Abstract
The pancreas-derived hormones, insulin and glucagon, are the two main regulators of glucose homeostasis. However, their actions can be modulated by the presence of other circulating factors including cytokines. Pancreatic-derived factor (PANDER) is a novel cytokine-like molecule secreted from the endocrine pancreas, but its biological function is currently unknown. To address this, we employed adenoviral gene delivery to develop a novel murine model of PANDER overexpression, which we used to study PANDER’s effect on glucose homeostasis. Although serum metabolites in fed mice were unaffected by PANDER overexpression, fasting glucose, insulin, and corticosterone levels were significantly elevated. Additionally, PANDER-overexpressing mice displayed elevated glucose and insulin levels during a glucose tolerance test, indicating that glucose tolerance was impaired. However, there were no defects in glucose-stimulated insulin secretion or peripheral insulin sensitivity. Elevated transcription of hepatic gluconeogenic genes, PEPCK and G6Pase accompanied the fasting hyperglycemia observed in PANDER-overexpressing animals. Similarly, treatment of primary hepatocytes with PANDER-expressing adenovirus or PANDER-enriched conditioned medium elevated gluconeogenic gene expression and glucose output. PANDER treatment also resulted in higher levels of Ser133-phosphorylated cAMP-response element-binding protein in hepatocytes stimulated with 8-bromo-cAMP and dexamethasone and higher levels of intracellular cAMP upon stimulation with forskolin. In summary, we provide the first report that identifies PANDER as a regulator of hepatic glucose metabolism, where it serves as a novel factor that amplifies hepatic cAMP and cAMP-response element-binding protein signaling to induce gluconeogenic gene expression and glucose output.
Investigation of the biological role of PANDER reveals that it induces fasting hyperglycemia via cAMP and CREB pathways.
Pancreatic-derived factor (PANDER; formerly FAM3B), is a 235-amino-acid secreted protein that was discovered through a screen for novel cytokine families based on a highly conserved four-helix bundle secondary structure (1). Interestingly, of the four members in the FAM3 family, immunohistochemical analysis showed that PANDER was localized to the endocrine pancreas, specifically to the islets of Langerhans, and present in both pancreatic α- and β-cells (2). In the β-cell, PANDER transcription and secretion are increased in response to increasing glucose concentrations (3,4), whereas in the α-cell, secretion is increased with the glucagon secretagogue arginine (5). Furthermore, ligand-binding studies demonstrated that [125I]PANDER bound specifically to liver membrane compared with other tissues that were screened (6), suggesting that there is a hepatic PANDER receptor and supporting the hypothesis that once secreted, PANDER acts in an endocrine or paracrine manner at target organs, particularly the liver. Additionally, treatment of a human hepatoma cell line with murine PANDER resulted in an inhibitory effect on insulin signaling (6).
The liver is a critical organ for regulating glucose levels especially in the fasted state where hepatic glucose production (HGP) from glycogen breakdown (glycogenolysis) and de novo glucose synthesis (gluconeogenesis) is increased to provide glucose for peripheral tissue (7). These hepatic processes are controlled by counterregulatory hormones secreted from the pancreas, namely glucagon, which stimulates HGP during fasting, and insulin, which suppresses HGP in the fed state (8,9,10). Tight control of HGP maintains normoglycemia, whereas dysregulated or inappropriate HGP contributes to fasting hyperglycemia in diseases such as type 2 diabetes mellitus (T2DM) (11,12). HGP can be elevated by increasing stimuli that promote gluconeogenesis or by a failure of insulin to suppress HGP, as in the case of hepatic insulin resistance. Circulating factors are known to contribute to the development of elevated HGP by promoting one or both of these hepatic defects. For example, excess glucocorticoid receptor signaling causes hyperglycemia by increasing gluconeogenesis and insulin resistance (13,14). Cytokine molecules such as IL-6 and resistin can also promote hyperglycemia by inducing insulin resistance (15,16,17). As a pancreas-derived secreted factor that targets the liver, PANDER may serve a biological role in hepatic glucose regulation. Targeted disruption of the PANDER gene in mice resulted in decreased HGP during a hyperinsulinemic-euglycemic clamp (18). In addition, experiments in mice overexpressing PANDER selectively in the β-cells showed that PANDER caused an earlier onset of high-fat diet-induced glucose intolerance (19).
To investigate the effect of PANDER on hepatic glucose regulation, we created a model with increased circulating PANDER levels in mice. Here we report that PANDER overexpression caused fasting hyperglycemia and elevated fasting corticosterone levels. In these Ad-PANDER-injected mice, fasting hyperglycemia was accompanied by elevated fasting insulin levels and glucose intolerance. Similarly, treatment of primary hepatocytes with PANDER resulted in increased glucose output. The elevated glucose levels observed both in vivo and in vitro correlated with elevated transcription of gluconeogenic genes. Furthermore, the increase in hepatic gene expression was the result of PANDER-induced amplification of cAMP and cAMP-response element (CRE)-binding protein (CREB) signaling. These data suggest that PANDER is a novel circulating hormone that positively regulates glucose output by the liver.
Materials and Methods
Animals and adenoviruses
Male C57BL/6 mice were purchased from Charles Rivers Laboratories, maintained in a constant 12-h light, 12-h dark cycle, and fed a standard rodent chow and water ad libitum. Adenoviral vector encoding for PANDER was constructed and created as previously described (20). LacZ and enhanced green fluorescent protein (eGFP) control viruses were obtained from the Vector Core of the University of Pennsylvania. Mice were injected with 0.75–1.5 × 108 plaque-forming units (pfu) of adenovirus via the tail vein at 8–13 wk of age and used for experiments 6–7 d after injection. PANDER knockout (KO) mice were obtained as described (18) and used for gene expression analysis at 7 months of age. All animal studies were performed in compliance with the approved protocols of the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Measurement of serum metabolites
Glucose levels were measured with a Freestyle glucometer via tail vein blood sampling. For determination of serum metabolites, trunk blood was collected from killed animals in a Microvette CB 300 (Sarstedt AG & Co., Nümbrecht, Germany), placed on ice, and centrifuged at 12,000 rpm for 10 min at 4 C. Plasma was removed and stored at −80 C until further analysis. Insulin, glucagon, and corticosterone levels were quantified using commercially available ELISAs (Chrystal Chem, Downers Grove, IL; ALPCO Diagnostics, Salem, NH; and Assay Designs, respectively) according to the manufacturers’ instructions. Nonesterified fatty acids, hepatic triglycerides, and liver aspartate aminotransferase and alanine aminotransferase, were measured using commercially available kits (Wako Chemicals, Richmond, VA, and Stanbio Laboratory, Boerne, TX, respectively). Liver glycogen was extracted by homogenization of tissue in acid followed by acid hydrolysis of the homogenate. The glucose released was measured using glucose LiquiColor test reagent (Stanbio).
Metabolic tests and fasting experiments
Mice were fasted overnight (18 h) and injected ip with 2 g glucose (Fisher Scientific, Pittsburgh, PA) per kilogram body weight for glucose tolerance tests (GTT). Glucose and insulin levels at indicated time points were measured from tail vein blood as described. For insulin tolerance tests (ITT), mice fasted for 4 h were given an ip bolus of 0.75 U insulin (NovoLog; Novo Nordisk, Bagsvaerd, Denmark) per kilogram of body weight and glucose measured as described above. For fasting experiments, mice were fasted for 18 h or fasted and then refed for 4 h before they were killed for blood and tissue collection.
Primary hepatocyte isolation and treatment
Hepatocytes were isolated from male mice by noncirculating collagenase perfusion. The livers of anesthetized mice were perfused via the inferior vena cava with liver perfusion medium (Invitrogen, Carlsbad, CA) followed by digestion with liver digest medium (Invitrogen) supplemented with 5000 U collagenase (Worthington Biochemical Corp., Lakewood, NJ). After mincing, the cell suspension was filtered through a 70-μm mesh filter and hepatocytes collected on a Percoll gradient (Sigma-Aldrich Corp., St. Louis, MO). Cell viability was assessed by Trypan Blue staining (Sigma-Aldrich Corp.). Cells were cultured at 37 C in 5% CO2, 95% air in DMEM supplemented with 10% FBS, 2 mm glutamine, 100 μg/ml streptomycin, and 100 IU/ml penicillin. One day after isolation, hepatocytes were transduced with the appropriate number of viral particles as previously described (20) and used for experiments 48 h later. Cells were serum starved in 5 mm glucose, 0.5% FBS DMEM (pretreatment medium) for 2 h before stimulation with 1 μm dexamethasone (DEX) and 1 mm 8-bromoadenosine-cAMP, 100 nm glucagon, or 10 μm forskolin (Sigma-Aldrich Corp.).
Glucose production assay
The 48-h-transduced hepatocytes were incubated overnight in pretreatment medium with or without 1 μm DEX and 1 mm 8-bromo-cAMP. Next day, cells were washed twice and then incubated in glucose production medium [glucose-free, phenol red-free DMEM, 2 mm pyruvate, 20 mm lactate (all from Sigma-Aldrich Corp.) with or without 1 μm DEX, and 1 mm 8-bromo-cAMP] for 5 h before collection of medium and cell lysate or total RNA. Glucose secreted into the medium was measured using the Amplex Red glucose assay kit (Invitrogen) and normalized to total protein content determined by bicinchoninic acid assay for each sample.
Conditioned medium (CM) experiments
Primary hepatocytes were isolated and plated on either collagen-coated six-well plates or 0.45-μm cell culture inserts in separate dishes (BD Biosciences, San Jose, CA). Only hepatocytes plated on the inserts were transduced with 42 pfu of Ad-PANDER or Ad-LacZ virus. After transduction, cells were washed twice and then incubated in 1% FBS DMEM. The inserts were then added to the six-well plates and the cells cocultured for 48 h. After overnight incubation in cell culture medium with or without 1 μm DEX and 1 mm 8-bromo-cAMP, glucose production assay was performed, and samples were collected and analyzed as described above.
RNA isolation and quantitative real-time RT-PCR
On the day of killing, tissue was immediately snap frozen in liquid nitrogen and later homogenized in TRIzol reagent (Invitrogen). Total RNA from tissue and cultured hepatocytes was extracted using RNeasy mini kit (QIAGEN Inc., Valencia, CA) and analyzed for quality by an electrophoresis bioanalyzer (Agilent, Santa Clara, CA). TaqMan real-time quantitative RT-PCR was performed with the One Step RT-PCR kit (Applied Biosystems, Inc., Foster City, CA) using total RNA (200–250 ng for tissue and 100–200 ng for hepatocytes) for each sample in duplicate. Primer and probe sets for detection of G6Pase catalytic subunit, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α), GK, HNF4α, and tyrosine aminotransferase (TAT) were commercially available (Applied Biosystems). To detect phosphoenolpyruvate carboxykinase (PEPCK), we used the following: forward, 5′-CCACAGCTGCTGCAGAACAC-3′; reverse, 5′-GAAGGGTCGCATGGCAAA-3′; and probe, 5′/56-FAM/AGGGCAAGATCATCATGCACGACC/36-TAMSp/-3′. Detection of PANDER mRNA has been described (20). Expression levels were normalized to either β-actin or 18S rRNA, and relative levels compared with control were determined using the 2−ΔΔCt method (21).
Immunoblotting
Whole-cell lysates and liver homogenates were collected in fresh Roth lysis buffer as described (6). Lysate (25 μg) or homogenate protein (100 μg) was analyzed by SDS-PAGE for detection of Ser133-phosphorylated and total forms of CREB (Cell Signaling Technology, Danvers, MA), actin (Sigma-Aldrich Corp.), and PANDER (Alpha Diagnostics, San Antonio, TX). Medium from transduced hepatocytes was concentrated 5-fold using ammonium sulfate precipitation, and 25–50 μg total protein was used for PANDER detection (3,20). Proteins transferred to nitrocellulose membrane using the iBlot semi-dry transfer system (Invitrogen) were detected as previously described using 2% blocking powder (GE Healthcare, Piscataway, NJ) for primary and secondary antibody dilutions (6,20). Advanced enhanced chemiluminescence detection reagents (GE Healthcare) were used to detect enhanced chemiluminescence, and signals were visualized and evaluated using LAS 3000 Intelligent Dark Box (Fujifilm, Stamford, CT). Blots were stripped and reprobed for confirmation of equivalent loaded total protein.
For detection of PANDER protein in serum, 2.5 μl serum from fasted mice was analyzed by SDS-PAGE using 12% Pierce (Thermoscientific, Rockford, IL) gels and then transferred onto polyvinylidene fluoride membrane using the iBlot system, followed by detection as described above using PANDER polyclonal antibody (Strategic Biosolutions, Windham, ME) at a dilution of 1:1000 and peroxidase-conjugated secondary antibody at 1:10,000. Blots were developed as described above. The relative amount of target protein was determined using Multi Gauge software (version 3.0; Fujifilm) and normalized to the control value.
Intracellular cAMP content
Intracellular cAMP content in hepatocytes treated with 10 μm forskolin or dimethyl sulfoxide for 15 min was measured using a cAMP assay kit (Biovision, Mountain View, CA) and normalized to total protein content.
Statistical analysis
Data are presented as means ± sem. Statistical significance was determined by unpaired, two-tailed Student’s t test. Prism (GraphPad, La Jolla, CA) was used to perform all analyses of PANDER-treated levels compared with controls. A P value <0.05 was considered statistically significant.
Results
Validation of adenoviral PANDER overexpression in mice
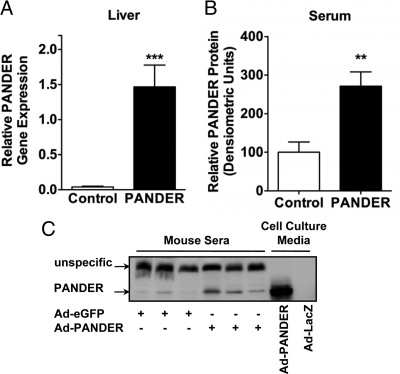
To study the effect of PANDER in vivo, we used adenovirus to overexpress PANDER in male C57BL/6 mice. Adenoviruses are known to specifically target the liver and offer a reliable way to increase serum levels of secreted proteins (22,23). The major advantage of this approach was the ability to increase PANDER levels in a normal adult mouse thereby avoiding compensatory developmental effects. Mice injected with either PANDER or eGFP control adenovirus showed specific expression of these proteins in the liver with no expression in spleen, kidney, fat, and muscle (data not shown). Correspondingly, Ad-PANDER-injected mice had robust levels of PANDER mRNA in the liver (Fig. 1A), and consistent with experiments describing PANDER as a secreted cytokine-like molecule (1,3), circulating PANDER levels were increased 2.7-fold in sera compared with mice injected with control adenovirus (Fig. 1, B and C). No differences in body weight or serum nonesterified fatty acids were observed between the two groups. Serum aspartate aminotransferase and alanine aminotransferase levels were comparable and within the normal range (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), indicating that adenoviral injection did not impair liver function. Furthermore, expression of endoplasmic reticulum stress marker genes ATF6 and GRP78 was similar between Ad-PANDER and control mice (Supplemental Fig. 1A). Overall, adenoviral delivery resulted in significantly elevated circulating PANDER levels.
Figure 1.
Overxpression of PANDER in C57BL/6 mice. C57BL/6 mice were injected with 0.75–1.5 × 108 pfu of PANDER or control eGFP adenovirus via the tail vein. Blood and tissue samples were collected 7 d after injection. A, Quantitative real-time RT-PCR was used to assess PANDER mRNA expression in the liver (n = 20–22); B, quantification of relative amount of PANDER in the sera of adenovirus-injected mice (n = 11); C, representative blot of serum PANDER with medium from transduced hepatocytes as controls. Data represent mean and sem. **, P < 0.01; ***, P < 0.001.
PANDER-overexpressing mice exhibit increased fasting glucose levels
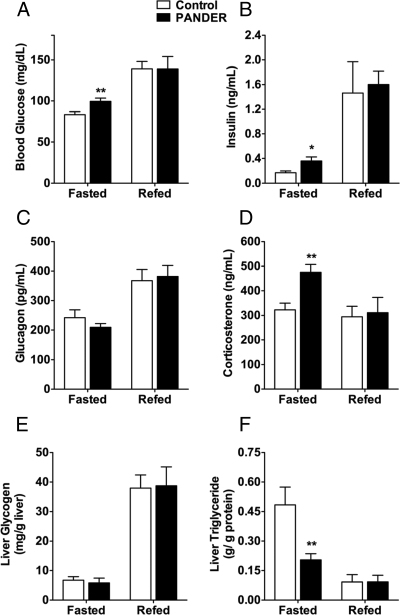
To assess any effect on glycemia, we measured glucose levels in mice 1 wk after adenoviral injection. Examination of fed blood metabolites showed no differences compared with control mice; however, significant differences emerged when samples from overnight fasted mice were analyzed (Fig. 2A). PANDER-overexpressing mice had a mean fasting glucose level of 100 mg/dl compared with 83 mg/dl in the controls (Fig. 2A). In addition, fasting insulin levels in the Ad-PANDER-injected mice were 2-fold higher than in control mice (0.36 ± 0.06 vs. 0.17 ± 0.03 ng/ml). The elevated insulin levels were likely a compensatory response to the high glucose levels. In light of this, we investigated the levels of hormones known to promote elevated glucose levels in the fasted state. Increased glucagon levels have been implicated in T2DM-related hyperglycemia, and glucocorticoids are known to induce elevated glucose and insulin levels in both humans and animals (13,24,25). We found that glucagon levels were comparable between both groups (Fig. 2C), but corticosterone, the active glucocorticoid in mice, was significantly elevated in fasted PANDER-overexpressing mice (Fig. 2D). PANDER may therefore promote hyperglycemia in these mice either directly or indirectly via increased glucocorticoid receptor signaling. To determine whether glycemic differences were restricted to circulating glucose, we measured hepatic glycogen levels and found them to be comparable between groups (Fig. 2E). Surprisingly, although fed hepatic triglyceride levels in the PANDER-overexpressing mice were similar to control levels, fasted triglyceride content was markedly decreased (Fig. 2F).
Figure 2.
PANDER overexpression results in fasted hyperglycemia and hyperinsulinemia. Metabolic parameters were assessed in overnight fasted mice or in mice refed for 4 h after fasting. A–D, Glucose (n = 5–17) (A), insulin (n = 3–15) (B), glucagon (n = 5–14) (C), and corticosterone (D) (n = 4–6) levels were measured 7 d after injection; E and F, liver glycogen (E) and triglyceride (F) (n = 4–6) content were also determined. Data represent mean and sem. *, P < 0.05; **, P < 0.01.
PANDER overexpressers are glucose intolerant due to increased fasting glucose
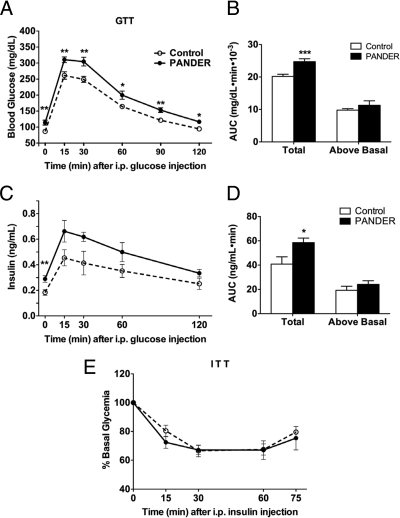
GTTs were then performed to evaluate whole-body glucose utilization. PANDER mice displayed glucose intolerance with glucose levels significantly higher than control levels over the course of the assay (Fig. 3A). Assessment of the area under curve (AUC) revealed that although total AUC was significantly larger in the PANDER-overexpressing mice, the AUC above baseline was comparable between the two groups (Fig. 3B). This suggests that the glycemic response to the glucose injection was similar in both groups and that glucose intolerance in the Ad-PANDER-injected mice was most likely due to an increase in basal glucose levels. However, to rule out the possibility that the glucose intolerance was due to impaired glucose-stimulated insulin secretion (GSIS), we measured insulin levels during the GTT and found higher levels compared with controls (Fig. 3C). Moreover, the insulin response to the glucose load (area under insulin curve above basal) was only slightly higher with PANDER overexpression, indicating that the hyperinsulinemia during the GTT could also be attributed to higher fasting insulin levels. We also measured glucose-stimulated insulin release in isolated islets (26) from control and PANDER-overexpressing mice and found no difference in fractional insulin secretion in response to 16.7 mm glucose or 30 mm KCl (Supplemental Fig. 1B). This confirmed that impaired insulin secretion was not the cause of the glucose intolerance in the Ad-PANDER-injected mice and that the elevated insulin levels were likely a compensatory response by the pancreas to counteract the hyperglycemia. Still, to assess any potential defects in insulin sensitivity, we next measured sensitivity to the glucose-lowering effects of exogenously administered insulin by ITT. Mice showed comparable decreases in glucose levels with insulin injection (Fig. 3E), demonstrating that peripheral insulin sensitivity was not impaired. Taken together, these results demonstrate that in the PANDER-overexpressing mice, fasting hyperglycemia results in the development of impaired glucose tolerance despite elevated insulin levels and normal peripheral insulin sensitivity.
Figure 3.
PANDER overexpression promotes glucose intolerance. A, Intraperitoneal GTT was performed by injecting fasted mice with 2 g glucose/kg body weight and measuring glucose levels at the indicated time points (n = 10–13); B, AUC over the course of the GTT; C, GSIS was determined by measuring insulin levels during the GTT (n = 8); D, AUC GSIS; E, ITT (0.75U/kg body weight) was performed after a 4-h fast (n = 8). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
PANDER increases gluconeogenic gene expression
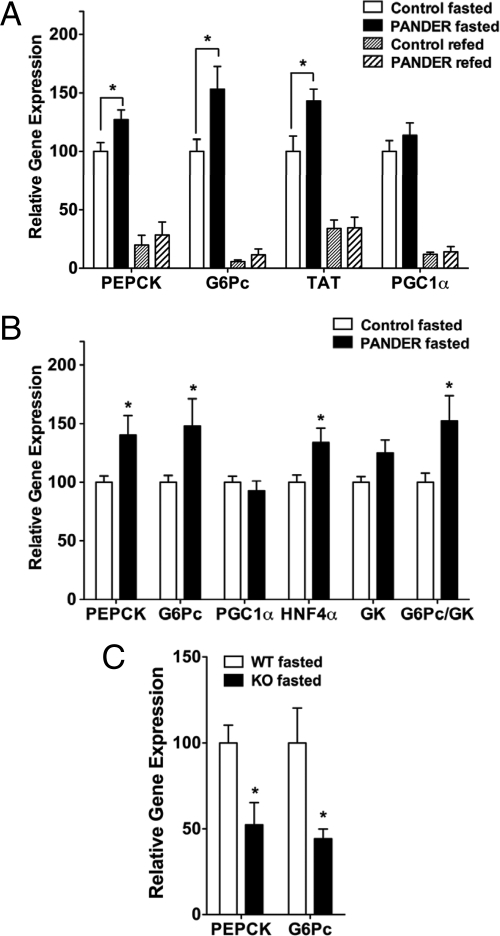
During fasting, glucose released from the liver is derived from hepatic glycogen storage or from de novo glucose synthesis (gluconeogenesis). Whereas glycogenolysis occurs rapidly (2–3 h after a meal), gluconeogenesis is the main source of glucose during prolonged fasting (27). An increase in gluconeogenesis usually indicates elevated transcription of key enzymes that control unidirectional reactions of glucose production (PEPCK) and glucose release (G6Pase) (28,29). We therefore measured expression of these gluconeogenic genes in overnight fasted mice. PEPCK and G6Pase gene expression was significantly elevated in fasted PANDER mice compared with controls (Fig. 4A). We also measured the expression of PGC1α, a fasting inducible transcriptional coactivator that stimulates the expression of gluconeogenic genes (29,30), but no difference was observed. However, expression of tyrosine aminotransferase (TAT), an enzyme strongly induced by cAMP and glucocorticoid signaling (31,32), was also significantly increased compared with control mice.
Figure 4.
Gluconeogenic gene expression is increased in Ad-PANDER-injected mice. Total RNA isolated from the livers of mice was used to determine relative gene expression by quantitative real-time RT-PCR using 18S rRNA expression as an endogenous control A, Gluconeogenic gene expression in overnight fasted and refed mice (n = 5–14); B, gene expression in mice fasted 4 h at end of GTT (n = 8); C, PEPCK and G6Pase expression in overnight fasted PANDER−/− and wild-type mice (n = 5). *, P < 0.05. G6Pc, G6Pase-catalytic subunit.
Although these genes are up-regulated by glucagon and glucocorticoids via the activation of CRE and glucocorticoid response units in their promoters, they are also sensitive to strong inhibition by insulin (33,34,35). Previous studies have shown that in cases of insulin deficiency or hepatic insulin resistance, elevations in mRNA levels of PEPCK and G6Pase are usually observed (36,37). Because high glucocorticoid levels are known to cause insulin resistance (14,38), we wanted to determine whether the transcriptional regulation of these genes in the PANDER-overexpressing mice was still sensitive to insulin. Therefore, we measured their expression in mice refed for 4 h after an overnight fast. We first noted that refed insulin levels were similarly increased between the two groups (Fig. 2B). Consequently, gluconeogenic gene expression was significantly reduced but comparable between control and PANDER-overexpressing mice. Additionally, similar levels of insulin-induced inhibition were observed for both groups: 80 and 78% inhibition of PEPCK and 94 and 93% inhibition of G6Pase gene expression for control and Ad-PANDER-injected mice, respectively, compared with fasted levels (Fig. 4A). This demonstrated that despite elevations in fasting corticosterone, the mice overexpressing PANDER still maintained their hepatic sensitivity to gene suppression by insulin. Because the serine/threonine kinase Akt mediates the effect of insulin on gene expression, we measured hepatic levels of Ser473-phosphorylated Akt in refed mice as well in mice injected with insulin and found no difference between control and Ad-PANDER-injected mice (Supplemental Fig. 2, A and D). No significant difference in hepatic glycogen synthase kinase 3 activation was observed (Supplemental Fig. 2, B and E), supporting our observation of no impact of PANDER on glycogen levels.
We also measured hepatic gene expression in mice that were killed at the end of the GTT after 3–4 h without refeeding. PEPCK and G6Pase levels were also elevated in these PANDER-overexpressing mice compared with controls (Fig. 4B). Furthermore, the ratio of G6Pase to GK was significantly larger in the Ad-PANDER-injected mice (1.52 ± 0.20 vs. 1.00 ± 0.08), indicating a higher net efflux of glucose from the liver (39) and supporting the observation of higher glucose levels in the PANDER mice during the GTT.
Next, we sought to determine whether an absence of PANDER in the serum would have any implications for gluconeogenic gene expression. Interestingly, in mice lacking the PANDER gene, although there was no defect in fasted glucose levels, PEPCK and G6Pase gene expression was significantly decreased compared with wild-type mice (Fig. 4C). Therefore, we conclude that PANDER positively regulates gluconeogenic gene expression that may cause fasting hyperglycemia in these mice.
PANDER increases glucose output in vitro
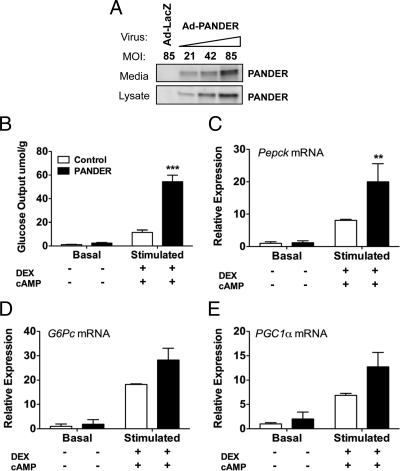
To address the molecular mechanism of PANDER-induced increase in PEPCK and G6Pase, we established an in vitro model in primary murine hepatocytes using adenoviral gene delivery. Transduction of primary hepatocytes with PANDER adenovirus resulted in a dose-dependent increase in PANDER protein in medium and lysate (Fig. 5A). Hepatocytes infected with a dose of 85 pfu/cell (Fig. 5A, lane 4) were used for experiments 48 h after transduction. First, we determined whether PANDER had any impact on glucose output in vitro. Hepatocytes were incubated with the gluconeogenic substrates, 2 mm pyruvate and 20 mm lactate, in the absence (basal) or presence (stimulated) of DEX and 8-bromo-cAMP, and glucose secreted into the medium after 5 h was measured. We detected higher glucose output in the PANDER-treated cells compared with controls under both basal and stimulated conditions (Fig. 5B). Although basal glucose output was 2.4-fold higher in the PANDER samples, the difference was not statistically significant. However, stimulation caused a robust increase in glucose output for both groups (54.432 μmol/g for PANDER-treated and 11.492 μmol/g for control-treated samples) with PANDER-treated cells producing almost 5-fold more glucose than controls. This demonstrates that PANDER increases DEX- and cAMP-induced glucose output from primary hepatocytes.
Figure 5.
PANDER increases glucose production from primary hepatocytes. Primary hepatocytes were injected with either PANDER or LacZ control adenovirus and used for experiments 48 h after transduction. A, Adenoviral delivery resulted in PANDER expression in cell lysate and medium. MOI, Multiplicity of infection. B, Glucose output in transduced hepatocytes was determined by incubating cells for 5 h in glucose- and phenol red-free DMEM containing 2 mm pyruvate and 20 mm lactate with or without 1 mm cAMP and 1 μm DEX and after an overnight incubation in low-glucose, low-serum medium. Glucose secreted into medium was detected using fluorometric assay and normalized to total protein content in samples. C–E, Relative mRNA expression for PEPCK, G6Pase-catalytic subunit, and PGC1α was determined by quantitative real-time RT-PCR using 100–200 ng total RNA isolated at the end of the glucose production assay. Data represent mean and sem; n = 4–7 per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
PANDER increases gluconeogenic gene expression in vitro
Next we wanted to determine whether PANDER also induced gluconeogenic gene expression in this model. Total RNA isolated from hepatocytes at the end of the glucose production assay was analyzed by quantitative real-time RT-PCR. We found that gene expression levels of PEPCK, G6Pase, and PGC1α were highest in the PANDER-treated cells (Fig. 5, C–E). Stimulated PEPCK gene expression was 2.5-fold higher in PANDER-treated samples compared with controls, and similar results were observed for G6Pase and PGC1α. In addition, insulin treatment was able to suppress gene expression to control levels (data not shown) demonstrating that the insulin-dependent repression of these genes was not impaired.
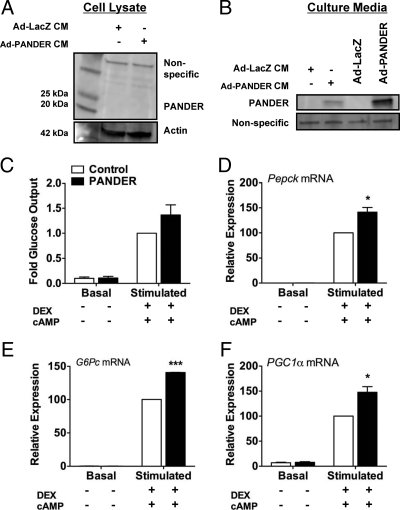
To verify that the effect of PANDER on the hepatocytes was mediated by increased PANDER levels in the medium and not due to intracellular expression, we performed CM experiments in which nontransduced hepatocytes were cocultured with either PANDER or control adenovirus-transduced hepatocytes, plated on permeable cell culture inserts. Using this system, PANDER was detected in the culture medium but not in the cell lysate of the nontransduced cells (Fig. 6, compare B with A for Ad-PANDER CM-treated cells). PANDER CM treatment increased glucose output from primary hepatocytes and also significantly increased expression of PEPCK, G6Pase, and PGC1α compared with cells incubated in control CM (Fig. 6, C–F). The less robust effect of PANDER CM on glucose output, compared with adenoviral transduction, is possibly due to lower PANDER concentration in the CM compared with the medium of transduced cells (Fig. 6B).
Figure 6.
PANDER CM increases glucose output and gluconeogenic gene expression. Nontransduced primary hepatocytes in six-well dishes were cocultured with hepatocytes plated on cell culture inserts and transduced with 42 pfu of either Ad-PANDER or Ad-LacZ control virus. After 48 h, the hepatocytes were incubated overnight in culture medium with or without 1 mm cAMP and 1 μm DEX instead of O/N incubation in low-serum, low-glucose DMEM. The next day, the inserts were removed from the six-well plates, and the glucose assay and sample collection were performed as described. A, Representative blot of PANDER in the cell lysate of hepatocytes treated with CM. B, PANDER in the culture medium of CM-treated and transduced hepatocytes. C, Glucose output; D–F, expression of PEPCK (D), G6Pase-catalytic subunit (E), and PGC1α (F) were measured as described. Data represent mean and sem; n = 2 per group. *, P < 0.05; ***, P < 0.001.
cAMP and phosphorylated-CREB levels are increased with PANDER treatment
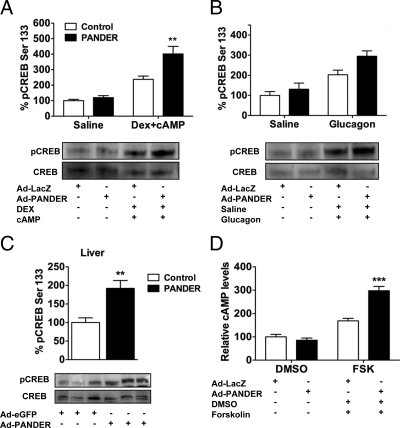
Glucagon-induced gluconeogenic gene transcription occurs via a G protein-coupled mechanism that results in elevated intracellular cAMP levels (10,40) and subsequent activation of cAMP-dependent protein kinase A (41). Active protein kinase A phosphorylates CREB on Ser133, causing its translocation to the nucleus where it activates gluconeogenic gene transcription by binding to CRE in promoter regions of G6Pase and PEPCK (42,43). Furthermore, knockdown of CREB via antisense oligonucleotide prevented fasting hyperglycemia in rodent models of diabetes (44). CREB is therefore a key regulator of gluconeogenic gene expression. We determined the effect of PANDER on Ser133-phosphorylated CREB in hepatocytes stimulated with DEX and 8-bromo-cAMP. Basal levels were similar for both groups, but stimulation resulted in a robust increase in CREB phosphorylation (238 and 402% in the controls and PANDER-treated cells, respectively) (Fig. 7A). To test the response to a more physiological stimulus, cells were treated with 100 nm glucagon. Glucagon stimulation also caused a robust increase in CREB phosphorylation with higher levels in the PANDER- vs. control-treated cells (295 ± 27.1 vs. 203 ± 23%) (Fig. 7B). Similarly, phospho-Ser133 CREB levels were elevated 2-fold in the livers of short-term fasted PANDER-overexpressing mice (Fig. 7C), indicating that CREB is a target of PANDER’s action. Because increased cAMP levels promote CREB activation, and previous studies have shown that cAMP signaling is synergistic with corticosteroids in activating the gluconeogenic pathway (45), we determined whether PANDER had any impact on cAMP levels. Basal cAMP levels were comparable between both groups but PANDER-treated cells had significantly more intracellular cAMP compared with controls upon stimulation with forskolin (298 ± 18.6 vs. 167 ± 10.7%) (Fig. 7D). These data therefore suggest a mechanism by which PANDER can directly augment glucose output from hepatocytes in vivo, i.e. by amplifying cAMP and CREB signaling.
Figure 7.
PANDER treatment enhances CREB and cAMP signaling. A and B, Cell extract (25 μg) was used for Western blot analysis to detect relative levels of phospho-Ser133 CREB (pCREB) in transduced hepatocytes after stimulation with 1 μm DEX plus 1 mm cAMP or 100 nm glucagon for 30 min. Blots were stripped and reprobed for detection of total CREB levels. C, Liver homogenates (100 μg) from 3-h-fasted mice was used to detect total and phospho-Ser133 CREB. D, Intracellular cAMP levels were measured in cells after treatment with 10 μm forskolin (FSK) for 15 min. DMSO, Dimethylsulfoxide. Data represent mean and sem; n = 3–7 per group. *, P < 0.05; **, P < 0.01.
Discussion
Fasting hyperglycemia is a risk factor for the development of T2DM (11). In patients and animal models of diabetes, elevated glucose levels are the result of increased HGP (12), of which gluconeogenesis is the major contributor (46,47). Because fasting hyperglycemia predicts the magnitude of post-meal hyperglycemia in humans (48), the development of drugs to treat this condition has strong clinical relevance. Previous studies have shown that decreasing the expression or activity of key regulators of gluconeogenesis such as glucocorticoids and CREB can prove successful in controlling glycemia in diabetic models (44,49). Preliminary experiments in fasted wild-type mice demonstrate that old, fat mice are hyperglycemic and have 6-fold higher PANDER levels compared with young, lean mice (our unpublished data). This indicates that 1) there are physiological conditions under which fasting PANDER levels are significantly increased and 2) that elevated fasting PANDER levels may correlate with high fasting glucose levels in wild-type mice. We have shown that PANDER overexpression induces fasting hyperglycemia and gluconeogenic gene expression in mice. However, no effects on fed glucose are observed even though circulating PANDER levels are expected to be high in fed mice due to its glucose-responsive secretion from β-cells. Our data suggest that PANDER can increase glucose levels only by enhancing stimulated hepatic glucose output, in which case PANDER’s effect will be more potent in the context of low circulating insulin levels and during fasting. Furthermore, because PANDER amplifies stimulated levels of cAMP and phospho-CREB, no effect of PANDER is observed under nonstimulated conditions; i.e. an effect is seen only if these pathways are activated.
Physiologically, high levels of PANDER during feeding may be less important for hepatic glucose regulation but important for maintaining β-cell homeostasis. Recent findings have implicated a role for PANDER in maintaining β-cell function such that disruption of the PANDER gene in mice impairs β-cell function as measured by decreased glucose-stimulated insulin release and impaired Ca2+ homeostasis (18). As a result, these mice are also glucose intolerant compared with wild-type mice. In addition to this β-cell-specific phenotype, the PANDER KO mice have decreased HGP during a hyperinsulinemic-euglycemic clamp and, as we have shown, decreased hepatic gluconeogenic gene expression, confirming the effects of PANDER on the liver. One potential common point of regulation of these pathways is CREB. CREB signaling is critical for maintaining fuel-induced β-cell insulin secretion (50). Both glucose and glucagon-like peptide-1, which promote insulin secretion, activate CREB by increasing Ser133 phosphorylation via Ca2+ and cAMP pathways, respectively (51). Furthermore, disruption of CREB activity decreases β-cell function (52,53). It is therefore possible that loss of PANDER-induced CREB signaling may contribute to the β-cell dysfunction in the KO mice.
Notably, the development of fasting hyperglycemia and hyperinsulinemia after 1 wk of PANDER overexpression is similar to corticosteroid-induced impaired glucose tolerance and hyperinsulinemia after 1 wk of DEX dosing compared with other models that require 8–12 wk of high-fat diet feeding (37). Glucocorticoids are the most common hormones known to cause insulin resistance and worsen diabetes (36,38). Additionally, many rodent models of T2DM are associated with elevated corticosterone levels (54), and antagonism of hepatic glucocorticoid signaling normalizes elevated HGP and improves glycemic control (49,55). Therefore, elevated corticosterone levels in the PANDER-overexpressing mice likely contribute to the development of hyperglycemia. Corticosterone levels are regulated by the hypothalamic-pituitary-adrenal axis, and secretion of ACTH from proopiomelanocortin neurons stimulates corticosterone release from adrenal cells via a cAMP-mediated mechanism (56). cAMP or an adenylate cyclase agonist such as forskolin and 3-isobutyl-1-methylxanthine can on their own, or synergistically with ACTH, increase corticosterone release (57,58). We have shown that PANDER increases forskolin-induced cAMP levels in hepatocytes. Whether PANDER can also increase cAMP levels in adrenal cells is a question of great interest. Furthermore, although PANDER is predominantly expressed in the endocrine pancreas, immunohistochemical staining with PANDER antibody detected selective immunoreactivity in the Purkinje cell layer of the cerebellum and the nerve cell bodies of the brainstem (1). Although the PANDER receptor has not been cloned, and hypothalamic, pituitary, and adrenal membrane were not screened in the [125I]PANDER binding studies (6), it is possible that PANDER may have a direct impact on the hypothalamic-pituitary-adrenal axis, leading to an increase in corticosterone levels.
One difference between corticosteroid-induced hyperglycemia and PANDER overexpression is that although glucocorticoids also induce insulin resistance in mice, Ad-PANDER-injected mice are insulin sensitive. After refeeding, glucose levels were normalized in the PANDER-overexpressing mice compared with controls due to a strong inhibition of hepatic gluconeogenic gene expression by insulin. In addition, insulin-induced hepatic phospho-Akt levels are comparable between both groups, and ITT demonstrated that peripheral insulin sensitivity was not impaired. Furthermore, we believe that even in fasted Ad-PANDER-injected mice, hepatic insulin sensitivity is maintained. Although not statistically significant, the expression of insulin-induced genes, glucokinase (GK) and fatty acid synthase (FAS), was slightly higher in the fasted livers of PANDER-overexpressing mice (data not shown), and this was likely in response to the elevated insulin levels in these mice. This suggests that some suppression of HGP by insulin was possible in the fasted Ad-PANDER-injected mice but that this suppression could not overcome the combined stimulus of PANDER and corticosterone on HGP. It also suggests that the expression of gluconeogenic enzymes in the PANDER-overexpressing mice might have been higher if the hyperglycemia was not accompanied by hyperinsulinemia. This idea is supported by our in vitro studies where, in the absence of insulin, much higher fold gene expression levels were observed in the PANDER-treated hepatocytes compared with controls. The protection from insulin resistance in these mice may be due to the fact that 1) their corticosterone levels are not chronically elevated because they are increased only in fasted mice, and 2) mice are exposed to PANDER for only 1 wk. As a result, some of the complications of glucocorticoid treatment that promote insulin resistance, such as increased body weight and elevated hepatic triglycerides and serum free fatty acids (14,59,60), are not observed in the Ad-PANDER-injected mice. On the contrary, hepatic triglycerides were decreased with PANDER overexpression. The absence of these complicating factors therefore allowed us to uncover the primary metabolic defects that arise as a result of increased circulating PANDER levels. Secondary metabolic defects may arise in a model of chronic PANDER overexpression.
Acute PANDER overexpression has allowed us to identify a specific role for PANDER in regulating glucose homeostasis. Our data strongly suggest that PANDER promotes gluconeogenic gene expression and glucose output by increasing CREB and cAMP signaling. Concordantly, PANDER overexpression in mice results in fasting hyperglycemia, impaired glucose tolerance, and compensatory hyperinsulinemia. PANDER may therefore be a novel drug target for the control of fasting hyperglycemia.
Acknowledgments
We thank Dr. Mitch Lazar and Dr. Roy Kim for their valuable discussions regarding the manuscript. We also acknowledge Erik Walp from the Lee-May lab for his expertise in isolating the primary islets.
Footnotes
This work was supported by Grant K01-DK070744 (to B.R.B.) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Juvenile Diabetes Research Foundation (to B.A.W.).
The work presented here was in partial fulfillment of the doctor of philosophy at the University of Pennsylvania to C.G.W.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 15, 2010
Abbreviations: AUC, Area under the curve; CM, conditioned medium; CRE, cAMP-response element; CREB, CRE-binding protein; DEX, dexamethasone; eGFP, enhanced green fluorescent protein; GK, glucokinase; G6Pase, glucose-6-phosphatase; GSIS, glucose-stimulated insulin secretion; GTT, glucose tolerance test; HGP, hepatic glucose production; ITT, insulin tolerance test; KO, knockout; PANDER, pancreatic-derived factor; PEPCK, phosphoenolpyruvate carboxykinase; pfu, plaque-forming units; PGC1α, peroxisome proliferator-activated receptor-γ coactivator-1α; T2DM, type 2 diabetes mellitus.
References
- Zhu Y, Xu G, Patel A, McLaughlin MM, Silverman C, Knecht K, Sweitzer S, Li X, McDonnell P, Mirabile R, Zimmerman D, Boyce R, Tierney LA, Hu E, Livi GP, Wolf B, Abdel-Meguid SS, Rose GD, Aurora R, Hensley P, Briggs M, Young PR 2002 Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics 80:144–150 [DOI] [PubMed] [Google Scholar]
- Cao X, Gao Z, Robert CE, Greene S, Xu G, Xu W, Bell E, Campbell D, Zhu Y, Young R, Trucco M, Markmann JF, Naji A, Wolf BA 2003 Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells. Diabetes 52:2296–2303 [DOI] [PubMed] [Google Scholar]
- Yang J, Robert CE, Burkhardt BR, Young RA, Wu J, Gao Z, Wolf BA 2005 Mechanisms of glucose-induced secretion of pancreatic-derived factor (PANDER or FAM3B) in pancreatic beta-cells. Diabetes 54:3217–3228 [DOI] [PubMed] [Google Scholar]
- Burkhardt BR, Yang MC, Robert CE, Greene SR, McFadden KK, Yang J, Wu J, Gao Z, Wolf BA 2005 Tissue-specific and glucose-responsive expression of the pancreatic derived factor (PANDER) promoter. Biochim Biophys Acta 1730:215–225 [DOI] [PubMed] [Google Scholar]
- Carnegie JR, Robert-Cooperman CE, Wu J, Young RA, Wolf BA, Burkhardt BR 2010 Characterization of the expression, localization, and secretion of PANDER in α-cells. Mol Cell Endocrinol 325:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wang C, Li J, Burkhardt BR, Robert-Cooperman CE, Wilson C, Gao Z, Wolf BA 2009 PANDER binds to the liver cell membrane and inhibits insulin signaling in HepG2 cells. FEBS Lett 583:3009–3015 [DOI] [PubMed] [Google Scholar]
- Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI 1991 Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254:573–576 [DOI] [PubMed] [Google Scholar]
- Kahn CR, Goldstein BJ 1989 Molecular defects in insulin action. Science 245:13 [DOI] [PubMed] [Google Scholar]
- Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD 2002 Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 51:1663–1671 [DOI] [PubMed] [Google Scholar]
- Jiang G, Zhang BB 2003 Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284:E671–E678 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L 2009 Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 32:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy JL 2005 Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- West KM 1959 Response of the blood glucose to glucocorticoids in man; determination of the hyperglycemic potencies of glucocorticoids. Diabetes 8:22–28 [DOI] [PubMed] [Google Scholar]
- Qi D, Rodrigues B 2007 Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab 292:E654–E667 [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Rich AS, Rhoades B, Shapiro JS, Obici S, Rossetti L, Lazar MA 2004 Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes 53:1937–1941 [DOI] [PubMed] [Google Scholar]
- Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y 1998 Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83:859–862 [DOI] [PubMed] [Google Scholar]
- Pickup JC, Chusney GD, Thomas SM, Burt D 2000 Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci 67:291–300 [DOI] [PubMed] [Google Scholar]
- Robert-Cooperman CE, Carnegie JR, Wilson CG, Yang J, Cook JR, Wu J, Young RA, Wolf BA, Burkhardt BR 21 June 2010 Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic β-cell function. Diabetes 10.2337/db09-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert CE, Wu J, Burkhardt BR, Wolf BA 2005 Transgenic mice overexpressing the novel islet specific cytokine, PANDER, exhibit glucose intolerance. Diabetes 54(Suppl 1):74-LB (Abstract) [Google Scholar]
- Yang J, Gao Z, Robert CE, Burkhardt BR, Gaweska H, Wagner A, Wu J, Greene SR, Young RA, Wolf BA 2005 Structure-function studies of PANDER, an islet specific cytokine inducing cell death of insulin-secreting beta cells. Biochemistry 44:11342–11352 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Herrmann J, Abriss B, van de Leur E, Weiskirchen S, Gressner AM, Weiskirchen R 2004 Comparative analysis of adenoviral transgene delivery via tail or portal vein into rat liver. Arch Virol 149:1611–1617 [DOI] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM 2004 Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest 114:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Kimmerling G 1976 Effects of glucocorticoids on carbohydrate metabolism. Am J Med Sci 271:202–210 [DOI] [PubMed] [Google Scholar]
- Perley M, Kipnis DM 1966 Effect of glucocorticoids on plasma insulin. N Engl J Med 274:1237–1241 [DOI] [PubMed] [Google Scholar]
- Gu C, Stein GH, Pan N, Goebbels S, Hörnberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, Lee JE 2010 Pancreatic β-cells require NeuroD to achieve and maintain functional maturity. Cell Metab 11:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB 1975 Muscle amino acid metabolism and gluconeogenesis. Annu Rev Med 26:245–258 [DOI] [PubMed] [Google Scholar]
- Klover PJ, Mooney RA 2004 Hepatocytes: critical for glucose homeostasis. Int J Biochem Cell Biol 36:753–758 [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM 2001 Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M 2001 CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- Iynedjian PB, Auberger P, Guigoz Y, Le Cam A 1985 Pretranslational regulation of tyrosine aminotransferase and phosphoenolpyruvate carboxykinase (GTP) synthesis by glucagon and dexamethasone in adult rat hepatocytes. Biochem J 225:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RY, Birely LA, Lum NL, Perella CM, Cherry JM, Bhat NK, Kasprzak KS, Powell DA, Alvord WG, Anderson LM 2004 Expressions of hepatic genes, especially IGF-binding protein-1, correlating with serum corticosterone in microarray analysis. J Mol Endocrinol 32:257–278 [DOI] [PubMed] [Google Scholar]
- Angrand PO, Coffinier C, Weiss MC 1994 Response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids depends on the integrity of the cAMP pathway. Cell Growth Differ 5:957–966 [PubMed] [Google Scholar]
- O'Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA 2001 Insulin-regulated gene expression. Biochem Soc Trans 29:552–558 [DOI] [PubMed] [Google Scholar]
- Schoneveld OJ, Gaemers IC, Lamers WH 2004 Mechanisms of glucocorticoid signalling. Biochim Biophys Acta 1680:114–128 [DOI] [PubMed] [Google Scholar]
- Korach-André M, Gao J, Gounarides JS, Deacon R, Islam A, Laurent D 2005 Relationship between visceral adiposity and intramyocellular lipid content in two rat models of insulin resistance. Am J Physiol Endocrinol Metab 288:E106–116 [DOI] [PubMed] [Google Scholar]
- Ai J, Wang N, Yang M, Du ZM, Zhang YC, Yang BF 2005 Development of Wistar rat model of insulin resistance. World J Gastroenterol 11:3675–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovska L, Rosella G, Proietto J 1990 Evolution of dexamethasone-induced insulin resistance in rats. Am J Physiol 258:E748–E756 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L 1993 Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem 268:25019–25025 [PubMed] [Google Scholar]
- Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ 2003 International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 55:167–194 [DOI] [PubMed] [Google Scholar]
- Jelinek LJ, Lok S, Rosenberg GB, Smith RA, Grant FJ, Biggs S, Bensch PA, Kuijper JL, Sheppard PO, Sprecher CA, O'Hara PJ, Foster D, Walker KM, Chen LHJ, McKernan PA, Kindsvogel W 1993 Expression cloning and signaling properties of the rat glucagon receptor. Science 259:1614–1616 [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR 1989 Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675–680 [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M 2001 Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609 [DOI] [PubMed] [Google Scholar]
- Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D, Hsiao JJ, Zhang D, Iwasaki T, Stark R, Flannery C, Kahn M, Carmean CM, Yu XX, Murray SF, Bhanot S, Monia BP, Cline GW, Samuel VT, Shulman GI 2009 Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab 10:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Cripe TP, Koch SR, Andreone TL, Petersen DD, Beale EG, Granner DK 1984 Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem 259:15242–15251 [PubMed] [Google Scholar]
- Consoli A, Nurjhan N, Capani F, Gerich J 1989 Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 38:550–557 [DOI] [PubMed] [Google Scholar]
- Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI 1992 Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MF, Izard A, Riboni K, Burge MR, Schade DS 2002 Fasting hyperglycemia predicts the magnitude of postprandial hyperglycemia: implications for diabetes therapy. Diabetes Care 25:1247–1248 [DOI] [PubMed] [Google Scholar]
- Edgerton DS, Jacobson PB, Opgenorth TJ, Zinker B, Beno D, von Geldern T, Ohman L, Scott M, Neal D, Cherrington AD 2006 Selective antagonism of the hepatic glucocorticoid receptor reduces hepatic glucose production. Metabolism 55:1255–1262 [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM 1987 Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67:1185–1248 [DOI] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M 2003 cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev 17:1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, Dalle S 2009 Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in β-cells and human pancreatic islets. Diabetes 58:1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A, Hamamoto Y, Tsuura Y, Miyazaki J, Toyokuni S, Ihara Y, Nagai K, Yamada Y, Bonner-Weir S, Seino Y 2004 Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic β-cells. Mol Cell Biol 24:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone DE, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ, Walker BR 2000 Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology 141:560–563 [DOI] [PubMed] [Google Scholar]
- Jacobson PB, von Geldern TW, Ohman L, Osterland M, Wang J, Zinker B, Wilcox D, Nguyen PT, Mika A, Fung S, Fey T, Goos-Nilsson A, Grynfarb M, Barkhem T, Marsh K, Beno DW, Nga-Nguyen B, Kym PR, Link JT, Tu N, Edgerton DS, Cherrington A, Efendic S, Lane BC, Opgenorth TJ 2005 Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. J Pharmacol Exp Ther 314:191–200 [DOI] [PubMed] [Google Scholar]
- Karpac J, Czyzewska K, Kern A, Brush RS, Anderson RE, Hochgeschwender U 2008 Failure of adrenal corticosterone production in POMC-deficient mice results from lack of integrated effects of POMC peptides on multiple factors. Am J Physiol Endocrinol Metab 295:E446–E455 [DOI] [PubMed] [Google Scholar]
- Lo MJ, Wang PS 2002 Involvement of cAMP but not PKA in the increase of corticosterone secretion in rat zona fasciculata-reticularis cells by aging. J Cell Biochem 85:35–41 [PubMed] [Google Scholar]
- Rodbard D 1974 Apparent positive cooperative effects in cyclic AMP and corticosterone production by isolated adrenal cells in response to ACTH analogues. Endocrinology 94:1427–1437 [DOI] [PubMed] [Google Scholar]
- Friedman TC, Mastorakos G, Newman TD, Mullen NM, Horton EG, Costello R, Papadopoulos NM, Chrousos GP 1996 Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J 43:645–655 [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS 2010 Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151:2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]