Abstract
TSH receptor (TSHR) antibodies (Abs) may be stimulating, blocking, or neutral in their functional influences and are found in patients with autoimmune thyroid disease, especially Graves’ disease (GD). Stimulators are known to activate the thyroid epithelial cells via both Gs- and Gq-coupled signaling pathways, whereas blockers inhibit the action of TSH and may act as weak agonists. However, TSHR neutral Abs do not block TSH binding and are unable to induce cAMP via Gsα. The importance of such neutral Abs in GD remains unclear because their functional consequence has been assumed to be zero. We hypothesized that: 1) neutral TSHR Abs are more common to GD than generally recognized; 2) they may induce distinct signaling imprints at the TSHR not seen with TSH itself; and 3) these signaling events may alter cellular function. To evaluate these hypotheses, we first confirmed the presence of neutral TSHR Abs in sera from patients with GD and then, using mouse and hamster neutral TSHR monoclonal Abs (N-mAbs) performed detailed signaling studies, including a proteomic Ab array, with rat thyrocytes (FRTL-5) as targets. This allowed us to examine a battery of signaling cascades and their downstream effectors. Neutral TSHR Abs were indeed frequently present in sera from patients with GD. Sixteen of 27 patients (59%) had detectable neutral TSHR Abs by competition assay with N-mAbs. On examining signaling cascades, we found that N-mAbs induced signal transduction, primarily via the protein kinase A II cascade. In addition to the activation of phosphatidylinositol 3K/Akt, N-mAbs, unlike TSH, had the ability to exclusively activate the mammalian target of rapamycin/p70 S6K, nuclear factor-κB, and MAPK-ERK1/2/p38α signaling cascades and their downstream effectors p90 ribosomal kinase/MAPK-interacting kinase-1/mitogen and stress-activated kinase-1 and N-mAbs activated all forms of protein kinase C isozymes. To define the downstream effector mechanisms produced by these signaling cascades, cytokine production, proliferation, and apoptosis in thyrocytes were investigated. Although N-mAbs produced less cytokines and proliferation compared with TSH, they had the distinction of inducing thyroid cell apoptosis under the experimental conditions used. When dissecting out possible mechanisms of apoptosis, we found that activation of multiple oxidative stress markers was the primary mechanism orchestrating the death signals. Therefore, using oxidative stress-induced apoptosis, N-mAbs may be capable of exacerbating the autoimmune response in GD via apoptotic cells inducing antigen-driven mechanisms. This may help explain the inflammatory nature of this common disorder.
TSH receptor neutral antibodies may be capable of exacerbating the autoimmune response in Graves’ disease.
The TSH receptor (TSHR) is a member of the seven-transmembrane receptor subfamily and activates the classical G protein-coupled receptor (GPCR) effectors, Gαs and Gαq and their complex signaling systems (1,2). The TSHR has constitutive signaling activity and is further activated by TSH ligand binding or autoantibodies to the TSHR seen in patients with Graves’ disease (GD) and less commonly in Hashimoto’s thyroiditis (HT). Therefore, inappropriate activation and/or inactivation of signaling cascades triggered by these different TSHR antibodies (Abs) may contribute to thyroid pathophysiology. Whereas Abs to the TSHR, which either stimulate as TSH agonists or block TSH action, have been well characterized (1,2,3,4,5,6,7,8), TSHR Abs of the neutral type recognizing linear epitope(s) have also been recognized by binding to the receptor without any influence on cAMP generation (9,10,11,12,13).
Activation, modulation, or suppression of protein phosphorylation in signaling cascades via cognate receptor-ligand or TSHR-Ab interactions are key mechanisms that regulate thyroid function in autoimmune thyroid disease (14,15,16). Typically, GPCR signaling involves recruitment of multiple G proteins to activate signal transduction pathways that act synergistically and in a combinatorial manner to relay signals to the nucleus and promote transcription and translation of specific functions. GPCR signal transduction pathways involve multiple G proteins that include αs, αq, βγ, i/o, α12, and α13 and their subsequent downstream effectors. Each effector has characteristic downstream signaling events that induce crossover activation, modulation, and/or suppressive functions. Depending on signal strengths and duration of action, the resulting net effects specify the ultimate outcome. Major signaling cascades in TSHR activation include cAMP/protein kinase A (PKA)/cAMP response element-binding protein (CREB), phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR)/S6K, MAPK-ERK1/2/p38α/c-Jun N-terminal kinase (JNK), PI3K/protein kinase C (PKC), nuclear factor-κB (NF-κB), and Janus kinase (JAK)/signal transducer and activator of transcription (STAT)/suppressor of cytokine signaling (SOCS), and their downstream effectors. These cascades have been well characterized in different cellular model systems and are implicated in cell growth, cell survival, cell differentiation, secretion of cytokines and chemokines, and induction of apoptosis. cAMP/PKA/CREB, PI3K/Akt/PKC, and MAPK-ERK1/2/p38/JNK have been described in the TSH activation of thyrocytes (16,17,18,19,20,21,22,23,24,25,26). However, their upstream and downstream effectors have not been thoroughly studied.
The conformational binding site for stimulating and some blocking TSHR Abs involves mainly the leucine rich repeat region of the α-subunit (4,7,27). In contrast, the linear epitopes recognized by TSHR neutral Abs (N-Abs) are often confined to the cleaved region of the ectodomain (residues 316–366) (4). The pathophysiological significance of these Abs remains unclear, but we have previously shown the following: 1) their ability to inhibit TSHR cleavage and enhance TSHR expression and thereby influence antigen processing (14,28) and 2) N-Abs may act as weak TSH agonists (14). Although human monoclonal neutral TSHR Abs are not available for study, the development of hamster and mouse neutral TSHR monoclonal Abs (N-mAbs) has enabled us to further dissect their potential signaling events. Recently we characterized TSHR activation signaling cascades using TSHR mAbs in rat (FRTL-5) thyrocytes (16) as a model system and the present work is an amplification of these ongoing signaling studies. In the present study, we hypothesized that: 1) N-Abs that recognize the TSHR cleavage region may be more common in Graves’ patients than previously assumed; 2) monoclonal neutral Abs may induce distinct signaling imprints at the TSHR; and 3) these signaling events may alter cellular function.
Our observations demonstrated that neutral TSHR Abs were frequently present in sera from patients with GD. They were more frequent in GD than other autoimmune disorders including HT. Although neutral TSHR Abs failed to generate cAMP via Gαs effectors, they initiated unique molecular signatures, possibly via recruitment of multiple G proteins (17,29), and thus influenced multiple downstream signal transduction cascades including PKC/MAPK, mTOR/S6K, NF-κB, certain cytokines, and oxidative stress signaling and ultimately caused rat thyroid cell apoptosis on chronic exposure. Our findings suggest that oxidative stress may play a significant role in such Ab-induced thyrocyte death and thus exacerbate the chronic inflammatory process via antigen-driven mechanisms seen in autoimmune thyroid disease.
Materials and Methods
Patients and controls
GD was defined as increased thyroid hormone levels and suppressed serum TSH in patients with diffuse glands and the presence of TSHR Abs. HT was defined as thyroid failure with a previously increased TSH greater than 10 μU/ml in a patient receiving thyroid hormone replacement and positive Abs to thyroid peroxidase and/or thyroglobulin. We examined unselected serum samples from 27 untreated adult patients with GD, 15 with HT, 10 with self-reported rheumatoid arthritis, and 11 healthy individuals. Informed consent was obtained from all patients and controls who participated in the present study.
Human IgGs recognizing the linear TSHR cleavage region
Based on our previous reports (4,28), we used a competitive Ab inhibition assay to identify patient serum Abs to the two major linear epitopes (P1: residues 322–341 and P2: 337–356) determined by N-mAbs. Serum IgG was prepared by protein G columns (Amersham Biosciences, Piscataway, NJ). ELISA plates coated with TSHR peptides at a concentration of 10 μg/ml (4,28) and incubated with purified biotinylated neutral hamster mAbs (Tab-16, 200 ng/ml; or Tab-6, 500 ng/ml) in the presence of the patients’ serum IgG at a concentration of 10 μg/ml. The percent binding inhibition was expressed by the reduction of OD by serum IgG based on the biotinylated mAb alone. Seropositivity was defined as an inhibition greater than 2 sd from the mean of control IgGs.
To confirm ELISA reactivities by human IgG, a solid-phase indirect ELISA was also developed using those peptides. Briefly, 96-well plates were coated with specific peptides (2 μg/ml) overnight, blocked with 5% BSA, and incubated with selected IgG (1 μg/ml) samples in blocking buffer and a known mAb (Tab-16) was also run dose dependently in the same plate. Incubation with the horseradish peroxidase-conjugated antihuman or antihamster, washings, substrate reactions, and plate reading were performed according to a standard ELISA protocol. A 2-fold serial dilution of a known N-mAb (Tab-16) or a positive serum IgG that recognized the cleavage epitope (P1) was linear in each assay performed. A mean ± 2 sd of OD values was used to determine a cutoff level to generate seropositivity.
Confirmation of Ab binding to TSHR cleavage region by flow cytometry
To confirm specific binding of purified IgG from the patient serum to the TSHR, two further independent assays were performed. Binding of human IgGs to 4% paraformaldehyde-fixed Chinese hamster ovary (CHO) cells expressing human TSHR (JPO9, kindly supplied by Dr. G. Vassart, Bruxelles, Belgium) or TSHR lacking the cleavage region (the construct to generate this stable CHO cell lines, NC35, was kindly provided by Dr. B. Rappaport, Cedars-Sinai Research Institute and University of California, Los Angeles, CA) was visualized by flow cytometry assay. IgG that recognized the cleavage region showed positive reactivities with full-length TSHR (JPO9) but not with TSHR lacking the cleavage region (NC35). To further confirm the specific binding of purified human IgGs to the regions of interest, IgGs were incubated with JPO9 cells in the presence or absence of specific peptides and assayed for binding by flow cytometry. A reduction of mean fluorescent intensity in the presence of peptide compared with an irrelevant peptide indicated specific binding of IgG. Finally, immunoblot assays were performed using JPO9 cell lysates as before (4,28).
Cell culture and lysates for signaling study
Synchronized FRTL-5 rat thyroid cells were used as the model system (16,21,30). Cells were grown and maintained as we described recently (16). Before any stimulation experiments, synchronized cells were made quiescent by starvation in bovine calf serum-free basal medium (modified Ham’s F12) containing 0.3% BSA for 2 d (16). Before stimulation, medium was discarded from the 60-mm culture dishes, and the cells were washed three times with a fresh medium containing Hanks’ balanced salt solution (Life Technologies, Inc. Laboratories, Grand Island, NY). Using a fixed concentration of TSH (1 mU/ml) or N-mAbs (1 μg/ml) (Table 1) in fresh modified Ham’s F12 basal medium, cells were stimulated for an hour at 37 C in the incubator, washed twice with ice-cold PBS (pH 7.2) without calcium and magnesium (Mediatech Inc., Herndon, VA), scraped into cold lysis buffer (provided by the Kinexus Co., Toronto, Ontario, Canada) containing different phosphatase and protease inhibitor cocktail (Complete, Mini; Roche Applied Science, Indianapolis, IN; phenylmethanesulfonyl fluoride; Sigma, St. Louis, MO; and 1% Triton X-100), and sonicated. Lysates were then centrifuged and supernatants were collected. After measuring protein concentrations by the Bradford method (Bio-Rad Laboratories, Hercules, CA), equal amount of various protein samples were used for proteomics array analysis. Because culture conditions may influence signaling events, the same conditions were applied to all treatments and strictly maintained in each experiment.
Table 1.
Characteristics of Abs used to study signaling mechanisms
| Type | Species | Isotype | λ/κ-chain | NC35a | Epitope |
|---|---|---|---|---|---|
| Neutrals | |||||
| Tab-16 | Hamster | IgG2 | κ | No | 322–341 |
| IC8 | Hamster | IgG2 | κ | No | 322–341 |
| RSR4 | Mouse | IgG2 | λ | No | 322–341 |
| 7G10 | Hamster | IgG2 | λ | No | 337–356 |
| Control | |||||
| Isotype | Mouse | IgG2 | κ | No | No binding |
Fold stimulation was determined by intracellular cAMP assay using CHO cells expressing human TSHR, and blocking was expressed as the percent reduction of cAMP produced by test Abs in the presence of TSH divided by cAMP produced by medium (29, 30, 54). RSR4 Ab was kindly supplied by Dr. B. Rees Smith (RSR Ltd., Cardiff, Wales, UK).
NC35 indicates that the Ab binding target was CHO-NC35 cells expressing a TSHR variant lacking residues 316–366 and also included amino acid substitutions at 367–369.
Signaling molecules by proteomic array
All known GPCR signaling pathways were investigated by a proteomic array performed at the Kinexus Co. Arrays were performed with TSH, control mAbs, and neutral TSHR-mAbs (Tab-16). These arrays were based on Ab-coated slides for capturing specific signaling molecules. The Ab array comprised a total of 650 proteins including 257 phosphorylated and 346 nonphosphorylated signaling proteins.
Confirmation of signaling molecules by immunoblots
Immunoblots were performed as described (16). Following pathway phospho-specific Abs were used: ERK (c-Raf, Ser338; MAPK/ERK kinase (MEK1/2), Ser217/221; ERK1/2, Thr202/Tyr204; p90 ribosomal kinase (RSK), Ser380) and PKCζ/λ (Thr410/403), Akt (Ser473), mTOR (Ser2448), S6K (Thr389), PKA-C (Thr197), CREB (Ser133), p38 (Thr202/Tyr204), and NF-κB (p65, Ser276) (Cell Signaling Technology, Beverly, MA). Mouse mAb to β-actin and unrelated control mAbs (IgG2, κ-chain) were from Sigma and BD Biosciences (Franklin Lakes, NJ), respectively.
Multiplex assay for cytokine and chemokine profiling
Procarta cytokine profiling kits from Panomics (Fremont, CA) were used to measure 18 different cytokines and chemokines. Procarta protein assays use mutlianalyte profiling beads to enable the detection and quantitation of multiple protein targets simultaneously. A Luminex reader from PerkinElmer (CS1000 autoplex analyzer; Waltham, MA) was used for detection. The detection limit for each cytokine was 1.28 pg/ml or less.
Apoptotic assays
Synchronized FRTL-5 cells were grown as described and cultured with increasing concentrations of N-mAbs. Annexin V with propium iodide (PI) and MitoProbe JC-1 flow cytometry assay kits from BD Biosciences and Invitrogen (Carlsbad, CA), respectively, were used to determine apoptotic cells. In the apoptosis assays, we extended the induction time by changing to new medium containing Ab on d 3 and observed them microscopically every day. Apoptosis became apparent on d 5. These cells were trypsinized and stained with fluorescein isothiocyanate-conjugated annexin V with PI or a JC-1 kit according to the manufacturer’s protocols. To confirm apoptosis being induced either on adherent or on trypsinized live FRTL-5 cells, both kits were used for the microscopic analyses of these treated cells.
Fluorometric detection of reactive oxygen species (ROS) by dichlorodihydrofluorescein diacetate (H2DCF-DA) or dihydrorhodamine 123 (H2R123)
In these assays, H2DCF-DA or H2R123 oxidizes a nonfluorescent probe to a fluorescent one that is detected by either flow cytometry or spectrofluorometry (31). Briefly, synchronized adherent cells were loaded with 50 μm H2DCF-DA (Invitrogen) or H2R123 (AnaSpec Inc., Fremont, CA) fluorescent dyes by incubating them at 37 C for 15 min. Cells were then washed twice with PBS and stimulated with Abs or H2O2 for the indicated time in the incubator. Cells were washed again with PBS and harvested with trypsin. After washing twice, cells were lysed by ultrasonication, centrifuged, and the supernatants analyzed by a spectrofluorometer (Coulter, Miami, FL).
Statistical analysis
The paired t test was used to evaluate the significance of differences in means for continuous variables. P ≤ 0.05 was used to determine statistical significance. A 30% increase in proteins over controls in the microarray was considered significant. Data are presented as the mean ± sd.
Results
Neutral TSHR-Abs in patients with GD
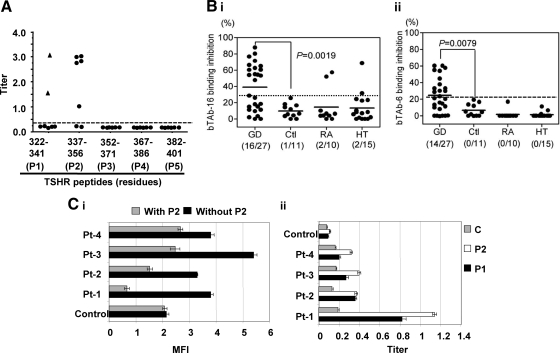
Among 26 overlapping human TSHR synthetic peptides, representing the entire TSHR, five of seven sera from TSHR-immunized hamsters recognized one or the other or both of the two major linear epitopes in the cleavage region, which we had previously identified as the major epitopes for neutral TSHR mAbs (Fig. 1A) (4,28). To examine whether the sera from Graves’ patients bound to these same neutral TSHR Ab epitopes, we used the same two peptides (P1 and P2) and competed the patient samples for binding with neutral-labeled TSHR-mAbs (Tab-16 and Tab-6). We found 16 of 27 GD patients with TSHR neutral Abs (59%) (Fig. 1, B and C) not seen in normal controls. Three positive sera were also found in patients with HT or rheumatoid arthritis.
Figure 1.
Hamster mAbs recognized TSHR linear epitopes. A, Titers of mAbs indicated that the major epitopes were directed against the peptide sequence of 322–341 (P1) and 337–356 (P2) amino acid residues by a competitive inhibition ELISA with specific mAbs against the epitopes (Tab-16 and Tab-6, respectively). B, These two epitopes were also recognized by human IgGs from patients with GD. The frequency of these Abs was significantly higher in Grave’s patients compared with IgG from healthy individuals (P < 0.008). Ctl, Control; RA, rheumatoid arthritis; HT: Hashimoto’s thyroiditis. C, Flow cytometry recognized Abs against the human TSHR in CHO cells (i) and the indirect peptide ELISA (ii) also confirmed the presence of serum IgGs against peptides P1 and P2. A TSHR unrelated control peptide (C) failed to produce any significant binding (ii). Flow cytometry indicated that the binding of Abs was apparently inhibited by peptide (P2) as deduced by the reduction in binding of individual patient’s IgGs when incubated with the specific peptide (i). Inhibition ELISA and indirect ELISA were performed in duplicate and repeated twice. Similarly flow cytometry was repeated twice with different IgG preparations. MFI, Mean fluorescent intensity.
To confirm binding of Graves’ IgGs with the cleavage region, we also performed flow cytometry and an additional indirect peptide ELISA on four randomly chosen Graves’ IgGs from these groups (Fig. 1C). For flow cytometry, we used fixed (4% paraformaldehyde in PBS, pH 7.4) TSHR-expressing cells (JPO9) to prevent the binding of conformationally dependent stimulating and blocking TSHR Abs. Using four different purified Graves’ IgGs known to contain TSHR Abs, we showed significant nonconformationally dependent binding, which could be partly inhibited by cleavage region P1 (data not shown) and P2 [Fig. 1C (i)]. Similarly, the indirect ELISAs showed highly significant binding to both peptides [Fig. 1C (ii)]. Hence, all three methods confirmed the presence of neutral TSHR Abs directed at the cleavage region in patients with GD. Even more evidence was obtained using flow cytometry based on fixed cells expressing the TSHR devoid of the cleavage region (NC35 cells). These cells showed no IgG binding (28).
Rodent neutral TSHR mAbs do not induce cAMP generation but do use the PKA II pathway
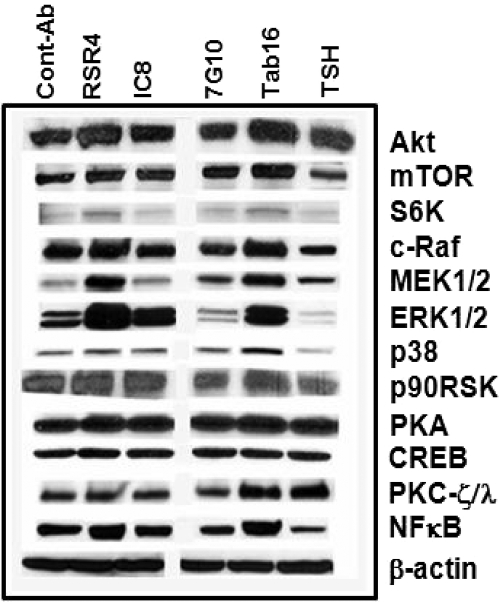
To examine the functional characteristics of neutral Abs to the TSHR, as found in patients with GD, we used a panel of mAbs generated from hamsters and mice (Table 1) (4,28). We previously characterized TSH and N-mAb responses in rat thyroid FRTL-5 cells and CHO-TSHR cells, and both dose and time response studies confirmed that TSH induced cAMP generation but not these N-mAbs (16). Furthermore, to examine the possibility of different signaling pathway activation, we then studied cAMP-dependent protein kinase (PKA). We have previously shown, and now confirm (Fig. 2), that many neutral TSHR mAbs can induce the phosphorylation of PKA (16). This enzyme exists as two regulatory isoforms, R1 and R2, that distinguish PKA type I (PKA-I) and type II (PKA-II) isozymes. Their spatiotemporal regulation is known to ensure signaling specificity. Using a protein microarray, we found that a neutral TSHR Ab induced the PKA-II pathway and primarily activated R2α (S98) and R2β (S114) (Table 2). Interestingly, TSH failed to activate PKA-II but did activate PKA-α/β(T197) and PKA-β (S338) catalytic subunits, possibly via activation of a PKA-II inhibitory loop.
Figure 2.
Representative immunoblots of signaling molecules. Quiescent, starved cells were stimulated with a fixed concentration (1.0 μg/ml) of different N-mAbs and TSH (1.0 mU/ml) over 60 min of incubation. They showed variable changes in signaling molecules as shown in these immunoblots. RSR4, with an epitope similar to Tab-16 (P1), induced a robust effect on most of the signaling molecules with increased activities of Akt/mTOR/S6K, c-Raf/MEK1/2/p38/p90RSK, PKA/CREB, PKC-ζ/λ, and NF-κB. 7G10, which recognized a different epitope (P2), did not induce such effects, whereas TSH ligand activated Akt but not mTOR/S6K. TSH also activated PKC-ζ/λ and most importantly it suppressed c-Raf/MEK1/2/p38/p90RSK signaling cascade. By contrast, isotype control mAb repeatedly did not produce any significant activity. The experiments were repeated twice and confirmed the illustrated findings.
Table 2.
Changes in representative proteins in Ab- and TSH-treated thyroid cells
| PKA
|
PIK/Akt/mTOR/S6K/eIF
|
PKC
|
MAPK
|
||||
|---|---|---|---|---|---|---|---|
| Proteins | N-mAb/TSH | Proteins | N-mAb/TSH | Proteins | N-mAb/TSH | Proteins | N-mAb/TSH |
| Cα/β (T197) | −43/64 | PI3K-δ (110, pan) | 44/2a | α (S657) | 19/−26a | MEK1/2 (S217/221, S4) | 87/−34a |
| Cβ (S338) | −3/29 | PI3K-α (pan) | 23/49 | β1/2 (T500) | 86/−51a | MEK1 (T291) | 29/−46a |
| R2α (S98) | 94/5a | PI3KR4 (pan) | −21/64 | γ (T514) | 44/7a | ERK1/2 (T+Y; T/Y) | 45/−17a |
| R2β (S114) | 80/−16a | PI4K-2β (pan) | 13/43 | γ (T655) | 104/3a | RSK1/2 (S363/369) | 93/−43a |
| PIP5K2α (pan) | 35/27 | γ (T674) | 82/10a | RSK1/3 (T573/577) | 74/−9a | ||
| PDK1 (S244) | 0/51 | δ (Y313) | 79/−23a | MSK1 (S376) | 26/−44a | ||
|
JAK/STATs/SOCS4
|
Akt1 (T308) | 38/76 | δ (T507) | 28/8a | p38α (T180+Y182) | 188/−33a | |
| JAK2 (Y1007/1008) | −58/−31 | Akt1 (S473) | 131/48a | δ (S645) | 28/−13a | Mnk1 (T209/214) | 115/1a |
| STAT1 (Y701) | −9/24 | mTOR (S2448) | 173/−5a | δ (S664) | 112/−46a | JNK (T183/Y185) | −32/−16a |
| STAT2 (Y690) | 180/−10a | S6K-40S (S235) | 104/−29a | η (T655) | 72/−4a | Jun (S73) | −66/−5 |
| STAT3 (S727) | 114/107 | S6K (T229) | 43/−39a | θ (S676) | 31/−19a | Jun (S63) | 61/−15a |
| STAT3 (S705) | −14/43 | elF2Bε (S540) | 45/−1a | ζ/λ (T+T) | 60/46 | Fos (T232) | −18/33 |
| STAT4 (pan) | −23/0 | elF4G (S1107) | −34/27 | ||||
| STAT5A (Y694) | 49/−25a | AMPK-α (T174/172) | −45/−27 |
NF-κB
|
|||
| STAT6 (pan) | 44/−2 | GSK3 (S21/9) | −39/−17 | I-κBα (pan) | −3/17 | ||
| SOCS4 (pan) | −13/−41 | I-κBβ (pan) | 1/0 | ||||
| IKKα (pan) | 69/−33a | ||||||
|
Protein-serine phosphatases
|
ρ/Rac
|
IKKα (S180/181) | −20/−40 | ||||
| PP1/Ca (pan) | 49/4a | ROCK1 (pan) | 188/−8a | IKKβ (pan) | −9/6 | ||
| PP2A/Aα/β (pan) | 123/−4a | ROCK2 (pan) | 249/−28a | IKKγ (NEMO, pan) | 109/−14a | ||
| PP2A/Cα (pan) | 22/−33a | RAC1 (pan) | 38/146 | NF-κB p50 (pan) | 35/30 | ||
| PP2B/Aα(pan) | 31/5a | RAC1 (S71) | 15/36 | NF-κB p65 (pan) | 10/35 | ||
| NF-κB p65 (S276) | 114/−33a | ||||||
A change of 30% increase or decrease over control indicates significant.
Significant activation or suppression.
Neutral TSHR mAbs also activate the PI3K/Akt/mTOR/S6K signaling cascade
Different PI3Ks were found to be elevated by certain TSHR N-mAbs and TSH. The Akt/mTOR/S6K cascade was increased by two of the four mAbs tested (Fig. 2). In the microarray, the N-mAb was able to produce high activities of PI3K-p110, PI3KR4, and PIP5K2α, whereas TSH increased total PI3K and PI4K2β (Table 2). In contrast, the N-mAb did not activate PDK1-S244 phosphorylation, whereas TSH showed a significant effect. Both activated two phosphorylation sites on Akt1. Interestingly, only the N-mAb significantly activated the Akt1-S473 site (Table 2). A strong significant increase in mTOR activation was also observed but, again, not seen in these cells by TSH (Table 2). In parallel with Akt1/mTOR activation, the specific Akt downstream effector S6K was also activated as judged by binding to phosphorylation-specific Abs (S235 and T229). These observations indicated that selected N-mAbs were capable of producing a robust effect on the PI3K/Akt/mTOR/S6K signaling cascade, an important arm of the GPCR signaling cascade.
To further analyze the mTOR signaling activation pathway, we examined both upstream activators and downstream effectors of this cascade. Under baseline conditions, the tuberous sclerosis complex (TSC)-1-TSC2 produced downstream inhibitory signals to Ras homolog enriched in brain to suppress mTOR activity, whereas MAPK-ERK1/2 and Akt gave suppressive effects on the TSC1-TSC2 complex (data not shown). It is known that once MAPK and Akt become activated, they release the inhibitory effect on the mTOR/S6K pathway (32). Furthermore, one of the MAPK downstream effectors, p90RSK, is known to feed back and activate mTOR directly. As demonstrated below, Akt, MAPK, and p90RSK all were activated by the N-mAb in the array (see below).
AMP-activated protein kinase and glycogen synthase kinase-3, two important upstream molecules that activate the TSC1-TSC2 complex and inhibit mTOR, were suppressed in the protein array (Table 2), inducing a net activating effect on mTOR. One downstream effect of mTOR is an increase in the p70S6K/elF4B module and coinhibition of eukaryotic translation initiation factor 4 binding protein. As expected, both p70S6K and its downstream molecule eukaryotic translation initiation factor 4B were activated in parallel with the reduced eukaryotic translation initiation factor 4 epsilon binding protein activity, further confirming activation of the mTOR/S6K pathway by the N-mAb.
N-mAbs induce both PKC and ERK1/2/p38α pathway modules but not JNK in MAPK signaling cascades
The microarray comprised all PKC isozymes including classical, novel, and atypical, and the N-mAb activated most of their phosphorylation sites (Table 2), whereas TSH activated only an atypical isozyme, PKCζ/λ, similar to our previous data (16). The phosphorylation of the MAPKs is of major importance to cell function and was examined extensively in the microarray approach. These kinases include the ERKs, ERK1 and ERK2 (p44-MAPK and p42-MAPK, respectively), the stress-activated protein kinases (stress-activated protein kinases/JNKs), and p38 kinase. Recent data have shown that the activation of MAPK/ERK induced by GPCRs such as the TSHR is mediated by both Gαq and Gβγ subunits and involve a common signaling pathway with receptor-tyrosine kinases (32). Gβγ-induced MAPK is mediated by activation of PI3K, followed by a series of tyrosine phosphorylation events that involve functional association among the adaptor proteins Shc, Grb2, and Sos. Subsequently stress-activated protein kinases/JNKs and p38 are able to be activated by Gβγ proteins in a pathway involving Rho family proteins including RhoA, Rac1, and Cdc42 (17). As described earlier (16), TSH- and TSHR-stimulating Abs did not induce the cRaf/ERK1/2 module but two of the four N-mAbs activated it (Fig. 2). Consistent with these results, activating upstream molecules of ERK1/2/p38α and their downstream effectors RSK/MNK1/MSK1/MAPKAPK2 were all activated by the N-mAb (Table 2). In contrast, TSH suppressed most of these elements. Indirect confirmation of ERK1/2 (T202+Y204; T185/Y187) activation also came from the higher activities of immediate upstream activators MEK1/2 (S217+S221, S4). Similar step-wise pathway module activation was also seen for p38α (Fig. 2). Upstream activators and downstream effectors of p38α are known to be MEK3 and MNK1/MAPKAPK2, respectively, and were increased by the N-mAb but not TSH. Similarly, whereas JNK activity was reduced by both TSH and N-mAb, Jun, an immediate early gene marker that binds with activator protein 1 and one of the downstream effectors of MAPK/JNK phosphorylation was also activated only by the N-mAb and not TSH (Table 2). Whereas TSH lacked such effect, it was able to activate Fos, another early gene product downstream to MAPK/ERK1/2. This suggested that these differences between N-mAb and TSH were indeed ligand dependent.
We then analyzed certain signaling molecules that might contribute to the higher activities of MAPK family signaling proteins. Shc1 (Y349+Y350) activity was not significantly affected by either TSH or N-mAb. Grb2 and Sos were not included in the array. However, we found increasing activities of Rho family proteins by both TSH and N-mAb. TSH induced Rac1/cdc42 activity (data not shown) and suppressed RhoA kinase-β, whereas N-mAb activated both RhoA protein kinase-α (2.5-fold) and -β (1.8-fold) very significantly.
Neutral mAbs activate NF-κB signaling
In the nucleus, NF-κB regulates genes encoding cytokines, cytokine receptors, cell adhesion molecules, proteins involved in coagulation, and genes involved in cell growth control. Because these are important end points of thyroid cell activity, we also examined this important pathway. Activation of NF-κB is initiated by the signal-induced degradation of inhibitory-κB (I-κB) proteins. This occurs primarily via activation of a kinase called the I-κB kinase (IKK). IKK is composed of a heterodimer of the catalytic IKK-α and IKK-β subunits and a master regulatory protein termed NEMO (NF-κB essential modulator) or IKK-γ (33). A variety of stimuli lead to the rapid nuclear accumulation of NF-κB by the induced phosphorylation and subsequent degradation of I-κB. The Tab-16 N-mAb activated NF-κBp65 (S276) (Fig. 2) and two I-κB kinases, IKK-α and NEMO, whereas it suppressed IKK-α phosphorylation (Table 2). In contrast, TSH suppressed most of these signals (Table 2).
Differential activation of JAK/STAT and SOCS-4 molecules by TSH and N-mAbs
TSH is known to activate Stat3 and so did the N-mAb (Table 2). However, the N-mAb also activated STAT2 and -5a (Y690 and Y694), robustly suggesting a wider effect on cytokine production. In keeping with this, SOCS-4, an important regulatory protein that suppresses cytokines, was not activated (Table 2).
Modulation of cytokines and chemokines by N-mAb
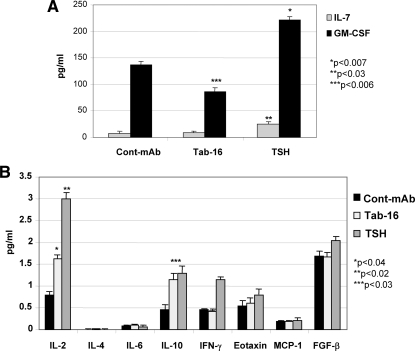
Having shown that STAT and NF-κB were activated, we hypothesized that certain thyroid cell responses produced by the Ab-TSHR signalosome activity would result in important immune regulation. Among 18 cytokines and chemokines assessed by multiplex bead array, responses were observed in six (Fig. 3, A and B). Granulocyte macrophage colony-stimulating factor (GM-CSF), IL-7, and interferon-γ were increased by TSH but not by the Tab-16 N-mAb, which inhibited these cytokines in the cell lysate compared with the isotype control Ab. In contrast, IL-2 and IL-10 were induced by both TSH and N-mAb, although the Ab effect was small. IL-12 (p70), IL-13, IL-17, IL-1β, IL-8, macrophage inflammatory protein-1β, TNF-α, and vascular endothelial growth factor were present at concentrations less than 1.28 pg/ml and no significant changes were evident (data not shown).
Figure 3.
Multiplex cytokines in the cell lysates. A, Tab-16 neutral mAb attenuated GM-CSF induction significantly compared with control Ab, whereas TSH induced both IL-7 and GM-CSF significantly higher than control mAb (Cont-mAb). B, Both Tab-16 and TSH induced IL-2 and IL-10 significantly higher than control. There was no other significant induction of cytokines either by Tab-16 or TSH. The assay was performed twice in duplicate. IFN, Interferon; MCP, monocyte chemotactic protein; FGF, fibroblast growth factor.
N-mAbs induce apoptosis
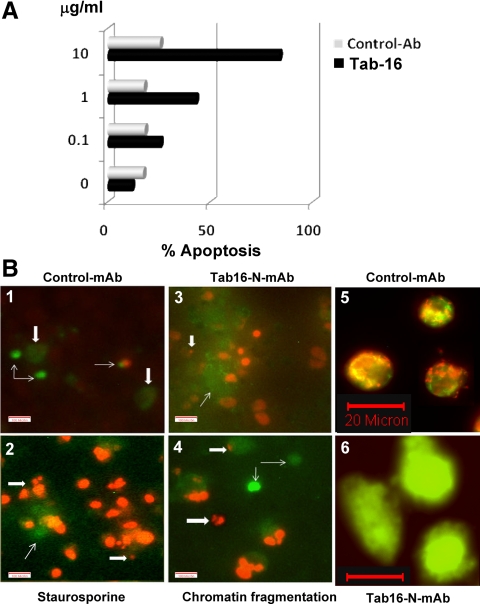
Overactivation of multiple signaling cascades along with the NF-κB activation including downstream effectors IL-7 and GM-CSF levels in cell lysates led us to examine cell viability in the cultures under investigation. We performed both proliferation and apoptosis assays as our end point readouts. From our previous studies, we knew that Tab-16 N-mAb induced some proliferation but markedly less than TSH or TSHR stimulating Abs (16). In the present studies, the FRTL-5 rat thyroid cells started to show apoptotic features after d 5 of culture, and marked dose-related apoptosis induced by Tab-16 was confirmed by both flow cytometry and microscopy of annexin V plus PI staining and JC-1 staining (Fig. 4). Similar results were obtained by IC8 and RSR4 mAbs (data not shown).
Figure 4.
Annexin V binding to apoptotic cells by flow cytometry and microscopy. A, Increasing concentrations of Tab-16, but not the control Ab, induced apoptosis in thyrocytes. Cells were exposed to Tab-16 (1 μg/ml) and induced apoptosis that was stained on d 5 for flow cytometry. Four mAbs to similar epitope were used twice to ensure reproducibility. B, Apoptosis was also confirmed by microscopic observation of annexin V-positive cells (thin arrow) and propium iodide (thick arrow) staining of nucleus in the treated cells. Staurosporine, a known PKC inhibitor that induces apoptosis at 10 μm, was a positive control. Both the Tab-16 and staurosporine induced nuclear DNA fragmentation (thick arrow) as shown in B-2, B-3, and B-4. By contrast, control Ab induced significantly less effect (B-1). JC-1 staining (a mitochondrial dye or mitotracker) in live cells showed green fluorescence, as another indicator of apoptosis (B-6), whereas untreated cells exhibited both green and red fluorescence (B-5), indicating preserved mitochondrial function.
N-mAbs activate oxidative stress signaling cascades
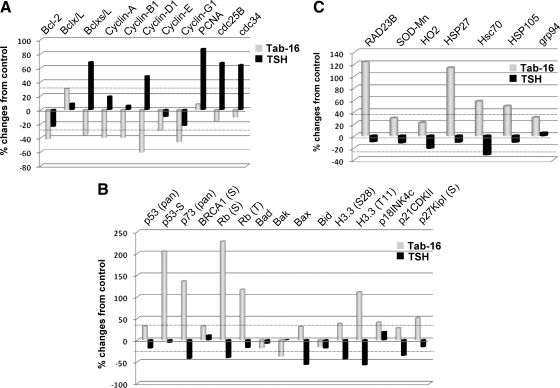
To explore possible mechanisms of apoptosis by N-mAbs, we then analyzed the proteomic array for pro- and antiapoptotic-, cell cycle-, DNA-damage-, TNF/Fas-, caspase-, and oxidative stress-related proteins. In the case of antiapoptotic responses, TSH induced Bclxs/L and to a lesser degree Bclx/L, whereas the N-mAb induced mainly Bclx/L and the Ab suppressed Bclxs/L. Both failed to activate Bcl-2, an important antiapoptotic protein. Regarding proapoptotic proteins, the Ab activated Bcl-2-associated X protein, and TSH inhibited all of them. In the proliferative response, TSH activated most of the cyclins, including proliferating cell nuclear antigen (PCNA). In contrast, N-mAb suppressed all of them and showed no significant effect on PCNA. Cell cycle-related proteins revealed activation of certain cyclin-dependent kinase-1/2 phosphorylation (T14+Y15) by the N-mAb, a negative regulator of cell cycle progression, whereas TSH did the opposite. In parallel with the positive regulator of cell cycle progression, TSH induced cyclin-dependent kinase-1, -2, and -6, cdc25B, and cdc34, whereas the N-mAb inhibited them. Surprisingly, the N-mAb activated most of the cell cycle-related kinase inhibitor proteins (Fig. 5A), and TSH produced suppressive effects. Both TSH and N-mAb inhibited caspase activities, and the Ab showed more significant effects. There were no significant responses in the TNF/Fas signaling molecules when TSH was compared with the Ab.
Figure 5.
Antiapoptotic and stress-induced proapoptotic signaling molecules by protein array. A, TSH but not N-mAb activated multiple antiapoptotic proteins including Bclx/L, Bclxs/L, cyclins (A, B1, and D1), PCNA, and cell cycle-related proteins. Both TSH and Tab-16 failed to activate Bcl-2. B, N-mAb activated stress-induced DNA damage proteins including p53, p73, breast cancer type 1 susceptibility protein (BRCA1), and retinoblastoma (Rb). Tab-16 also activated many proapoptotic proteins, importantly Bcl-2-associated X protein (Bax) and cyclin-dependent kinase inhibitors. TSH did the opposite. C, N-mAb activated DNA stress (Rad23B), mitochondrial stress (SOD-Mn), hemeoxygenase (HO2), heat-shock p70-related protein (Hsc70), and stress-induced endoplasmic reticulum protein (glucose regulated protein 94). The TSH effect was minor.
N-mAb activated many DNA damage-related proteins including p53, p73, BRCA1, Rb, and stress-related phosphatases, whereas TSH inhibited most of them (Fig. 5B). As expected and in parallel with these findings, N-mAb activated Rad23B, superoxide dismutase (Mn-SOD), and hemeoxygenase 2 and also activated heat shock 70-kDa protein 8 and glucose-regulated protein 94, a stress-related protein in the endoplasmic reticulum (Fig. 5C). TSH did not induce any of them. These findings indicate that oxidative stress-related proteins may be responsible for the activation of the DNA damage signaling cascades. Because ROS are oxidative stress molecules and are one of the important key effectors in DNA damage responses, we studied ROS induction in these FRTL-5 thyrocytes.
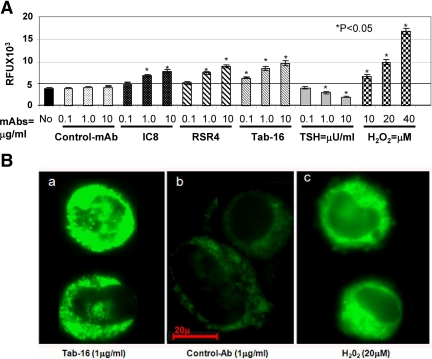
To explore ROS induced by the Abs, we performed a series of time- and dose-dependent assays. A dose-dependent increase in ROS activity was observed by the N-mAbs but not by TSH or control Ab (Fig. 6) and was further confirmed by the microscopic observations. In fact, TSH suppressed ROS activity in a dose-dependent manner.
Figure 6.
Oxidative stress or ROS in cells detected by spectrofluorometry. A, N-mAbs and H2O2 activated ROS, whereas control Ab and medium alone did not. ROS was observed by relative fluorescent units (RFU) stained with H2DCF-DA. In contrast, TSH reduced ROS activity dose dependently. The highest activity of ROS was observed at the highest concentration of the N-mAbs (10 μg/ml) and H2O2 (40 μm). B, FRTL-5 cells were also stained with H2R123 (a ROS indicator) and observed under the microscope. Tab-16 (a) and H2O2 (c) activated ROS as determined by a clear increase in green fluorescent intensity, whereas such change was not seen with control mAb (b). Both spectrofluorometry and microscopy were repeated twice in duplicate.
Discussion
Autoantibodies to the TSHR are keys to the disease process in autoimmune thyroid disease, particularly GD. The repertoire of TSHR Abs includes stimulating, blocking, and neutral varieties. Both stimulating and many blocking Abs are conformational and exert variable effects on thyrocyte growth (16). Our recent study clearly indicated, using monoclonal hamster TSHR Abs of the neutral variety, that they induced certain signaling cascades with unclear downstream effectors (16). The present study was undertaken to confirm the existence of such Abs in Graves’ sera and to further characterize signaling events relating to downstream effector mechanisms. These data confirmed and extended the earlier observations (16) that neutral Abs are indeed present in human GD using three independent immunoassays (Fig. 1). Moreover, our findings of signaling mechanistics, in a broader sense, implicate that such N-mAbs play an important pathogenic role in the disease process.
Methodological differences can affect the detection of TSHR neutral Abs and the assessment of their pathogenic significance. Nonspecific binding by cross-reactive epitope(s) may result in false-positive results. Differences in Ab avidity and class may also influence detection. We were also concerned that ELISAs may detect cell surface Abs of low avidity that may predominate in many diseases other than GD. However, flow cytometry-based assays detect only high avidity Abs of greater pathogenic importance. Indeed, using fixed cells expressing the full-length TSHR for flow cytometry, in the presence of target peptides, we saw a significant reduction in the binding of neutral Abs to the TSHR cleavage region that confirmed the specificity of peptide binding in the competitive inhibition ELISA or the indirect ELISA.
The presence of neutral Abs is common to autoimmune thyroid disease because they are also present in HT and possibly in rheumatoid arthritis with concurrent immunological disorders. Animal models of autoimmune thyroid disease are in agreement with such a notion (34) and further substantiate that most neutral Abs to the TSHR are directed against the cleavage region (4). Although such Abs have no influence on TSH binding, they induced distinct signaling imprints at the TSHR (16) and thus represented a novel class of TSHR Abs.
To clarify what role neutral Abs may play in the myriad of signaling cascades, we performed a series of investigations using well-characterized TSHR N-mAbs, including proteomic arrays, immunoblots, multiplex cytokine and chemokine detection, cell proliferation, and cell apoptosis assays. Because TSH is an agonistic ligand for the TSHR and gives positive physiological responses, we used this as a common denominator for normal responses and thus compared TSH signaling effects to that of the N-mAbs. Our proteomic array results of signaling molecules confirmed our recent published study (16). The array findings were in agreement with the multitude of different upstream and downstream molecules in each signaling cascade. This was true particularly in the case of PI3K/Akt/mTOR/S6 and PKC/MAPK signaling pathways (Fig. 2). However, the array was able to demonstrate involvement of many additional GPCR signaling cascades initiated by the TSHR including NF-κB/STAT, apoptosis/proliferative/cell-cycle, and stress/DNA-damage signaling cascades.
A preferential use of PI3Ks was seen by N-mAbs with the activation of downstream effectors Akt/mTOR/S6/elF that was absent in the case of TSH, which induced the PI3K/PKD/Akt activation loop. At present, it is not clear whether this usage is responsible for the downstream activation of the cascade or whether other signaling cascades may have contributed to this effect. It is more likely that the signaling strength together with PKC/MAPK activation may have induced the mTOR/S6/elF cascade by the Ab. Signal inhibitors and/or knockdown experiments with short hairpin RNA are needed to help resolve these critical connections.
Whereas the proteomic array confirmed our earlier observations of MAPK activation, the hyperphosphorylation of multiple PKC isozymes by N-mAb was also revealed, and our immunoblots were also in agreement with these findings (Fig. 2). Because PKC classical and novel isozymes were not activated by TSH, it is likely that classical and some novel isozymes may be involved in the activation of ERK1/2/p90RSK or p38/MSK1, as noted by others in different cells (19). It seems unavoidable to illustrate ERK1/2 activation loops by PI3K/PKC because it has also been under the influence of cAMP/PKA activation loops via exchange proteins activated by cAMP/Rap1 (23,35). Our array results indicated that N-mAbs activated the PKA-II compartment (regulatory unit 2) instead of PKA-I (regulatory unit 1), which was induced by TSH. These observations may have been impacted by the availability of kinases and their substrates to phosphorylate other signaling proteins and thus may explain why the spatiotemporal dynamic association is so important to define cAMP/PKA signaling compartments via activation of specific GPCR for specific functions. Recent findings on PKA-I and PKA-II compartmentalization in a different model system are congruent to this hypothesis (36).
N-mAb activated NF-κB via IKKα/IKKγ kinases and was inhibited by TSH. NF-κB activation is known to be induced by cytokines (TNF and interferons) in FRTL-5 cells, and those cytokines have been implicated in innate immune activation as well as major histocompatibility complex protein expression (37,38). It is also well known that activated NF-κB is a potent inducer of certain cytokines and is important for cell survival (39,40). Interestingly, induction of certain cytokines induced by the N-mAb is in line with NF-κB activation. Activation of certain JAK/STAT/SOCS signaling cascades may have contributed to this event (37,38). Interestingly, N-mAb activated most of the STATs and TSH activated only STAT3, and both of them inhibited JAK2 and SOCS4. These findings may have been evolved by the overactivation of certain signaling cascades to overcome harmful signaling responses to keep cells functioning normally. Alternatively, N-mAb may form an immune complex tethered onto the surface and therefore may activate other receptors present on the thyrocyte via their Fc region. Moreover, if thyrocytes are unable to degrade these internalized complexes, this process may perturb normal signaling responses and thus bring deleterious effects to cells. Further study is indeed required to help evaluate these hypotheses.
In GD, the thyroid tissue is under chronic exposure to Ab that is associated with the immune pathology. Here we set out to answer the question of whether chronic stimulation of cells by N-Abs can bring good or bad responses. Thyrocytes were exposed to N-mAbs using an in vitro culture system time dependently. Chronic stimulation demonstrated the distinct morphological feature of thyrocyte death resembling apoptosis, and the observation was subsequently confirmed by the apoptotic assays. In keeping with this observation, we found activation or suppression of multiple apoptotic- and antiapoptotic-related signaling cascades induced by the N-mAb including the exclusive activation of stress-induced DNA damage signaling molecules.
DNA damage has been known to be activated by metabolic stress, irradiation, UV rays, and toxic chemicals (41). Because cultured thyrocytes were treated only with the Abs, we reasonably assumed that metabolic stress induced in a receptor-specific manner must have caused the DNA damage as also noted by activated multiple suppressor oncoproteins and their downstream effectors. Remarkably, the p53 protein, the guardian of the genome, demonstrated a significant increase in phosphorylation (42). Besides the induction of multiple oncoproteins, the microarray also revealed increased activation of certain stress-induced proteins (43), most importantly Mn-SOD. Oxidative stress or ROS has been well known to cause stress-induced DNA damage (44), and our results provide evidence for oxidative stress activation induced by the TSHR N-Abs. To confirm ROS was being induced by the thyrocytes when exposed to the N-mAbs, a dose-dependent increase in ROS production was evident (Fig. 6). Clearly these findings confirmed our array results of ROS production by the Ab. Although overactivation of multiple signaling mechanisms (Ca+/PKC/MAPK, Akt/mTOR, NF-κB) (45,46,47,48,49,50,51), either individually or synergistically, may be instrumental in cell death, our findings of ROS activation indicate that it may be the key player for the DNA damage that caused apoptosis of the rat FRTL-5 cells. Oxidative stress itself can regulate ERK/p38, and NF-κB activities and hence N-mAbs must be able to modulate such downstream effects. Further evidence for ROS activation came from significant NEMO activation, which has been shown to cause DNA damage that activates complex damage recognition and signaling cascades including cell-cycle arrest, DNA repair, and apoptosis (52,53). Our findings shed a new light on the activation of stress signaling by Abs targeting the TSHR, and further studies are necessary to delineate key signaling events in the thyrocyte oxidative stress networks.
These findings are no different from those that target other GPCRs. For example, Abs directed to a glutamate receptor-induced apoptosis in animal models (54,55,56,57) of autoimmunity. In mice, passive transfer of TSHR stimulating mAbs induced hyperthyroidism with apoptotic thyrocytes on histology, suggesting that such TSHR stimulating Abs were also able to cause cell death (58). The presence of apoptotic thyrocytes in GD thyroid tissue has been demonstrated (59), and because apoptosis is thought to be a key player in autoimmune disease, such findings indicate that Ab-induced stress signaling may be one of the novel mechanisms that is involved in thyrocyte death in autoimmune thyroid disease.
In conclusion, although TSHR N-Abs activated multiple signaling cascades, multiple lines of evidence indicate that Ab-induced cell death occurred via oxidative stress signaling mechanisms in thyrocytes. This finding has important implications for the generation and persistence of chronic inflammation in autoimmune thyroid disease. Because apoptosis is a viable mechanism for continuous antigen production and antigen presentation by macrophages and dendritic cells, it may well contribute to the immune response. Thus, Abs directed against TSHR linear epitopes in autoimmune thyroid disease may initiate an inflammatory process by the activation of selective signaling pathways and subsequent oxidative stress-induced thyrocyte death, which may further sustain the vicious cycle of chronic inflammation.
Acknowledgments
Special thanks go to Drs. Julia Kaufman and Salina Parveen (Rockefeller University, New York, NY) for cytokine array analysis and Drs. Bernard Rees Smith and Jane Sanders (RSR Inc., Cardiff, Wales, UK) for provision of certain TSHR mAbs.
Footnotes
This work was supported by National Institutes of Health Grants DK069713 and DK052464 and the Veterans Affairs Merit Award Program.
Disclosure Summary: S.A.M., T.A., and R.L. have nothing to declare. T.F.D. is a member of the Board of Kronus Inc. (Boise, ID), which markets diagnostic kits including those for thyroid autoantibodies.
First Published Online September 15, 2010
Abbreviations: Ab, Antibody; CHO, Chinese hamster ovary; CREB, cAMP response element-binding protein; GD, Graves’ disease; GM-CSF, granulocyte macrophage colony-stimulating factor; GPCR, G protein-coupled receptor; HT, Hashimoto’s thyroiditis; I-κB, inhibitory-κB; IKK, I-κB kinase; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; mAb, monoclonal Ab; MEK, MAPK/ERK kinase; Mn-SOD, superoxide dismutase; mTOR, mammalian target of rapamycin; N-Ab, neutral Ab; NEMO, NF-κB essential modulator; NF-κB, nuclear factor-κB; N-mAb, neutral TSHR monoclonal antibody; PCNA, proliferating cell nuclear antigen; PI, propium iodide; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PKC, protein kinase C; ROS, reactive oxygen species; H2DCF-DA, dichlorodihydrofluorescein diacetate; RSK, p90 ribosomal kinase; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TSC, tuberous sclerosis complex; TSHR, TSH receptor.
References
- Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP 2001 Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22:631–656 [DOI] [PubMed] [Google Scholar]
- Medina DL, Santisteban P 2000 Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur J Endocrinol 143:161–178 [DOI] [PubMed] [Google Scholar]
- Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF 2002 A monoclonal thyroid-stimulating antibody. J Clin Invest 110: 1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Latif R, Daniel S, Eguchi K, Davies TF 2004 Dissecting linear and conformational epitopes on the native thyrotropin receptor. Endocrinology 145:5185–5193 [DOI] [PubMed] [Google Scholar]
- Ando T, Latif R, Davies TF 2004 Concentration-dependent regulation of thyrotropin receptor function by thyroid-stimulating antibody. J Clin Invest 113:1589–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Jeffreys J, Depraetere H, Richards T, Evans M, Kiddie A, Brereton K, Groenen M, Oda Y, Furmaniak J, Rees Smith B 2002 Thyroid-stimulating monoclonal antibodies. Thyroid 12:1043–1050 [DOI] [PubMed] [Google Scholar]
- Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Núñez Miguel R, Blundell TL, Furmaniak J, Rees Smith B 2004 Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14:560–570 [DOI] [PubMed] [Google Scholar]
- Sanders J, Allen F, Jeffreys J, Bolton J, Richards T, Depraetere H, Nakatake N, Evans M, Kiddie A, Premawardhana LD, Chirgadze DY, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2005 Characteristics of a monoclonal antibody to the thyrotropin receptor that acts as a powerful thyroid-stimulating autoantibody antagonist. Thyroid 15:672–682 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Costagliola S, Cetani F, Ducobu J, Stordeur P, Vassart G, Ludgate M 1996 Patient with monoclonal gammopathy, thyrotoxicosis, pretibial myxedema and thyroid-associated ophthalmopathy; demonstration of direct binding of autoantibodies to the thyrotropin receptor. Eur J Endocrinol 134:97–103 [DOI] [PubMed] [Google Scholar]
- Minich WB, Loos U 2000 Detection of functionally different types of pathological autoantibodies against thyrotropin receptor in Graves’ patients sera by luminescent immunoprecipitation analysis. Exp Clin Endocrinol Diabetes 108:110–119 [DOI] [PubMed] [Google Scholar]
- Nagy EV, Burch HB, Mahoney K, Lukes YG, Morris 3rd JC, Burman KD 1992 Graves’ IgG recognizes linear epitopes in the human thyrotropin receptor. Biochem Biophys Res Commun 188:28–33 [DOI] [PubMed] [Google Scholar]
- Vlase H, Graves PN, Magnusson RP, Davies TF 1995 Human autoantibodies to the thyrotropin receptor: recognition of linear, folded, and glycosylated recombinant extracellular domain. J Clin Endocrinol Metab 80:46–53 [DOI] [PubMed] [Google Scholar]
- Latif R, Morshed SA, Zaidi M, Davies TF 2009 The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am 38:319–341, viii [DOI] [PubMed] [Google Scholar]
- Michalek K, Morshed SA, Latif R, Davies TF 2009 TSH receptor autoantibodies. Autoimmun Rev 9:113–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed SA, Latif R, Davies TF 2009 Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology 150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büch TR, Biebermann H, Kalwa H, Pinkenburg O, Hager D, Barth H, Aktories K, Breit A, Gudermann T 2008 G13-dependent activation of MAPK by thyrotropin. J Biol Chem 283:20330–20341 [DOI] [PubMed] [Google Scholar]
- Ciullo I, Diez-Roux G, Di Domenico M, Migliaccio A, Avvedimento EV 2001 cAMP signaling selectively influences Ras effectors pathways. Oncogene 20:1186–1192 [DOI] [PubMed] [Google Scholar]
- Corrèze C, Blondeau JP, Pomérance M 2005 p38 mitogen-activated protein kinase contributes to cell cycle regulation by cAMP in FRTL-5 thyroid cells. Eur J Endocrinol 153:123–133 [DOI] [PubMed] [Google Scholar]
- Devlin MA, Das S, Singh I, Bourgoin S, Brindley DN, Ginsberg J 2000 The characterization of phospholipase D in FRTL-5 thyroid cells. Mol Cell Endocrinol 167:107–115 [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Capobianco L, Salvatore L, Sallese M, D'Ancona GM, De Blasi A 2001 Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60:924–933 [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK 2007 Integrating signals from RTKs to ERK/MAPK. Oncogene 26:3113–3121 [DOI] [PubMed] [Google Scholar]
- Saavedra AP, Tsygankova OM, Prendergast GV, Dworet JH, Cheng G, Meinkoth JL 2002 Role of cAMP, PKA and Rap1A in thyroid follicular cell survival. Oncogene 21:778–788 [DOI] [PubMed] [Google Scholar]
- Suh JM, Song JH, Kim DW, Kim H, Chung HK, Hwang JH, Kim JM, Hwang ES, Chung J, Han JH, Cho BY, Ro HK, Shong M 2003 Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem 278:21960–21971 [DOI] [PubMed] [Google Scholar]
- Urso L, Muscella A, Calabriso N, Ciccarese A, Fanizzi FP, Migoni D, Di Jeso B, Storelli C, Marsigliante S 2005 Differential functions of PKC-Δ and PKC-ζ in cisplatin response of normal and transformed thyroid cells. Biochem Biophys Res Commun 337:297–305 [DOI] [PubMed] [Google Scholar]
- Woloshin PI, Walton KM, Rehfuss RP, Goodman RH, Cone RD 1992 3′,5′-cyclic adenosine monophosphate-regulated enhancer binding (CREB) activity is required for normal growth and differentiated phenotype in the FRTL5 thyroid follicular cell line. Mol Endocrinol 6:1725–1733 [DOI] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Ando T, Latif R, Davies TF 2007 Antibody-induced modulation of TSH receptor post-translational processing. J Endocrinol 195:179–186 [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G 1996 The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambesi-Impiombato FS, Parks LA, Coon HG 1980 Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA 77:3455–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan PK, Ragheb K, Lawler G, Robinson JP 1997 Defects in intracellular oxidative metabolism of neutrophils undergoing apoptosis. J Leukoc Biol 61:481–488 [DOI] [PubMed] [Google Scholar]
- Rozengurt E 2007 Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213:589–602 [DOI] [PubMed] [Google Scholar]
- Salminen A, Suuronen T, Huuskonen J, Kaarniranta K 2008 NEMO shuttle: a link between DNA damage and NF-κB activation in progeroid syndromes? Biochem Biophys Res Commun 367:715–718 [DOI] [PubMed] [Google Scholar]
- Takai O, Desai RK, Seetharamaiah GS, Jones CA, Allaway GP, Akamizu T, Kohn LD, Prabhakar BS 1991 Prokaryotic expression of the thyrotropin receptor and identification of an immunogenic region of the protein using synthetic peptides. Biochem Biophys Res Commun 179:319–326 [DOI] [PubMed] [Google Scholar]
- Dremier S, Vandeput F, Zwartkruis FJ, Bos JL, Dumont JE, Maenhaut C 2000 Activation of the small G protein Rap1 in dog thyroid cells by both cAMP-dependent and -independent pathways. Biochem Biophys Res Commun 267:7–11 [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M 2008 Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res 103:836–844 [DOI] [PubMed] [Google Scholar]
- Kikumori T, Kambe F, Nagaya T, Funahashi H, Seo H 2001 Thyrotropin modifies activation of nuclear factor κB by tumour necrosis factor α in rat thyroid cell line. Biochem J 354:573–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Suh JM, Hwang ES, Kim DW, Chung HK, Song JH, Hwang JH, Park KC, Ro HK, Jo EK, Chang JS, Lee TH, Lee MS, Kohn LD, Shong M 2003 Thyrotropin-mediated repression of class II trans-activator expression in thyroid cells: involvement of STAT3 and suppressor of cytokine signaling. J Immunol 171:616–627 [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM 2002 NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734 [DOI] [PubMed] [Google Scholar]
- Perkins ND 2007 Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol 8:49–62 [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F 2007 Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740 [DOI] [PubMed] [Google Scholar]
- Lane DP 1992 Cancer. p53, guardian of the genome. Nature 358:15–16 [DOI] [PubMed] [Google Scholar]
- Valdivia A, Pérez-Alvarez S, Aroca-Aguilar JD, Ikuta I, Jordán J 2009 Superoxide dismutases: a physiopharmacological update. J Physiol Biochem 65:195–208 [DOI] [PubMed] [Google Scholar]
- Barreto G, Madureira D, Capani F, Aon-Bertolino L, Saraceno E, Alvarez-Giraldez LD 2009 The role of catechols and free radicals in benzene toxicity: an oxidative DNA damage pathway. Environ Mol Mutagen 50:771–780 [DOI] [PubMed] [Google Scholar]
- He J, Takano T, Ding J, Gao S, Noda C, Sada K, Yanagi S, Yamamura H 2002 Syk is required for p38 activation and G2/M arrest in B cells exposed to oxidative stress. Antioxid Redox Signal 4:509–515 [DOI] [PubMed] [Google Scholar]
- Li PF, Dietz R, von Harsdorf R 1999 p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J 18:6027–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PF, Maasch C, Haller H, Dietz R, von Harsdorf R 1999 Requirement for protein kinase C in reactive oxygen species-induced apoptosis of vascular smooth muscle cells. Circulation 100:967–973 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL 2003 Calcium orchestrates apoptosis. Nat Cell Biol 5:1041–1043 [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Mustafa FB, Pervaiz S, Ng FS, Lim LH 2008 ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int J Oncol 33:81–92 [PubMed] [Google Scholar]
- Wang T, Lao U, Edgar BA 2009 TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol 186:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Zhuang L, Luo X, Wei P 2003 TL1A-induced NF-κB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem 278:39251–39258 [DOI] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH 2000 Role of NF-κB in p53-mediated programmed cell death. Nature 404:892–897 [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S 2004 Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73:39–85 [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B 2001 A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med 7:1189–1193 [DOI] [PubMed] [Google Scholar]
- He XP, Patel M, Whitney KD, Janumpalli S, Tenner A, McNamara JO 1998 Glutamate receptor GluR3 antibodies and death of cortical cells. Neuron 20:153–163 [DOI] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT 2004 Cognition and immunity; antibody impairs memory. Immunity 21:179–188 [DOI] [PubMed] [Google Scholar]
- Liang CS, Mao W, Liu J 2008 Pro-apoptotic effects of anti-β1-adrenergic receptor antibodies in cultured rat cardiomyocytes: actions on endoplasmic reticulum and the prosurvival PI3K-Akt pathway. Autoimmunity 41:434–441 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Gianoukakis AG, Salehi S, Moorhead J, Rao PV, Khan MZ, McGregor AM, Smith TJ, Banga JP 2006 Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in Graves’ disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways. J Immunol 176:5084–5092 [DOI] [PubMed] [Google Scholar]
- Bossowski A, Czarnocka B, Bardadin K, Stasiak-Barmuta A, Urban M, Dadan J, Ratomski K, Bossowska A 2008 Identification of apoptotic proteins in thyroid gland from patients with Graves’ disease and Hashimoto’s thyroiditis. Autoimmunity 41:163–173 [DOI] [PubMed] [Google Scholar]