeled motoneurons was measured in an average of 27.3 motoneurons for each animal using a video-based morphometry system (Stereo Investigator; MBF Bioscience, Inc., Williston, VT) at a final magnification of ×1350. Soma areas within each animal were averaged for statistical analysis. The OD of labeled somata was also measured under bright-field illumination to confirm the equivalence of BHRP labeling density.
Dendritic length
For each animal, dendritic lengths in a single representative set of alternate sections were measured under dark-field illumination. Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the SNB dendritic field was assessed in every other section (320 μm apart) in three dimensions using a computer-based morphometry system (Neurolucida; MBF Bioscience; final magnification ×250) to yield both composite illustrations of the arbor and measurements of individual fiber lengths. All BHRP-labeled fibers were drawn regardless of location, size, or contiguity with labeled cell bodies to ensure a complete assessment of dendritic length. Because the entire rostrocaudal range of the SNB dendritic field in each animal was sampled, this method allows for a complete assessment of SNB dendrites in both the transverse and horizontal planes. Average dendritic arbor per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by 2 to correct for sampling, and then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons (79) but has been shown to be a sensitive and reliable indicator of changes in dendritic morphology during normal development (18), after hormonal or surgical manipulation (18,28,77,79,80,81,82,83,84) due to dendritic interactions (85) or after N-methyl-d-aspartate receptor blockade (86,87).
To assess potential redistributions of dendrites across treatment groups, for each animal the composite dendritic arbor created in the length analysis was divided using a set of axes radially oriented around the central canal, dividing the spinal cord into 12 bins of 30° each. The portion of each animal’s dendritic arbor per labeled motoneuron contained within each location was then determined.
Dendritic extent
The comparability of BHRP labeling across groups was assessed by quantifying both the rostrocaudal and radial dendritic extent of SNB arbors. The rostrocaudal extent of the dendritic arbor was determined by recording the distance between the first and last section in which labeled SNB dendrites were present for each animal. In the transverse plane, for each animal the maximal radial extent of the dendritic arbor for each section throughout the rostrocaudal extent of the SNB dendritic field was measured using the same set of axes and 30° bins used for the dendritic distribution analysis. For each bin, the distance between the central canal and the most distal BHRP-filled process was measured.
Statistics
Statistical analysis consisted of analyses of variance (one or two way with repeated measures) followed by appropriate planned comparisons [Fisher’s protected least significant difference (LSD)] as described below. Digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Co., Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop (San Jose, CA).
Results
BC/LA muscle weight
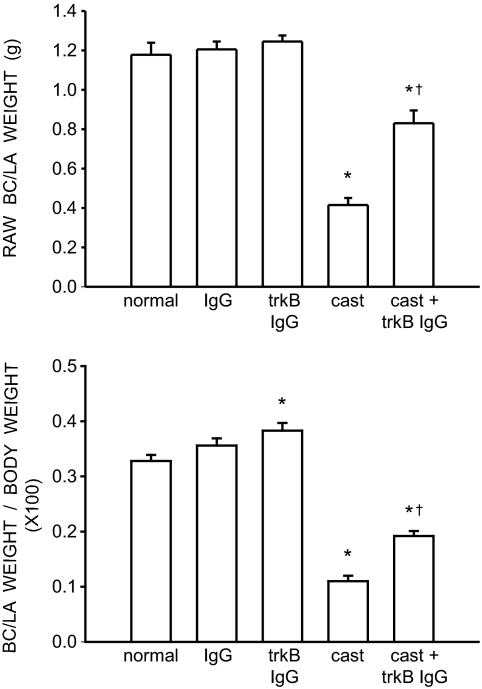
The weights of BC/LA musculature showed a significant effect of group [F (4,26) = 48.49, P < 0.0001; Fig. 1, top panel]. The weight of the BC/LA muscles in normal males (1.18 ± 0.061 g), IgG-treated males (1.21 ± 0.040 g), and trkB IgG-treated males (1.25 ± 0.031 g) was typical and did not differ across groups (LSDs, all P = n.s.). However, the weight of BC/LA muscles in castrates (0.42 ± 0.036 g) was 64% less than that of normal males (LSD, P < 0.0001). The BC/LA muscle weights in castrates treated with trkB IgG (0.83 ± 0.065 g) were increased 100% relative to those of castrated males (LSDs, P < 0.0001) but were still 30% less than BC/LA muscle weights in normal males (LSD, P < 0.0001).
Figure 1.
Weights of BC/LA muscles expressed as raw weights (top panel) and relative to individual body weights (bottom panel) of normal, IgG-treated, trkB IgG-treated, castrated, and trkB IgG-treated castrated males. Bar heights represent means ± sem for six to seven animals per group. *, Significantly different from normal males; †, significantly different from castrated males.
To determine whether the altered BC/LA muscle weights reflected a general somatic effect of treatment, we assessed overall body weights across groups and found a significant effect of treatment [F (4,26) = 12.58, P < 0.0001]. Body weights in normal males (358.00 ± 9.99 g), IgG-treated males (339.00 ± 4.83 g), and trkB IgG-treated males (326.00 ± 8.274 g) did not differ across groups (LSDs, all P = n.s.). However, the body weight of castrates treated with trkB IgG were on average 18.5% greater than all other treatment groups (LSDs, P < 0.01). In addition, body weights of untreated castrates were 15% greater than those of trkB IgG-treated males (LSD, P < 0.02).
After correcting for the differences in body weight (Fig. 1, bottom panel), differences in BC/LA muscle weight remained [F (4,26) = 107.51, P < 0.0001]. Whereas corrected BC/LA muscle weights in normal and IgG-treated males were still not different from each other (LSD, P = n.s.), trkB IgG-treated males had muscle weights that were 17% greater than those of normal males (LSD, P < 0.003). However, corrected BC/LA muscle weights in trkB IgG-treated males and IgG-treated males still did not differ from each other (LSD, P = n.s.). Similarly, corrected muscle weights in castrates continued to be significantly decreased relative to those of normal males (LSD, P < 0.0001), and muscle weights in trkB IgG-treated castrates were still greater than those of castrated males (LSD, P < 0.0001). In addition, corrected BC/LA muscle weights in trkB IgG-treated castrates were still significantly less than that of normal males (LSD, P < 0.0001).
Morphometry
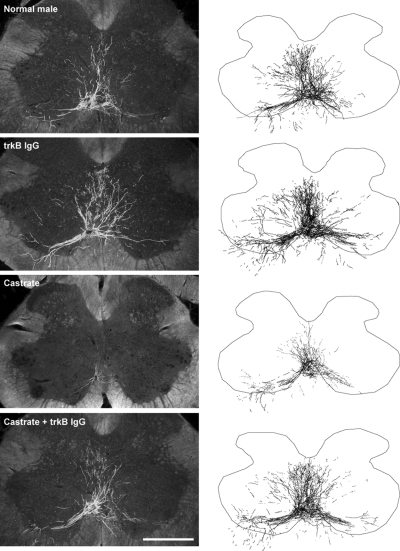
Injection of BHRP into the left BC successfully labeled ipsilateral SNB motoneurons of all animals (72.00 ± 5.06 per animal) in a manner consistent with previous studies (27,28,74,88). SNB motoneurons displayed their characteristic multipolar morphologies, with dendritic arbors projecting ventrolaterally and dorsomedially and across the midline into the area of the contralateral SNB (Fig. 2).
Figure 2.
Left panels, Dark-field photomicrographs of transverse sections through the lumbar spinal cord of normal (top panel), trkB IgG-treated (middle and upper panels), castrated (middle and lower panels), and trkB IgG-treated castrated (bottom panel) males after BHRP injection into the left BC muscle. Scale bar, 500 μm. Right panels, Computer-generated composites of BHRP-labeled SNB somata and processes drawn at 320-μm intervals through the entire rostrocaudal extent of the SNB; these composites were selected because they are representative of their respective group average dendritic lengths.
Soma area
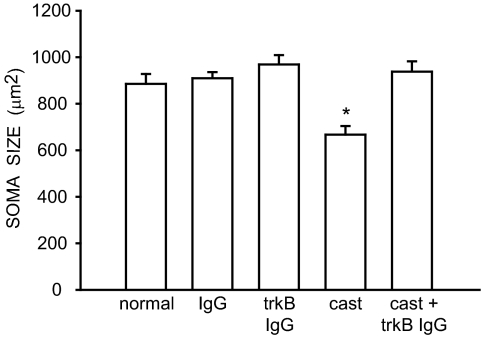
The size of SNB somata differed across groups [F (4,26) = 9.167, P < 0.0001; Fig. 3]. The mean cross-sectional area of SNB somata in normal males (884.41 ± 42.43 μm2), IgG-treated males (908.44 ± 26.23 μm2), trkB IgG-treated males (967.72 ± 40.10 μm2), and trkB IgG-treated castrated (936.90 ± 44.31 μm2) males were typical and did not differ from one another (LSDs, all P = n.s.). However, the SNB somata in castrates (665.97 ± 37.10 μm2) were, on average, 28% smaller than those of all other treatment groups (LSDs, P < 0.0007).
Figure 3.
Soma areas of SNB motoneurons in normal, IgG-treated, trkB IgG-treated, castrated, and trkB IgG-treated castrated males. Bar heights represent means ± sem for six to seven animals per group. *, Significantly different from normal males.
Dendritic length
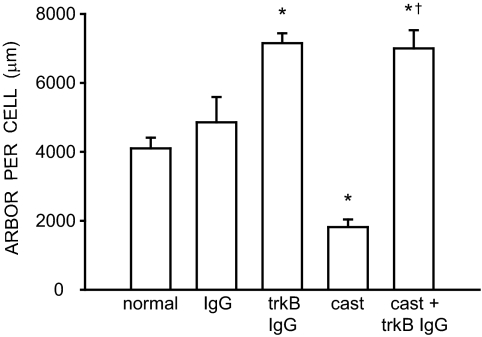
The overall length of SNB dendrites differed across groups [F (4,26) = 23.56, P < 0.0001; Fig. 4]. SNB dendritic lengths in normal (4089.80 ± 253.55 μm) and IgG-treated males (4856.11 ± 733.65 μm) did not differ from each other (LSD, P = n.s.). However, SNB dendritic length in trkB IgG-treated males (7151.97 ± 283.90 μm) was 75% larger than that of normal males (LSD, P < 0.0001). SNB dendritic length in castrates (1814.87 ± 223.64 μm) was typical and 56% smaller than those of normal males (LSD, P < 0.002). Interestingly, dendritic length in trkB IgG-treated castrates (6999.72 ± 525.07 μm) was 285% larger than that of castrated males (LSD, P < 0.0001) and did not differ from trkB IgG-treated males (LSD, P = n.s.).
Figure 4.
Dendritic lengths expressed as length of arbor per labeled motoneuron in normal, IgG-treated, trkB IgG-treated, castrated, and trkB IgG-treated castrated males. Bar heights represent means ± sem for six to seven animals per group. *, Significantly different from normal males; †, significantly different from castrated males.
Dendritic distribution
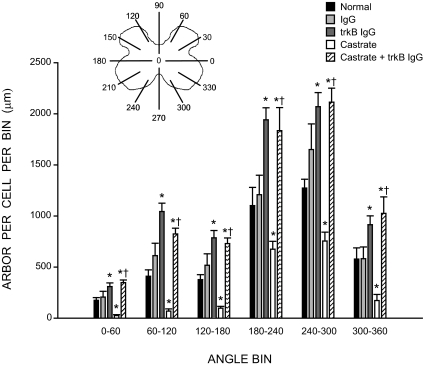
As previously noted (85), the SNB dendritic arbor of normal males is radially organized but not uniformly distributed, with greater than 50% of the arbor concentrated ventrolaterally between 180° and 300° (Fig. 5). The distribution of SNB dendrites showed the typical significant effects of location [F (11,286) = 144.67, P < 0.0001] as well as a main effect of group [F (4,286) = 24.33, P < 0.0001]. In intact males, trkB IgG treatment increased dendritic length at all locations, ranging from 58 to 154% per bin [trkB IgG treated males compared with normal males; F (1,110) = 78.13, P < 0.0001]. On the other hand, castration of normal males decreased dendritic length at all locations, ranging from 39 to 85% per bin [untreated castrates compared with normal males; F (1,110) = 44.80, P < 0.0001], whereas treatment of castrates with trkB IgG increased dendritic length across all radial bins, ranging from 172 to 1275% per bin [trkB IgG treated castrates compared with untreated castrates; F (1,121) = 73.12, P < 0.0001]. Castrates treated with trkB IgG showed the same hypertrophy at all locations relative to normal males [F (1, 121) = 24.67, P < 0.0004] and did not differ from those seen in intact, trkB IgG-treated males [F (1, 121) = 0.09, P = n.s.].
Figure 5.
Inset, Schematic drawing of spinal gray matter divided into radial sectors for measure of SNB dendritic distribution. Length per radial bin of SNB dendrites in normal, IgG-treated, trkB IgG-treated, castrated, and trkB IgG-treated castrated males; for graphical purposes length per radial bin measures have been collapsed into six bins of 60° each. Bar heights represent means ± sem for six to seven animals per group. *, Significantly different from normal males; †, significantly different from castrated males.
Dendritic extent
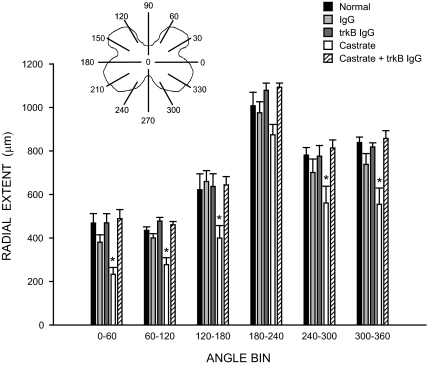
As with dendritic length, the radial dendritic extent of SNB motoneurons was also nonuniformly distributed (Fig. 6), and repeated-measures ANOVA showed a significant effect of location [F (11,275) = 110.41, P < 0.0001]. There was also a significant effect of group on radial dendritic extent [F (4,25) = 12.42, P < 0.0001]. This effect on radial extent was due to decreases in untreated castrates [F (1,99) = 38.49, P < 0.0002]; radial extents in all other groups did not differ [F (3,231) = 1.43, P = n.s.]. Furthermore, the distance spanned by SNB dendrites throughout the rostrocaudal axis did not differ across treatment groups [F (4,26) = 0.08, P = n.s.; normal males = 3173.33 ± 177.29 μm, IgG-treated males = 3360.00 ± 160.00 μm, trkB IgG-treated males = 3386.67 ± 76.36 μm, castrates = 2906.67 ± 76.36 μm, trkB IgG-treated castrates = 3360.00 ± 130.64 μm].
Figure 6.
Inset, Schematic drawing of spinal gray matter divided into radial sectors for measure of SNB radial extent. Radial extents of SNB dendrites in normal, IgG-treated, trkB IgG-treated, castrated, and trkB IgG-treated castrated males; for graphical purposes dendritic extent measures have been collapsed into six bins of 60° each. Bar heights represent means ± sem for six to seven animals per group. *, Significantly different from normal males.
Discussion
In the present study, blockade or interruption of BDNF signaling using the fusion protein trkB IgG resulted in hypertrophy of BC/LA musculature and SNB dendritic arbors in gonadally intact animals; SNB soma size was unaffected. Treatment of gonadally intact animals with the IgG vehicle did not affect BC/LA musculature or SNB dendritic morphology; thus, we believe the changes we observed in these structures were specifically due to trkB IgG treatment. These results suggest that normal levels of BDNF may have inhibitory effects on the SNB neuromuscular system. In agreement with previous findings (25,28,38), castration resulted in atrophy of SNB somata and dendritic arbors as well as decreases in BC/LA muscle weight. Interestingly, after castration BDNF levels are sharply increased in the BC muscle (46), a proposed site of action for the regulation of BC muscle weight and SNB dendritic morphology (75,89). Blocking or interrupting BDNF signaling in castrated adult male rats prevented or reversed the castration-induced atrophy observed in SNB motoneuron somata and dendrites and attenuated decreases in BC/LA muscle weight. These data demonstrate that various elements of the SNB neuromuscular system can be maintained in the absence of androgens. In addition, these results also represent the first demonstration to our knowledge that blocking or interfering with BDNF signaling can have trophic effects on skeletal muscle.
trkB IgG effects in gonadally intact animals
Treatment of gonadally intact males with trkB IgG resulted in hypertrophy of BC/LA muscle weight. The expression of BDNF and its receptor trkB in skeletal muscle tissue has been well documented (46,47,50) and was originally thought to serve as a source of retrograde signaling to spinal motoneurons (50,51). More recently the role of neurotrophins in the development and maintenance of muscle tissue under both normal and pathological conditions has been elucidated (90,91). Although the exact mechanism for BDNF’s influence on skeletal muscle remains unclear, it has been suggested that BDNF plays an important role in the regulation of muscle homeostasis (90). Our current data are in agreement with these findings and support the idea that BDNF is involved in the maintenance of skeletal muscle in adulthood, albeit in an inhibitory manner.
Similarly, treatment of gonadally intact males with trkB IgG resulted in hypertrophy of dendritic arbors. Several studies have demonstrated that BDNF can have inhibitory effects on dendritic morphology in other neuronal populations. For example, BDNF inhibits dendritic growth in developing layer VI cortical neurons (1), decreases dendritic complexity in retinal ganglion cells (52), and decreases dendritic length in neurons of the nucleus tractus solitarius (71). Although it is not known whether direct application or overexpression of BDNF in SNB motoneurons would result in decreased dendritic arbor, in the current study, blockade or interruption of BDNF signaling did result in hypertrophied dendritic arbors, suggesting that BDNF may be acting in an inhibitory manner.
On the other hand, the size of SNB somata was not affected by trkB IgG treatment, demonstrating that under normal hormonal conditions, interruption of BDNF signaling does not affect SNB somal morphology. Previous work from our laboratory has demonstrated that the different elements of the SNB neuromuscular system can respond differentially, depending on the experimental manipulation. For example, treatment of adult castrates with dihydrotestosterone fully supports SNB dendritic length but only partially supports somal size (27). As described above, castration decreases BDNF protein in SNB motoneurons but significantly increases BDNF protein in the BC musculature (46). Thus, the current results may reflect differences in the responsivity of BC muscles and SNB somata and dendrites to interruptions in BDNF signaling.
trkB IgG effects after castration
Previous reports have demonstrated that the maintenance of BC/LA weight and SNB dendritic morphology in adulthood is dependent on the action of androgens, specifically testosterone (26,27). Castration of adult males resulted in a significant reduction in BC/LA muscle weight, and this decrease was attenuated by trkB IgG treatment. However, trkB IgG treatment did not maintain BC muscle weight to that of gonadally intact males, suggesting that whereas BDNF contributes to the regulation of BC/LA muscle weight, other potentially androgen-sensitive factors are likely involved.
Similarly, SNB soma size was protected from castration-induced atrophy by trkB IgG treatment. It has been suggested that the site of action for the maintenance of SNB soma size is the motoneurons themselves (75,92). Given that BDNF is present at multiple, androgen-sensitive sites (i.e. motoneurons and target muscles) in the SNB neuromuscular system, it is possible that BDNF is part of a general signaling cascade mediating androgenic effects. Alternatively, the maintenance of SNB soma size with trkB IgG after castration could reflect the requirements of larger somata necessary to support large dendritic arbors. Further studies will be needed to address the molecular mechanisms involved in the androgenic regulation of SNB soma size.
Treatment of castrated males with trkB IgG completely prevented the typical castration-induced atrophy of SNB dendrites and in fact resulted in dendritic hypertrophy similar to that seen in trkB IgG-treated intact males. This protection from castration-induced atrophy by blockade or interruption of BDNF signaling is consistent with the hypothesis that BDNF can act in an inhibitory manner (see above text). Interestingly, BDNF has previously been shown to support SNB somata (93) and dendritic morphology (94) after axotomy. However, axotomy is known to change the trophic factor responsivity of neurons (95,96), and the beneficial effects of the BDNF application to the cut SNB axons may reflect this change. In addition, it appears that there are different signaling pathways mediating the castration- and axotomy-induced decreases in SNB morphology because the effects of axotomy on SNB motoneurons are not equivalent to those of castration (93).
Previous studies have demonstrated that neither axonal transport of BHRP (97) nor dendritic transport as demonstrated by the rostrocaudal or transverse extent of dendritic labeling (79) is affected by hormone levels. Thus, the decreases in extent we observed after castration likely reflect decreases in overall dendritic length. The possibility that BDNF blockade could affect retrograde transport is also an important consideration because such an artifact could potentially result in apparent increases in dendritic length. However, no differences in either the rostrocaudal or radial extents of dendrites were observed in any of the trkB IgG or IgG groups, indicating that the ability of SNB dendrites to transport BHRP out to the most distal, highest-order branches was not affected. Moreover, there were no differences in the labeling density of SNB motoneurons between any groups (data not shown). Thus, we believe the dendritic labeling across groups was comparable, allowing direct comparisons of dendritic length and distribution across groups.
Potential mechanisms for BDNF regulation of SNB dendritic morphology
Together these data suggest that BDNF may be part of a signaling cascade mediating the androgenic support of BC/LA muscle weight and dendritic length in adulthood. It has been suggested that androgenic regulation of BC/LA weight and SNB dendritic morphology occurs locally at the target muscles (75,89). If BDNF is indeed acting in an inhibitory manner on BC/LA muscles and SNB dendrites, these data support the idea that increased levels of BDNF in the SNB target musculature after castration may be responsible for the castration-induced atrophy in the SNB neuromuscular system. Furthermore, prevention of castration-induced atrophy of the SNB system with androgen treatment could result through the maintenance of BDNF at gonadally intact male levels in the target musculature (46).
Recently an increasingly complex role for neurotrophins and their receptors in shaping neuronal morphologies has emerged (3,57,98). Neurotrophins were previously considered to serve in survival or growth-promoting roles (55,56,57,99). This view has shifted, and it is now recognized that the role of neurotrophins in shaping neuronal morphologies is far more highly coordinated and dynamic, dependent on the actions of several molecular and activity-dependent cues that are cell type specific (1,2,3). For example, BDNF limits dendritic growth in layer VI cortical neurons, whereas NT-3 promotes dendritic growth (1). On the other hand, BDNF promotes the dendritic growth of layer IV cortical neurons, whereas NT-3 inhibits it (1). Similarly, the biological actions of neurotrophins are also regulated by cleavage from their pro-form to mature form (3,100) and can exert opposing effects, depending on whether the low-affinity p75 receptor or the high-affinity trk receptors are activated (100,101). These opposing actions of neurotrophins on neuronal morphologies have been described as yin and yang (3) or push and pull (1) and serve to underscore the complexity of neurotrophin action in the regulation of dendritic morphology. It is possible that similar dynamic processes underlie the regulation of SNB dendritic morphology. By blocking or interrupting the potential inhibitory effects of BDNF signaling in SNB motoneurons, we may have allowed counteractive processes to predominate, resulting in the expansion of dendritic arbors.
In addition, BDNF can be released from neurons or their targets in an autocrine and/or paracrine manner (50,54), and depending on the source of BDNF, its influence on neuronal morphology can have varying effects. For example, retinally derived BDNF decreases retinal ganglion cell dendritic arborization, whereas BDNF derived from the tectum increases retinal ganglion cell dendritic arborization (52,53). Similarly, BDNF released in an autocrine manner from layer II/III pyramidal neurons significantly increased the number of basilar dendrites and decreased spine density (2). On the other hand, BDNF released in a paracrine manner from layer II/III cortical neurons increased dendritic branching in nearby neurons in a highly localized manner that was dependent on proximity to the BDNF source (2,65). Given that BDNF is produced in both SNB motoneurons (46,72) and the BC target musculature (46), it is possible that BDNF can act differentially in the SNB neuromuscular system, depending on its source. Because we used a systemic treatment of trkB IgG, we cannot definitively say where the blockade or interruption of BDNF signaling occurred. It is possible that our current results are due to a blockade or interruption either at the motoneuron itself and/ or the BC muscles. Alternatively, the effects we observed could be due to decreases in peripheral BDNF, resulting in an indirect effect on the SNB motoneurons and target muscles. Similarly, treatment with trkB IgG in gonadally intact animals could potentially have altered circulating levels of testosterone or GH, resulting in the hypertrophies in SNB dendrites or BC muscle weight. However, given that the hypertrophies were observed in castrated males, this explanation is at the very least not parsimonious because treatment-induced increases in gonadal testosterone would not be possible after castration, and there is no evidence that GH effects motoneuron morphology.
BDNF has been shown to activate calmodulin-dependent protein kinase II (CamKII) via stimulation of phospholipase C (102), and intracellular signaling involving CamKII is thought to limit the growth of dendritic arbors in spinal motoneurons (103). It is possible that blocking or interrupting BDNF signaling alters this CamKII pathway, allowing for the elongation of SNB dendritic arbor. Alternatively, it has been hypothesized that SNB dendritic arbors may be stabilized via an N-methyl-d-aspartate -stimulated, BDNF activation of trkB (72). However, previous reports have also demonstrated that BDNF-induced activation of trkB can render motoneurons vulnerable to glutamate excitotoxicity (69), and this effect can be reduced by inhibiting downstream mediators or transactivation pathways of trkB activation (69,70). If similar excitotoxic effects of trkB activation are involved in the regulation of SNB dendrites after castration, it is possible we have diminished this excitotoxic vulnerability and prevented or reversed the typical castration-induced atrophy of SNB dendrites. Clearly, further studies will be needed to address the molecular mechanisms involved in the maintenance of SNB dendritic morphology in adulthood.
Conclusion
The present study demonstrates that blockade or interruption of BDNF signaling can have trophic effects on several morphological features of the SNB neuromuscular system. These findings represent the first demonstration that the highly androgen-sensitive SNB motoneuron dendrites and target muscles can be maintained in the absence of gonadal hormones. Furthermore, these trophic effects were observed in gonadally intact as well as gonadectomized animals, suggesting that BDNF may act in an inhibitory manner in the SNB system in adulthood, regardless of hormonal condition. Preventing or attenuating the castration-induced atrophy in the SNB neuromuscular system via BDNF blockade suggests that BDNF is part of the signaling cascade mediating the androgenic support of SNB neuromuscular morphology in adulthood.
The current results may have relevance to therapeutic approaches in the treatment of neurodegenerative diseases or other human myopathies because previous reports have suggested that abnormal expression of trophic factors and/or their receptors may play a role. For example, BDNF concentrations are elevated in human biceps muscles of patients with amyotrophic lateral sclerosis, a progressive motoneuron disease (47). After the castration of adult males, we observed a similar increase in BDNF concentration in the BC musculature (46) and a concomitant atrophy in SNB neuronal morphology (27,28). In the current study, we were able to prevent or reverse this castration-induced atrophy in SNB motoneurons by treating animals with trkB IgG, suggesting that blockade of the excess BDNF production may be neuroprotective. It is possible that such a therapeutic strategy may be useful in treating degenerative neuromuscular diseases in which abnormal expression of trophic factors is a suspected cause. Indeed, BDNF has been shown to render motoneurons vulnerable to excitotoxic insult through activation of the trkB receptor (69,70), and furthermore, clinical tests using neurotrophic factors in a therapeutic role have met with only mixed results (70,104).
Acknowledgments
We thank Dr. Cara Wellman for helpful comments on this manuscript.
Footnotes
This work was supported by National Institutes of Health-National Institute of Neurological Disorders and Stroke Grant NS047264 to D.R.S.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
Abbreviations: BC, Bulbocavernosus; BDNF, brain-derived neurotrophic factor; BHRP, horseradish peroxidase conjugated to the cholera toxin B subunit; CamKII, calmodulin-dependent protein kinase II; LA, levator ani; LSD, least significant difference; SNB, spinal nucleus of the BC; trkB, tyrosine kinase receptor type B.
References
- McAllister AK, Katz LC, Lo DC 1997 Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18:767–778 [DOI] [PubMed] [Google Scholar]
- Horch HW 2004 Local effects of BDNF on dendritic growth. Rev Neurosci 15:117–129 [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH 2005 The yin and yang of neurotrophin action. Nat Rev Neurosci 6:603–614 [DOI] [PubMed] [Google Scholar]
- Ueyama T, Arakawa H, Mizuno N 1987 Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol 177:37–49 [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Cullheim S 1988 Postnatal development of the cat hindlimb: II. In vivo morphology of dendritic growth cones and the maturation of dendritic morphology. J Comp Neurol 278:88–102 [DOI] [PubMed] [Google Scholar]
- Ulfhake B, CullHeim S, Franson P 1988 Postnatal development of cat hind limb motoneurons. I: Changes in length, branching structure, and spatial distribution of dendrites of cat triceps surae motoneurons. J Comp Neurol 278:69–87 [DOI] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ 1985 Quantitative analysis of the dendrites of cat phrenic motoneurons stained intracellularly with horseradish peroxidase. J Comp Neurol 231:91–101 [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE 1987 Membrane area and dendritic structure in type-identified triceps surae α motoneurons. J Comp Neurol 255:68–81 [DOI] [PubMed] [Google Scholar]
- Furicchia JV, Goshgarian HG 1987 Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol 96:621–634 [DOI] [PubMed] [Google Scholar]
- Ritz LA, Bailey SM, Murray CR, Sparkes ML 1992 Organizational and morphological features of cat sacrocaudal motoneurons. J Comp Neurol 318:209–221 [DOI] [PubMed] [Google Scholar]
- Schroder HD 1980 Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol 192:567–587 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1980 Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science 210:564–566 [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I 1986 Organization of the pudendal nerve in the male and female rat. J Comp Neurol 248:532–549 [DOI] [PubMed] [Google Scholar]
- Sachs BD 1982 Role of striated penile muscle in penile reflexes, copulation and induction of pregnancy in the rat. J Reprod Fertil 66:433–443 [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D'Hospital PY 1983 Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav 31:807–813 [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP 1985 Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science 229:671–673 [DOI] [PubMed] [Google Scholar]
- Lee JH, Jordan CL, Arnold AP 1989 Critical period for androgenic regulation of soma size of sexually dimorphic motoneurons in rat lumbar spinal cord. Neurosci Lett 98:79–84 [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR 1990 Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci 10:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR 1992 Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J Comp Neurol 326:147–157 [DOI] [PubMed] [Google Scholar]
- Cihák R, Gutman E, Hanzliková V 1970 Involution and hormone-induced persistence of the muscle sphincter (levator ani) in female rats. J Anat (Lond) 106:93–110 [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP 1989 The role of gonadal hormones in neuromuscular synapse elimination in rats. I. Androgen delays the loss of multiple innervation in the levator ani muscle. J Neurosci 9:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP 1989 The role of gonadal hormones in neuromuscular synapse elimination in rats. II. Multiple innervation persists in the adult levator ani muscle after juvenile androgen treatment. J Neurosci 9:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL 1967 Testosterone regulation of sexual reflexes in spinal male rats. Science 155:1283–1284 [DOI] [PubMed] [Google Scholar]
- Davidson JM, Stefanick ML, Sachs BD, Smith ER 1978 Role of androgen in sexual reflexes of the male rat. Physiol Behav 21:141–146 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1981 Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormonal manipulation, absence in androgen-insensitive rats. Brain Res 225:297–307 [DOI] [PubMed] [Google Scholar]
- Forger NG, Fishman RB, Breedlove SM 1992 Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav 26:204–213 [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Buckley KE, Sergent MA, Sengelaub DR 2010 Testosterone Metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol 70:206–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP 1986 Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science 232:395–398 [DOI] [PubMed] [Google Scholar]
- Leedy MG, Beatiie MS, Bresnahan JC 1987 Testosterone induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res 424:386–390 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi GA, Micevych PE 1988 Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci 8:4177–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Micevych PE, Arnold AP 1988 Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J Neurosci 8:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Wagner CK, Contois M, Bengston L, MacLennan AJ 1998 Ciliary neurotrophic factor receptor α in spinal motoneurons is regulated by gonadal hormones. J Neurosci 18:8720–8729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DA, Getsios S, MacCalman CD, Watson NV 2001 N-cadherin is regulated by gonadal steroids in adult sexually dimorphic spinal motoneurons. J Neurobiol 47:255–264 [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE 1990 Steroid regulation of calcitonin gene-related peptide mRNA expression in motoneurons of the spinal nucleus of the bulbocavernosus. Mol Brain Res 8:159–166 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Prins GS 1996 Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol 8:553–559 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S 1992 Effect of androgen on the expression of gap junction and B-actin mRNAs in adult rat motoneurons. Neurosci Res 14:133–144 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Hyodo S 1993 Androgenic regulation of expression of B-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J Neuroendocrinol 5:357–363 [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff G 1941 The effects of castration and testosterone propionate on the striated perineal musculature in the rat. Endocrinology 29:975–978 [Google Scholar]
- Venable JH 1966 Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat 119:271–301 [DOI] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson A 1989 Androgens modulate endplate size and Ach receptor density at synapses in rat levator ani muscle. J Neurobiol 20:189–202 [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Breedlove SM, Bernstein S, Lichtman JW 1990 Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci 10:2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson AL, Luine VN 1982 Testosterone increases acetylcholine receptor number in the “levator ani” muscle of the rat. J Neurobiol 13:153–161 [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA 1997 Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78:431–448 [DOI] [PubMed] [Google Scholar]
- Matsuoka I, Meyer M, Hofer M, Thoenen H 1991 Differential regulation of nerve growth factor and brain-derived neurotrophic factor expression in the peripheral nervous system. Ann NY Acad Sci 633:550–552 [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H 1995 Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res 42:21–33 [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR 2010 Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology 151:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küst BM, Copray JC, Brouwer N, Troost D, Boddeke HW 2002 Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol 177:419–427 [DOI] [PubMed] [Google Scholar]
- Mousavi K, Parry DJ, Jasmin BJ 2004 BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am J Physiol Cell Physiol 287:C22–C29 [DOI] [PubMed] [Google Scholar]
- Thoenen H 1991 The changing scene of neurotrophic factors. Trends Neurosci 14:165–170 [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL 1993 Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10:359–367 [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Friedman C, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ 1992 The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron 8:983–993 [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S 1999 Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci 19:9928–9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S 2002 Local and target derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci 22:7639–7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CR, Sebum KL, Cope TC 2000 Neurotrophin expression by spinal motoneurons in adult and developing rats. J Comp Neurol 416:309–318 [DOI] [PubMed] [Google Scholar]
- Snider WD 1994 Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627–638 [DOI] [PubMed] [Google Scholar]
- Pitts E, Potluri S, Hess D, Balice-Gordan R 2006 Neurotrophin and Trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin 44:21–76 [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC 1999 Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295–318 [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillips H, Rosenthal A 1993 Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 363:266–270 [DOI] [PubMed] [Google Scholar]
- Xu J, Gingras KM, Bengston L, Di Marco A, Forger NG 2001 Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons. J Neurosci 21:4366–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F 1999 BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron 22:53–62 [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA 2009 The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci 29:6461–6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE 1995 Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature 378:192–196 [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ 1995 Inhibition of ocular dominance column formation of NT-4/5 or BDNF. Science 267:1662–1666 [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE 2000 BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci 20:771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Katz LC 2002 BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci 5:1177–1184 [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC 1995 Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15:791–803 [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Fiumelli H, Martin J Role of trkB-mediated signaling pathways in the regulation of cortical dendritic development by BDNF. 39th Meeting of the Society for Neuroscience, Chicago, IL, 2009, 321.11 (Abstract) [Google Scholar]
- Fryer HJ, Wolf DH, Knox RJ, Strittmatter SM, Pennica D, O'Leary RM, Russell DS, Kalb RG 2000 Brain-derived neurotrophic factor induces excitotoxic sensitivity in cultured embryonic rat spinal motor neurons through activation of the phosphatidylinositol 3-kinase pathway. J Neurochem 74:582–595 [DOI] [PubMed] [Google Scholar]
- Hu P, Kalb RG 2003 BDNF heightens the sensitivity of motor neurons to excitotoxic insults through activation of trkB. J Neurochem 84:1421–1430 [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG 2006 Protecting motor neurons from toxic insult by antagonism of adenosine A2a and trk receptors. J Neurosci 26:9250–9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Balkoweic A BDNF reduces the dendritic length of tyrosine hydroxylase-immunoreactive neurons from the caudal nucleus tractus solitarius. 39th Meeting of the Society for Neuroscience, Chicago, IL, 2009, 467.410 (Abstract) [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM 2007 Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology 148:3655–3665 [DOI] [PubMed] [Google Scholar]
- Arnold A, Yang LY The bulbocavernosus and levator ani (BC/LA) muscle complex expresses BDNF protein. 29th Meeting of the Society for Neuroscience, Miami Beach, FL, 1999, pp 1269 (Abstract 507.6) [Google Scholar]
- Fargo KN, Sengelaub DR 2004 Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol 469:96–106 [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM 1995 Androgens alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci 15:4408–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ 1997 Blockade of endogenous ligands of the trkB inhibits formation of ocular dominance columns. Neuron 19:63–76 [DOI] [PubMed] [Google Scholar]
- Nowacek AS, Sengelaub DR 2006 Estrogenic support of motoneuron dendritic growth via the neuromuscular periphery in a sexually dimorphic motor system. J Neurobiol 66:962–976 [DOI] [PubMed] [Google Scholar]
- Mesulam N 1982 Tracing neural connections with horseradish peroxidase. Chichester, NY: John Wiley and Sons [Google Scholar]
- Kurz EM, Brewer RG, Sengelaub DR 1991 Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol 22:976–988 [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM 1987 Seasonal variation in striated muscle mass and motoneuron morphology. J Neurobiol 18:155–165 [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR 1994 Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol 25:878–892 [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Mills AC, Sengelaub DR 1996 Motoneuron development after deafferentation. I. Dorsal rhizotomy does not alter growth in the spinal nucleus of the bulboavernosus. Dev Brain Res 91:11–19 [DOI] [PubMed] [Google Scholar]
- Hays TC, Goldstein LA, Mills AC, Sengelaub DR 1996 Motoneuron development after deafferentation: II. Dorsal rhizotomy does not block estrogen-supported growth in the dorsolateral nucleus (DLN). Dev Brain Res 91:20–28 [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR 2003 Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: morphological changes and implications for estrogen sites of action. J Comp Neurol 467:80–96 [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Kalkbrenner AE, Sengelaub DR 1993 Changes in dendritic morphology of rat spinal motoneurons during development after unilateral target deletion. Dev Brain Res 73:151–163 [DOI] [PubMed] [Google Scholar]
- Kalb RG 1994 Regulation of motor neuron dendrite growth by NMDA receptor activation. Development 120:3063–3071 [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR 2002 N-methyl-d-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol 451:142–152 [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR 2004 Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J Neurobiol 60:348–359 [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM 1992 Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol 23:17–30 [DOI] [PubMed] [Google Scholar]
- Chevrel G, Hohlfeld R, Sendtner M 2006 The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve 33:462–476 [DOI] [PubMed] [Google Scholar]
- Angelucci F, Colantoni L 2010 Facioscapulohumeral muscular dystrophy: do neurotrophins play a role? Muscle Nerve 41:120–127 [DOI] [PubMed] [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM 2001 Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci 21:1062–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Arnold AP 2000 Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol 44:308–319 [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR 2004 Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology 145:161–168 [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Price DL, Clatterbuck RE 1994 Motor neurons in Onuf’s nucleus and its rat homologues express the p75 nerve growth factor receptor: sexual dimorphism and regulation by axotomy. J Comp Neurol 345:510–527 [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H 1993 Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M, Forger NG, Breedlove SM 1991 Does androgen affect axonal transport of cholera toxin HRP in spinal motoneurons? Neurosci Lett 126:199–202 [DOI] [PubMed] [Google Scholar]
- McAllister AK 2000 Cellular and molecular mechanisms of dendritic growth. Cereb Cortex 10:963–973 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R 1987 The nerve growth factor 35 years later. Science 237:1154–1162 [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL 2001 Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948 [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M 2005 The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 25:9989–9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet PR, Lamour Y 1997 Brain-derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J Biol Chem 272:24133–24136 [DOI] [PubMed] [Google Scholar]
- Metzger F 2010 Molecular and cellular control of dendrite maturation during brain development. Curr Mol Pharmacol 3:1–11 [DOI] [PubMed] [Google Scholar]
- The BDNF Study Group Phase III 1999 A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 52:1427–1433 [DOI] [PubMed] [Google Scholar]