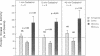Abstract
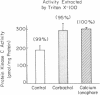
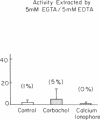
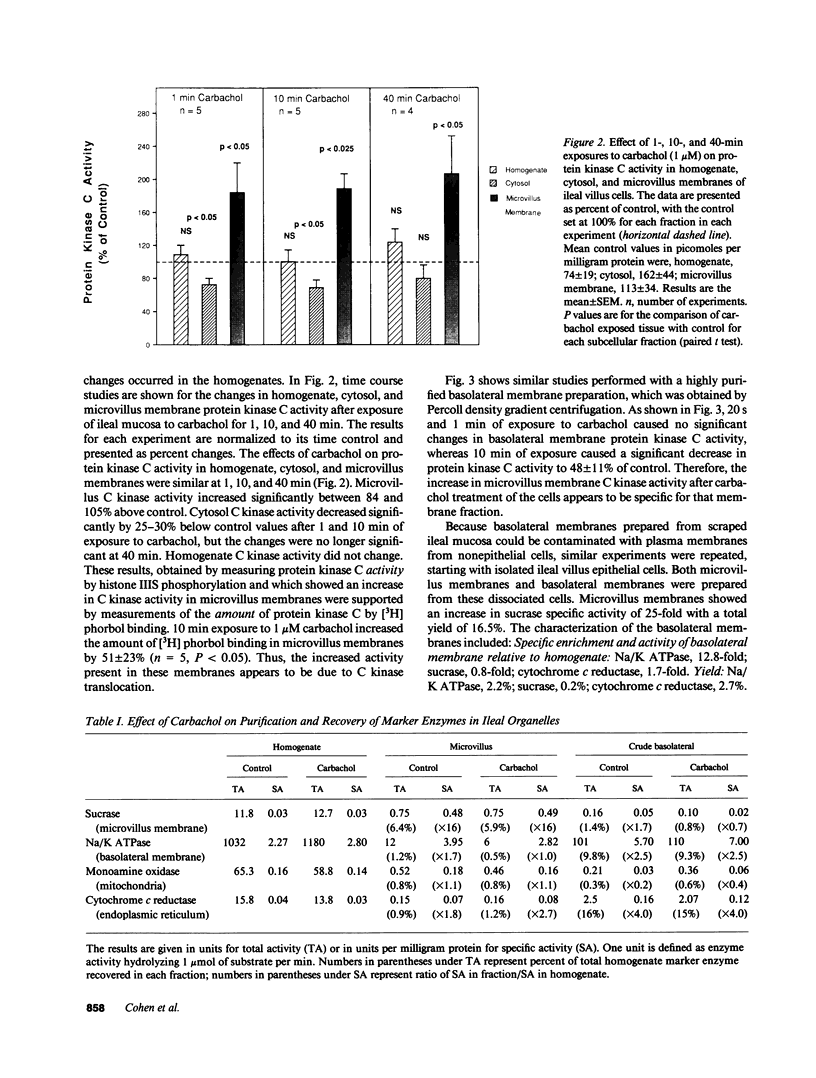
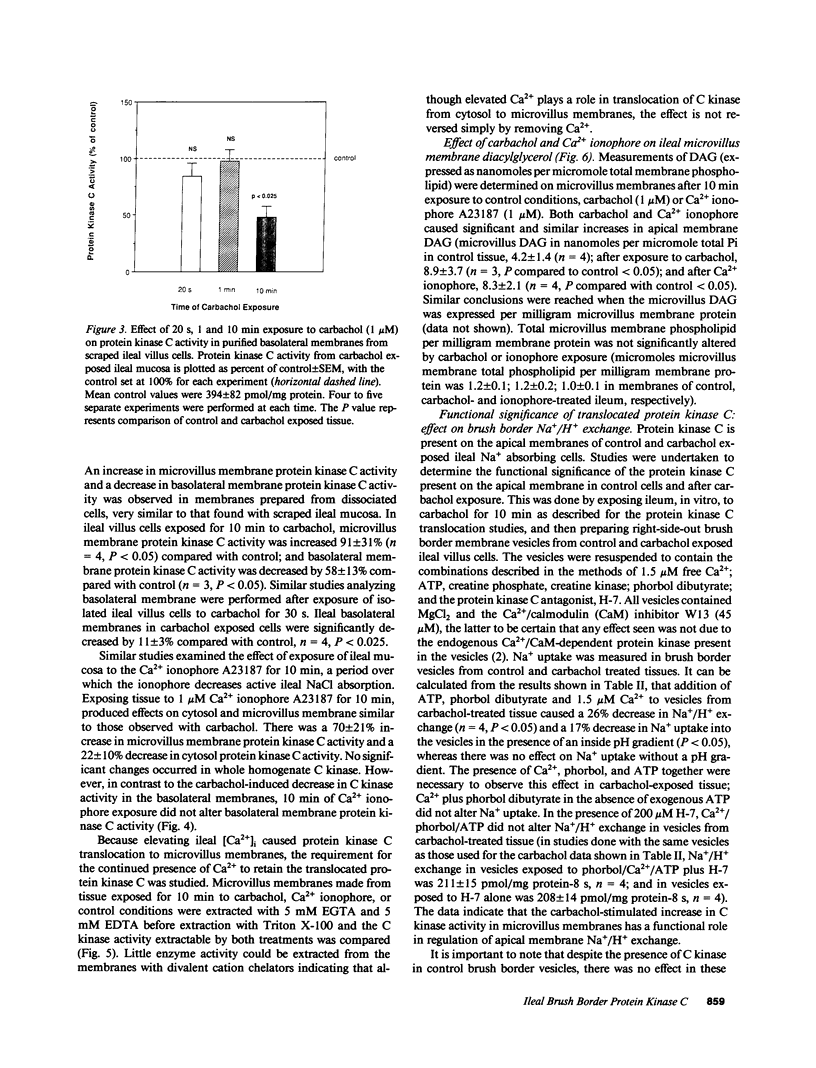
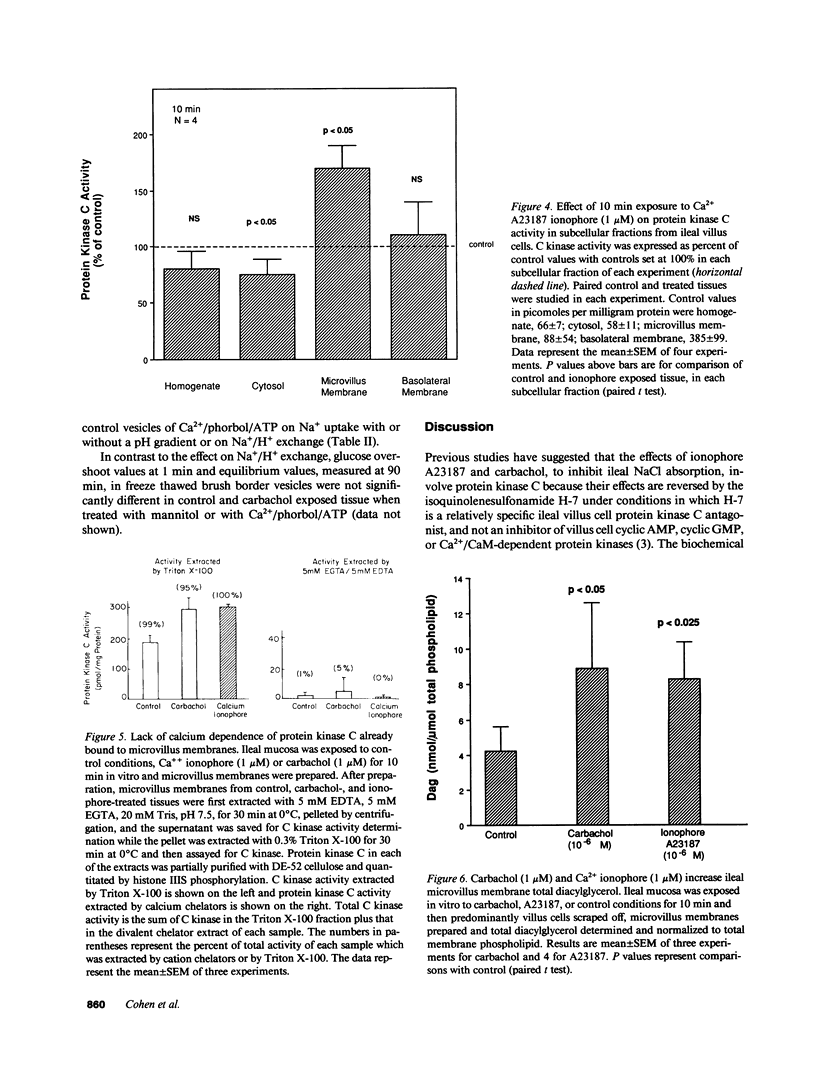
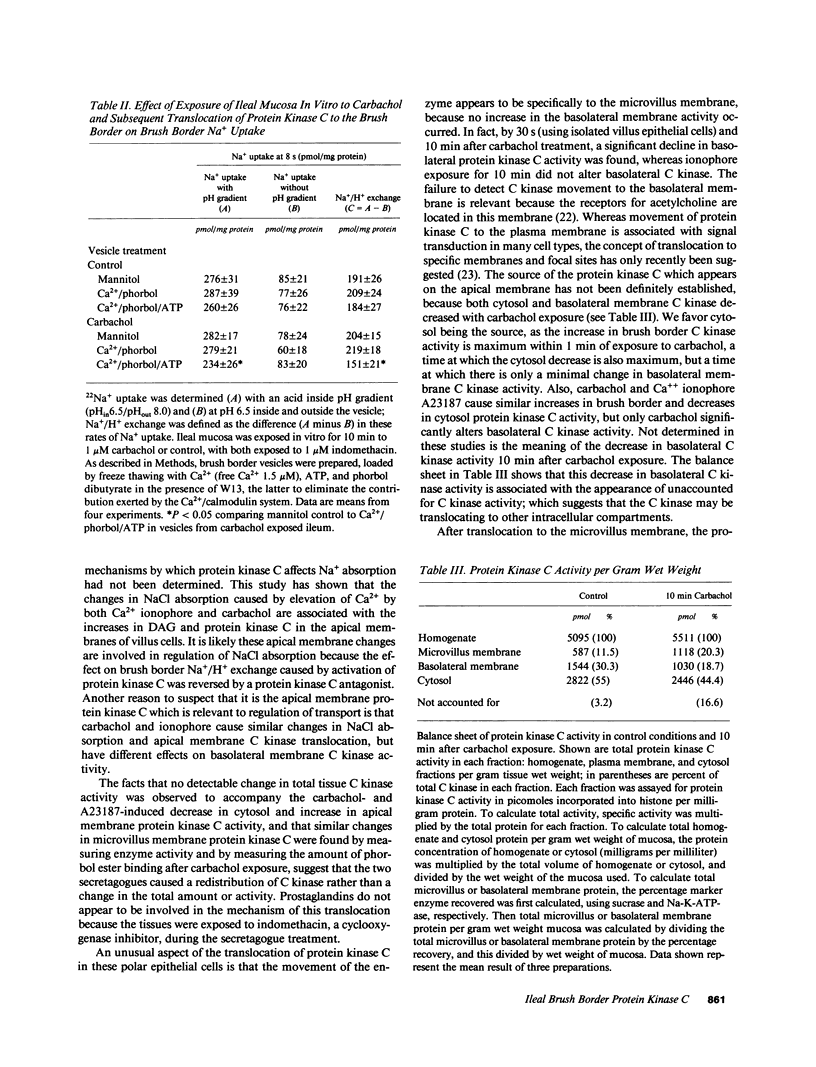
Protein kinase C is involved in mediating the effects of elevated Ca2+ in ileal villus Na+ absorbing cells to inhibit NaCl absorption. The present studies were undertaken to understand the mechanism by which this occurs. The effects of carbachol and the calcium ionophore A23187, agents which elevate intracellular Ca2+ and inhibit NaCl absorption in ileal villus cells, were studied. Carbachol treatment of villus cells caused a rapid decrease in protein kinase C activity in cytosol, with an accompanying increase in microvillus membrane C kinase. Exposure of the villus cells to calcium ionophore also caused a quantitatively similar decrease in cytosol C kinase and increase in C kinase activity in the microvillus membrane. This increase caused by carbachol and Ca2+ ionophore was specific for the microvillus membrane. In fact, 30 s and 10 min after exposure of the cells to carbachol, basolateral membrane protein kinase C decreased, in a time-dependent manner; whereas 10 min of Ca2+ ionophore exposure did not alter basolateral C kinase. Exposure of villus cells to Ca2+ ionophore or carbachol caused similar increases in microvillus membrane diacylglycerol content. As judged by the ability to inhibit Na+/H+ exchange measured in ileal villus cell brush border membrane vesicles, the protein kinase C which translocated to the microvillus membrane was functionally significant. Inhibition of Na+/H+ exchange required ATP and was reversed by the protein kinase C antagonist H-7. In conclusion, the effect of carbachol and Ca2+ ionophore in regulation of ileal NaCl absorption is associated with an increase in microvillus membrane diacylglycerol content and functionally active protein kinase C. The effects of both carbachol and Ca2+ ionophore are different on brush border and basolateral membrane distribution of protein kinase C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERS R. W., RODRIGUEZDE LORES, DEROBERTIS E. SODIUM-POTASSIUM-ACTIVATED ATPASE AND POTASSIUM-ACTIVATED P-NITROPHENYLPHOSPHATASE: A COMPARISON OF THEIR SUBCELLULAR LOCALIZATIONS IN RAT BRAIN. Proc Natl Acad Sci U S A. 1965 Mar;53:557–564. doi: 10.1073/pnas.53.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casavola V., Helmle-Kolb C., Murer H. Separate regulatory control of apical and basolateral Na+/H+ exchange in renal epithelial cells. Biochem Biophys Res Commun. 1989 Dec 15;165(2):833–837. doi: 10.1016/s0006-291x(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Wang N. S., Rao M. C. Phorbol ester stimulation of active anion secretion in intestine. Am J Physiol. 1985 Sep;249(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1985.249.3.C356. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cheng H. Y., Sharp G. W. Effects of phorbol esters on sodium and chloride transport in rat colon. Am J Physiol. 1986 Oct;251(4 Pt 1):G509–G517. doi: 10.1152/ajpgi.1986.251.4.G509. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cohen M. E., Gudewich R., Taylor L., Sharp G. W. Ca2+-calmodulin-, cyclic AMP- and cyclic GMP-induced phosphorylation of proteins in purified microvillus membranes of rabbit ileum. Biochem J. 1984 Apr 15;219(2):573–581. doi: 10.1042/bj2190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Emmer E., McCullen J., Reinlib L., Cohen M. E., Rood R. P., Madara J., Sharp G. W., Murer H., Malmstrom K. Freeze-thaw and high-voltage discharge allow macromolecule uptake into ileal brush-border vesicles. Am J Physiol. 1987 Jun;252(6 Pt 1):G723–G735. doi: 10.1152/ajpgi.1987.252.6.G723. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Halsey D. L., Girard P. R., Kuo J. F., Blackshear P. J. Protein kinase C in fibroblasts. Characteristics of its intracellular location during growth and after exposure to phorbol esters and other mitogens. J Biol Chem. 1987 Feb 15;262(5):2234–2243. [PubMed] [Google Scholar]
- Ho A. K., Thomas T. P., Chik C. L., Anderson W. B., Klein D. C. Protein kinase C: subcellular redistribution by increased Ca2+ influx. Evidence that Ca2+-dependent subcellular redistribution of protein kinase C is involved in potentiation of beta-adrenergic stimulation of pineal cAMP and cGMP by K+ and A23187. J Biol Chem. 1988 Jul 5;263(19):9292–9297. [PubMed] [Google Scholar]
- Kandel G., Donohue-Rolfe A., Donowitz M., Keusch G. T. Pathogenesis of Shigella diarrhea. XVI. Selective targetting of Shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J Clin Invest. 1989 Nov;84(5):1509–1517. doi: 10.1172/JCI114327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S. 1,2-Diacylglycerol, protein kinase C, and pancreatic enzyme secretion. J Biol Chem. 1986 Apr 5;261(10):4438–4444. [PubMed] [Google Scholar]
- Pandol S. J., Thomas M. W., Schoeffield M. S., Sachs G., Muallem S. Role of calcium in cholecystokinin-stimulated phosphoinositide breakdown in exocrine pancreas. Am J Physiol. 1985 May;248(5 Pt 1):G551–G560. doi: 10.1152/ajpgi.1985.248.5.G551. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Michetti M., Salamino F., Horecker B. L. Isozymes of protein kinase C in human neutrophils and their modification by two endogenous proteinases. J Biol Chem. 1990 Jan 15;265(2):706–712. [PubMed] [Google Scholar]
- Rood R. P., Emmer E., Wesolek J., McCullen J., Husain Z., Cohen M. E., Braithwaite R. S., Murer H., Sharp G. W., Donowitz M. Regulation of the rabbit ileal brush-border Na+/H+ exchanger by an ATP-requiring Ca++/calmodulin-mediated process. J Clin Invest. 1988 Sep;82(3):1091–1097. doi: 10.1172/JCI113665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalera V., Storelli C., Storelli-Joss C., Haase W., Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J. 1980 Jan 15;186(1):177–181. doi: 10.1042/bj1860177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper E. J., Powell D. W., Morris S. M. Cholinergic-adrenergic interactions on intestinal ion transport. Am J Physiol. 1978 Oct;235(4):E402–E409. doi: 10.1152/ajpendo.1978.235.4.E402. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]
- Velasco G., Iglesias C. F., Domínguez P., Barros F., Gascón S., Lazo P. S. Protein kinase C from small intestine epithelial cells. Biochem Biophys Res Commun. 1986 Sep 30;139(3):875–882. doi: 10.1016/s0006-291x(86)80259-5. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Weikel C. S., Sando J. J., Guerrant R. L. Stimulation of porcine jejunal ion secretion in vivo by protein kinase-C activators. J Clin Invest. 1985 Dec;76(6):2430–2435. doi: 10.1172/JCI112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman E. J., Dubinsky W. P., Fisher K., Steplock D., Dinh Q., Chang L., Shenolikar S. Regulation of reconstituted renal Na+/H+ exchanger by calcium-dependent protein kinases. J Membr Biol. 1988 Aug;103(3):237–244. doi: 10.1007/BF01993983. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Wooten M. W., Wrenn R. W. Redistribution of phospholipid/calcium-dependent protein kinase and altered phosphorylation of its soluble and particulate substrate proteins in phorbol ester-treated rat pancreatic acini. Cancer Res. 1985 Aug;45(8):3912–3917. [PubMed] [Google Scholar]