Abstract
Hormone-dependent breast cancers respond to inhibitors of estrogen synthesis or action with tumor regression and with a reduction of new metastases. The mechanisms underlying the effects of estrogen on metastasis likely differ from those on tumor regression. Cell migration is a key first step in the metastatic process. Based on our prior work and other published data, we designed and tested a working model that suggested that estrogen receptor α, epidermal growth factor receptor, focal adhesion kinase (FAK), paxillin, phosphatidylinositol 3 kinase, p60 Src tyrosine kinase (c-Src), c-Jun N-terminal kinase, and MAPK interact to facilitate estradiol (E2)-induced cell migration. Accordingly, we examined the effect of E2 on activation of these pathways and demonstrated mechanistic effects by blocking each component and assessing cell migration as a biologic endpoint. Initial studies validated a robust cell migration assay characterized by highly reproducible, dose-dependent responses to E2. Examining various mechanisms involved in migration, we showed that E2 induced activation of c-Src, FAK, and paxillin with early peaks within 5–30 min and later peaks at 24 h. ERK and protein kinase B phosphorylation exhibited only early peaks. Blockade of various steps in these signaling pathways with use of small interfering RNA or specific inhibitors demonstrated mechanistic effects of these signaling molecules on cell migration. Our results suggest that the effects of E2 on cell migration involve multiple, interacting signaling pathways. Important effects are mediated by the MAPK, phosphatidylinositol 3 kinase, and c-Jun N-terminal kinase pathways and use FAK, paxillin, and c-Src for activation. Each pathway represents a potential target for blocking cell migration and metastasis of breast cancer cells.
Estradiol, acting through the estrogen receptor, induces breast cancer cell migration through a complex interplay involving the MAP kinase, PI-3-kinase, JNK, and c-Src kinase signaling pathways as well as the EGF receptor and paxillin.
Estrogens stimulate the growth of hormone-dependent breast cancers by increasing cellular proliferation and inhibiting apoptosis. By abrogating these effects, administration of aromatase inhibitors or antiestrogens delays tumor recurrence in the adjuvant treatment setting and causes regression of advanced breast cancer (1). These therapeutic strategies also reduce the frequency of distant metastases, presumably through interruption of additional pathways (1). The metastatic process is complex and requires several steps, including cellular invasion through blood vessels, travel through the circulation to distant organs, reentry into tissue, and growth at the new sites (2,3,4,5). The ability of cells to migrate in tissue is essential to the metastatic process and represents its initial step (3). In our earlier studies, we observed effects of estradiol (E2) on the morphology of hormone-dependent breast cancer cells with formation of filopodia, lamellipodia, and pseudopodia within 20 min of administration (6). Such dynamic cell membrane changes are hallmarks of cells that are preparing for migration (7). These observations initially led us to question whether estrogen exerts effects on cell migration and to determine the precise molecular mechanisms involved.
Four steps are required for cell migration: protrusion of cytoplasm at the leading edge of the migration front, formation of adhesions with the underlying cell matrix, disassembly of the adhesions at the rear of the cell, and actin-myosin fiber contractions to pull the bulk of the cytoplasm forward (8). The first step involves the protrusion of lamellipodia and filopodia at the cell’s leading edge. At these points of extension, adhesions to the underlying matrix form and serve a function similar to that of suction cups. The adhesions at the rear of the cell are then disassembled and contractile forces move the bulk of the cell forward. Additional adhesions then reform at the rear. Protrusion, adhesion, and disassembly then reoccur serially, allowing movement in a given direction. This process requires a finely tuned sequence of molecular changes involving more than 125 proteins and suggests that multiple pathways are involved (9). The more important components coordinating the process of migration include the p60 Src tyrosine kinase (c-Src), focal adhesion kinase (FAK), paxillin, epidermal growth factor receptor (EGF-R), phosphatidylinositol (PI)-3-kinase, c-Jun N-terminal kinase (JNK), and MAPK. We considered it important to fully understand the role of estrogens in this process as a means to identify potentially useful drug targets to prevent metastases.
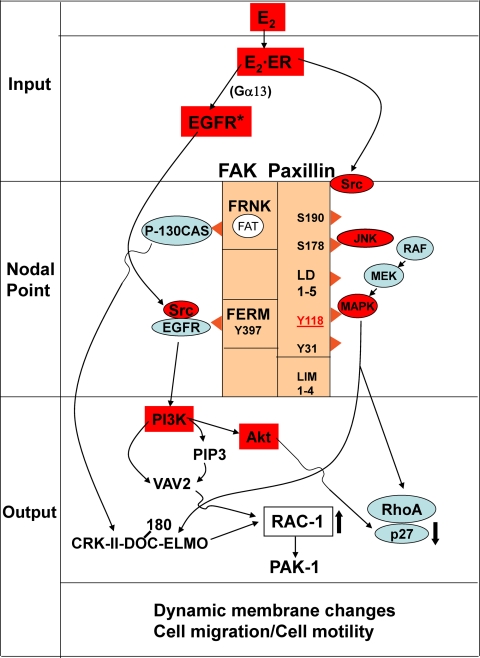
We have reported previously that extranuclear effects of E2, in addition to direct nuclear components, activate several signaling pathways in MCF-7 cells in culture. Based on a review of the literature and our prior studies, we developed a working model that could be used as a framework for dissecting out the molecular mechanisms whereby E2 stimulates cell migration (7,9,10,11,12,13,14,15,16,17,18,19,20). As shown in Fig. 1, this model consists of input, nodal point, and output components. The model proposes that E2 binds to estrogen receptor (ER)α and activates c-Src as the input component. This intracellular kinase in turn results in activation of signaling cascades, involving FAK, MAPK, PI-3-kinase, JNK, and the EGF-R. As a result, a complex forms between FAK and paxillin, which acts as a nodal point to integrate downstream signaling. Pathways involving PI-3-kinase and v-crk sarcoma virus CT10 oncogene homolog (CRKII)-dedicator of cytokinesis (DOCK180)-engulfment and cell motility (ELMO) ultimately interact with Rho family GTPase (Rac) and Rho small GTPase proteins, which are known to regulate cell migration.
Figure 1.
Working model of the effects of E2 on cell migration. This model is a hypothetical construct based on a comprehensive review of the literature and was used as a means of designing and interpreting our experiments. Components of the cell migration signaling pathways are divided into an input phase, a nodal point, and an output phase. This model provides us with a means of integrating the multiple signaling pathways involved in cell migration. In the diagram, we have highlighted the molecules examined in this study in red. Molecules highlighted in blue are upstream kinases or downstream mediators of cell migration not studied in this paper. In this model, E2 transactivates the EGF-R, perhaps through a Gα13 mediated GTP-coupled protein, and at the same time causes the phosphorylation and activation of c-Src. FAK and paxillin form a complex that acts as a nodal point to initiate downstream effects on the Rac-1, PAK-1, and Rho proteins responsible for migration. c-Src plays a central role in phosphorylating paxillin, in transactivation of the EGF-R, and in binding to FAK (9). MAPK (ERK1/2) is one of the kinases that phosphorylates and activates paxillin. Downstream, PI-3 kinase activates Akt and VAV2, a guanosine triphosphatase protein exchange factor that is involved in activation of RAC-1. P-130CAS is activated by FAK, which results in the formation of a complex consisting of CRK II, DOCK180, and ELMO. This complex activates RAC-1, leading to activation of PAK-1. MAPK is involved in the regulation of RHO. RAF (MAPK kinase kinase) and MEK (MAPK kinase) are upstream kinases that activate MAPK.
In this study, we validated a highly reproducible cell migration assay for breast cancer cells and dissected out several components of the molecular steps required for migration. Our results indicate that multiple signaling pathways interact to regulate cell migration and that paxillin and FAK provide a platform to integrate downstream events. A key component is the effect of E2 to stimulate phosphorylation of paxillin at the tyrosine 118 position (9). Knock down of paxillin or blockade of its phosphorylation with the c-Src kinase inhibitor, 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), completely abrogated the effects of E2 on migration. As evidence of the complexity of this process, we demonstrated a role for the EGF-R, PI-3-kinase, JNK, and MAPK on cell migration. Taken together, these studies emphasize the important effect of E2 on cell migration in addition to its known actions to enhance cell proliferation and prevent cell death. Targeting of the specific steps involved could provide a means to prevent metastasis.
Materials and Methods
Reagents
17ß-Estradiol was obtained from Steraloids, Inc. (Newport, RI). SP600125, PP2, LY 294002, U0126, and SB202580 were purchased from Calbiochem (San Diego, CA). Sources of antibodies were as follows: polyclonal antibodies against phospho-ERK1/2, c-Src (p-Tyr416), FAK (p-Tyr576/577), paxillin (p-Tyr118), protein kinase B (Akt) (p-Ser473), phospho-JNK, and antibodies against total proteins mentioned above were from Cell Signaling Technology (Danvers, MA); monoclonal antibody against moesin (p-Thr558) and polyclonal antibody against total FAK were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); horseradish peroxidase-conjugated secondary antibodies were from GE Health Care Bio-Sciences Corp. (Piscataway, NJ).
Cell cultures and treatments
Hormone-dependent T47D cells were obtained from M.B.A. Djamgoz (Imperial College, London, UK). These cells were routinely grown in an incubator with 95% humidified air 5% CO2 at 37 C in RPMI 1640 containing phenol red and supplemented with l-glutamine (2 mm) and 10% fetal bovine serum (FBS). In preparation for cell migration assays, the protocol involved initial plating of 20,000 cells/cm2 in six-well dishes with growth for 4–5 d until 90–100% confluence was reached as judged by visual assessment. The cells were then stepped down into phenol red-free media containing 5% charcoal-stripped serum [dextran-coated charcoal (DCC)] and grown for 2 d to allow adaptation. After this, various concentrations of E2 or specific signaling pathway inhibitors were added.
Cell migration assay
Cell migration assay was developed by slightly modifying the method described by the group of Simoncini et al. (21). A vertical line was cut with a razor blade in six-well dishes containing confluent cells stepped down to phenol red-free media as described above. The remainder of the cells to the right of the line were scraped away using a rubber policeman. The vertical boundary thus represented the “starting line,” and the endpoint of the experiments represented the number of cells crossing this line over 24 h (see example in Fig. 2A). After scraping away cells, the plates were washed with Hanks’ solution, and 2.0 ml of phenol red-free RPMI 1640 medium containing 5% DCC-FBS and gelatin (1 mg/ml) were added. To prevent the confounding effect of cell proliferation, cytosine ß-d-arabinofuranoside hydrochloride (10 μm; Sigma, St. Louis, MO), a selective inhibitor of DNA synthesis that does not inhibit RNA synthesis, was added 1 h before the test substances. Media were changed every 12 h, and fresh media containing cytosine ß-d-arabinofuranoside hydrochloride, gelatin, and the various concentrations of E2, vehicle, or inhibitors were added. Cells were digitally imaged, and migration distances from the starting line were visualized using phase contrast microscopy every 24 h. Photographs were made from an average of six fields, and the number of cells crossing the line were counted and expressed as mean ± sem. Initial validation experiments involved counting cells at 24, 48, and 72 h, but 24 h counts provided the most reproducible and efficient time point for routine experiments.
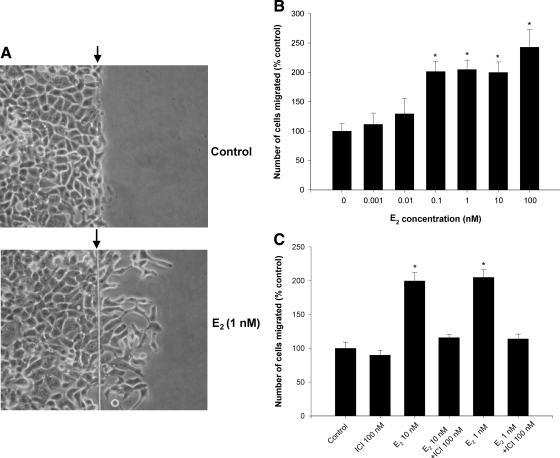
Figure 2.
A, Photomicrographs to illustrate the effect of E2 on migration. In each panel, the vertical line (arrow) represents the starting line for migration, and the right hand side of the panel is the area initially scraped free of cells. T47D cells have been exposed to vehicle or to 1 nm E2 for 24 h. The cells crossing the starting line were counted. B, E2 induced cell migration as a function of dose in T47D cells. The specific step down culture conditions are described in Materials and Methods. Vehicle as well as E2 concentrations from 0.001 to 100 nm are present. C, Effect of the antiestrogen fulvestrant (ICI) at a concentration of 100 nm on the migration of cells stimulated by 10 and 1 nm E2. Results are expressed as mean ± sem. *, P < 0.05 compared with vehicle controls by Student’s t test.
Western blotting
Cells were lysed in lysis buffer, and lysates were sonicated and cleared by centrifugation at 10,000 × g for 10 min. Protein concentrations were determined using Bio-Rad (Richmond, CA) protein assay dye reagent according to the manufacturer’s specification. Equal amounts of protein (50 μg) were electrophoresed on 10% polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were probed with the indicated antibodies for 2 h at 25 C in 5% BSA in 1× Tris-buffered saline with Tween 20 [50 mm Tris (pH 7.4), 150 mm NaCl, and 0.5% Tween 20]. Target proteins were detected with horseradish peroxidase-coupled secondary antibodies combined with chemiluminescence and exposure to x-ray film. X-ray film was scanned, and densities of the bands of tested molecules were quantified and normalized by the density of total protein using the TotalLab (Newcastle, UK) program. All data presented have been confirmed in at least two other experiments.
Small interfering RNA (siRNA) knockdown of paxillin
T47D cells were plated at a density of 4 × 105 in 60-mm plates and cultured for 3 d to 80% confluence. Cells were then transfected with 5 pm paxillin siRNA (Dharmacon RNAi Technologies, Lafayette, CO) duplex (target sequence, CAACUGGAAACCACACAUA, GGACGUGGCACCCUGAACA, CCAAACGGCCUGUGUUCUU, UGACGAAAGAGAAGCCUAA) using DharmaFECT transfection reagent (Dharmacon RNAi Technologies) according to manufacturers’ recommendations. After incubation with siRNA for 24 h, the cells were incubated in phenol red-free RPMI 1640 medium containing 5% DCC-FBS overnight. Cells were treated and assayed for cell migration and protein expression. Control siRNAs consisted of Dharmacon’s nontargeting duplex.
Statistical analyses
All data are expressed as the mean ± se. Statistical significance was determined by Student’s t test. The majority of statistical comparisons determined if the various treatments differed statistically significantly from the effects of E2 at a 1 nm concentration. In the figures showing these results, the E2-alone bars lack asterisk, whereas the comparisons with E2 alone show asterisks if the differences are statistically significant at P ≤ 0.05.
Results
Effect of E2 on migration
The initial set of experiments validated the cell migration assay. As shown in Fig. 2, A and B, 0.1–100 nm of E2 stimulated migration of T47D cells in a dose-responsive fashion with excellent reproducibility as illustrated by the sems at each dose. The increases achieved statistical significance with concentrations of E2 of 100 pm to 100 nm. Additional experiments assessed migration at 48 and 72 h (data not shown) with similar results. However, data obtained at 24 h were more reproducible and allowed performance of the assay over a shorter period of time. For those reasons, all subsequent experiments used the 24-h time period. The effect of E2 was mediated by ERs as evidenced by ability to block migration with the antiestrogen fulvestrant (Fig. 2C). Multiple experiments (see figures 3–8 below) demonstrated the high degree of reproducibility of this cell migration bioassay. We sought to confirm that these responses were not limited to T47D cells nor to wound assays. Accordingly, additional experiments in wild-type cells (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) and long-term-estrogen-deprived MCF-7 cells (data not shown) demonstrated similar effects in the wound assay. E2 also stimulated migration of T47D cells in Boyden chamber assays (Supplemental Fig. 2).
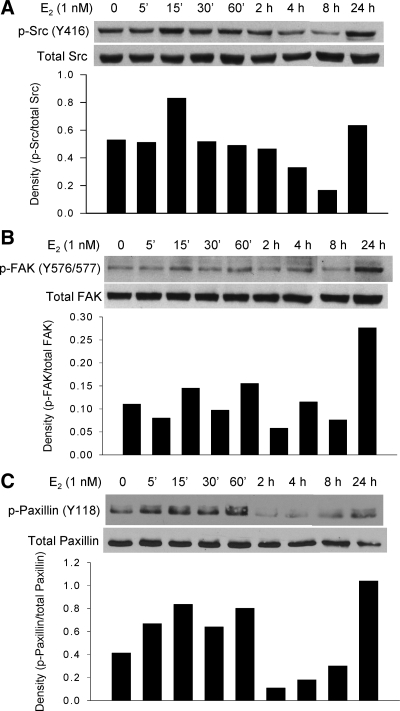
Figure 3.
Time course of E2 induced phosphorylation of c-Src (A), FAK (B), and paxillin (C). T47D cells grown in 60-mm dishes were stepped down to RPMI 1640 medium containing 5% DCC-FBS for 48 h before addition of E2 (1 nm). Whole-cell lysates (50 μg proteins) were used for Western blot analysis using specific antibodies against phosphor-c-Src (Y416), phospho-FAK (Y576/577), and phospho-paxillin (Y118). The densitometry of phosphorylated molecules were quantified and normalized by the total protein. The experiments were repeated three times with similar results. Shown in the figure is a representative of three experiments.
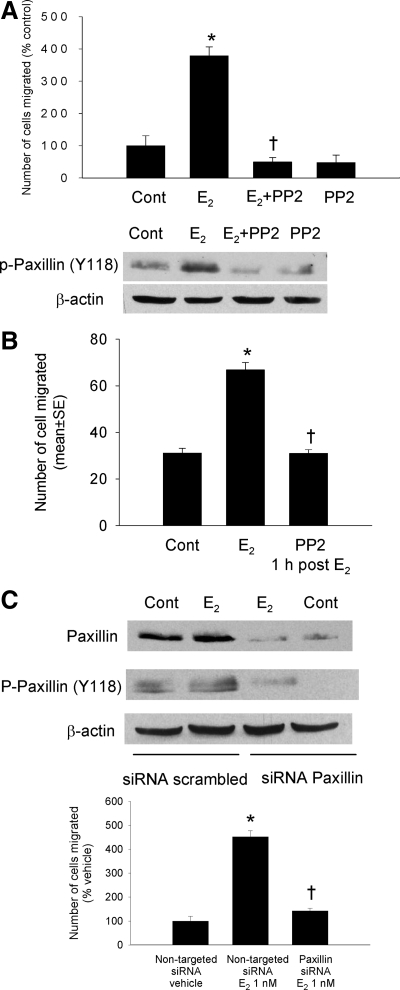
Figure 4.
A, Effect of the c-Src inhibitor PP2 on cell migration (top) and paxillin phosphorylation at Tyr118 (bottom). T47D cells were pretreated with PP2 (5 μm) for 1 h before the addition of 1 nm E2. Incubation time with E2 is 24 h for cell migration assay and 1 h for Western blot analysis. Tyr118 phosphoryated paxillin was quantified by densitometric scanning and normalized by total paxillin. B, Effect of posttreatment of PP2 on cell migration. T47D cells were exposed to E2 for 1 h, and then PP2 (5 μm) was added. Cell migration was assessed at 24 h of E2 treatment. C, Effects of siRNA targeted to paxillin on paxillin protein and E2-induced paxillin phorphorylation (top) and migration (bottom). β-Actin served as a loading control and off target protein. Results from scrambled, nontarget siRNA were compared with siRNA directed against paxillin. Data represent the relative migration of cells expressed as a percent of control values. Data shown are means ± sem. *, P < 0.05 compared with the nontarget siRNA; and †, P < 0.05 compared with E2 + paxillin siRNA with Student’s t test. Cont, Vehicle control.
Figure 5.
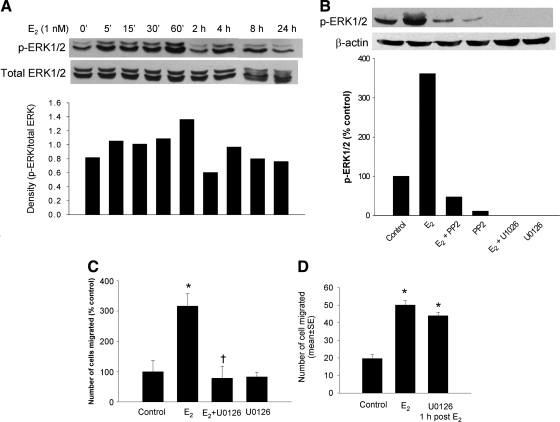
A, Western blot analysis of cells exposed to 1 nm E2 for 5 min through 24 h with an antibody against phospho-ERK1/2 at the Thr202/Tyr204 positions. Total ERK1/2 is blotted for loading controls. Bottom panel, Densitometric quantification of the Western blot analyses shown in the top panel. B, Western blot analysis of cells exposed to 1 nm E2 for 1 h and blotted for phosphorylated ERK1/2. E2 and the ERK inhibitors U0126 (10 μm) and PP2 (5 μm) were added alone and together as shown. The lower lanes show β-actin as the loading controls. Bottom panel, Densitometric quantification of phospho-ERK1/2 determined from the Western blot analyses shown in the top panel. C, Effect of MEK inhibitor U0126 on E2-induced migration. Data represent the relative migration of cells expressed as a percent of control values. Cells are grown in the presence of vehicle, 1 nm E2 with or without U0126 (10 μm) for 24 h. D, Effect of posttreatment with U0126 on E2 induced cell migration. T47D cells were exposed to E2 for 1 h, and then U0126 (10 μm) was added. Cell migration was assessed at 24 h of E2 treatment. Data are means ± sem. *, P < 0.05 compared with vehicle control; and †, P < 0.05 compared with the E2-stimulated group by Student’s t test.
Figure 6.
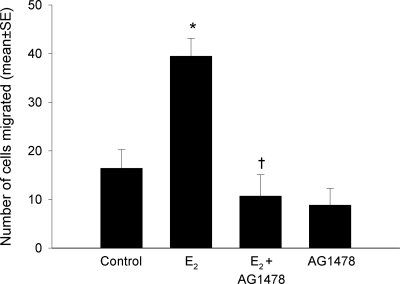
Effect of the EGF-R inhibitor, AG 1478, on the migration of cells exposed to 1 nm E2 alone or E2 plus AG 1478 (5 μm). *, P < 0.05 compared with vehicle control; and †, P < 0.05 compared with the E2-stimulated group by Student’s t test.
Figure 7.
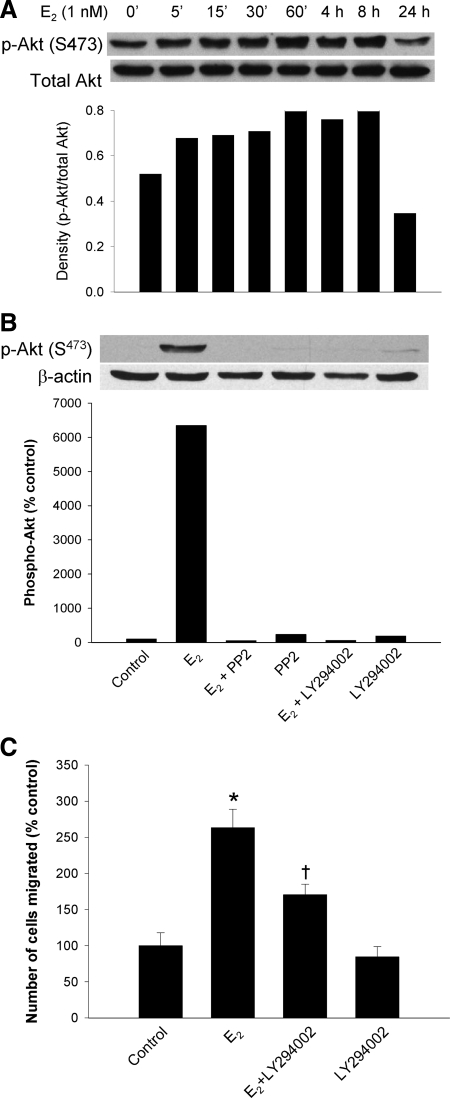
A, Western blot analysis of cells exposed to 1 nm E2 for various time points and blotted for Ser473-phosphorylated Akt. The lower lanes represent total Akt. Bottom panel, Densitometric analysis of the Western blot analyses shown above. B, Western blot analyses for Ser473 phosphorylated Akt in cells exposed to 1 nm E2 or vehicle and combinations of LY 294002 (10 μm) to block PI-3-kinase or PP2 to block c-Src. Bottom panel, Densitometric analysis of the Western blot analyses shown in the top panel. C, Effect of the PI-3-kinase inhibitor LY 294002 on the migration of cells exposed to 1 nm E2. Dimethyl sulfoxide and LY 294002 controls are shown. *, P < 0.05 compared with vehicle control; and †, P < 0.05 compared with the E2-stimulated group by Student’s t test.
Figure 8.
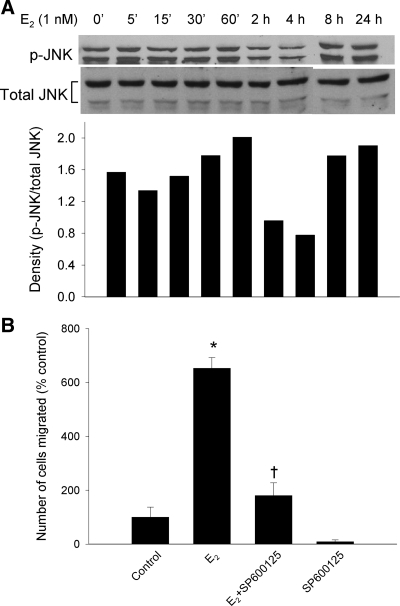
A, Western blot analyses for phosphorylated JNK in cells exposed to 1 nm E2 for various times. Total JNK is used in the lower lanes as loading controls. Bottom panel, Densitometric analysis of the Western blot analyses shown in the top panels. B, Effect of the JNK inhibitor SP 600125 (10 μm) on the migration of cells exposed to 1 nm E2 or dimethyl sulfoxide as vehicle. Data are expressed as mean ± sem. *, P < 0.05 compared with vehicle control; and †, P < 0.05 compared with the E2-stimulated group by Student’s t test.
Role of c-Src, FAK, and paxillin on migration
Our prior data demonstrated that E2 can activate c-Src through rapid, extranuclear effects in MCF-7 cells (22). Our working model (Fig. 1) suggests that c-Src activation results in the phosphorylation of FAK and paxillin and can influence cell migration. For these reasons, we initially assessed the effects of E2 on phosphorylation and activation of c-Src, FAK, and paxillin. Cells were exposed to 1 nm E2 for 5, 15, and 30 min and 1, 2, 4, 8, and 24 h before harvesting cell lysates. c-Src phosphorylation increased at 15 min of E2 exposure and went down between 30 min and 8 h. Phosphorylation of FAK increased slightly at early time points (15 and 60 min) and then oscillated at base line levels. Phosphorylation of paxillin increased gradually from 5 to 60 min and then fell to levels below base line between 2 and 8 h. At 24 h of E2 treatment, phosphorylation of all three molecules increased again. The second peaks of FAK and paxillin were higher than the early ones (Fig. 3, A–C).
We next questioned whether blockade of c-Src would abrogate the effects of E2 on cell migration and on downstream kinases. T47D cells were pretreated with vehicle or the c-Src inhibitor, PP2 (5 μm), for 60 min before E2 was added, and then E2-induced migration was measured at 24 h. As shown in Fig. 4A, PP2 completely inhibited E2-induced cell migration and blocked E2-induced paxillin phosphorylation (Fig. 4A, bottom), demonstrating that c-Src is upstream of paxillin, whose kinase is activated by E2 treatment.
To determine whether later activation of c-Src contributed to cell migration, we conducted additional wound assays with delayed addition of PP2, 1 h after adding E2. Similar to the results shown in Fig. 4A, PP2 1 h after E2 completely abrogated E2-induced migration, indicating that sustained activation of this pathway is required for E2-stimulated migration of T47D cells (Fig. 4B).
To directly evaluate the requirement of paxillin for E2-mediated migration, we used a siRNA to knock down paxillin and examined the effect of this maneuver on E2-induced cell migration. First, we examined the efficiency of knock down. After siRNA transfection, the levels of total paxillin protein and E2-induced paxillin phosphorylated protein (but not the nontarget protein β-actin) were substantially reduced compared with scrambled (nontarget) siRNA-treated cells (Fig. 4C, top). We then examined the effect of paxillin knock down on cell migration. T47D cells treated with paxillin siRNA no longer responded to E2 with increased cell migration (Fig. 4C, bottom). Taken together, these data strongly support a role for c-Src, FAK, and paxillin in the process of E2-induced T47D cell migration and the requirement of sustained activation of c-Src.
Role of ERK1/2 (MAPK)
The ERK subfamily of the MAPK plays a crucial role in the regulation of cell migration (20). To systematically investigate the role of ERK1/2 on E2-induced migration in T47D cells, we initially examined the degree of stimulation of ERK1/2 with E2. The cells were treated with 1 nm E2 for, 5, 15, 30, 60 min and 2, 4, 8, and 24 h. Activation of ERK1/2 was determined by the amount of phospho-ERK1/2 using Western blotting. ERK1/2 activation increased at 5 min, peaked at the 60-min time point, and then fell to the control levels thereafter (Fig. 5A). The ERK1/2 inhibitor (U0126) blocked this activation (Fig. 5B).
E2 stimulated MAPK only during the first 60 min but not later in contrast to the early and late effects on c-Src, FAK, and paxillin. Accordingly, we questioned if blockade of MAPK early but not late might inhibit migration. T47D cells were treated with U0126 1 h before 1 nm E2 and compared with cells where the U0126 was delayed by 60 min. Early but not late inhibition of ERK by U0126 exhibited an inhibitory effect on cell migration (Fig. 5D). These results suggest that for MAPK, the rapid signaling event is the predominant driving force for E2-induced cell migration. Once the rapidly induced signal is transmitted, the cell migration process is not stopped by blocking ERK activation.
Role of EGF-R
Our working model suggests that EGF-R is activated by E2 and that blockade of this receptor should inhibit migration. Our prior studies had demonstrated in MCF-7 cells that E2 transactivates EGF-R in a rapid, ER-dependent, extranuclear fashion (23). As shown in Fig. 6, AG1478, a specific EGF-R tyrosine kinase inhibitor, blocked cell migration.
Role of PI-3-kinase
In a variety of cellular systems, E2 activates PI-3-kinase in a rapid, extranuclear fashion. We assessed the rapid effect of E2 in our model by examining the activation of the downstream target of PI-3-kinase, Akt. As shown in Fig. 7A, top and bottom, E2 caused the phosphorylation and activation of Akt at Ser473, with initial stimulation at 5 min and maximal stimulation at 60 min. The peak levels of phosphorylated Akt were maintained for at least 8 h before reduction, but a peak at 24 h was not observed. This effect was blocked by the PI-3-kinase inhibitor, LY 294002, and by the c-Src inhibitor PP2 (Fig. 7B, top and bottom). As evidence of the biologic effects on cell migration, LY 294002 partially inhibited cell migration (Fig. 7C). The lack of complete blockade of E2 stimulation suggested that the PI-3 kinase pathway is only a partial mediator of cell migration, as predicted by working model in Fig. 1.
Role of JNK
JNK is one of panoply of kinases that can phosphorylate paxillin at both serine and tyrosine sites and enhance its ability to mediate cell migration. These various kinases include p21-activated kinase (PAK), c-Src, MAPK, p38 kinase, p90 ribosomal S6 kinase, CDC5, ABL tyrosine kinase, and RACK1, as well as JNK (9). To examine whether E2 stimulated JNK phosphorylation, we collected lysates of T47D cells stimulated with E2 for 5, 15, 30, and 60 min and 2, 4, 8, and 24 h. The amount of phosphorylation of JNK was determined by Western blottings. E2 increased the level of JNK phosphorylation, which reached a maximum at 1 h and then was reduced. A second increase in phosphorylated JNK occurred at 8 h and remained high at 24 h (Fig. 8A, top and bottom). To determine the biologic effect of JNK on E2-induced cell migration, we used the specific JNK inhibitor, SP600125. We preexposed cells to 10 μm SP600125 for 60 min and then added 1 nm E2 for 24 h. Specific inhibition of JNK by SP600125 significantly reduced E2-induced migration (Fig. 8B). The result suggested that E2 mediates T47D cell migration via JNK signaling pathways.
Role of moesin
The group of Simoncini and co-workers (24) demonstrated a role for moesin in estrogen and progestin-stimulated breast cell migration by examining moesin phosphorylation and blockade of migration by the Rho-associated protein kinase (ROCK) inhibitor Y27632. In our cellular system, we were unable to demonstrate an effect of E2 either to enhance phosphorylation of moesin or to block cell migration with Y27632 (10 μm) (Supplemental Fig. 3, top and bottom).
Discussion
Aromatase inhibitors and antiestrogens block metastatic cancer spread as well as tumor growth in women with hormone-dependent breast cancer (1,25,26,27). Demonstration of an effect of E2 on cell migration in a model system provided evidence of the mechanisms whereby specific targeted agents could potentially modulate the metastatic process. Our studies clearly demonstrated that E2 stimulates cell migration in a model of hormone-dependent breast cancer and identified several key signaling pathways involved. The effects of E2 were mediated through ERα and resulted in both early and late phosphorylation and activation of c-Src. The function of c-Src persisted beyond the early phase, because delayed addition of a c-Src inhibitor 60 min after addition of E2 still blocked cell migration. E2 also produced downstream effects on FAK, ERK, PI-3-kinase, JNK, and paxillin, which appeared to be mediated by an upstream effect of c-Src. As evidence of the biologic relevance of each of these steps, blockade with specifically targeted inhibitors abrogated cell migration. These results are consistent with our working model, as shown in Fig 1, which suggests that each of these steps is interlinked and represents a complex regulatory system controlling cell migration.
E2 induced only early but not late effects on MAPK (ERK1/2) as opposed to both early and late effects on c-Src, FAK, and paxillin phosphorylation. Inhibitor studies demonstrated that late inhibition of MAPK did not affect cell migration, whereas early inhibition did. These observations clearly dissociate the mechanistic effects of MAPK and c-Src. These results suggest that E2 can stimulate an integrated series of events affecting both rapid, extranuclear, as well as nuclear signaling. Madak-Erdogan et al. (28) recently demonstrated that the extranuclear actions of E2 can result in later initiation of transcriptional events. They showed that the rapid E2 signals result in transcription of a subset of E2-regulated genes that are not associated with ER binding to nuclear estrogen response elements. These observations suggest that rapid effects of E2 can be integrated later with more classical transcriptional events. Based on this reasoning, we cannot readily dissociate extranuclear from nuclear actions of E2 on cell migration. However, our data highlight the contrast between the late actions of E2 on c-Src, FAK, and paxillin with the early actions on MAPK. These observations suggest that the effects of E2 on migration probably involve both rapid extranuclear and later transcriptional events.
From a cancer biology perspective, our findings illustrate the ability of neoplastic cells to use multiple signaling pathways to mediate a specific effect. Cancer cells are known to exhibit a high degree of plasticity and have the potential of adapting to various therapeutic strategies with up-regulation of alternative pathways not specifically targeted (29). Because several different inhibitors used in our studies blocked cell migration, the steps involved serve as potential targets for prevention of metastases in women with breast cancer.
These studies, to our knowledge, are the first to demonstrate that E2 causes phosphorylation and activation of paxillin in breast cancer cells. Paxillin, a scaffold protein present in focal adhesions, has four LIM domains (LIM 1-LIM 4), two of which, LIM 2 and LIM 3, mediate focal adhesion targeting (9). At the N-terminal portion of the paxillin molecule is a polyproline-rich area that binds Src. Five LD domains also reside in the N-terminal and midportions of paxillin. Particularly important is the LD 4 domain, which regulates focal adhesion turnover and localized lamellipodia formation through Rho guanosine triphosphatase proteins. Paxillin can be phosphorylated at multiple tyrosine, serine, and threonine sites throughout the paxillin molecule by PAK, FAK, Src, JNK, MAPK, p38 kinase, p90 RSK, CDC5, ABL tyrosine kinase, and RACK1 (9). The tyrosine 118 domain (Y118) of paxillin can be considered an integrator region (30,31,32,33). Through this site, multiple signaling pathways can alter cell migration through downstream events. Both Src and FAK phosphorate Y118 and Y31, key sites for interaction with CRKII-DOCK180-ELMO, which activate the Rac and Rho proteins that ultimately regulate migration (9).
The role of E2 on cell migration has been a controversial issue for some time. Rochefort et al. (34) and Rochefort and co-workers (35) initially reported that E2 blocked cell migration and wound healing in hormone-dependent, MCF-7 breast cancer cells. Other reports also suggested an antimigratory effect of estrogen (36). However, our prior publication (22) demonstrating that E2 caused a rapid increase in dynamic membrane structures, including filopodia, lamellipodia, and pseudopodia and localization of ERα near the plasma membrane, stimulated others to look for stimulatory effects on migration. The Planas-Silva, Simoncini, Chakravarty, and other laboratories have studied the effects of E2 on the migration of vascular epithelial cells, endometrial cells, and breast cancer cells (21,37,38,39,40). The Simoncini group demonstrated a rapid, extranuclear effect of E2 on migration in vascular endothelial cells and identified the phosphorylation of moesin as a key event in FAK activation (21). FAK contains several functional components, including the FERM (erythrocyte band four, 1-ezrin-radixin-moesin) domain, which binds growth factor receptors such as the EGF-R and integrin subunits (10,11,12). The Simoncini group demonstrated that a Rho kinase family member, ROCK, is responsible for moesin phosphorylation, and blockade of ROCK with Y27632 inhibits cell migration (24,41,42). His group demonstrated this effect in T47D breast cancer cells as well as in vascular endothelium. We have not been able to replicate this finding in T47D and MCF-7 cells (24) and currently have not provided a mechanistic reason for the divergence of results between our laboratories. A likely explanation is that we used T47D cells of different lineage or under slightly altered conditions. It may be that with continuous culture, T47D cells from various sources depend to a greater or lesser extent on moesin for cell migration. With so many pathways involved, it is not surprising that some cell lines might use differing pathways to a greater extent than others.
We have not yet examined the downstream mediators of cell migration such as guanine nucleotide exchange factor vav2 (VAV2), CRKII-DOCK180-ELMO, RAC-1, PAK-1, and RhoA. PI-3-kinase, which is activated by E2, should result in the phosphorylation of VAV2, which acts as a guanosine triphosphatase protein exchange factor and catalyzes the conversion of GDP-Rac to GTP-Rac (active RAC-1) (14). PAK-1 is then activated by Rac-1, and dynamic membrane changes are initiated by more downstream effectors (16). Rac-1 stimulates formation of lamellipodia, cdc42 filopodia, and RhoA influences the maturation of adhesions at the leading edge and disassembly of adhesions at the rear. P130CAS is also a key downstream mediator of migration. After phosphorylation by c-Src, this protein binds to CRKII-DOCK180-ELMO and activates Rac-1. The next step, activation of PAK-1 controls stress fiber formation, focal adhesion maintenance, MAPK and JNK signaling cascades, and vascular endothelial growth factor expression (9).
A critical question regarding cell culture studies is whether similar results can be reproduced in other cell lines. We initially began to develop the cell migration assay in MCF-7 cells and found the degree of stimulation with E2 to be less robust than in T47D cells (see Supplemental data). Nonetheless, we demonstrated stimulation of MCF-7 cell migration with 1–100 pm E2 in a dose-dependent fashion, which could be blocked by fulvestrant, PP2, and AG 1478. As noted above, Simoncini and co-workers (24) also demonstrated estrogen stimulation of vascular endothelial as well as MCF-7 cells in their studies, and Kumar and co-workers (39) showed stimulation of endometrial cancer cells. Taken together, it is likely that the stimulation of cell migration by E2 is a phenomenon inherent in multiple benign and malignant cell types and is not limited to the T47D line.
The studies described considerably extend those of Simoncini et al. (21) and Simoncini and co-workers (24) by demonstrating a mechanistic role for MAPK, PI-3-kinase, JNK, and paxillin in estrogen-induced cell migration. The Simoncini group did demonstrate effects of MAPK and PI-3-kinase inhibitors on moesin phosphorylation induced by progesterone and medroxyprogesteron 17-acetate (38), but they did not examine such effects in estrogen-stimulated breast cancer cells. From all of the available data, it would appear that both estrogen and progestins alter migration via multiple mechanisms and that further study will be required to fully understand this complex process.
Our studies and those of others have demonstrated the key role of c-Src in activation of the MAPK and PI-3-kinase pathways and in increasing the aggressiveness of breast cancers (43). These observations suggest that a c-Src inhibitor might be an effective agent in the treatment of breast cancer. AstraZeneca (Macclesfield, UK) have developed such an inhibitor, which is currently entering clinical trial (44). Other potential targets would be inhibitors of ERK or of paxillin. Further studies are being pursued to more precisely determine the role of each of the components of cell migration and the relationship of each of these to estrogen activation.
Footnotes
Present addresses: Beijing Friendship Hospital Affiliated to the Capital University of Medical Sciences, 95 YongAn Road Xuanwu District, Beijing 100050, China (Y.L.); and Department of General Surgery, Busan Paik Hospital, Inje University, Busan 614-735, Republic of Korea (T.-H.K.).
This work was supported by National Institutes of Health Grant CA65622.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
Abbreviations: Akt, Protein kinase B; CRKII, V-crk sarcoma virus CT10 oncogene homolog; c-Src, p60 Src tyrosine kinase; DCC, dextran-coated charcoal; DOCK180, dedicator of cytokinesis; E2, estradiol; EGF-R, epidermal growth factor receptor; ELMO, engulfment and cell motility; ER, estrogen receptor; FAK, focal adhesion kinase; FBS, fetal bovine serum; JNK, c-Jun N-terminal kinase; PAK, p21-activated kinase; PI, phosphatidylinositol; PP2, 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; Rac, Rho family GTPase; ROCK, Rho-associated protein kinase; siRNA, small interfering RNA; VAV2, guanine nucleotide exchange factor vav2; Y118, tyrosine 118 residual.
References
- Santen RJ, Manni A, Harvey H, Redmond C 1990 Endocrine treatment of breast cancer in women. Endocr Rev 11:221–265 [DOI] [PubMed] [Google Scholar]
- Cowin P, Welch DR 2007 Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia 12:99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T 2005 Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5:744–749 [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA 2008 The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch DR, Cooper CR, Hurst DR, Lynch CC, Martin MD, Vaidya KS, VanSaun MN, Mastro AM 2008 Metastasis research society—American association for cancer research joint conference on metastasis. Cancer Res 68:9578–9582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ 2002 Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol Endocrinol 16:116–127 [DOI] [PubMed] [Google Scholar]
- Parsons JT 2003 Focal adhesion kinase: the first ten years. J Cell Sci 116:8–16 [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K 2003 Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374 [DOI] [PubMed] [Google Scholar]
- Deakin NO, Turner CE 2008 Paxillin comes of age. J Cell Sci 121:15–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya V, Beviglia L, Xu LH, Earp 3rd HS, Craven R, Cance W 2002 Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem 277:38978–38987 [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D 1998 Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558–560 [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kiyokawa E, Hara S, Iemura S, Natsume T, Manabe T, Matsuda M 2009 Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp Cell Res 315:863–876 [DOI] [PubMed] [Google Scholar]
- Li S, Hua ZC 2008 FAK expression regulation and therapeutic potential. Adv Cancer Res 101:45–61 [DOI] [PubMed] [Google Scholar]
- Bernards A 2003 GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 1603:47–82 [DOI] [PubMed] [Google Scholar]
- Bernards R 2003 Cancer: cues for migration. Nature 425:247–248 [DOI] [PubMed] [Google Scholar]
- Eswaran J, Soundararajan M, Kumar R, Knapp S 2008 UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci 33:394–403 [DOI] [PubMed] [Google Scholar]
- Schrecengost RS, Riggins RB, Thomas KS, Guerrero MS, Bouton AH 2007 Breast cancer antiestrogen resistance-3 expression regulates breast cancer cell migration through promotion of p130Cas membrane localization and membrane ruffling. Cancer Res 67:6174–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin ME, Muthuswamy SK 2009 ErbB receptors and cell polarity: new pathways and paradigms for understanding cell migration and invasion. Exp Cell Res 315:707–716 [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R 2004 p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 5:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF 2004 FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6:154–161 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR 2006 Estrogen receptor α interacts with Gα13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol 20:1756–1771 [DOI] [PubMed] [Google Scholar]
- Song RX, Santen RJ 2006 Membrane initiated estrogen signaling in breast cancer. Biol Reprod 75:9–16 [DOI] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ 2007 Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology 148:4091–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giretti MS, Fu XD, De Rosa G, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T 2008 Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE 3:e2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A 2009 History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev 30:343–375 [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Coombes RC, Goss PE, Winer EP 2008 Summary of aromatase inhibitor clinical trials in postmenopausal women with early breast cancer. Cancer 112:700–709 [DOI] [PubMed] [Google Scholar]
- Jordan VC, Brodie AM 2007 Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids 72:7–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ, Lobenhofer EK, Afshari CA, Bao Y, Song RX 2005 Adaptation of estrogen-regulated genes in long-term estradiol deprived MCF-7 breast cancer cells. Breast Cancer Res Treat 94:213–223 [DOI] [PubMed] [Google Scholar]
- Turner CE 2000 Paxillin and focal adhesion signalling. Nat Cell Biol 2:E231–E236 [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE 2004 Paxillin: adapting to change. Physiol Rev 84:1315–1339 [DOI] [PubMed] [Google Scholar]
- Bellis SL, Miller JT, Turner CE 1995 Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem 270:17437–17441 [DOI] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Vallés AM 2000 Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol 148:957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platet N, Cunat S, Chalbos D, Rochefort H, Garcia M 2000 Unliganded and liganded estrogen receptors protect against cancer invasion via different mechanisms. Mol Endocrinol 14:999–1009 [DOI] [PubMed] [Google Scholar]
- Rochefort H, Platet N, Hayashido Y, Derocq D, Lucas A, Cunat S, Garcia M 1998 Estrogen receptor mediated inhibition of cancer cell invasion and motility: an overview. J Steroid Biochem Mol Biol 65:163–168 [DOI] [PubMed] [Google Scholar]
- Sisci D, Aquila S, Middea E, Gentile M, Maggiolini M, Mastroianni F, Montanaro D, Andò S 2004 Fibronectin and type IV collagen activate ERα AF-1 by c-Src pathway: effect on breast cancer cell motility. Oncogene 23:8920–8930 [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Waltz PK 2007 Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid BiochemMol Biol 104:11–21 [DOI] [PubMed] [Google Scholar]
- Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T 2008 Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS ONE 3:e2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acconcia F, Barnes CJ, Kumar R 2006 Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology 147:1203–1212 [DOI] [PubMed] [Google Scholar]
- Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK 2010 Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res 70:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XD, Flamini M, Sanchez AM, Goglia L, Giretti MS, Genazzani AR, Simoncini T 2008 Progestogens regulate endothelial actin cytoskeleton and cell movement via the actin-binding protein moesin. Mol Hum Reprod 14:225–234 [DOI] [PubMed] [Google Scholar]
- Giretti MS, Simoncini T 2008 Rapid regulatory actions of sex steroids on cell movement through the actin cytoskeleton. Steroids 73:895–900 [DOI] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Santen RJ 2005 Estrogen rapid action via protein complex formation involving ERα and Src. Trends Endocrinol Metab 16:347–353 [DOI] [PubMed] [Google Scholar]
- Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, Messersmith WA, Hidalgo M 2009 Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res 15:4138–4146 ff [DOI] [PubMed] [Google Scholar]