Abstract
Alterations in sphingolipid metabolism have been shown to contribute to the development of endocrine resistance and breast cancer tumor survival. Sphingosine kinase (SK), in particular, is overexpressed in breast cancer and is a promising target for breast cancer drug development. In this study, we used the novel SK inhibitor ABC294640 as a tool to explore the relationship between SK and estrogen (E2) receptor (ER) signaling in breast cancer cells. Treatment with ABC294640 decreased E2-stimulated ERE-luciferase activity in both MCF-7 and ER-transfected HEK293 cells. Furthermore, the inhibitor reduced E2-mediated transcription of the ER-regulated genes progesterone receptor and SDF-1. Competitive receptor-binding assays revealed that ABC294640 binds in the antagonist ligand-binding domain of the ER, acting as a partial antagonist similar to tamoxifen. Finally, treatment with ABC294640 inhibited ER-positive breast cancer tumor formation in vivo. After 15 d of treatment with ABC294640, tumor volume was reduced by 68.4% (P < 0.05; n = 5) compared with control tumors, with no marked weight loss or illness. Taken together, these results provide strong evidence that this novel SK inhibitor, which had not previously been known to interact with E2 signaling pathways, has therapeutic potential in treating ER-positive breast cancer via inhibition of both SK and ER signaling.
The novel sphingosine kinase-2 selective inhibitor, ABC294640, binds the estrogen receptor in an antagonistic manner and inhibits estrogen receptor signaling in vitro and in vivo.
Estrogen (E2) and E2 receptor (ER) signaling are critically important in promoting breast cancer tumorigenesis, proliferation, and survival. E2 affects the development and progression of breast cancer through its binding of the ER and activation of ER-mediated signaling. Blocking this interaction has been the target of various chemotherapeutic and anti-E2 drugs (1). However, a large number of breast cancers that are initially hormone sensitive become hormone independent (resistant to endocrine therapy) as the disease progresses, and these treatment options are no longer viable. Cross talk between sphingolipid- and ER-mediated signaling has previously been suggested in the literature, but there have not been adequate pharmacological tools to explore the direct relationship between the inhibition of sphingosine kinase and ER signaling events in breast cancer (2,3,4).
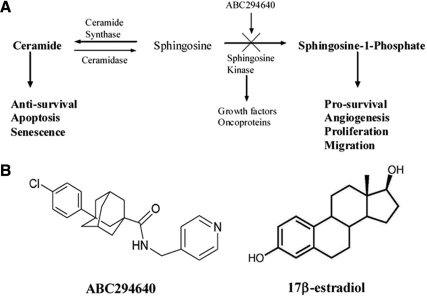
The ceramide-sphingosine-1-phosphate (S1P) pathway (Fig. 1A) plays a significant role in cellular regulation of apoptosis and proliferation in many biological systems, including endocrine-regulated tissues such as breast, prostate, thyroid, and ovarian systems (5,6,7,8,9,10,11). The enzyme sphingosine kinase regulates the conversion of ceramide (proapoptotic) into S1P (proliferative, prosurvival), thus simultaneously removing an apoptotic signal and triggering a proliferative one. Therefore, sphingosine kinase is viewed as a potential switch for cells in an antiproliferative state to transition to a prosurvival and proliferative state (12). S1P functions to stimulate proliferation and cell survival and suppress apoptosis through activation of specific downstream signaling pathways including AKT and members of the MAPK family, such as ERK and p38 (13,14). Both phosphatidylinositol 3-kinase (PI3K)/AKT and MAPK signaling are implicated in endocrine and chemotherapy resistance in breast cancer (3,15,16). Recent evidence suggests that ER-mediated transactivation of the Edg-3 receptor (S1P3 receptor) may be involved in breast cancer tumorigenesis (17). Estrogen has been associated with the up-regulation of sphingosine kinase, and sphingosine kinase is required for E2-dependent ERK activation, thereby establishing a link between E2 and sphingolipids in breast carcinoma cells (2,18). Interestingly, sphingosine kinase was recently shown to be involved in endocrine resistance, although its ability to regulate ER activity and gene expression has not been thoroughly investigated (19). S1P has been shown to stimulate levels of circulating steroid hormones, such as E2, through increased expression of CYP19 and CYP17 (10). Estrogen is known to induce sphingosine kinase activity, resulting in decreased apoptosis and increased MAPK activation, EGFR transactivation, and calcium mobilization (10,11). Estrogen also induces S1P export from the cell, thus allowing it to act in an autocrine and paracrine manner (21). Sphingosine kinase-1 (SK1) was recently shown to be important in the development of endocrine resistance, specifically promoting development of tamoxifen resistance in MCF-7 cells (19). However, to date, there are no published papers on the ability of pharmacological inhibitors of sphingosine kinase to block E2-mediated signaling in ER-positive breast cancer.
Figure 1.
A, Ceramide-S1P signaling pathway; B, structures of ABC294640 and 17β-estradiol (E2).
There have been few studies on the pharmacological targeting of sphingosine kinase as a strategy for breast cancer treatment, largely due to the lack of specific, small-molecule inhibitors to block sphingosine kinase activity. The novel sphingosine kinase inhibitor (SKI)-2 selective inhibitor ABC294640 was recently shown to have a greater antiproliferative effect in ER-positive than ER-negative breast cancer cells (22). Given that this inhibitor is set to enter clinical trials in 2010, a thorough understanding of its role in endocrine signaling is of utmost importance to interpret potential clinical benefits and adverse events. Furthermore, the ability of ABC294640 to affect E2 signaling may be of therapeutic use in the treatment of endocrine-related diseases where steroid hormones and sphingolipids are known to be dysregulated, such as uterine fibroids and cancers of the thyroid, ovaries, prostate, and breast (23,24,25). Therefore, in this study, we test the hypothesis that the novel SK2 inhibitor ABC294640 cannot only inhibit sphingosine kinase but can also alter E2 signaling. With resistance to first-line treatment that targets the ER on the rise, the development of novel therapeutics that affect E2 signaling pathways is of growing importance.
Materials and Methods
Reagents
ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)-amide] was provided by Apogee Biotechnology Corp. (Hummelstown, PA) (22). ICI 182,780 was purchased from Tocris Bioscience (Ellisville, MO). Dimethylsulfoxide (DMSO) and estradiol were purchased from Fisher Scientific (Waltham, MA).
Cell culture
ER-positive MCF-7 cells were cultured as previously described (26). Briefly, the MCF-7 cell line used is a subclone of MCF-7 cells obtained from the American Type Culture Collection (Manassas, VA) generously provided by Louise Nutter (University of Minnesota, Minneapolis, MN) (27). The culture flasks were maintained in a tissue culture incubator in a humidified atmosphere of 5% CO2 and 95% air at 37 C. For E2 studies, cells were washed with PBS three times and grown in phenol red-free DMEM supplemented with 5% dextran-coated charcoal-treated fetal bovine serum (FBS) for 72 h before plating for each particular experiment.
Real-time RT-PCR
Real-time RT-PCR was performed similar to previously reported studies (28,29). In brief, total cellular RNA was extracted using the RNeasy mini column (QIAGEN, Valencia, CA), following the manufacturer’s instructions. The concentration of RNA was determined using an UV spectrophotometer. Reverse transcription was performed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). The level of stromal derived factor-1 (SDF-1) and progesterone receptor (PgR) transcripts was determined using the iQ5 real-time quantitative PCR detection system (Bio-Rad Inc., Hercules, CA). Primers for PCR were designed to span intron/exon junctions to minimize amplification of residual genomic DNA. The primer sequences for PgR, SDF-1, and ER are (sense and antisense, respectively): PgR (5′-TACCCGCCCTATCTCAACTACC-3′ and 5′-TGCTTCATCCCCACAGATTAAACA-3′), SDF-1 (5′-AGTCAGGTGGTGGCTTAACAG-3′ and 5′-AGAGGAGGTGAAGGCAGTGG-3′), and ER (5′-GCGATGGTGGAGATCTTCGA-3′ and 5′-CCTCTCCCTGCAGATTCATCA-3′).
The PCR mix contained optimal concentrations of primers, cDNA, and SYBR Green PCR Master Mix (Bio-Rad). Quantification and relative gene expression were calculated with internal controls. The ratio between these values obtained provided the relative gene expression levels.
Clonogenic survival assay
Colony assays were performed similar to previously published methods (30). MCF-7 cells were plated in six-well plates at a density of 1000 cells per well in full DMEM. Twenty-four hours later, cells were treated with ABC294640 (0.1–10 μm) and then monitored for colony growth. Ten days later, the cells were fixed with 3% glutaraldehyde. After fixation for 15 min, the plates were washed and stained with a 0.4% solution of crystal violet in 20% methanol for 30 min, washed with PBS, and dried. Colonies of at least 30 cells were counted as positive. Results were normalized to DMSO vehicle-treated control cells. Statistical analysis of IC50 values were calculated from concentration-response curves using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Cell proliferation immunofluorescence assay
Cells were plated at a density of 10,000 cells per well in a 96-well plate in 10% DMEM and allowed to attach over night. he following day, cells were treated with DMSO or ABC294640 for 24 h. At endpoint, cells were fixed using 100 μl 3.7% formaldehyde in PBS for 10 min. Formaldehyde was removed, and cells were permeabilized using cold methanol for 5 min at room temperature and washed twice with PBS. One hundred microliters of 3% FBS in PBS blocking buffer was then added. After 30 min, blocking buffer was removed and cells were incubated for 1 h with Ki-67 (BD PharMingen, San Diego, CA) antibody. Cells were then washed with PBS and stained with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain for 5 min before imaging. For staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view per image. The vehicle control was then set to 1 for comparison with ABC294640 treatment.
Cell viability assay
Viability assays were performed as previously described (30). Briefly, cells were plated at a density of 7.5 × 105 cells per well in a 96-well plate in phenol-free DMEM supplemented with 5% FBS and allowed to attach overnight. Cells were then treated with ABC294640 (ranging from 10 nm to 100 μm) for 24 h. After treatment, 20 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml) reagent was incubated in each well for 4 h. Cells were lysed with 20% sodium dodecyl sulfate in 50% dimethylformamide. The pH and absorbance values were read on an ELx808 Microtek plate reader (Bio-Tek Instruments, Winooski, VT) at 550 nm, with a reference wavelength of 630 nm.
E2 response element (ERE)-luciferase assay
As previously described (28,31), the cells were seeded in 24-well plates at a density of 5 × 105 cells per well in the same medium and allowed to attach overnight. After 18 h, cells were transfected for 5 h in serum-free DMEM with 300 μg pGL2-ERE2X-TK-luciferase plasmid, using 6 μl Effectene (QIAGEN) per microgram of DNA. After 5 h, the transfection medium was removed and replaced with phenol red-free DMEM supplemented with 5% dextran-coated charcoal-treated FBS-containing vehicle, E2, SKI, or E2 plus SKI and incubated at 37 C. After 18 h, the medium was removed, and 100 μl lysis buffer was added per well and then incubated for 15 min at room temperature. Cell debris was pelleted by centrifugation at 15,000 × g for 5 min. Cell extracts were normalized for protein concentration using reagent according to the manufacturer’s protocol (Bio-Rad). Luciferase activity for the cell extracts was determined using luciferase substrate (Promega Corp., Madison, WI) in an Autoluminat Plus luminometer (Berthhold Technologies, Bad Wildbad, Germany).
ERα-binding assays
Receptor binding assays were performed as previously described (28). In this method, recombinant ER is in equilibrium with a fluorescent ligand (ES2) and a concentration of the competitor (ABC294640). The relative displacement of the ES2 is measured as a change in polarization anisotropy. Serial dilutions of competitors (ABC294640 and estradiol) were prepared from DMSO stock solutions in screening buffer at the desired concentrations. The ER and ES2 were combined with each competitor aliquot to a final concentration of 2 nm ER and 3 nm ES2, respectively. In addition, both a no-binding control (ER plus ES2, equivalent to 0% competitor inhibition) and a 100% binding control (only free ES2, no ER, equivalent to 100% competitor inhibition) were prepared. All competitors and controls were prepared in duplicate within a binding experiment. After 2 h incubation at room temperature, the anisotropy value for each sample and control were measured using the Beacon 2000. Anisotropy values were converted to percent inhibition using the following formula: I% = (A0 − A)/(A0 − A100) × 100, where I% is the percent inhibition, A0 is 0% inhibition, A100 is 100% inhibition, and A represents the observed value. This conversion to percent inhibition makes the data more intuitive and normalizes the experiment-to-experiment differences in the range of anisotropy values. The percent inhibition vs. competitor concentration curves were analyzed by nonlinear least-squares curve fitting (Prism version 5.0a; GraphPad Software) to yield IC50 values (the concentration of competitor needed to displace half of the bound ligand). To compare binding affinities of the test compounds to those reported in the literature, IC50 values were converted to relative binding affinities (RBA) using E2 as a standard. The E2 RBA was set equal to 100 RBA = (IC50/IC50 of E2) × 100.
Molecular modeling
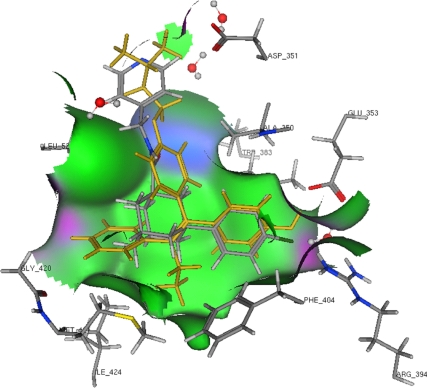
The structure of ABC294640 was converted to unique SMILE strings with ChemDraw (CambridgeSoft, Cambridge, MA) and then converted to three-dimensional structures using MOE 2008.10 (Chemical Computing Group, Montreal, Quebec, Canada). The initial three-dimensional models were then optimized in MOE using the MMFF94 force field with the conjugated gradient method using a termination of 0.005 kcal/mol. The ABC294640 model was added to a database containing an optimized model of 4-OH-tamoxifen. Docking and scoring of ABC294640 were performed using the crystal structure of the human ERα ligand-binding domain in complex with 4-OH-tamoxifen (Protein Data Bank: 3ERT, antagonist configuration) and the docking function of MOE. The A chain, including associated waters, was extracted from the 3ERT crystal structure, and then hydrogens were added and optimized for docking with the protonate three-dimensional function of MOE. The models of ABC294640 and 4-OH-tamoxifen were then docked into the 3ERT chain A model with MOE dock using the triangle matcher placement (default settings), London dG rescoring 1, Forcefield refinement (default settings), and London dG rescoring 2. This docking procedure was previously defined in this study as the MOE docking method that produced the best root mean square deviation (0.758) replacement of the 4-OH-tamoxifen model into this crystal structure. Up to 30 scoring poses were obtained for docking into the tamoxifen-induced, antagonist form of the ERα. Fewer than 10 of these were found to have very similar, favorable docking scores (S). Of these high scoring poses, one representative pose was selected to depict possible ER-binding modes for the ABC294640 compound.
Animals
Xenograft models were performed similar to previously reported studies (32). In brief, nu/nu immunocompromised female ovariectomized mice (29–32 d old) were obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment ad libitum. Placebo or estradiol pellets (0.72 mg, 60-d release; Innovative Research of America, Sarasota, FL) were implanted sc in the lateral area of the neck in the middle point between the ear and shoulder using a precision trochar (10 gauge). MCF-7 cells in the exponential phase of growth were harvested using PBS/EDTA solution and washed. Viable cells (5 × 106) in a 50-μl sterile PBS suspension were mixed with 100 μl Matrigel reduced factors (BD Biosciences, Bedford, MA). MCF-7 cells were injected in the mammary fat pad through a 5-mm incision in the hypogastric region, and the incision was closed using staples. All the procedures in animals were performed under anesthesia using a mix of isoflurane and oxygen delivered by mask. Tumors were allowed to form over 10 d, and mice were randomized to two treatment groups with five mice per group: vehicle control and ABC294640. The ABC294640 mixture was suspended in a solution of DMSO and PBS and was given ip at 100 mg/kg · mouse · d for 15 d starting after tumors were measureable. Control mice were injected with vehicle daily for 15 d. Tumor size was measured every 2 d using a digital caliper. The volume of the tumor was calculated using the following formula: 4/3π LS2 (L = larger radius; S = shorter radius). At necropsy on d 24, animals were euthanized by cervical dislocation after exposure to a CO2 chamber. Tumors, uteri, livers, and lungs were removed and either frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Tulane University Animal Care and Use Committee. The facilities and laboratory animal program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Immunohistochemistry
Immunohistochemistry was performed as described in previously published methods (29). Tumor explants were collected at necropsy and fixed in 10% buffered formalin phosphate. Formalin-fixed paraffin-embedded (FFPE) 4-μm-thick tumor sections were analyzed by immunohistochemistry using primary monoclonal antibodies against human PgR (Dako North America, Inc., Carpinteria, CA). The mouse antibodies on mouse tissue polymer detection kit (Biocare Medical, LLC, Concord, CA) were used to perform immunohistochemistry. Briefly, FFPE sections were deparaffinized and hydrated in a graded series of ethanol solutions followed by 3% H2O2 for 5 min to inactivate endogenous peroxides and then rinsed. Slides were subjected to 10 min incubation in avidin followed by 10 min incubation in biotin. For antigen retrieval, sections were exposed to rodent decloaker (Biocare Medical) at 95 C for 25 min, rinsed, and allowed to cool to room temperature for 20 min. Slides were incubated with rodent block for 30 min and then with primary antibodies (PgR; Dako) or serum alone (negative control) for 75 min. Mouse-on-mouse horseradish peroxidase-polymer secondary antibody was added to the sections and incubated for 15 min. After rinsing, diaminobenzidine solution (Biocare Medical) was applied and incubated for 1 min, and sections were counterstained with hematoxylin (Biocare Medical) followed by Tacha Blueing reagent (Biocare Medical) for 30 sec each. Slides were then allowed to air dry and then coverslipped using Acrymount (Fisher Scientific). Sections were viewed and photographed using the Leica DM IRB inverted research microscope and SPOT RT color camera. Five images at ×40 were taken of each tumor with care to avoid areas of necrosis. For PgR staining quantification, numbers of positively stained cells were expressed as a percentage of the total number of cells per field of view per image.
Lipidomics analysis
Endogenous lipid levels were quantified by mass spectrometry (Lipidomics Core, Medical University of South Carolina, Charleston, SC) according to published methods (34). Briefly, cells were collected, fortified with internal standards, and extracted with ethyl acetate/isopropyl alcohol. Electrospray ionization followed by tandem mass spectrometry (ESI/MS/MS) analyses of sphingoid bases, sphingoid base 1-phosphates, ceramides, and sphingomyelins were performed on a Thermo Finnigan (San Jose, CA) TSQ 7000 triple quadrupole mass spectrometer.
Western blot analysis
Protein analysis was performed as described previously (27). Briefly, cells were plated at 50–60% confluency in 10-cm2 culture flasks in 10% DMEM for 48 h. Cells were then treated with DMSO or ABC294640 for 1 h. After treatment, cells were detached with PBS-EDTA and centrifuged. After removing supernatant, cells were lysed in 60–100 μl lysis buffer [mammalian protein extraction reagent and Halt protease inhibitor, (Pierce, Rockford, IL) and PhoSTOP phosphatase inhibitor (Roche, Boulder, CO]. Lysed cells were centrifuged for 10 min at 12,000 × g at 4 C to separate protein from cell debris. The supernatants were combined with loading buffer (5% 2-mercaptoethanol in 4× lithium dodecyl sulfate loading buffer; Invitrogen), boiled for 5 min, and loaded onto a 4–12% bis-tris polyacrylamide gels (Invitrogen) followed by polyacrylamide gel electrophoresis at 150 V for 1.25 h. Protein was transferred to nitrocellulose membranes using the iBlot (Invitrogen) transfer unit. Nitrocellulose membranes were blocked in 5% milk (Bio-Rad) Tris-buffered saline-Tween 20 (TBST) for 1 h at room temperature. Cells were washed briefly with 1× TBST (USB, Cleveland OH) and primary antibodies were diluted in 5% BSA (Sigma-Aldrich, St. Louis, MO) and TBST according to manufacturer’s recommended dilutions. Antibodies for tubulin, AKT, and phospho-AKT were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Membranes were incubated in primary antibody overnight at 4 C with gentle agitation. Secondary infrared conjugated antibodies (LI-COR Biosciences, Lincoln, NE) were diluted in 5% milk-TBST solution at 1:10,000, and membranes were incubated for 1 h under gentle agitation at room temperature. Membranes were scanned using the LI-COR Odyssey imager and software (LI-COR) to detect total and phosphorylated protein levels in cell lysates. Protein levels were quantified using densitometry analyses.
Statistical analysis
Statistical analysis of IC50 values were calculated from concentration-response curves using GraphPad Prism version 5.0 (GraphPad Software), using the equation Y = bottom + (top − Bottom)/1 + 10 logEC50 − X, assuming a standard slope, where the response goes from 10–90% of maximal as X increases over two log units. Differences in IC50 were compared using Student’s unpaired t test with P < 0.05 as the limit of statistical significance. Experiments comparing multiple concentrations to the control were tested with one-way ANOVA with Bonferroni post-test to compare individual concentrations. All statistical analyses were done using GraphPad Prism version 5.0 (GraphPad Software).
Results
ABC294640 decreases E2 signaling and MCF-7 tumor proliferation in vivo
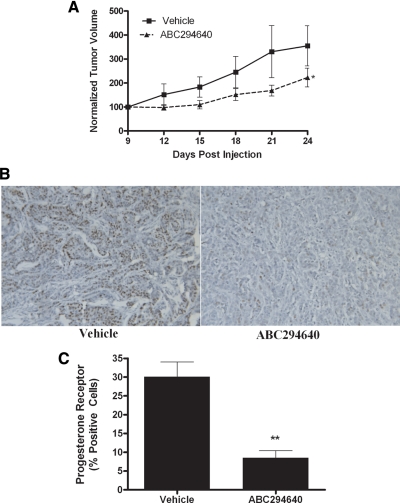
The most comprehensively studied ER-positive breast cancer cell line, MCF-7, was used as our model system to explore the effects of a novel SK2 inhibitor, ABC294640, on tumor growth. Using well-established immunocompromised mouse xenograft models for tumor growth, MCF-7 cells were injected into the mammary fat pads of female ovariectomized mice with exogenous E2 pellets, and tumor formation and size were documented over the course of 24 d. As seen in Fig. 2A, ABC294640 showed marked antitumor activity, with a statistically significant 68.4 ± 10.95% (P < 0.05) decrease in tumor volume at endpoint (n = 5). Furthermore, there were no obvious signs of weight loss or illness in these mice at a dose of 100 mg/kg. Here, we demonstrate for the first time that the SKI ABC294640 can block breast cancer tumor growth in vivo.
Figure 2.
ABC294640 decreases MCF-7 tumor growth and ER signaling in vivo. A, MCF-7 cells (5 × 106) were injected in the mammary fat pads of female ovariectomized mice with exogenous E2 pellets. Tumors were allowed to form over 9 d. Mice were treated ip with 100 mg/kg ABC294640 for 15 d. Tumor volume was measured every 2 d. Treatment tumors at endpoint were statistically significantly different (Student’s t test) from vehicle (P < 0.05). B, Tumors from vehicle- and ABC294640-treated mice were processed and stained for PgR. Representative images of human PgR staining in tumor sections are shown. C, Quantitation of PgR staining is expressed as percent positive of total number of cells per field of view. **, P < 0.01.
Given the increased efficacy of ABC294640 in ER-positive compared with ER-negative breast cancer, we examined the effect of ABC294640 on E2 signaling in vivo. We further studied the effect of ABC294640 on ER signaling using immunohistochemical staining for PgR in tumors excised at endpoint from the xenograft-treated mice (Fig. 2B). Expression of PgR is regulated through ER-mediated pathways and thus serves as a marker of E2 exposure within larger target tissues. Treatment with ABC294640 correlated with a 71.33 ± 6.40% (P < 0.01) decrease in PgR staining compared with vehicle control (Fig. 2C). These results were quantified as a percentage of total cells per field of view, thus taking into account changes in cell density. Therefore, the decrease in progesterone staining can be attributed to an anti-E2 effect rather than a simultaneous decrease in tumor cell volume. Taken together, these data demonstrate the therapeutic potential of ABC294640 in treating ER-positive breast cancer.
SKI blocks ER activity
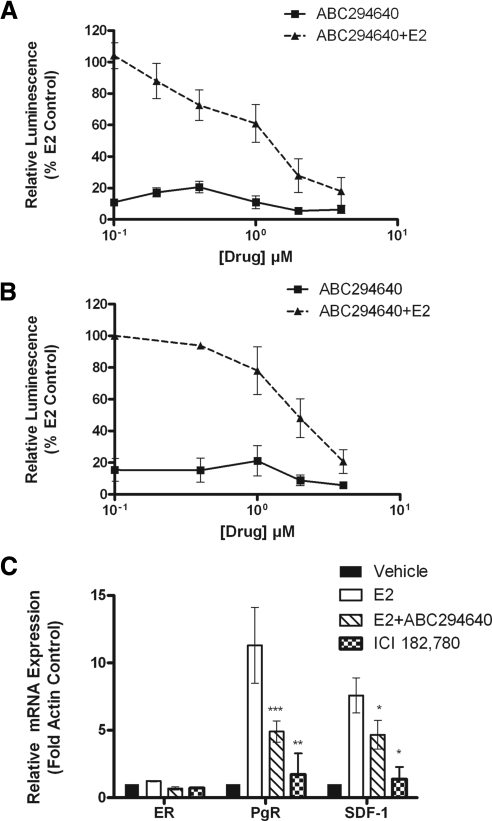
Given the decrease in PgR expression after ABC294640 treatment in vivo, we set out to determine the antiestrogenic mechanism of ABC29640 using cell model systems. We next examined the ability of ABC294640 to block ERE transcriptional activity in ER-negative HEK293 cells transfected with ERα. As seen in Fig. 3A, ABC294640 dose-dependently decreased ERE luciferase activity in these cells. To confirm that this finding occurs in an ER-positive breast cancer, the inhibitor was also tested for effects on ERE activity in MCF-7 cells, which correlated with the HEK293 results (Fig. 3B). These data suggest that ABC29640 can block ER transcriptional activity in human breast cancer.
Figure 3.
ABC294640 blocks ER signaling in vitro. A and B, HEK293 (A) or MCF-7 (B) cells were transiently transfected with pGl2-ERE2X-TK-luciferase plasmid. HEK293 cells were additionally transfected with pcDNA3.1B-ERα plasmid. Subsequently, cells were treated with DMSO (control), E2, or ABC294640. Cells treated with E2 were set to 1. C, Effect of ABC294640 on ERα, PgR, and SDF-1 mRNA gene expression. E2 (1 nm) increased PgR and SDF-1, whereas ABC294640 (6 μm) and ICI 182,780 (100 nm) decreased E2-induced PgR and SDF-1. Mean values ± sem of three independent experiments are reported. Statistical analysis was performed comparing treatment groups with E2 control: ***, P < 0.001; **, P < 0.01, *, P < 0.05.
We further investigated the inhibitor for its effect on downstream ER-mediated gene expression. Two differentially expressed genes were selected for quantitative real-time RT-PCR analysis: PgR and SDF-1. These genes are known to be regulated by the ER and play a role in E2-induced proliferation and tumorigenesis. Interestingly, ABC294640 decreased E2-stimulated mRNA levels of PgR and SDF-1 in MCF-7 cells, whereas levels of ER remained constant (Fig. 3C). ABC294640 decreased PgR and SDF-1 mRNA expression 61.5 ± 14.08% (P < 0.001) and 38.3 ± 3.11% (P < 0.05). However, this decrease in PgR and SDF-1 expression was lower compared with the pure anti-E2 ICI 182,780. These results demonstrate that ABC294640 can block ER activity without affecting total transcriptional levels of ER.
The SKI ABC294640 directly binds the ER
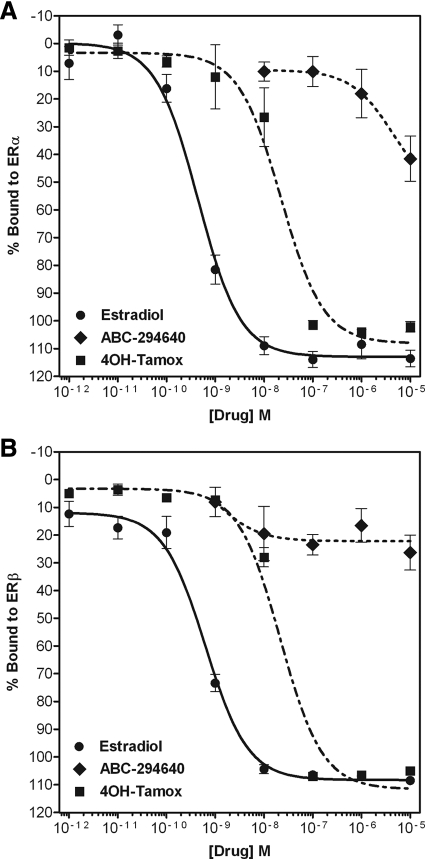
The effect of the SKI ABC294640 on ER signaling in vitro and in vivo led us to investigate possible mechanisms through which this drug blocks E2 signaling. Using an exogenous competitive binding assay, we found that ABC294640 can act as an anti-E2, directly binding ERα, but not ERβ, and displaces E2 in situ (Fig. 4). This ERα binding was relatively weak compared with E2, with an ERα-binding IC50 of 4.51 ± 6.35 μm (P < 0.05). In comparison, E2 has an IC50 of 0.44 ± 1.21 nm (P < 0.05), and the ER antagonist 4OH-tamoxifen has an IC50 of 22.2 ± 1.34 nm (P < 0.05). The low micromolar binding of ABC294640 compared with E2 correlates with the antisurvival IC50 of this compound, suggesting that there may be a duel mechanism of action, blocking both sphingosine kinase and E2 signaling in vitro.
Figure 4.
Competitive binding of ABC294640 to the ER. Increasing concentrations of ABC294640 or 4-OH-tamoxifen were added to ERα (A) or ERβ (B) complex and compared with E2. Data points and error bars represent the mean ± sem of seven independent experiments for ABC294640 and E2 treatment for each concentration tested.
To further characterize the anti-E2 mechanism of this SKI, molecular modeling, docking, and scoring to ERα were performed. As seen in Fig. 5, the binding cavity of the ER is depicted using three essential amino acids located within the binding pocket: arginine 394 and glutamate 353 on the right and histidine 524 on the left. The binding model of tamoxifen is displayed in yellow, whereas those of ABC294640 are in atom colors. ABC294640 can prevent association of helix 12 with helix 11, as needed for an agonist configuration. This drug is too long to fit into the ER ligand-binding cavity in the agonist configuration and can dock only into the ER crystal structure that is formed from the binding of the antagonist. Only one ER-binding mode is proposed by docking for ABC294640. This pose, shown in Fig. 5, has the halogenated phenyl ring of ABC294640 binding the same way that the phenolic group of tamoxifen binds to ER. The halogen can form pseudo H-bonds to Arg 395 and Glu 353 and water, which, although weak, may be strong enough to stabilize binding. The complete H-bonding network can be accomplished with the phenolic ring binding of ABC294640. Because the pyridine ring of ABC294640 projects out of the ER-binding cavity in a similar way to the aryl amino side chain of 4-hydroxy-tamoxifen, it may interact with Asp 351 (H-bond) to stabilize ABC294640 ER binding.
Figure 5.
Molecular modeling and docking of ABC294640 to the ER, showing 4-OH-tamoxifen and ABC294640 in the binding cavity of the ERα ligand-binding domain interacting with arginine 394, glutamate 353, and histidine 524.
ABC294640 inhibits breast cancer clonogenic survival, proliferation, and viability in vitro
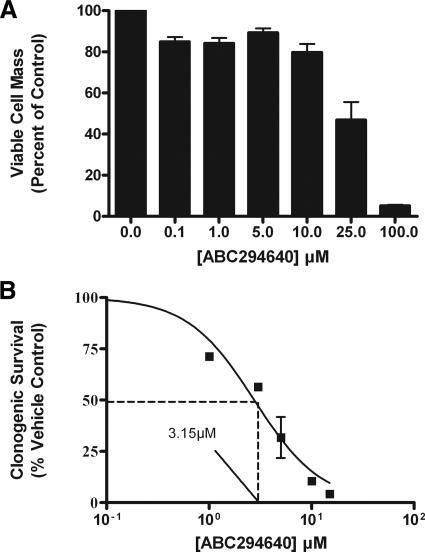
Both the sphingolipid and E2 pathways are known to promote cancer growth and survival. Pharmacological inhibition of both S1P and the ER may be an advantageous characteristic for potential experimental therapeutics targeting ER-positive breast cancer. Therefore, we examined the effect of ABC294640 on MCF-7 cell viability. Using MTT assays, we report here that ABC294640 dose-dependently decreases MCF-7 cell viability, with an IC50 value of 25.8 ± 3.24 μm (P < 0.001) (Fig. 6A). There is disagreement in the literature as to whether short-term viability assays accurately reflect chemotherapeutic potential because cancer treatment is usually over weeks and not hours (35). To investigate the effect of this SKI on long-term breast cancer clonogenic survival, we tested the effects of ABC294640 on MCF-7 colony formation. As seen in Fig. 6B, treatment with ABC294640 caused a dose-dependent decrease in clonogenic survival, with an IC50 value of 3.15 ± 1.16 μm (P < 0.001). This low micromolar antisurvival potency places this drug within clinical treatment range with current approved chemotherapeutic agents such as paclitaxel and etoposide (36,37).
Figure 6.
ABC294640 decreases MCF-7 clonogenic viability and survival. A, MCF-7 cells were plated at 7.5 × 105 cells per 96-well plate. The following day, cells were treated with indicated concentrations of ABC294640 for 24 h. Data are presented as percentage of vehicle-treated samples. Mean values of ± sem of three different experiments in quadruplicate are reported. B, MCF-7 cells were plated at 1000 cells per well in six-well plates, and 24 h later, cells were treated with DMSO (vehicle) or ABC294640 (0.1–15 μm). Colonies of at least 30 cells were counted as positive. Results were normalized to percent clonogenic survival from vehicle control cells. Data points and error bars represent the mean ± sem of three independent experiments.
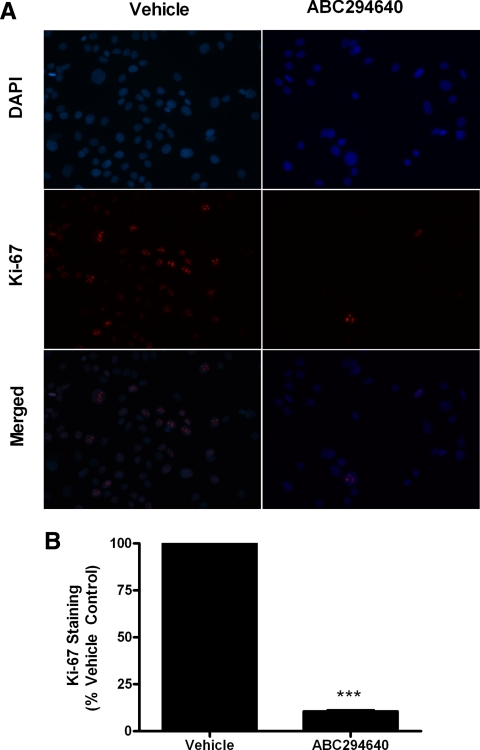
The ability to block both survival and proliferation is of therapeutic interest in the treatment of breast cancer. Therefore, we examined the ability of ABC294640 to alter the Ki-67 proliferative index. The Ki-67 protein is a known marker of proliferation and is present only during active phases of the cell cycle (38,39). Levels of Ki-67 have been correlated with breast cancer prognosis, and this protein remains the preferred molecular marker for breast cancer proliferation (39,40). Therefore, we stained MCF-7 cells for both Ki-67 and the nuclear stain DAPI to determine whether ABC294640 could decrease cancer cell proliferation. As seen in Fig. 7A, ABC294640 has potent antiproliferative properties, significantly abolishing Ki-67 in vitro immunostaining. Quantitative analysis revealed that treatment with 10 μm ABC294640 decreased Ki-67 staining by 89.34 ± 1.79% (P < 0.001) compared with vehicle control (Fig. 7B).
Figure 7.
SKI blocks MCF-7 proliferation. A, MCF-7 cells treated with DMSO (vehicle) or ABC294640 for 48 h. Cells were then fixed using 3.7% formaldehyde in PBS, permeabilized using cold methanol, and incubated with anti-Ki-67 antibody (red). Cells were then washed with PBS and DAPI (blue) nuclear stain before imaging. Three independent experiments were performed with representative pictures shown here. B, Quantitation of Ki-67 staining was determined as a percentage of total positive cells per image. The vehicle control was then set to 1 for comparison with ABC294640 treatment: ***, P < 0.001. Data points and error bars represent the mean ± sem of three independent experiments.
ABC294640 blocks SK/S1P signaling
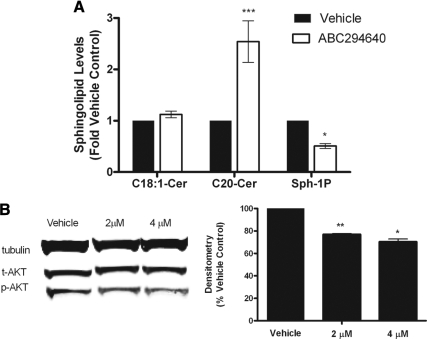
Finally, we determined whether ABC294640 could block formation of S1P and induce formation of endogenous, long-chain ceramides. A potential advantage of sphingosine kinase inhibition as a cancer treatment is blockade of the prosurvival compound S1P while simultaneously decreasing the metabolism of endogenous, proapoptotic ceramides. Thus, blocking sphingosine kinase can decrease a proliferative signal while increasing levels of proapoptotic and antiproliferative long-chain ceramides. Using ESI/MS/MS analyses, we analyzed levels of C18-ceramide, C20-ceramide, and S1P in MCF-7 cells with and without treatment with the inhibitor. As expected, ABC294640 blocked formation of S1P, with a concomitant increase in endogenous ceramide levels (Fig. 8A). Specifically, 24 h treatment with ABC294640 decreased cellular S1P levels by 50.8% (P < 0.05). Long-chain ceramide levels of C20-ceramide were increased by 154.2% (P < 0.001); however, the increase in C18-ceramide was not statistically significant.
Figure 8.
ABC294640 blocks SK/S1P signaling. A, MCF-7 cells were treated with either DMSO vehicle control or ABC294640 (10 μm) for 24 h and measured for cellular levels of C18-ceramide (Cer), C20-ceramide, and S1P (Sph-1P) using ESI/MS/MS. Data points and error bars represent the mean ± range of two independent experiments. ***, P < 0.001; **, P < 0.01; *, P < 0.05. B, MCF-7 cells were treated with either DMSO vehicle control or ABC294640 and analyzed by Western blot for phosphorylated (p-) and total (t-) AKT proteins with densitometry analysis. Phosphorylated AKT levels were normalized to corresponding tubulin and vehicle control set to 1. Data shown are representative of three independent experiments.
We then further examined ABC294640 to determine whether the inhibitor could diminish signaling pathways known to be mediated by SK/S1P signaling. Sphingosine kinase has been shown to induce phosphorylation of AKT in a variety of cell systems (13). The PI3K/AKT pathway is known to induce proliferation in the MCF-7 cell system and has been shown to be an important mediator of endocrine resistance (16). Blockade of this pathway would provide an additional benefit in the treatment of breast cancer. Here, we found that treatment with ABC294640 inhibited phosphorylation of AKT (Fig. 8B). Taken together, these data provide proof of principle that ABC294640 blocks sphingosine kinase and S1P activity in MCF-7 breast cancer cells.
Discussion
Sphingosine kinase has been increasingly studied as a potential therapeutic target in various hematological and solid tumors because it is an important mediator of proliferation, survival, apoptosis, differentiation, and cell senescence (9). In ER-positive breast cancer, sphingosine kinase is a critical promoter of endocrine therapy resistance. Overexpression of sphingosine kinase has been shown to increase MCF-7 cell proliferation and confer resistance to tamoxifen-induced cell death. Correspondingly, tamoxifen-resistant MCF-7 variants exhibit increased concentrations of sphingosine kinase (19). A recent study found a positive correlation between SK1 expression and breast tumor aggressiveness and metastasis in patient tumor samples (41). With the dearth of selective inhibitors of sphingosine kinase available, few studies exist examining cross talk between sphingosine kinase and ER signaling in ER-positive breast cancer beyond biologically altering sphingosine kinase expression in ER-positive cell systems.
The two isoforms of sphingosine kinase have various functions, tissue expression, and subcellular localization within the cell. Endogenous SK1 expression is highest in lung, spleen, kidney, and blood, whereas SK2 is found in highest concentration in the liver, kidney, brain, and heart (42,43,44). It should be noted that sphingosine kinase activity is measureable in all tissue types (45). The isoforms of a sphingosine are differentially located within the cell, depending on the tissue and pathological state. For example, SK2 is found mainly in the nucleus of MCF-7 breast cancer cells but in the cytosol of MDA-MB-453 breast cancer cells. SK1 is found primarily in the cytoplasm of most cells. SK2 shares five conserved domains with SK1 but has an additional 200 amino acids, and both SKs contain a conserved ATP-binding motif (46). Unlike SK1, SK2 has an extended NH2 terminus and possesses a putative Bcl-2 homology domain 3 (BH3)-binding domain. The BH3-binding motif of SK2 is unusual in that it possesses a leucine rather than a glycine at the position immediately before a conserved aspartic acid sequence. It is believed that the BH3 domain in SK2 is at least partially responsible for the differing physiological effects between SK1 and SK2 (47,48,49). Interestingly, some have suggested overexpression of SK2 can change its cellular distribution, possibly accounting for nonendogenous physiological effects (50). Because SK2 contains a BH3 motif, it is possible that proteolysis of excess Sphk2 may be responsible for increases in apoptosis due to free BH3 peptide domains inducing programmed cell death.
Although SK1 has been well studied in the literature, the role of SK2 on cell growth and drug resistance has only recently been elucidated. It is believed that the catalytic activity of SK2, as opposed to overall protein levels, are increased in breast cancer (22,48,49). Small interfering RNA knockdown of SK2 has been shown to restore chemosensitivity to drug-resistant breast cancer cells. It was recently reported that pharmacological inhibition of SK2 induces autophagy in certain solid tumor cancers (50,51). A recent study has shown that SK2 is bound to the histone deacetylases HDAC1 and HDAC2 in a repressor complex on specific genes, such as p21 and c-fos, directly affecting gene transcription and regulating epigenetic modifications in the nucleus (52).
In this study, we describe for the first time the effects of the SKI ABC294640 on ERE transcriptional activity, ER gene expression, ER binding, and ER docking. This inhibitor dose-dependently decreased ERE transcriptional activity and downstream ER-mediated gene expression of PgR and SDF-1 in human breast cancer cells. Furthermore, it can directly bind to the ER in an antagonist fashion similar to tamoxifen. Taken together, our data demonstrate a dual mechanism of action for ABC29640 in human breast cancer, because it blocks both sphingosine kinase and E2 signaling. Both of these features are advantageous in the treatment of ER-positive breast cancer.
Interestingly, there is a disparity in the in vitro and in vivo E2 signaling blockade with SKI treatment and the affinity for this drug to bind the ER. Although the trend is the same, the binding and docking of ABC294640 is weaker than the ER signaling knockdown would suggest. For example, ABC294640 binds with 100-fold less affinity to the ER than does E2, yet there is a 71% decrease in PgR staining with ABC294640 treatment. Indeed, there are several other factors that may be involved other than direct binding to the ER. It is known that sphingosine kinase can regulate phosphorylation of ERK, AKT, and p38 kinases, and we show here that ABC294640 can affect the PI3K/AKT signaling cascade. The ERK and p38-MAPK signaling pathways, as well as AKT, are known to phosphorylate and activate the ER (53,54,55). Therefore, it is likely that there is a secondary effect of ABC294640 on ER activity through inhibition of ER phosphorylation by MAPK and PI3K/AKT signaling pathways. Furthermore, sphingosine kinase is involved in nuclear factor-κB signaling, which can regulate superoxide dismutase (SOD1) (20,56). Superoxide dismutase is known to stabilize the ER complex, interact with DNA-bound ER, and differentially influence E2 response gene expression (33). Further study is needed to determine the effect of nuclear factor-κB and MAPK abrogation by ABC294640 on ER phosphorylation, translocation, and gene transcription.
We also determined the therapeutic potential of ABC294640 in targeting ER-positive breast cancer, documenting its effects on proliferation, survival, viability, and tumor growth. We found that ABC294640 decreases clonogenic survival and viability in MCF-7 cells. This inhibitor also blocked proliferation as measured by Ki-67 immunofluorescence staining in vitro. Finally, we found that ABC29640 significantly decreased breast cancer tumorigenesis in a xenograft mouse model. Taken together, these results suggest that SKI, such as ABC294640, can act as novel therapeutic agents targeting E2 signaling in the treatment of human breast cancer.
Acknowledgments
We thank Dr. Syreeta Tilghman and Dr. Melyssa Bratton for critical reading and review of this manuscript.
Footnotes
This work was supported by The Center for Bioenvironmental Research at Tulane and Xavier Universities, National Institutes of Health Grant NIDDK DK 059389, and the PhRMA Foundation Paul Calabresi Research Fellowship (to J.W.A.).
Disclosure Summary: J.W.A., M.D.W., W.D.M., E.M.S., S.E.M., S.E., L.V.R., H.B.A., T.E.W., M.E.B., and B.S.B. have nothing to declare. C.D.S. is Chief Executive Officer of Apogee Corp. and President of Apogee Biotechnology Corp. and owns stock in this entity.
First Published Online September 22, 2010
Abbreviations: BH3, Bcl-2 homology domain 3; DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethylsulfoxide; E2, estrogen; ER, E2 receptor; ERE, E2 response element; ESI/MS/MS, electrospray ionization followed by tandem mass spectrometry; PgR, progesterone receptor; PI3K, phosphatidylinositol 3-kinase; SDF-1, stromal derived factor-1; SK1, sphingosine kinase-1; SKI, sphingosine kinase inhibitor; S1P, sphingosine-1-phosphate; TBST, Tris-buffered saline-Tween 20.
References
- Fuqua SA, Cui Y 2002 Targeting the estrogen receptor in clinical breast cancer. Breast Dis 15:3–11 [DOI] [PubMed] [Google Scholar]
- Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S 2002 Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res 281:115–127 [DOI] [PubMed] [Google Scholar]
- Burow ME, Weldon CB, Collins-Burow BM, Ramsey N, McKee A, Klippel A, McLachlan JA, Clejan S, Beckman BS 2000 Cross-talk between phosphatidylinositol 3-kinase and sphingomyelinase pathways as a mechanism for cell survival/death decisions. J Biol Chem 275:9628–9635 [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P 2009 Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 150:4484–4492 [DOI] [PubMed] [Google Scholar]
- Hong G, Baudhuin LM, Xu Y 1999 Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Lett 460:513–518 [DOI] [PubMed] [Google Scholar]
- Gratschev D, Löf C, Heikkilä J, Björkbom A, Sukumaran P, Hinkkanen A, Slotte JP, Törnquist K 2009 Sphingosine kinase as a regulator of calcium entry through autocrine sphingosine 1-phosphate signaling in thyroid FRTL-5 cells. Endocrinology 150:5125–5134 [DOI] [PubMed] [Google Scholar]
- Balthasar S, Samulin J, Ahlgren H, Bergelin N, Lundqvist M, Toescu EC, Eggo MC, Törnquist K 2006 Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem J 398:547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham WD, Antoon JW, Burow ME, Struckhoff AP, Beckman BS 2009 Sphingolipids as determinants of apoptosis and chemoresistance in the MCF-7 cell model system. Exp Biol Med (Maywood) 234:1253–1263 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM 2008 Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev 9:139–150 [DOI] [PubMed] [Google Scholar]
- Lucki NC, Sewer MB 2008 Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem 49:387–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki NC, Sewer MB 2010 The interplay between bioactive sphingolipids and steroid hormones. Steroids 75:390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S 2001 Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins 64:123–134 [DOI] [PubMed] [Google Scholar]
- Baudhuin LM, Cristina KL, Lu J, Xu Y 2002 Akt activation induced by lysophosphatidic acid and sphingosine-1-phosphate requires both mitogen-activated protein kinase kinase and p38 mitogen-activated protein kinase and is cell-line specific. Mol Pharmacol 62:660–671 [DOI] [PubMed] [Google Scholar]
- Kim DS, Hwang ES, Lee JE, Kim SY, Park KC 2003 Sphingosine-1-phosphate promotes mouse melanocyte survival via ERK and Akt activation. Cell Signal 15:919–926 [DOI] [PubMed] [Google Scholar]
- Weldon CB, Parker AP, Patten D, Elliott S, Tang Y, Frigo DE, Dugan CM, Coakley EL, Butler NN, Clayton JL, Alam J, Curiel TJ, Beckman BS, Jaffe BM, Burow ME 2004 Sensitization of apoptotically-resistant breast carcinoma cells to TNF and TRAIL by inhibition of p38 mitogen-activated protein kinase signaling. Int J Oncol 24:1473–1480 [PubMed] [Google Scholar]
- Weldon CB, Scandurro AB, Rolfe KW, Clayton JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe BM, Beckman BS, Burow ME 2002 Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray. Surgery 132:293–301 [DOI] [PubMed] [Google Scholar]
- Filardo EJ 2002 Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80:231–238 [DOI] [PubMed] [Google Scholar]
- Sukocheva OA, Wang L, Albanese N, Pitson SM, Vadas MA, Xia P 2003 Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol 17:2002–2012 [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P 2009 Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 150:4184–4192 [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D'Andrea RJ, Gamble JR, Vadas MA 2002 Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J Biol Chem 277:7996–8003 [DOI] [PubMed] [Google Scholar]
- Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S 2010 Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem 285:10477–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD 2010 Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther 333:129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond MN, Bole-Feysot C, Banno Y, Tanfin Z, Robin P 2006 Endothelin-1 inhibits apoptosis through a sphingosine kinase 1-dependent mechanism in uterine leiomyoma ELT3 cells. Endocrinology 147:5873–5882 [DOI] [PubMed] [Google Scholar]
- Morales A, Fernandez-Checa JC 2007 Pharmacological modulation of sphingolipids and role in disease and cancer cell biology. Mini Rev Med Chem 7:371–382 [DOI] [PubMed] [Google Scholar]
- Gangoiti P, Camacho L, Arana L, Ouro A, Granado MH, Brizuela L, Casas J, Fabrias G, Abad JL, Delgado A, Gomez-Munoz A 2010 Control of metabolism and signaling of simple bioactive sphingolipids: Implications in disease. Prog Lipid Res [DOI] [PubMed] [Google Scholar]
- Antoon JW, Liu J, Gestaut MM, Burow ME, Beckman BS, Foroozesh M 2009 Design, synthesis, and biological activity of a family of novel ceramide analogues in chemoresistant breast cancer cells. J Med Chem 52:5748–5752 [DOI] [PubMed] [Google Scholar]
- Burow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, Hammond TG, Clejan S, Beckman BS 1998 Differences in susceptibility to tumor necrosis factor α-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Res 58:4940–4946 [PubMed] [Google Scholar]
- Boué SM, Tilghman SL, Elliott S, Zimmerman MC, Williams KY, Payton-Stewart F, Miraflor AP, Howell MH, Shih BY, Carter-Wientjes CH, Segar C, Beckman BS, Wiese TE, Cleveland TE, McLachlan JA, Burow ME 2009 Identification of the potent phytoestrogen glycinol in elicited soybean (Glycine max). Endocrinology 150:2446–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey MR, Beckman BS, McLachlan JA, Rowan BG, Pochampally R, Burow ME 2010 Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat 121:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS 2004 Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther 309:523–532 [DOI] [PubMed] [Google Scholar]
- Bratton MR, Frigo DE, Vigh-Conrad KA, Fan D, Wadsworth S, McLachlan JA, Burow ME 2009 Organochlorine-mediated potentiation of the general coactivator p300 through p38 mitogen-activated protein kinase. Carcinogenesis 30:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carter-Wientjes C, Wood CE, Erhardt PW, Beckman BS, McLachlan JA, Cleveland TE, Burow ME 2006 Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res 12:7159–7164 [DOI] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM 2008 Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol Endocrinol 22:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J, Szulc ZM, Hannun YA, Bielawska A 2006 Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39:82–91 [DOI] [PubMed] [Google Scholar]
- Brown JM, Wouters BG 1999 Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 59:1391–1399 [PubMed] [Google Scholar]
- Michalakis J, Georgatos SD, de Bree E, Polioudaki H, Romanos J, Georgoulias V, Tsiftsis DD, Theodoropoulos PA 2007 Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann Surg Oncol 14:1220–1228 [DOI] [PubMed] [Google Scholar]
- Weldon CB, Burow ME, Rolfe KW, Clayton JL, Jaffe BM, Beckman BS 2001 NF-κB-mediated chemoresistance in breast cancer cells. Surgery 130:143–150 [DOI] [PubMed] [Google Scholar]
- Starborg M, Gell K, Brundell E, Hoog C 1996 The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci 109(Pt 1):143–153 [DOI] [PubMed] [Google Scholar]
- Beresford MJ, Wilson GD, Makris A 2006 Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res 8:216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Harris R, Caldas C, Pinder SE, Pharoah P 2008 Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 17:323–334 [DOI] [PubMed] [Google Scholar]
- Ruckhäberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grösch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M 2008 Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat 112:41–52 [DOI] [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S 1998 Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem 273:23722–23728 [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T 2003 Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278:47408–47415 [DOI] [PubMed] [Google Scholar]
- Kihara A, Anada Y, Igarashi Y 2006 Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J Biol Chem 281:4532–4539 [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Kihara A, Igarashi Y 2003 Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem Biophys Res Commun 309:155–160 [DOI] [PubMed] [Google Scholar]
- Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S 2000 Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 275:19513–19520 [DOI] [PubMed] [Google Scholar]
- Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S 2005 Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem 280:36318–36325 [DOI] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S 2003 Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278:40330–40336 [DOI] [PubMed] [Google Scholar]
- Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill Jr AH, Milstien S, Spiegel S 2005 SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280:37118–37129 [DOI] [PubMed] [Google Scholar]
- Sankala HM, Hait NC, Paugh SW, Shida D, Lépine S, Elmore LW, Dent P, Milstien S, Spiegel S 2007 Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res 67:10466–10474 [DOI] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Smith CD 2010 A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther 333:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S 2009 Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325:1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong BN, Elliott S, Frigo DE, Melnik LI, Vanhoy L, Tomchuck S, Lebeau HP, David O, Beckman BS, Alam J, Bratton MR, McLachlan JA, Burow ME 2006 AKT regulation of estrogen receptor β transcriptional activity in breast cancer. Cancer Res 66:8373–8381 [DOI] [PubMed] [Google Scholar]
- Frigo DE, Basu A, Nierth-Simpson EN, Weldon CB, Dugan CM, Elliott S, Collins-Burow BM, Salvo VA, Zhu Y, Melnik LI, Lopez GN, Kushner PJ, Curiel TJ, Rowan BG, McLachlan JA, Burow ME 2006 p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol 20:971–983 [DOI] [PubMed] [Google Scholar]
- Kuske B, Naughton C, Moore K, Macleod KG, Miller WR, Clarke R, Langdon SP, Cameron DA 2006 Endocrine therapy resistance can be associated with high estrogen receptor α (ERα) expression and reduced ERα phosphorylation in breast cancer models. Endocr Relat Cancer 13:1121–1133 [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Gamble JR, Vadas MA 1999 Activation of sphingosine kinase by tumor necrosis factor-α inhibits apoptosis in human endothelial cells. J Biol Chem 274:34499–34505 [DOI] [PubMed] [Google Scholar]