Abstract
Previous studies have shown that β-cell M3 muscarinic acetylcholine receptors (M3Rs) play a key role in maintaining blood glucose homeostasis by enhancing glucose-dependent insulin release. In this study, we tested the hypothesis that long-term, persistent activation of β-cell M3Rs can improve glucose tolerance and ameliorate the metabolic deficits associated with the consumption of a high-fat diet. To achieve the selective and persistent activation of β-cell M3Rs in vivo, we generated transgenic mice that expressed the Q490L mutant M3R in their pancreatic β-cells (β-M3-Q490L Tg mice). The Q490L point mutation is known to render the M3R constitutively active. The metabolic phenotypes of the transgenic mice were examined in several in vitro and in vivo metabolic tests. In the presence of 15 mm glucose and the absence of M3R ligands, isolated perifused islets prepared from β-M3-Q490L Tg mice released considerably more insulin than wild-type control islets. This effect could be completely blocked by incubation of the transgenic islets with atropine (10 μm), an inverse muscarinic agonist, indicating that the Q490L mutant M3R exhibited ligand-independent signaling (constitutive activity) in mouse β-cells. In vivo studies showed that β-M3-Q490L Tg mice displayed greatly improved glucose tolerance and increased serum insulin levels as well as resistance to diet-induced glucose intolerance and hyperglycemia. These results suggest that chronic activation of β-cell M3Rs may represent a useful approach to boost insulin output in the long-term treatment of type 2 diabetes.
Chronic activation of β-cell M3 muscarinic receptors may represent a useful approach to boost insulin output in the long-term treatment of type 2 diabetes.
One of the key pathological features of type 2 diabetes is that pancreatic β-cells fail to secrete sufficient amounts of insulin to overcome elevated blood glucose levels (1,2,3). The secretion of insulin from pancreatic β-cells is modulated by the activity of many neurotransmitters and hormones acting via binding to specific cell surface receptors (4,5,6,7). Several studies have shown that acetylcholine (ACh), the major neurotransmitter of peripheral parasympathetic nerves, exerts a pronounced stimulatory effect on pancreatic insulin release (5,6). Studies with isolated islets derived from M3 muscarinic receptor (M3R) knockout mice demonstrated that this stimulatory effect of ACh on insulin release is mediated by the M3R subtype (8,9), consistent with the outcome of classical pharmacological studies (10,11,12). The M3R is a prototypic class I G protein-coupled receptor, and ACh binding to this receptor subtype leads to the selective activation of G proteins of the Gq family (13).
In pancreatic β-cells, M3R-mediated activation of Gq-type G proteins initiates a series of signaling pathways that eventually enhance glucose (nutrient)-induced insulin secretion (5,6,7). The activated Gαq subunits stimulate the activity of different isoforms of phospholipase Cβ (PLCβ), resulting in the enzymatic breakdown of the membrane lipid phosphatidylinositol 4,5-bisphosphate and the generation of two second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (5,6,7). DAG activates protein kinase C, thus enhancing the effects of free cytosolic calcium on the exocytosis of insulin granules. IP3 triggers a rapid increase in intracellular calcium levels via release of calcium from endoplasmic reticulum storage sites, followed by a small capacitative calcium entry (6). It is well known that increased intracellular calcium levels, via a series of intermediate steps, efficiently activate the exocytotic machinery in pancreatic β-cells with high efficiency (5,6,7). It has also been demonstrated that stimulation of β-cell M3R can partially depolarize the plasma membrane via activation of a specific sodium channel, resulting in a more sustained increase in intracellular calcium levels after glucose (nutrient)-dependent membrane depolarization (6).
A recent study (14) involving the analysis of β-cell-specific M3R knockout mice suggested that approaches aimed at enhancing signaling through β-cell M3Rs could become therapeutically useful in the treatment of glucose intolerance and type 2 diabetes. However, it remains unclear at present whether the potential use of selective M3R agonists might offer therapeutic benefits and retain efficacy during the long-term treatment of type 2 diabetes (note that such agents are not available currently). As is the case with other G protein-coupled receptors (15,16,17), studies with cultured cells have shown that long-term agonist stimulation leads to M3R desensitization and down-regulation, thus causing greatly reduced cellular responses (18,19,20,21,22,23,24). The possibility therefore exists that these processes might compromise the potential therapeutic usefulness of chronic stimulation of β-cell M3Rs. To address this issue, we generated and analyzed transgenic mice that overexpressed the Q490L mutant M3Rs in their pancreatic β-cells (β-M3-Q490L Tg mice). Studies with cultured mammalian cells have shown that this mutant receptor containing the activating Q490L point mutation can efficiently activate G proteins of the Gq family even in the absence of activating ligands (25). We therefore speculated that expression of this constitutively active mutant M3R in β-cells of transgenic mice might mimic the physiological effects of chronic administration of an M3R agonist.
The β-M3-Q490L Tg mice, together with their wild-type (WT) control littermates, were subjected to a series of metabolic tests. The resulting data strongly support the novel concept that chronic activation of β-cell M3Rs may represent a useful strategy for the long-term treatment of type 2 diabetes.
Materials and Methods
Mouse maintenance and diet
Mice were housed in a specific pathogen-free barrier facility, maintained on a 12-h light, 12-h dark cycle. All experiments were carried out with male littermates maintained on a pure C57BL/6 background.
Mice were fed ad libitum with a standard mouse chow [4% (wt/wt) fat content; Zeigler, Gardners, PA]. To induce diet-induced obesity, hyperglycemia, and glucose intolerance, 5-wk-old male mice were put on a high-fat diet [35.5% (wt/wt) fat content, no. F3282; Bioserv, Frenchtown, NJ] for at least 8 wk. All experiments were carried out in a manner that complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Generation of transgenic mice
A transgene was constructed in which the expression of the constitutively active Q490L mutant M3R (species rat) was under the control of a 650-bp fragment of the rat insulin promoter II (RIP II) (26). A nine-amino-acid hemagglutinin epitope tag was added to the N terminus of the mutant receptor construct to better distinguish transgene-derived M3Rs from endogenous M3Rs. The transgene construct contained 3′-untranslated sequences derived from the human GH gene containing transcriptional termination, polyadenylation, and splicing signals (the RIP II vector used was kindly provided by Dr. Adolfo Garcia-Ocana, University of Pittsburgh, Pittsburgh, PA). The resulting 4.7-kb transgene was isolated, purified, and microinjected into the pronuclei of ova prepared from C57BL/6 mice (Taconic, Germantown, NY). Transgenic mice were identified by Southern blot analysis of BglII-digested mouse tail DNA, using a 1.36-kb BamHI-BglII fragment that comprises most of the 3′-untranslated human GH sequence as a probe. Following this protocol, we identified several founder mice that stably transmitted the transgene to their progeny. For all experiments, we used a mutant mouse line that expressed the Q490L mutant M3R at relatively low levels. Throughout the manuscript, we refer to these mice as β-M3-Q490L Tg mice.
Mouse genotyping studies
Mouse genotypes were determined via Southern blotting (see above) and/or PCR analysis. To confirm the presence of the M3R transgene in β-M3-Q490L Tg mice, tail DNA was amplified by the following PCR primers that anneal to the 3′-untranslated region of the transgene: 5′-CTACGGGCTGCTCTACTGCTTCAGG (forward) and 5′-GGCACTGGAGTGGCAACTTCCAAGG (reverse) (size of PCR product, 171 bp; PCR conditions were 94 C for 10 min followed by 30 cycles at 94 C for 30 sec, 60 C for 30 sec, and 72 C for 60 sec).
The introduction of the Q490L point mutation into the rat M3R sequence generates an extra SspI restriction site (25). To distinguish between WT M3 and Q490L mutant M3Rs, we carried out additional PCR studies using two primers flanking the Q490 codon: 5′-GGCGATCGCTGGCTTTTACATG (forward) and 5′-CAGGACCATGATGTTGGCGGGGGTCCACGT (reverse) (PCR conditions were 94 C for 10 min followed by 30 cycles at 94 C for 30 sec, 60 C for 30 sec, and 72 C for 60 sec). After digestion of the resulting 820-bp PCR products by SspI, two cleavage products, 60 and 760 bp in size, were obtained with DNA samples containing the Q490L-M3 transgene, whereas PCR products obtained with samples obtained from WT mice remained uncleaved.
RT-PCR analysis of transgene expression
To examine whether the mutant M3R transgene was selectively expressed in pancreatic islets (β-cells), we extracted total RNA from various peripheral and central tissues of transgenic mice using the QIAzol lysis reagent (QIAGEN, Valencia, CA). During total RNA preparation, an on-column deoxyribonuclease I treatment step was performed (RNeasy mini kit; QIAGEN). cDNA was generated using the Superscript III first-strand synthesis system for RT-PCR kit (Invitrogen, Carlsbad, CA). To test for the potential presence of contaminating genomic DNA, the RT step was omitted in a series of control samples. The RT products were amplified via PCR using a primer pair specific for the M3R transgene [M3HA-F, 5′-CCTACGACGTCCCCGACTAC (this primer anneals to the hemagglutinin epitope tag sequence present at the N terminus of the mutant receptor), and M3-R, 5′-TGATGTAGGTCGTGAACAGG; size of the PCR product, 405 bp]. The PCR cycling conditions were as follows: 94 C for 3 min followed by 32 cycles at 94 C for 25 sec, 54 C for 40 sec, and 72 C for 30 sec. PCRs were carried out in a final volume of 50 μl containing 2 μl of the RT reaction product (corresponding to ∼0.1 μg RNA), 5 μl 10× Taq buffer containing 200 mm (NH4)2SO4 (Fermentas, Glen Burnie, MD), 1.5 mm MgCl2, 1 mm of each dNTP, 200 nm of each PCR primer, and 1 U recombinant Taq DNA polymerase (Fermentas). Mouse GAPDH mRNA was amplified as an internal control (forward primer, 5′-CGTGGAGTCTACTGGTGTCTTCACC; reverse primer, 5′-GATGGCATGGACTGTGGTCATGAGC; size of the PCR product, 258 bp).
Radioligand binding studies
Membranes were prepared from isolated mouse pancreatic islets as described (27) and incubated with a saturating concentration (2 nm) of the non-subtype-selective muscarinic antagonist [3H]N-methylscopolamine (specific activity, 83 Ci/mmol; PerkinElmer, Waltham, MA), following a previously described protocol (28). Binding reactions were carried out for 1 h at room temperature (22 C). Nonspecific binding was defined as binding observed in the presence of 10 μm atropine.
Morphometric analysis of pancreata from transgenic and WT control mice
Pancreata were obtained from transgenic mice and their corresponding control littermates (12-wk-old males). From each pancreas, 10 sections (each 5 μm thick), 100 μm apart, were obtained. Sections were stained with an antiinsulin antibody and analyzed using a light microscope (Zeiss AxioImager D1) and the MosaiX module of the AxioVision 4.2 imaging software (Carl Zeiss MicroImaging Inc., Thornwood, NY). The β-cell mass (defined as all cells staining positive for the hormone insulin) was calculated by multiplying the pancreas weight by the percentage of β-cell area.
In vitro studies with perifused islets: insulin release and [3H]inositol efflux
Pancreatic islets were isolated from β-M3-Q490L Tg mice and WT littermates (adult males that had free access to food), and insulin release and [3H]inositol efflux were measured as described in detail previously (9). Islets were isolated by collagenase digestion and handpicked, using a glass loop pipette under a stereomicroscope, into Krebs-Ringer bicarbonate (KRB) buffer supplemented with 3 mm glucose (9). Islets were free of contamination from the exocrine pancreas.
After isolation, a process lasting no more than 90 min after initial surgical removal of the pancreas, groups of 15–28 islets were loaded onto nylon filters (Sefar America Inc., Kansas City, MO). To label phosphoinositide pools, the filters were placed in small glass vials and incubated for 3 h in a [3H]inositol-containing KRB solution. This solution was formulated as follows: 10 μCi [3H]inositol (specific activity, 21.7 Ci/mmol; PerkinElmer) was placed in a small culture tube. To this aliquot of label, 255 μl warmed and oxygenated KRB buffer containing 5 mm glucose was added. After mixing, 240 μl of this solution was gently added to the vial with islets. The vial was capped and gently oxygenated for 10 sec. All groups of islets were then incubated for 3 h at 37 C.
After the 3-h incubation period, all islets were washed gently with 5 ml warmed, fresh KRB perifusion medium. Islets were then perifused in KRB buffer at a flow rate of 1 ± 0.1 ml/min for 30 min in the presence of 3 mm glucose. After this 30-min stabilization period, islets were perifused with 15 mm glucose in the absence or presence of 10 μm atropine (Sigma Chemical Co., St. Louis, MO).
Perifusate solutions were gassed with 95% O2/5% CO2 and maintained at 37 C. Insulin released into the medium was measured by RIA (9). After termination of the [3H]inositol efflux experiments, the number of counts remaining in the islets was assessed, and [3H]inositol efflux was expressed as fractional efflux rate (9).
In vivo physiological studies
Oral and ip glucose tolerance tests (OGTT and IGTT, respectively) were carried out with mice that had been subjected to an overnight (10–12 h) fast. In the OGTT, mice were administered an oral load of glucose (2 mg/g body weight) via oral gavage. In the IGTT, mice received the same dose of glucose via ip injection. In both tests, blood samples were collected via retroorbital sinus puncture before (0 min) and 15, 30, 60, and 120 min after glucose administration. Blood glucose levels were determined using an automated blood glucose reader (Glucometer Elite Sensor; Bayer, Elkhart, IN). Serum insulin concentrations were determined via ELISA (Crystal Chem Inc., Downers Grove, IL). Plasma glucagon concentrations were measured via RIA (Linco, St. Charles, MO). Serum triglycerides and free fatty acids were measured using reagents from Thermo DMA (Louisville, CO) and Roche Applied Science (Indianapolis, IN), respectively, according to the manufacturers’ instructions.
For insulin tolerance (sensitivity) tests, human insulin (0.75 U/kg; Eli Lilly, Indianapolis, IN) was administered ip to mice that had been fasted overnight for 10–12 h. Blood glucose measurements were carried out immediately before and 15, 30, and 60 min after insulin injection. Blood samples were taken from the tail vein.
In acute atropine administration studies, mice were injected with a single dose of atropine sulfate (10 mg/kg, ip; Sigma).
Statistical analysis
Data are expressed as means ± se for the indicated number of observations. For comparisons between two groups, the unpaired Student’s t test (two-tailed) was used. For multiple comparisons, the one-way ANOVA was used, followed by appropriate post hoc tests. A P value of <0.05 was considered statistically significant.
Results
Generation of transgenic mice expressing the Q490L mutant M3R in pancreatic β-cells
To explore the in vivo effects of persistent activation of β-cell M3Rs, we generated transgenic mice that overexpressed Q490L mutant M3Rs in their pancreatic β-cells. To ensure that M3Rs were selectively expressed by pancreatic β-cells, transgene expression was placed under the control of a 0.65-kb fragment of the RIP II (26,29). Using standard transgenic techniques, we obtained several founder mice that stably transmitted the transgene to their progeny. All experiments described in the following were carried out with a mutant mouse line (β-M3-Q490L Tg) that expressed the transgene at relatively low levels, using male littermates maintained on an isogenic C57BL/6 background. Studies with a second transgenic line gave results similar to those described below.
To determine actual muscarinic receptor densities, we incubated membranes prepared from pancreatic islets from WT and β-M3-Q490L Tg mice with a saturating concentration (2 nm) of the non-subtype-selective muscarinic antagonist, [3H]N-methylscopolamine. These studies yielded muscarinic receptor densities of 1.6 ± 0.2 fmol/100 islets for WT mice (n = 8) and 7.4 ± 0.1 fmol/100 islets for β-M3-Q490L Tg mice (n = 3). Because islets from WT mice predominantly express M3Rs (5,6,7,8,9), β-M3-Q490L Tg mice express approximately 3- to 4-fold more mutant M3Rs (∼5.8 fmol/100 islets) than WT M3Rs.
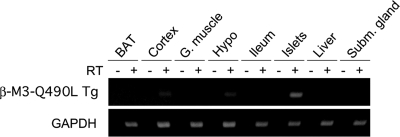
To confirm the β-cell selectivity of transgene expression, we carried out RT-PCR studies using total RNA prepared from several peripheral and central tissues of β-M3-Q490L Tg mice. Using transgene-specific primers, we could easily detect the expression of transgenic M3Rs in pancreatic islets from β-M3-Q490L Tg mice (Fig. 1). In contrast, transgene expression remained undetectable in other peripheral tissues (Fig. 1). Faint expression of the Q490L-M3 transgene was also observed with cDNA derived from cerebral cortex and hypothalamus (Fig. 1), consistent with previous studies that the RIP II promoter shows some degree of transcriptional activity in these brain areas (30,31).
Figure 1.
RT-PCR analysis indicating islet selectivity of transgene expression in β-M3-Q490L Tg mice. A transgene-specific PCR primer pair was used to amplify cDNA prepared from the indicated tissues of adult β-M3-Q490L Tg mice (see Materials and Methods for experimental details). As expected, transgene expression was readily detectable in islets. Transgene expression was undetectable in other peripheral tissues [brown adipose tissues (BAT), gastrocnemius muscle (G. muscle), ileum, liver, and submandibular gland]. A faint RT-PCR signal was found with cDNA prepared from cerebral cortex and hypothalamus of β-M3-Q490L Tg mice, consistent with published reports indicating that the RIP II promoter shows some degree of transcriptional activity in these brain areas. Control samples (indicated by − above the lanes) that had not been treated with reverse transcriptase (RT) did not give any detectable RT-PCR products, confirming the absence of contaminating genomic DNA. GAPDH cDNA was amplified in all samples as an internal control.
Functional studies with perifused islets
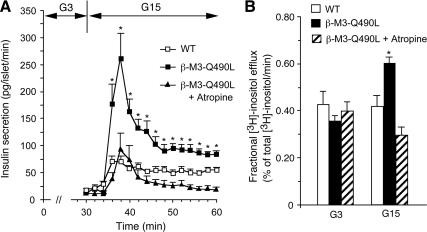
To confirm that the Q490L mutant M3R was indeed constitutively active in mouse pancreatic β-cells, we initially studied insulin release using perifused islets prepared from WT and β-M3-Q490L Tg mice. In the presence of 3 mm glucose, basal insulin release rates were similarly low in both mouse strains (∼10–20 pg/islet·min; Fig. 2A). Strikingly, in the presence of 15 mm glucose, islets expressing the Q490L mutant M3R released considerably more insulin than the WT control islets (Fig. 2A). This effect could be completely blocked by incubation of the transgenic islets with atropine (10 μm), an inverse muscarinic agonist that is able to prevent M3R signaling in the absence of activating ligands (32,33). Consistent with published reports (34,35), atropine (10 μm) had no significant effect on glucose-induced insulin secretion from WT islets (data not shown).
Figure 2.
Measurements of insulin secretion and [3H]inositol efflux from perifused isolated islets of β-M3-Q490L Tg mice and WT littermates. A, Insulin secretion studies; B, [3H]inositol efflux measurements. Groups of islets were isolated and incubated for 3 h in KRB buffer plus 5 mm glucose. In most experiments, [3H]inositol was included in the incubation buffer to label phosphoinositide pools. Subsequently, the islets were perifused for 30 min with 3 mm glucose (G3) and then stimulated with 15 mm glucose (G15) for 30 min in the absence or presence of atropine (10 μm). B shows [3H]inositol efflux rates at the end of the 3- and 15-mm glucose incubation periods. Asterisks indicate significant differences between the responses observed with β-M3-Q490L islets and the corresponding responses obtained with WT islets (P < 0.05). Data are presented as means ± se of at least four independent perifusion experiments.
At a biochemical level, activation of β-cell M3Rs results in the stimulation of PLCβ, which triggers the production of IP3 and DAG (5,6,7,9,13). The cellular actions of these two second messengers are thought to be primarily responsible for the ability of β-cell M3Rs to promote insulin release (5,6,7). In the present study, we therefore also determined the release of [3H]inositol from myo-[2-3H]inositol-prelabeled islets as a measure of PLCβ activation (9). Stimulation of perifused WT islets with 15 mm glucose had no significant effect on [3H]inositol efflux (Fig. 2B). In contrast, in β-M3-Q490L mutant islets, the same glucose stimulus resulted in a significant increase in [3H]inositol efflux (Fig. 2B). This stimulatory response was completely abolished in the presence of atropine (10 μm; Fig. 2B).
Both mutant and WT islets contained similar amounts of insulin (WT, 94 ± 8 ng per islet; β-M3-Q490L Tg, 86 ± 4 ng per islet; nine batches of ∼30 islets obtained from different mice were analyzed per group; P > 0.05). Morphometric studies indicated that β-cell mass and the average size and number of pancreatic islets were not significantly different between β-M3-Q490L Tg mice and their WT littermates (Table 1). Moreover, total pancreatic weight did not differ between the two mouse strains (data not shown).
Table 1.
Morphometric analysis of pancreata from β-M3-Q490L Tg mice and WT littermates
| β-Cell mass (mg) | Number of islets per mm2 | Mean islet size (mm2) | |
|---|---|---|---|
| Control (WT) | 2.10 ± 0.13 | 1.30 ± 0.09 | 10,610 ± 1,310 |
| β-M3-Q490L Tg | 1.97 ± 0.35 | 1.39 ± 0.02 | 11,820 ± 710 |
Pancreata were obtained from three transgenic mice and three WT littermates (12-wk-old males). From each pancreas, 10 sections (each 5 μm thick), 100 μm apart, were obtained (see Materials and Methods for experimental details).
β-M3-Q490L Tg mice display hypoglycemia, hyperinsulinemia, and greatly improved glucose tolerance
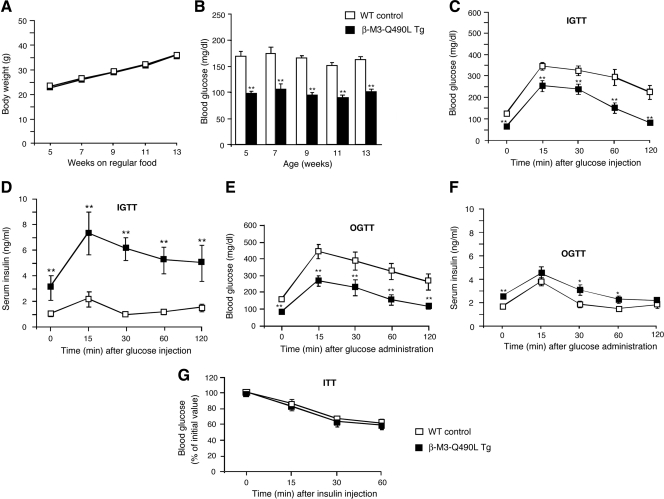
When maintained on standard mouse chow (Fig. 3A) or a high-fat diet (Fig. 4A), the body weight of β-M3-Q490L Tg mice did not differ significantly from that of their WT littermates. Remarkably, β-M3-Q490L Tg mice displayed an approximately 40–50% reduction in blood glucose levels in both the fed and fasting state (Table 2 and Fig. 3B), accompanied by an approximately 2- to 3-fold increase in fed and fasting serum insulin levels (Table 2; 16- to 24-wk-old males). In contrast, plasma glucagon levels were not significantly different between β-M3-Q490L Tg and WT control mice (β-M3-Q490L Tg, 92.0 ± 9.3 pg/ml; WT, 86.7 ± 8.7 pg/ml; n = 6 per group; 36-wk-old freely fed males).
Figure 3.
Physiological analysis of β-M3-Q490L Tg mice and control littermates maintained on regular mouse chow. A, Growth curves of male mice maintained on regular mouse chow (n = 10 per group). B, Fed blood glucose levels (WT, n = 8; β-M3-Q490L Tg, n = 10; male mice). C, IGTT. Blood glucose levels were measured at the indicated time points after ip administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). D, Serum insulin levels after ip administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). E, OGTT. Blood glucose levels were measured at the indicated time points after oral administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). F, Serum insulin levels after oral administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). G, Insulin tolerance test (ITT). Blood glucose levels were measured at the indicated time points after ip administration of insulin (0.75 U/kg; 20-wk-old males; n = 6 per group). Data are expressed as means ± se. *, P < 0.05; **, P < 0.01, compared with the corresponding WT value.
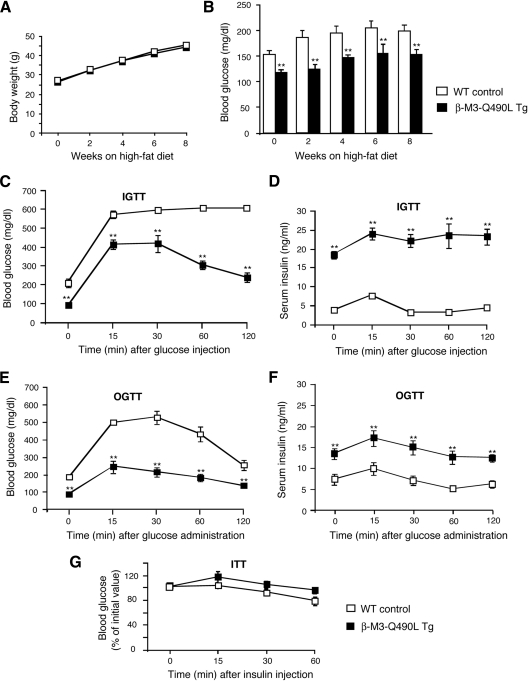
Figure 4.
Physiological analysis of β-M3-Q490L Tg mice and control littermates maintained on a high-fat diet. A, Growth curves of male mice maintained on a high-fat diet (n = 8 per group). Mice were put on the high-fat diet when they were 5 wk old. B, Fed blood glucose levels of male mice maintained on a high-fat diet (n = 7 per group). C, IGTT. Blood glucose levels were measured at the indicated time points after ip administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). D, Serum insulin levels after ip administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). E, OGTT. Blood glucose levels were measured at the indicated time points after oral administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). F, Serum insulin levels after oral administration of glucose (2 mg/g body weight; 16-wk-old males; n = 6 per group). G, Insulin tolerance test (ITT). Blood glucose levels were measured at the indicated time points after ip administration of insulin (0.75 U/kg; 20-wk-old males; n = 6 per group). Data are expressed as means ± se. *, P < 0.05; **, P < 0.01, compared with the corresponding WT value.
Table 2.
Fed and fasting blood glucose and serum insulin levels in male β-M3-Q490L Tg mutant mice and their corresponding control (WT) littermates
| WT | β-M3-Q490L Tg | |
|---|---|---|
| Regular chow | ||
| Blood glucose (mg/dl) | ||
| Fed | 149.9 ± 5.8 | 89.3 ± 5.6b |
| Fasted | 122.6 ± 9.0 | 61.5 ± 2.9b |
| Serum insulin (ng/ml) | ||
| Fed | 12.2 ± 1.0 | 26.0 ± 0.7b |
| Fasted | 0.94 ± 0.10 | 3.0 ± 0.9a |
| High-fat diet | ||
| Blood glucose (mg/dl) | ||
| Fed | 205.0 ± 5.7 | 155.1 ± 17.1b |
| Fasted | 179.8 ± 9.4 | 83.3 ± 5.1b |
| Serum insulin (ng/ml) | ||
| Fed | 22.8 ± 2.7 | 31.0 ± 0.6a |
| Fasted | 3.5 ± 0.4 | 18.3 ± 1.0b |
Measurements were carried out with 16- to 24-wk-old male mice (n = 6–10 per genotype). Data are given as means ± se. P values are as compared with the corresponding WT control group.
P < 0.05.
P < 0.01.
In both IGTT and OGTT (glucose dose, 2 mg/g body weight), β-M3-Q490L Tg mice exhibited greatly improved glucose tolerance during the entire 2-h observation period (Fig. 3, C and E) (16-wk-old males). In addition, β-M3-Q490L Tg mice displayed significantly increased serum insulin levels after ip or oral glucose administration (Fig. 3, D and F; 16-wk-old males).
Intraperitoneal administration of a fixed dose of insulin (0.75 U/kg ip) showed that β-M3-Q490L Tg mice and WT control mice (20-wk-old males) exhibited a similar degree of insulin sensitivity (Fig. 3G).
Atropine treatment reverses the changes in blood glucose and serum insulin levels displayed by β-M3-Q490L Tg mice
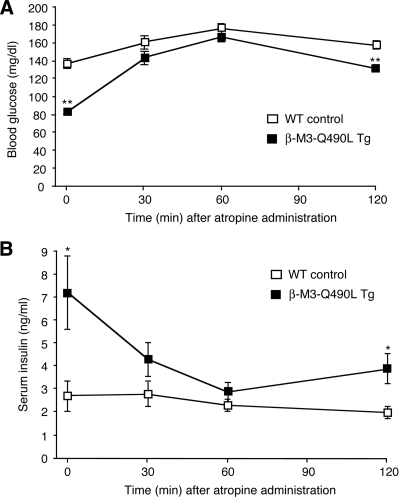
To examine whether the changes in blood glucose and serum insulin levels displayed by the β-M3-Q490L Tg mice were in fact due to persistent activation of the Q490L mutant M3R, we carried out a series of acute atropine injection experiments. Specifically, we injected freely fed β-M3-Q490L Tg mice and their WT littermates (adult males) maintained on regular mouse chow with atropine (10 mg/kg ip) and then measured blood glucose and serum insulin levels over a 2-h period. In WT mice, atropine treatment led to a small increase in blood glucose concentrations but had little effect on serum insulin levels (Fig. 5), consistent with the outcome of a previous study (36). Before atropine administration, β-M3-Q490L Tg mice showed significantly decreased blood glucose and greatly increased serum insulin levels (Fig. 5), consistent with the data shown in Fig. 3. Strikingly, after atropine treatment, β-M3-Q490L Tg mice exhibited a pronounced increase in blood glucose concentrations and a prominent reduction in serum insulin levels. As a result, the blood glucose and serum insulin levels of atropine-treated β-M3-Q490L Tg and WT mice did not differ significantly from each other at the 30- and 60-min time points (Fig. 5), strongly suggesting that persistent activation of the Q490L mutant M3R is responsible for the changes in blood glucose and serum insulin levels found with the β-M3-Q490L Tg mice.
Figure 5.
Effect of atropine treatment of β-M3-Q490L Tg mice and control littermates on blood glucose and serum insulin levels. For these experiments, freely fed adult male mice maintained on regular mouse chow were used (WT, n = 6; β-M3-Q490L Tg, n = 4). Mice were injected with atropine (10 mg/kg ip), and blood glucose (A) and serum insulin levels (B) were measured at the indicated time points. Data are expressed as means ± se. *, P < 0.05; **, P < 0.01, compared with the corresponding WT value.
β-M3-Q490L Tg mice are resistant against diet-induced hyperglycemia and glucose intolerance
An energy-rich, high-fat diet is known to trigger a number of metabolic changes including hyperglycemia and impaired glucose tolerance. To examine whether the severity of these metabolic deficits was reduced in β-M3-Q490L Tg mice, 5-wk-old β-M3-Q490L Tg mice and their WT littermates were fed a high-fat diet [fat content, 35.5% (wt/wt) fat content, no. F3282; Bioserv] and then monitored for 8 wk.
WT mice consuming the high-fat diet developed pronounced hyperglycemia (Fig. 4B). In contrast, average blood glucose levels of freely fed β-M3-Q490L Tg mice remained in a normal range (<150 mg/dl) during the entire observation period (Fig. 4B). Moreover, WT mice maintained on the high-fat diet showed markedly increased fasting blood glucose levels (∼200 mg/dl; Table 2) and severely impaired glucose tolerance (IGTT, Fig. 4C; OGTT, Fig. 4E). Remarkably, under the same experimental conditions, β-M3-Q490L Tg littermates displayed normal fasting blood glucose levels (∼80–90 mg/dl; Table 2) and glucose tolerance (IGTT, Fig. 4C; OGTT, Fig. 4E). Serum triglyceride and free fatty acid levels did not differ significantly between β-M3-Q490L Tg mice and their WT littermates (triglycerides: WT, 106.1 ± 6.4 mg/dl; Tg, 93.0 ± 9.9 mg/dl; free fatty acids: WT, 0.74 ± 0.06 mm; Tg, 0.74 ± 0.10 mm; n = 7 per group).
β-M3-Q490L Tg mice showed significantly elevated fed and fasting serum insulin levels, compared with WT control littermates (Table 2; 16- to 24-wk-old males). Moreover, after ip or oral glucose administration, β-M3-Q490L Tg mice exhibited significantly higher serum insulin levels throughout the entire 2-h observation period (Fig. 4, D and F), suggesting that the improved glucose tolerance displayed by the β-M3-Q490L Tg mice was due to enhanced insulin release. In an insulin sensitivity test, both β-M3-Q490L Tg mice and WT littermates showed comparable decreases in blood glucose levels (20-wk-old males; Fig. 4G).
Morphometric measurements indicated that β-cell mass did not differ significantly between β-M3-Q490L Tg mice and their WT littermates maintained on the high-fat diet [control mice (n = 3), 5.95 ± 3.27 mg; Tg mice (n = 3), 5.10 ± 1.82 mg; see Materials and Methods for experimental details].
Discussion
Activation of β-cell M3Rs triggers a series of biochemical events that are typically associated with the activation of Gq-type G proteins, including the activation of different isoforms of PLCβ, protein kinase C, and PLA2 and an increase in intracellular calcium levels (5,6,7,13). As discussed in detail elsewhere (6), M3R-induced insulin release is thought to be mediated by two major mechanisms, one depending on elevated intracellular calcium levels and the one involving a protein kinase C-mediated increase in the efficiency of calcium on insulin exocytosis.
Given the robust insulin release-promoting effects of β-cell M3R activation (5,6,7,8,9,14), it has been suggested that long-term stimulation of β-cell M3Rs might be beneficial in the treatment of glucose intolerance and type 2 diabetes (14). Currently, pharmacological tools that can be employed to selectively stimulate β-cell M3Rs in vivo are not available. As an alternative, we therefore generated transgenic mice that selectively expressed the constitutively active Q490L mutant M3R (25) in their pancreatic β-cells (β-M3-Q490L Tg mice).
Studies with isolated perifused islets confirmed that the Q490L mutant M3R exhibited pronounced ligand-independent signaling (constitutive activity) in mouse β-cells (Fig. 2). A characteristic feature of mouse islets is that the second phase of glucose-induced secretion tends to wane during sustained stimulation with glucose (37,38,39), consistent with the results of the in vitro insulin release data obtained with control and transgenic mice (Fig. 2). However, insulin release from transgenic islets was still greater during this period despite declining rates of secretion from both groups of islets. It is unclear why mouse islet secretory responses to glucose differ so dramatically from those routinely observed from rat islets. This diminution of release may reflect a decline in a glucose-derived signal, depletion of readily releasable pools of insulin granules, and/or some type of feedback inhibition of the secretory response.
In vivo studies showed that β-M3-Q490L Tg mice displayed an approximately 40–50% reduction in both fed and fasting blood glucose levels, but an approximately 2- to 3-fold increase in fed and fasting serum insulin levels (Table 2), compared with their WT littermates. β-M3-Q490L Tg mice exhibited similar sensitivity to exogenously administered insulin as their control littermates (Fig. 3G), indicating that the increase in serum insulin levels displayed by the β-M3-Q490L Tg mice was not a consequence of peripheral insulin resistance.
Moreover, the outcome of acute atropine injection experiments (Fig. 5) strongly supports the concept that the changes in blood glucose and serum insulin levels exhibited by the β-M3-Q490L Tg mice were caused by persistent muscarinic activation of pancreatic β-cells rather than by secondary changes in the function of β-cells or other cell types and tissues.
In addition, independent of the route of glucose administration (ip or oral), β-M3-Q490L Tg mice showed remarkable improvements in glucose tolerance, accompanied by elevated serum insulin levels (∼2- to 3-fold), compared with their WT littermates (Fig. 3). Strikingly, β-M3-Q490L Tg mice were also protected against diet-induced hyperglycemia and glucose intolerance, most likely due to enhanced insulin release (Fig. 4). Taken together, these findings strongly suggest that long-term, continuous activation of β-cell M3Rs does not lead to a loss or significant reduction in M3R activity caused by receptor desensitization or counterregulatory cellular events. Thus, a strategy aimed at long-term pharmacological stimulation of β-cell M3Rs might prove potentially useful in the treatment of type 2 diabetes.
It should be noted that we also detected low levels of Q490L-M3R transgene expression in cerebral cortex and hypothalamus (Fig. 1), consistent with the outcome of previous studies using the RIP II promoter to achieve β-cell-specific transgene expression (30,31). We can therefore not rule out the possibility that central mechanisms may have contributed to some of the phenotypic changes displayed by the β-M3-Q490L Tg mice.
Interestingly, a previous report demonstrated that activation of peripheral vagal nerves has a stimulatory effect on β-cell proliferation (40). Because ACh is the major neurotransmitter stored in vagal nerve endings and the M3R is the predominant cholinergic receptor expressed by pancreatic β-cells (5,6,7,8,9), we speculated that long-term activation of β-cell M3Rs might trigger an increase in β-cell mass and/or proliferation. However, we found that chronic activation of β-cell M3Rs in β-M3-Q490L Tg mice had no significant effect on β-cell mass and the average size and number of pancreatic islets (Table 1). One possibility therefore is that the reported stimulatory effects of vagal stimulation on β-cell proliferation are mediated by one or more other neurotransmitters known to be coreleased with ACh from pancreatic vagal nerve endings (5,6). However, it is also possible that more indirect mechanisms are involved in this activity.
The outcome of the present study clearly has important potential therapeutic implications. The pronounced metabolic changes observed with β-M3-Q490L Tg mice suggest that approaches aimed at enhancing signaling through β-cell M3Rs could become therapeutically useful for the long-term treatment of glucose intolerance or type 2 diabetes. The most direct approach would involve the administration of a peripherally acting, selective M3R agonist. The development of such agents is currently the focus of several laboratories (note that selective M3R agonists are not available at present). One caveat is that the potential administration of a selective M3R agonist might lead to significant side effects caused by stimulation of non-β-cell M3Rs expressed by smooth muscle and glandular tissues (13,41,42,43), including, for example, increased smooth muscle contractility and enhanced salivation. It is possible that such side effects could be avoided or ameliorated by the judicious use of partial M3R agonists or by the administration of so-called allosteric enhancers of M3R function that increase the affinity and/or efficacy of ACh at M3Rs without directly activating these receptors. The feasibility of such a strategy has been demonstrated recently by the development of positive allosteric enhancers for several other muscarinic receptor subtypes (44).
Acknowledgments
We thank Dr. Bo Li (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases) for carrying out the radioligand binding studies with islet membrane preparations.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. I.R.A. was the recipient of a Basque Government Fellowship.
Disclosure Summary: The authors have nothing to declare.
First Published Online September 15, 2010
Abbreviations: ACh, Acetylcholine; DAG, diacylglycerol; IGTT, ip glucose tolerance test; IP3, inositol 1,4,5-trisphosphate; KRB, Krebs-Ringer bicarbonate; M3R, M3 muscarinic receptor; OGTT, oral glucose tolerance test; PLCβ, phospholipase Cβ; RIP II, rat insulin promoter II; WT, wild type.
References
- Kahn CR 1994 Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43:1066–1084 [DOI] [PubMed] [Google Scholar]
- Kahn SE 2003 The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46:3–19 [DOI] [PubMed] [Google Scholar]
- Moller DE 2001 New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827 [DOI] [PubMed] [Google Scholar]
- Satin LS, Kinard TA 1998 Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine 8:213–223 [DOI] [PubMed] [Google Scholar]
- Ahrén B 2000 Autonomic regulation of islet hormone secretion: implications for health and disease. Diabetologia 43:393–410 [DOI] [PubMed] [Google Scholar]
- Gilon P, Henquin JC 2001 Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev 22:565–604 [DOI] [PubMed] [Google Scholar]
- Ahrén B 2009 Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8:369–385 [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J 2004 Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes 53:1714–1720 [DOI] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC, Tesz GJ, Taketo MM, Sterpka J, Philbrick W, Matsui M 2004 Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun 315:872–876 [DOI] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M 1988 The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett 236:89–92 [DOI] [PubMed] [Google Scholar]
- Verspohl EJ, Tacke R, Mutschler E, Lambrecht G 1990 Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol 178:303–311 [DOI] [PubMed] [Google Scholar]
- Karlsson S, Ahren BJ 1993 Muscarinic receptor subtypes in carbachol-stimulated insulin and glucagon secretion in the mouse. J Auton Pharmacol 13:439–446 [Google Scholar]
- Wess J 1996 Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol 10:69–99 [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J 2006 A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3:449–461 [DOI] [PubMed] [Google Scholar]
- Böhm SK, Grady EF, Bunnett NW 1997 Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J 322:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL 1998 The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38:289–319 [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR 2007 Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69:511–534 [DOI] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B 2003 Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther 98:197–220 [DOI] [PubMed] [Google Scholar]
- Bundey RA, Nahorski SR 2001 Homologous and heterologous uncoupling of muscarinic M3 and α1B adrenoceptors to αq/11 in SH-SY5Y human neuroblastoma cells. Br J Pharmacol 134:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willets JM, Challiss RA, Kelly E, Nahorski SR 2001 G protein-coupled receptor kinases 3 and 6 use different pathways to desensitize the endogenous M3 muscarinic acetylcholine receptor in human SH-SY5Y cells. Mol Pharmacol 60:321–330 [DOI] [PubMed] [Google Scholar]
- Willets JM, Mistry R, Nahorski SR, Challiss RA 2003 Specificity of g protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol Pharmacol 64:1059–1068 [DOI] [PubMed] [Google Scholar]
- Fukamauchi F, Saunders PA, Hough C, Chuang DM 1993 Agonist-induced down-regulation and antagonist-induced up-regulation of m2- and m3-muscarinic acetylcholine receptor mRNA and protein in cultured cerebellar granule cells. Mol Pharmacol 44:940–949 [PubMed] [Google Scholar]
- Steel MC, Buckley NJ 1993 Differential regulation of muscarinic receptor mRNA levels in neuroblastoma cells by chronic agonist exposure: a comparative polymerase chain reaction study. Mol Pharmacol 43:694–701 [PubMed] [Google Scholar]
- Yang J, Logsdon CD, Johansen TE, Williams JA 1993 Human m3 muscarinic acetylcholine receptor carboxyl-terminal threonine resides are required for agonist-induced receptor down-regulation. Mol Pharmacol 44:1158–1164 [PubMed] [Google Scholar]
- Schmidt C, Li B, Bloodworth L, Erlenbach I, Zeng FY, Wess J 2003 Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast. Identification of point mutations that “silence” a constitutively active mutant M3 receptor and greatly impair receptor/G protein coupling. J Biol Chem 278:30248–30260 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Cavaliere C, D'Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick W, Stewart AF 1996 Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem 271:1200–1208 [DOI] [PubMed] [Google Scholar]
- Ahrén B, Sauerberg P, Thomsen C 1999 Increased insulin secretion and normalization of glucose tolerance by cholinergic agonism in high fat-fed mice. Am J Physiol 277:E93–102 [DOI] [PubMed] [Google Scholar]
- Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR 1991 Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256:727–733 [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA 1999 Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274:305–315 [DOI] [PubMed] [Google Scholar]
- Gannon M, Shiota C, Postic C, Wright CV, Magnuson M 2000 Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26:139–142 [DOI] [PubMed] [Google Scholar]
- Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C 2004 Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24:258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding TA, Burstein ES, Brauner-Osborne H, Hill-Eubanks D, Brann MR 1995 Pharmacology of a constitutively active muscarinic receptor generated by random mutagenesis. J Pharmacol Exp Ther 275:1274–1279 [PubMed] [Google Scholar]
- Nelson CP, Nahorski SR, Challiss RA 2006 Constitutive activity and inverse agonism at the M2 muscarinic acetylcholine receptor. J Pharmacol Exp Ther 316:279–288 [DOI] [PubMed] [Google Scholar]
- Gao ZY, Gilon P, Henquin JC 1994 The role of protein kinase-C in signal transduction through vasopressin and acetylcholine receptors in pancreatic B-cells from normal mouse. Endocrinology 135:191–199 [DOI] [PubMed] [Google Scholar]
- Johnson DE, Yamazaki H, Ward KM, Schmidt AW, Lebel WS, Treadway JL, Gibbs EM, Zawalich WS, Rollema H 2005 Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets. Role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes 54:1552–1558 [DOI] [PubMed] [Google Scholar]
- Fukudo S, Virnelli S, Kuhn CM, Cochrane C, Feinglos MN, Surwit RS 1989 Muscarinic stimulation and antagonism and glucoregulation in nondiabetic and obese hyperglycemic mice. Diabetes 38:1433–1438 [DOI] [PubMed] [Google Scholar]
- Berglund O 1980 Different dynamics of insulin secretion in the perfused pancreas of the mouse and rat. Acta Endocrinol 93:54–60 [DOI] [PubMed] [Google Scholar]
- Lenzen S 1979 Insulin secretion by isolated perfused rat and mouse pancreas. Am J Physiol 236:E391–E400 [DOI] [PubMed] [Google Scholar]
- Ma YH, Wang J, Rodd GG, Bolaffi JL, Grodsky GM 1995 Differences in insulin secretion between rat and mouse islets: role of cAMP. Eur J Endocrinol 132:370–376 [DOI] [PubMed] [Google Scholar]
- Kiba T, Tanaka K, Numata K, Hoshino M, Misugi K, Inoue S 1996 Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology 110:885–893 [DOI] [PubMed] [Google Scholar]
- Eglen RM, Hegde SS, Watson N 1996 Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 48:531–565 [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJM 1998 International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290 [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D 2007 Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6:721–733 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW 2009 Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci 30:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]