Abstract
Androgens and the androgen receptor are important for both normal prostate development and progression of prostate cancer (PCa). However, the underlying mechanisms are not fully understood. The Polycomb protein enhancer of zeste homolog 2 (EZH2) functions as an epigenetic gene silencer and plays a role in oncogenesis by promoting cell proliferation and invasion. EZH2 has been implicated in human PCa progression, because its expression is often elevated in hormone-refractory PCa. Here, we demonstrated that expression of EZH2 is lower in androgen-sensitive LNCaP PCa cells compared with Rf and C4-2 cells, two androgen-refractory sublines that are derived from LNCaP cells. Androgen ablation by castration increased the level of EZH2 proteins in LNCaP xenografts in mice. In contrast, treatment of LNCaP cells in culture with the synthetic androgen methyltrieolone (R1881) at doses of 1 nm or higher suppressed EZH2 expression. Moreover, our data suggest that androgen repression of EZH2 requires a functional androgen receptor and this effect is mediated through the retinoblastoma protein and its related protein p130. We further showed that androgen treatment not only increases expression of EZH2 target genes DAB2IP and E-cadherin but also affects LNCaP cell migration. Our results reveal that androgens function as an epigenetic regulator in prostatic cells by repression of EZH2 expression through the retinoblastoma protein and p130-dependent pathways. Our findings also suggest that blockade of EZH2 derepression during androgen deprivation therapy may represent an effective tactic for the treatment of androgen-refractory PCa.
Expression of the oncoprotein EZH2 is down-regulated by androgens in prostate cancer cells through RB and its related protein p130-dependent pathways.
Androgens and the androgen receptor (AR) are essential for the development and function of the prostate (1). Androgen ablation via castration results in involution of the prostate, primarily due to apoptotic cell death in the androgen-dependent epithelium (2). The complex action of androgens in the prostate is further reflected in the fact that it also plays essential roles in proliferation and survival of prostate cancer (PCa) cells (3). Although it is not completely clear how androgens promote PCa cell growth and survival, recent findings suggest that androgens may induce prostatic cell transformation by up-regulating expression of oncogenes in the E-twenty six family, which are frequently found fused with androgen-regulated genes due to chromosomal translocations in PCas (4,5). Like normal prostatic epithelial cells, PCa cells undergo apoptosis after androgen deprivation therapy (ADT). After an initial disease remission, however, most patients relapse with a more aggressive form, which is termed androgen-refractory PCa or castration-resistant disease. Understanding how PCa develops into this lethal form of the disease provides promise for the prevention or cure of androgen-refractory PCa.
Enhancer of zeste homolog 2 (EZH2) belongs to the superfamily of Polycomb proteins and primarily functions as an epigenetic gene silencer (6,7,8). It is the only enzymatic subunit of the Polycomb repressive complex 2 and catalyzes trimethylation of lysine 27 on histone H3, thereby maintaining nucleosomes in the repressed state and promoting epigenetic gene silencing (6). Studies with embryonic stem cells demonstrate that EZH2 and other components in the Polycomb repressive complex 2 are important for stem cell self-renewal and global repression of undesirable differentiation (9,10,11). EZH2 has been implicated in human cancer progression, because its expression is often elevated in human cancers, including PCa (12,13). In line with this notion, overexpression of EZH2 induces neoplastic transformation of benign prostatic cells in vitro and in vivo and enhances PCa cell invasion in vitro and metastasis in animals (14,15,16,17).
Because EZH2 potentially has an important role in PCa progression, especially during relapse after ADT, it is critical to unravel the molecular mechanism governing EZH2 expression during disease progression. Expression of EZH2 was shown to be regulated by the retinoblastoma protein (RB)-E2F1 pathway in fibroblasts (18). It has been shown recently that expression of EZH2 is inhibited by microRNA-101 in PCa and bladder transitional cell carcinoma (15,19). In this study, we found that expression of EZH2 is repressed by androgens at concentrations of 1 nm or higher. We further demonstrated that repression of EZH2 by androgens requires a functional AR and that this effect is mediated primarily through the pocket proteins RB and p130. Findings from this study may, therefore, provide a plausible explanation for EZH2 overexpression in hormone-refractory PCa after ADT.
Materials and Methods
Cell lines and cell culture
LNCaP and DU145 cells were purchased from American Type Culture Collection (Manassas, VA), and C4-2 cells were purchased from UroCorporation (Oklahoma City, OK). The androgen-refractory LNCaP subline, Rf, was established as described previously (20,21). LNCaP and DU145 cells were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin B. Hormone-refractory Rf and C4-2 cells were cultured in RPMI 1640 containing 10% charcoal-stripped serum (CSS). LAPC-4 cells were kindly provided by C. L. Sawyers and maintained in Iscove’s Modified Dulbecco’s Media with 10% FBS. RB-deficient mouse prostate epithelial cells (RB−/− PrE) were a gift from M. L. Day and S. W. Hayward and maintained in RPMI 1640 containing 200 μg/ml G418, 5% FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin B (22). For androgen dose and time-course studies, cells were plated, and the next day, the media were changed to medium with 10% CSS. After 24 h, a synthetic androgen, methyltrieolone (R1881), or ethanol (EtOH) was added.
Plasmids and antibodies
Expression vectors for wild-type adenoviral E1A (12S) and the mutants, RG2 and YH47/CG124, were kindly provided by R. F. Kelm (23). Expression vectors for p107 and p130 were gifts from B. Dynlacht. Plasmids of E2F4 and E2F5 were kindly provided by J. R. Nevins. Antibodies against AR (N-20), E1A (M73), Erk2 (D-2), p107 (C-18), p130 (C-20), E2F4 (C20), and B-cell lymphoma 2 (Bcl-2) (N-19) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibody against EZH2 (AC22) was obtained from Cell Signaling Technology (Danvers, MA). The antibody to RB (no. 554136) was purchased from BD Biosciences (San Jose, CA) and phospho-p130 at S672 (p130-S672-p) from Abcam (Cambridge, MA).
Cell transfections
LNCaP or DU145 cells were transfected with plasmid DNA by electroporation. Cells were mixed with DNA and electroporated using a BTX T820 spare wave electroporator. SMARTpools of small interfering RNA (siRNA) specific for AR, RB, p107, p130, disabled homolog 2-interacting protein (DAB2IP), E-cadherin (E-Cad), and nonspecific (NS) control siRNA were purchased from Dharmacon (Lafayette, CO). Cells were transfected with 200 nm of the siRNA as described above. Cells were harvested 48–72 h after transfection. Approximately 75–90% transfection efficiencies were routinely achieved (20).
Western blot analysis
Protein samples were prepared by lysing cells in modified RIPA buffer [1× PBS, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO)]. Lysates (50–100 μg) were separated on a 7.5% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with the specific primary antibody and HRP-conjugated secondary antibody and then visualized by chemiluminescence.
Semiquantitative and real-time RT-PCR
Total RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen). PCR products were separated on a 2% agarose gel containing ethidium bromide. Quantitative real-time PCR was done with cDNA samples using the iQ SYBR Green Supermix and ABI Prism 7900 platform (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. The 2−ΔΔCt method was used to calculate the relative expression level by normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. The following primer sequences were used: EZH2, 5′-TGCAGTTGCTTCAGTACCCATAAT-3′ and 5′-ATCCCCGTGTACTTTCCCATCATAAT-3′; prostate specific antigen (PSA), 5′-AGGCCTTCCCTGTACACCAA-3′ and 5′-GTCTTGGCCTGGTCATTTCC-3′; GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′; beta-2 adrenergic receptor 5′-GTGACTTTATGCCCCTTTAGAGACAA-3′ and 5′-GAAGGGCTACAACTGGAACTGGAATA-3′; DAB2IP, 5′-ATTCCTCCAGGTGGGTGTGG-3′ and 5′-CTAAGCCGCTGTTGCCTTGGC-3′; E-Cad, 5′-TAGAGGGTCACCGCGTCTAT-3′ and 5′-TCACAGGTGCTTTGCAGTTC-3′; runt-related transcription factor 3, 5′-TGTCCCGGGATCCTCTTCT-3′ and 5′-TAGAGACGTTGGTGCGGAAAT-3′; and 2′-5′ oligoadenylate synthetase 2 (OAS2), 5′-TGAGAGCAATGGGAAATGGG-3′ and 5′-AGGTATTCCTGGATAAACCAACCC-3′.
Chromatin immunoprecipitation (ChIP) assay
LNCaP cells were treated with 10 nm R1881 or vehicle 24 h after plating. At 48 h after treatment, the ChIP assay was performed as described previously (24). The soluble chromatin was incubated with 1 μg of rabbit IgG, p130 (C-20), or E2F4 (C20) antibodies. PCR was performed using primers specific for the E2F binding region in the EZH2 promoter 5′-CGCCGGTTCCCGCCAAGAG-3′ and 5′-GTTCGCTGTAAGGGACGCCA-3′ or NS primers outside the E2F binding region in the EZH2 promoter 5′-CGGCCAGTGGCGTCCCTTAC-3′ and 5′-CCCAGCCCAATCAAGCGCCG-3′. Primers specific for the p130/E2F4 binding region in the E2F1 promoter were 5′-AGGAACCGCCGCCGTTGTTCCCGT-3′ and 5′-CTGCCTGCAAAGTCCCGGCCACTTT-3′. PCR products were separated on a 2% agarose gel containing ethidium bromide.
Xenografting
LNCaP cells (2 × 106) were mixed with equal volume of matrigel (BD Biosciences) and injected sc into nude mice. After 6 wk, mice were castrated. Mice were killed 1 wk later, and LNCaP grafts were collected. One part was fixed in 10% buffered formalin, washed with 70% EtOH, and paraffin embedded. The other part of the tissue was homogenized to extract protein for Western blot analysis. The Institutional Animal Care and Use Committee at the University of Minnesota approved all animal experiments.
Immunohistochemistry
Sections from the paraffin-embedded tissue were deparaffinized and rehydrated with xylene and graded alcohol dilutions. The endogenous peroxidase activity was destroyed by incubating 30 min with 0.5% hydrogen peroxide in methanol. A citric acid based unmasking solution (Vector Laboratories, Burlingame, CA) was added to the slides and heated to 95 C. After cooling to RT, the slides were washed with 1× PBS and incubated with blocking solution (Vector Laboratories) for 1 h. The sections were incubated with the primary antibody (EZH2) overnight at 4 C. The slides were washed and incubated with biotinylated antimouse secondary antibody. After washing, the ABC solution (Vector Laboratories) was added and staining was visualized with addition of 3,3′-diaminobenzidine (Sigma, St. Louis, MO). The sections were counterstained with 5% (wt/vol) Harris hematoxylin.
Wound healing assay
LNCaP cells were transfected with NS control siRNAs or DAB2IP and E-Cad-specific siRNAs. At 24 h after transfection, cells were treated with vehicle (EtOH) or 10 nm R1881, and then 24 h later, cells were plated in culture inserts (IBIDI, Martinsried, Germany). The culture inserts were removed 24 h later, which created a gap. The wound gap was visualized at 0 and 60 h. The area of the gaps and percent wound areas filled were determined.
Results
Increased expression of EZH2 in androgen-refractory sublines of LNCaP PCa cells
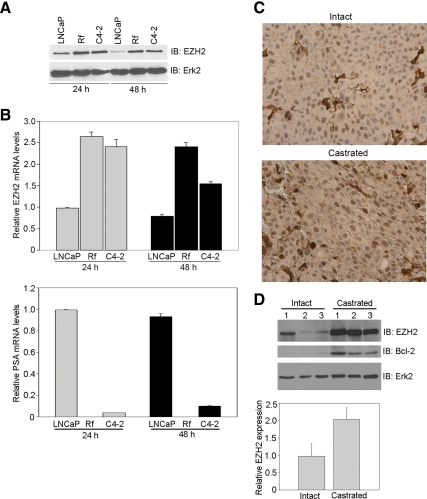
The finding that increased expression of EZH2 is associated with androgen-refractory progression of PCa (12,13) prompted us to determine whether EZH2 is regulated by androgens. To this end, we examined expression of EZH2 in the androgen-sensitive LNCaP cell line and its androgen-refractory sublines Rf and C4-2, which are derived from the parental LNCaP cell line under androgen ablation conditions in vitro and in vivo, respectively (20,21,25). Compared with LNCaP cells grown in regular media, expression of the EZH2 protein was much higher in Rf and C4-2 cells, which were routinely cultured in androgen-depleted media (Fig. 1A). In accordance with the Western blotting result, expression of EZH2 mRNA was much higher in Rf and C4-2 cells relative to LNCaP cells (Fig. 1B). Similar to previous reports (26,27), expression of PSA, an AR target gene, was not detected at the mRNA level in Rf cells and remained at a very low level in C4-2 cells under androgen-depleted conditions (Fig. 1B). Thus, by using the paired androgen-sensitive and androgen-refractory LNCaP cell models, we provide evidence that both EZH2 mRNA and protein are up-regulated in androgen-refractory sublines of LNCaP cells.
Figure 1.
EZH2 is overexpressed in PCa cells depleted of androgens. A, Cells were cultured in media as described in Materials and Methods. At the indicated time points after subculture, protein expression levels of EZH2 and Erk2 (loading control) were analyzed by immunoblotting (IB). B, Relative EZH2 and PSA mRNA levels were determined using real-time PCR. Error bars represent the sd of three experiments. C, Mice with LNCaP xenografts were either castrated or left intact. One week after castration, tumors from intact (n = 3) and castrated (n = 3) mice were harvested. Immunohistochemistry was performed with tumor tissues using the EZH2 antibody. D, Tumor tissues were subjected to homogenization, and 50 μg of total cell lysate were analyzed by Western blotting using the EZH2, Bcl-2, and Erk2 antibodies. The average level of EZH2 protein among the tumors from either intact or castrated mice was determined by the quantified value of EZH2 in each tumor normalized to Erk2 levels.
Castration increases expression of EZH2 protein in LNCaP xenografts in mice
We next examined whether acute androgen manipulation affects EZH2 expression in vivo. Androgen-sensitive LNCaP cells were injected sc into nude mice. After 6 wk, mice were castrated or left intact. One week later, mice were killed, and grafts from intact and castrated mice were collected. The level of EZH2 in the grafts was examined by both immunohistochemistry and Western blotting. As shown in Fig. 1, C and D, EZH2 levels increased after androgen withdrawal by castration. The effectiveness of castration was evident by the up-regulation of Bcl-2 (Fig. 1D), expression of which is known to increase in response to castration (28). These findings indicate that acute androgen removal results in an increase in EZH2 expression under in vivo conditions.
Androgens repress EZH2 expression in LNCaP PCa cells
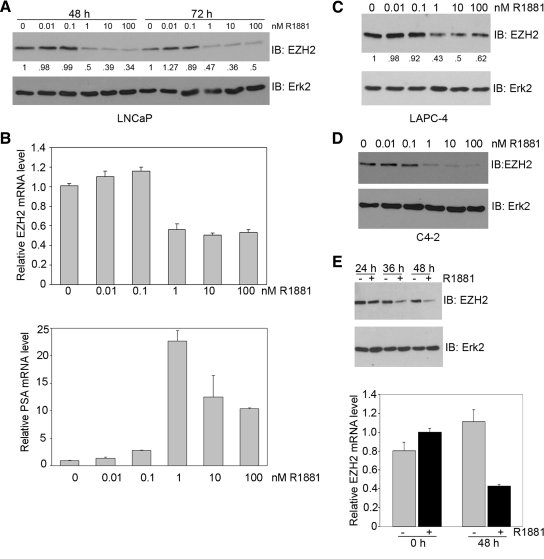
The results shown in Fig. 1 suggest that activation of the AR by androgens may repress EZH2 expression. To test this notion, LNCaP cells were cultured in charcoal-treated media supplied with different doses of R1881. As demonstrated in Fig. 2, A and B, upper panel, expression of EZH2 protein and mRNA was markedly repressed by treatment of LNCaP cells with concentrations of R1881 at 1 nm or higher, the levels of androgens which are detected under physiological conditions in humans (29). Androgen-induced activation of the AR was evident by increased expression of PSA mRNA (Fig. 2B, lower panel). Similar results were observed in LAPC-4 and C4-2 PCa cell lines (Fig. 2, C and D), suggesting that androgen-induced repression of EZH2 is not specific to the LNCaP cell line. We conclude that androgens repress EZH2 expression in PCa cells.
Figure 2.
The effect of androgen treatment on EZH2 expression. A, LNCaP cells were grown in CSS for 24 h and then incubated for an additional 48 or 72 h with the given concentrations of R1881. Western blottings were performed with antibodies to EZH2 and Erk2 for loading control. The level of EZH2 was quantified, normalized to Erk2 levels, and given relative to mock-treated cells for each time point. B, EZH2 and PSA mRNA levels after androgen treatment for 72 h were determined by real-time PCR. C and D, LAPC-4 (C) or C4-2 (D) cells were grown in CSS for 24 h and then incubated for an additional 48 h with the given concentrations of R1881. Western blottings were performed with antibodies to EZH2 and Erk2. The level of EZH2 was quantified as described above. E, Time-course study of LNCaP cells treated with 100 nm R1881. Protein expression of EZH2 and Erk2 was analyzed by Western blotting (upper panel). mRNA levels of EZH2 at 0 and 48 h were determined by real-time PCR (lower panel). Error bars represent the sd of three experiments. IB, Immunoblotting.
Time-course studies demonstrated that expression of the EZH2 protein began to decline at 36 h after treatment of LNCaP cells with 10 nm of R1881 (Fig. 2E), and at 48 h, both EZH2 protein and mRNA levels drastically decreased (Fig. 2E). Thus, androgen-mediated repression of EZH2 does not appear to be an immediate event.
Androgen repression of EZH2 is mediated through the AR
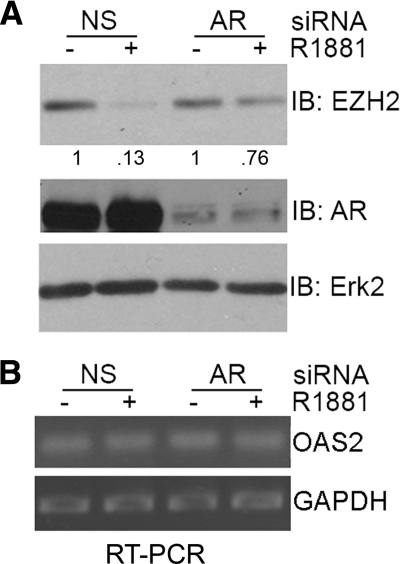
The time-course study (Fig. 2E) shows that EZH2 is a late response gene of androgen action, suggesting that it may not be a direct target of the AR. However, it is unclear whether AR plays a role in the androgenic repression of EZH2. To this end, LNCaP cells were cultured in regular media, in which the level of androgens is close to 0.1 nm (see Fig. 2A), and treated with a pool of NS siRNAs and a pool of AR siRNAs. At 24 h after siRNA treatment, cells were treated with or without 10 nm of R1881. Consistent with the finding that androgens at concentrations less than 1 nm (0.01 ∼ 0.1 nm) have a negligible effect on EZH2 expression (Fig. 2A), knockdown of AR had very limited impact on EZH2 expression (Fig. 3A). As expected, treatment with 10 nm of R1881 decreased expression of EZH2 protein in cells treated with NS siRNAs (Fig. 3A). However, androgen-induced repression of EZH2 expression was largely attenuated by silencing of endogenous AR (Fig. 3A). This effect is not likely mediated by off-target effects of siRNAs, because the expression of OAS2, a gene known to be activated by the interferon pathway in response to siRNA (30), was not affected in cells transfected with AR-specific siRNAs in comparison with NS control siRNAs (Fig. 3B). Thus, androgen-induced repression of EZH2 expression is mediated through the AR. Through in silico analysis, however, no androgen responsive element [AGAACA(NNN)AGAACA] (31) was found within the 5-kb region of the human EZH2 gene promoter (gene accession no. NM_004456). Thus, we speculate that AR may not regulate EZH2 expression by binding to an androgen responsive element site in its promoter but may require additional proteins to repress EZH2 expression.
Figure 3.
Role of AR in androgen regulation of EZH2. A, LNCaP cells were transfected with NS control (NS) or AR siRNA for 24 h and then treated with or without R1881 (10 nm) for an additional 48 h. EZH2, AR, and Erk2 protein expression was analyzed by Western blotting, and the level of EZH2 was quantified, normalized to Erk2 levels, and given relative to mock-treated cells. B, mRNA from LNCaP cells treated with NS or AR siRNA was analyzed by PCR to determine expression levels of OAS2 and GAPDH. IB, Immunoblotting.
The role of RB and its related protein p130 in androgen repression of EZH2
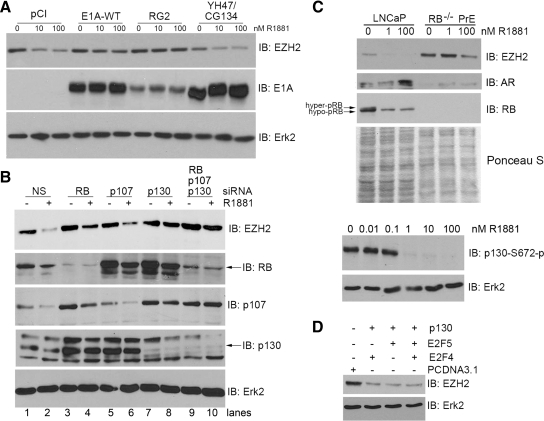
Next, we sought to determine which signaling pathway(s) is responsible for androgenic repression of EZH2 expression. It has been shown previously that androgenic regulation of other genes, such as Bcl-2, can be abolished with expression of the viral protein E1A in LNCaP cells (24). E1A binds to the members of the RB family and other proteins, such as cAMP binding protein-binding protein (CBP) and p300, and inhibits their functions (23). It has been reported that EZH2 is transcriptionally regulated by the RB-E2F1 pathway (18). Thus, we examined whether expression of E1A affects androgenic repression of EZH2. LNCaP cells were transfected with wild-type E1A, which interacts with both RB proteins and CBP/p300, and two mutants, RG2 and YH47/CG134, which cannot bind to CBP/p300 and RB proteins, respectively (23). At 24 h after transfection, cells were cultured in androgen-depleted media and treated with different doses of R1881. Expression of wild-type E1A blocked androgen-induced inhibition of EZH2 expression (Fig. 4A). A similar result was obtained in cells transfected with the RG2 mutant that can inhibit the functions of RB proteins but not CBP/p300 proteins. In contrast, expression of the YH47/CG134 mutant, which cannot bind to and inhibit members of the RB family, failed to block androgen-mediated repression of EZH2 expression (Fig. 4A). These data indicate that RB and its related proteins (p107 and p130) may be involved in androgenic repression of EZH2 expression.
Figure 4.
RB and its related proteins, p130, are required for androgen regulation of EZH2. A, LNCaP cells were transfected with the empty vector pCI, wild-type (WT) E1A, or two mutants of E1A, RG2, and YH47/CG134. At 24 h after transfection, the media were changed to CSS and then R1881 or vehicle was added. After 48 h of androgen treatment, cells were harvested, and protein expression of EZH2, E1A, and Erk2 was determined by Western blotting. B, LNCaP cells were treated with the indicated siRNA pools. At 48 h after transfection, 10 nm R1881 or vehicle was added and cells were incubated for an additional 48 h. Western blottings were performed to analyze protein expression of EZH2, RB, p107, p130, and Erk2. C, upper panel, LNCaP or RB−/− PrE cells were cultured in CSS for 24 h and then incubated for an additional 48 h with the given concentrations of R1881. Western blottings were performed with antibodies to EZH2, AR, and RB. The membrane was stained with Ponceau S for loading control. Lower panel, LNCaP cells were grown in CSS for 24 h and then incubated for an additional 72 h with the given concentrations of R1881. Western blottings were performed with antibodies to p130–S672-p and Erk2. D, DU145 cells were transfected with the given plasmids and harvested after 48 h. Protein levels for EZH2 and Erk2 were analyzed by Western blotting. IB, Immunoblotting.
To specifically determine whether RB and its related proteins play a role in androgen regulation of EZH2, genes encoding these proteins were knocked down by gene-specific siRNAs. As shown in Fig. 4B, endogenous RB protein was effectively silenced by RB-specific siRNAs. RB knockdown increased EZH2 proteins in nonandrogen-treated cells (Fig. 4B, lane 3 vs. lane 1), which is consistent with the previous report (18). However, RB knockdown only partially blocked androgen-induced repression of EZH2 (Fig. 4B, lane 4). Interestingly, expression of the RB-related protein p130 significantly increased in RB-knockdown cells (Fig. 4B). Thus, it is possible that increased p130 may compensate for RB function in mediating androgen repression of EZH2. Indeed, knockdown of p130 largely blocked the androgenic effect on EZH2 expression (Fig. 4B, lane 8), even though knockdown of p130 had no effect on EZH2 expression in LNCaP cells without androgen stimulation (Fig. 4B, lane 7). No significant decrease in the level of p107 protein was observed in cells transfected with p107 siRNAs (Fig. 4B), indicating that the knockdown efficiency of p107 is low. Not surprisingly, EZH2 was significantly repressed by androgens in these cells (Fig. 4B, lane 6). Therefore, this data failed to rule out the potential role of p107 in androgen-mediated regulation of EZH2. However, androgen-induced repression of EZH2 expression was almost completely blocked by knockdown of both RB and p130 (Fig. 4B, lane 10). Thus, RB and p130, but not p107, play a key role in mediating androgen repression of EZH2 expression in LNCaP cells. In line with this conclusion, androgen treatment of LNCaP cells resulted in activation of RB and p130 proteins as evident by loss of phosphorylation of these two proteins (Fig. 4C).
To further investigate the role of RB and p130 in androgenic regulation of EZH2, RB−/− PrE were treated with different concentrations of R1881. Different from the result obtained in LNCaP cells, treatment of RB−/− PrE with 1 nm of R1881 failed to suppress mouse EZH2 expression (Fig. 4C, upper panel). This data supports the conclusion that the RB protein is involved in androgen-induced inhibition of EZH2 expression in prostatic cells. However, treatment of RB−/− PrE with a high dose of R1881 (100 nm) resulted in a decrease in EZH2 expression (Fig. 4C, upper panel), therefore providing further support for the conclusion that both RB-dependent and RB-independent mechanisms are required for androgen-induced repression of EZH2 levels. Moreover, forced expression of constitutively active RB inhibited expression of EZH2 in LNCaP cells (data not shown), which is consistent with the previous report (18). In accordance with the results obtained in p130 knockdown cells, ectopic expression of p130 in combination with its partner proteins E2F4 or E4F5 repressed expression of EZH2 in DU145 PCa cells, which lack functional RB (Fig. 4D). Taken together, these data suggest that both RB and p130 proteins are responsible for androgen-induced repression of EZH2 expression in prostatic cells.
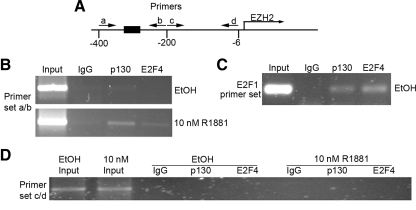
Androgens induce binding of p130/E2F4 to the EZH2 promoter in LNCaP cells
It has been shown previously that E2F1 binds to the EZH2 promoter and that this can be inhibited by its partner protein RB (18). However, it is unclear whether p130 and its partner proteins (E2F4 and E2F5) can bind to the EZH2 promoter. We employed a ChIP assay to assess the binding of p130 and E2F4 to the promoter of EZH2 in LNCaP cells in the presence or absence of androgen stimulation. LNCaP cells were treated with 10 nm R1881 or vehicle for 2 d, and cells were harvested and subjected to ChIP assays. As demonstrated in Fig. 5, A and B, there was little to no p130 and E2F4 proteins bound to the EZH2 promoter in LNCaP cells without androgen treatment. In contrast, both proteins bound to the EZH2 promoter in androgen-stimulated cells, although E2F4 binding was relatively weak (Fig. 5B). As a positive control (32), binding of both p130 and E2F4 to the promoter of their target gene E2F1 was readily detected in LNCaP cells (Fig. 5C). No binding of p130 and E2F4 proteins was observed in the region outside the E2F binding region in the promoter of EZH2 regardless of whether cells were treated with or without androgens (Fig. 5D). These data suggest that p130, and probably E2F4, can bind to the EZH2 promoter in response to androgen stimulation.
Figure 5.
Binding of p130 and E2F4 to the EZH2 promoter. A, Diagram of primers in the promoter of EZH2 used for ChIP assay. The black box between the primer set a and the primer set b represents four putative E2F1 binding sites. LNCaP cells were treated with 10 nm R1881 or vehicle for 48 h and then harvested. Chromatin proteins cross-linked to DNA were immunoprecipitated with p130, E2F4, or NS IgG. DNA that was pulled down was analyzed by PCR using primers that amplified the E2F binding region in the EZH2 promoter (B), p130/E2F binding site in E2F1 promoter (C), and a NS region of the EZH2 promoter (D).
Effect of androgens on expression and the biological consequences of EZH2 repression genes
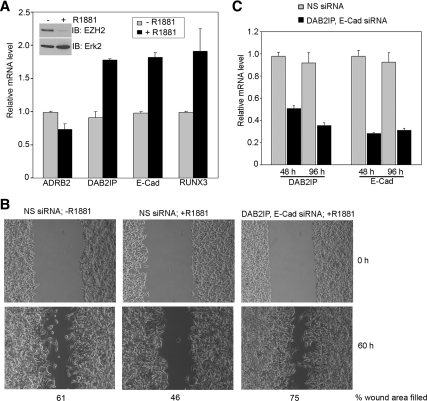
The biological functions of EZH2 are primarily mediated by its role in epigenetic gene silencing. A handful of tumor suppressor genes, such as ADRB2, DAB2IP, E-cadherin (E-Cad), and RUNX3, which are important for regulation of cell proliferation, migration, and invasion, are known to be repressed by EZH2 (12,15,17). Therefore, we examined the effect of androgens on expression of these EZH2 target genes. Expression of DAB2IP, E-Cad, and RUNX3, but not ADRB2, was much higher in LNCaP cells treated with androgens in comparison with mock-treated cells (Fig. 6A). Thus, except for ADRB2, the high level of three tumor suppressor genes (DAB2IP, E-Cad, and RUNX3) inversely correlates with the low level of EZH2 protein (Fig. 6A, inset) in LNCaP cells treated with androgens. Moreover, it has been shown recently that both DAB2IP and E-Cad proteins play an important role in PCa cell migration (17,33). Consistent with the result that treatment of LNCaP cells with 10 nm of R1881 increased expression of DAB2IP and E-Cad (Fig. 6A), androgen treatment decreased LNCaP cell migration (Fig. 6B). However, this effect was completely reversed in cells where both DAB2IP and E-Cad were knocked down (Fig. 6, B and C). Thus, androgens not only affect expression of EZH2 target genes but also regulate the biological impact of EZH2 target genes on PCa function.
Figure 6.
Effect of androgens on expression and biological consequences of EZH2 repression genes. A, LNCaP cells were grown in CSS for 24 h and then incubated for an additional 72 h with or without the addition of 10 nm R1881. mRNA levels of EZH2 target genes ADRB2, DAB2IP, E-Cad, and RUNX3 were determined by real-time PCR. Inset, Protein level of EZH2 and Erk2. B, LNCaP cells were transfected with NS or DAB2IP and E-Cad siRNA. On d 1, cells were treated with or without 10 nm R1881. Cells were plated in culture inserts on d 2, which were removed on d 3 to create a gap. The area of the gaps and percent wound area filled were determined at 0 and 60 h. A representative figure from two experiments is shown. C, mRNA levels of DAB2IP and E-Cad were determined by real time at 48 and 96 h after transfection. IB, Immunoblotting.
Discussion
EZH2 is known to be overexpressed in PCa, especially in hormone-refractory disease (12,13). Most importantly, overexpression of EZH2 promotes PCa growth and metastasis (12,16,17). However, the molecular mechanisms responsible for increased expression of EZH2 in hormone-refractory PCa are not fully understood. We demonstrated in this study that expression of EZH2 is repressed by physiological levels of androgens. Also, we provide evidence that removal of androgens by castration resulted in increased expression of EZH2 protein level in LNCaP xenografts in mice. Moreover, by using both in vitro and in vivo hormone-refractory progression models of LNCaP cells (20,21,25), we demonstrated that expression of EZH2 is higher in androgen-refractory Rf and C4-2 cells than in parental androgen-sensitive LNCaP cells. Thus, we demonstrate for the first time that androgens repress expression of EZH2 in PCa cells in vitro and in vivo. Additionally, the findings from this study support a model wherein androgen depletion caused by ADT leads to EZH2 overexpression in PCa cells, which thereby promotes proliferation and invasion of PCa cells under androgen deprivation conditions.
Previous studies show that expression of EZH2 is transcriptionally regulated by the RB-E2F1 pathway (18), and the RB-E2F1 pathway is known to be regulated by androgens in PCa cells (24,34). Treatment of PCa cells with physiological levels of androgens induce expression of cyclin-dependent kinase inhibitors p21WAF1, p15INKA, p27KIP1, hypophosphorylation of RB, and decreased transcriptional activity of E2F1 (24,29,34,35). In the model systems used in this study, androgen treatment decreased RB phosphorylation (Fig. 4C), implying that active RB may play a role in mediating androgenic inhibition of EZH2 expression in these cells. Accordingly, we provide evidence that homozygous deletion of both alleles of the Rb gene blocks decreased expression of EZH2 protein in cells treated with 1 nm of R1881. However, deletion of RB failed to block androgen-induced down-regulation of EZH2 at a high dose, suggesting a role of the RB-independent pathway in androgenic regulation of EZH2 in prostatic cells. This assumption is supported by our finding that knockdown of the RB-related protein p130 in combination with RB knockdown almost completely inhibited androgen-induced repression of EZH2. We provide further evidence that p130 and its partner protein E2F4 bind to the E2F binding region in the EZH2 promoter in androgen-treated, but not in mock-treated, cells. In agreement with this observation, forced expression of p130 in combination with E2F4 or E2F5 largely inhibited expression of EZH2 in PCa cells. Thus, the present study identifies RB and p130-dependent pathways by which androgens repress expression of EZH2 in PCa cells. Given the repressive nature of androgens on the expression of EZH2, elevated expression of EZH2 may inevitably occur during ADT. Identification of RB and p130 as the mediators of androgen repression of EZH2 opens a new avenue to intervene with androgen-refractory progression of PCa.
Increasing evidence suggests that EZH2 is important for oncogenesis by driving cancer cell proliferation, migration, and invasion (12,15), and this function is largely mediated by its role in gene silencing (36). We demonstrated that although treatment of LNCaP cells with androgens decreases the level of EZH2 protein, it also increases levels of EZH2 target genes, including DAB2IP, E-Cad, and RUNX3. In line with this observation, we further demonstrated that androgen treatment decreased LNCaP cell migration and that this effect was completely reversed in cells where DAB2IP and E-Cad were knocked down. Given that EZH2 target proteins play a pivotal role in suppression of tumor formation and progression (17,37), it is conceivable that removal of androgens not only elevates the levels of EZH2 protein itself in PCa cells, it also has the “ripple” effect on growth, survival, and migration of PCa by impairing expression of the tumor suppressor genes that are silencing targets of EZH2.
In summary, we demonstrated for the first time that expression of EZH2 is repressed by androgens at physiological levels. Conversely, removal of androgens by castration increases EZH2 expression in animals. We provide further evidence that this effect is mediated through the known, RB-dependent and the novel, p130/E2F4/5-dependent mechanisms. Given that EZH2 plays a critical role in promoting cell proliferation and invasion, our findings imply that androgen depletion therapy may adversely lead to overexpression of EZH2 in PCa cells, which thereby contribute to hormone-refractory progression of PCa after ADT. Development of therapeutic strategies to limit androgen depletion-induced overexpression of EZH2 may be beneficial when combined with ADT.
Acknowledgments
We thank R. F. Kelm, B. Dynlacht, and J. R. Nevins for plasmids; C. L. Sawyers, M. L. Day, and S. W. Hayward for cell lines; and D. J. Tindall and S. M. Dehm for critical reading of the manuscript.
Footnotes
This work was supported in part by funds from the National Institutes of Health (CA130908), the Department of Defense (W81XWH-07-1-0137 and W81XWH-09-1-0622), and the Brainstorm Award from University of Minnesota Masonic Cancer Center (H.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 29, 2010
Abbreviations: ADT, Androgen deprivation therapy; AR, androgen receptor; Bcl-2, B-cell lymphoma 2; CBP, cAMP binding protein-binding protein; ChIP, chromatin immunoprecipitation; CSS, charcoal-stripped serum; DAB2IP, disabled homolog 2-interacting protein; E-Cad, E-cadherin; EtOH, ethanol; EZH2, enhancer of zeste homolog 2; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, nonspecific; OAS2, 2′-5′ oligoadenylate synthetase 2; PCa, prostate cancer; PSA, prostate specific antigen; RB−/− PrE, RB-deficient mouse prostate epithelial cells; RB, retinoblastoma protein; RB−/− PrE, RB-deficient mouse prostate epithelial cells; siRNA, small interfering RNA.
References
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y 1987 The endocrinology and developmental biology of the prostate. Endocr Rev 8:338–362 [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT 1988 Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 122:552–562 [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ 2002 The role of the androgen receptor in prostate cancer. Crit Rev Eukar Gene 12:193–207 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM 2007 Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448:595–599 [DOI] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y 2002 Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298:1039–1043 [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D 2002 Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of Zeste protein. Genes Dev 16:2893–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE, Simon JA 2002 Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111:197–208 [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA 2006 Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R 2006 Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441:349–353 [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E 2009 Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136:1122–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM 2002 The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629 [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP 2006 Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 66:2815–2825 [DOI] [PubMed] [Google Scholar]
- Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, Morris DS, Marquez VE, Shah RB, Ghosh D, Varambally S, Chinnaiyan AM 2007 Integrative genomics analysis reveals silencing of β-adrenergic signaling by polycomb in prostate cancer. Cancer Cell 12:419–431 [DOI] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM 2008 Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322:1695–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikolas BD, Figueiredo ML, Wu L 2009 Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line. Mol Cancer Res 7:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE, Beroukhim R, Bronson RT, Ryeom S, Hahn WC, Loda M, Cichowski K 2010 An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-κB. Nat Med 16:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K 2003 EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 22:5323–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA 2009 The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res 69:2623–2629 [DOI] [PubMed] [Google Scholar]
- Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ 2001 PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem 276:38830–38836 [DOI] [PubMed] [Google Scholar]
- Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ 2001 Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 142:4795–4805 [DOI] [PubMed] [Google Scholar]
- Day KC, McCabe MT, Zhao X, Wang Y, Davis JN, Phillips J, Von Geldern M, Ried T, KuKuruga MA, Cunha GR, Hayward SW, Day ML 2002 Rescue of embryonic epithelium reveals that the homozygous deletion of the retinoblastoma gene confers growth factor independence and immortality but does not influence epithelial differentiation or tissue morphogenesis. J Biol Chem 277:44475–44484 [DOI] [PubMed] [Google Scholar]
- Liu SL, Rand A, Kelm Jr RJ, Getz MJ 2000 The retinoblastoma gene family members pRB and p107 coactivate the AP-1-dependent mouse tissue factor promoter in fibroblasts. Oncogene 19:3352–3362 [DOI] [PubMed] [Google Scholar]
- Huang H, Zegarra-Moro OL, Benson D, Tindall DJ 2004 Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene 23:2161–2176 [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW 1994 Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res 54:2577–2581 [PubMed] [Google Scholar]
- Li JJ, Li SA, Llombart-Bosch A, eds. 2005 Hormonal carcinogenesis IV. New York: Springer Verlag [Google Scholar]
- Liu P, Li S, Gan L, Kao TP, Huang H 2008 A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res 68:10290–10299 [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML 1992 Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52:6940–6944 [PubMed] [Google Scholar]
- Tsihlias J, Zhang W, Bhattacharya N, Flanagan M, Klotz L, Slingerland J 2000 Involvement of p27Kip1 in G1 arrest by high dose 5α-dihydrotestosterone in LNCaP human prostate cancer cells. Oncogene 19:670–679 [DOI] [PubMed] [Google Scholar]
- Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H 2007 Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res 67:6083–6091 [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ 2007 Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 21:2855–2863 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Rayman JB, Dynlacht BD 2000 Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev 14:804–816 [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, Kleer CG, Varambally S, Chinnaiyan AM 2008 Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27:7274–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman K, Swinnen JV, Verhoeven G, Heyns W 2001 E2F activity is biphasically regulated by androgens in LNCaP cells. Biochem Biophys Res Commun 283:97–101 [DOI] [PubMed] [Google Scholar]
- Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ 1999 Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol 13:376–384 [DOI] [PubMed] [Google Scholar]
- Simon JA, Kingston RE 2009 Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 10:697–708 [DOI] [PubMed] [Google Scholar]
- Xie D, Gore C, Liu J, Pong RC, Mason R, Hao G, Long M, Kabbani W, Yu L, Zhang H, Chen H, Sun X, Boothman DA, Min W, Hsieh JT 2010 Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci USA 107:2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]