Abstract
GnRH neurons are central regulators of fertility, and their activity is modulated by steroid feedback. In normal females, GnRH secretion is regulated by estradiol and progesterone (P). Excess androgens present in hyperandrogenemic fertility disorders may disrupt communication of negative feedback signals from P and/or independently stimulate GnRH release. Voltage-gated calcium channels (VGCCs) are important in regulating excitability and hormone release. Estradiol alters VGCCs in a time-of-day-dependent manner. To further elucidate ovarian steroid modulation of GnRH neuron VGCCs, we studied the effects of dihydrotestosterone (DHT) and P. Adult mice were ovariectomized (OVX) or OVX and treated with implants containing DHT (OVXD), estradiol (OVXE), estradiol and DHT (OVXED), estradiol and P (OVXEP), or estradiol, DHT, and P (OVXEDP). Macroscopic calcium current (ICa) was recorded in the morning or afternoon 8–12 d after surgery using whole-cell voltage-clamp. ICa was increased in afternoon vs. morning in GnRH neurons from OVXE mice but this increase was abolished in cells from OVXEP mice. ICa in cells from OVXD mice was increased regardless of time of day; there was no additional effect in OVXED mice. P reduced N-type and DHT potentiated N- and R-type VGCCs; P blocked the DHT potentiation of N-type-mediated current. These data suggest P and DHT have opposing actions on VGCCs in GnRH neurons, but in the presence of both steroids, P dominates. VGCCs are targets of ovarian steroid feedback modulation of GnRH neuron activity and, more specifically, a potential mechanism whereby androgens could activate GnRH neuronal function.
Circulating steroid hormones regulate voltage-gated calcium currents in GnRH neurons as a component of the mechanism for steroid feedback.
A pulsatile GnRH signal is required for secretion of the pituitary gonadotropins LH and FSH (1), which drive steroidogenesis and follicular development during the female reproductive cycle (2,3). Variations in GnRH pulse frequency during the cycle are critical for the differential synthesis and release of LH and FSH; low-frequency pulses favor FSH, and high frequencies favor LH (1,4,5). Steroid feedback regulates GnRH pulse frequency. During the luteal phase, progesterone (P)-mediated negative feedback reduces GnRH pulse frequency (6,7,8), favoring FSH synthesis; inhibin from the corpus luteum blocks FSH release at this time (9,10). After the demise of the corpus luteum, low-frequency GnRH release continues, allowing preferential release of FSH in the early follicular phase that is critical for follicular maturation.
In some hyperandrogenemic fertility disorders, including the common disorder polycystic ovary syndrome (PCOS), GnRH pulse frequency remains persistently high, impairing the preferential release of FSH and thus follicular maturation (11,12,13,14,15). Evidence suggests that the high levels of different androgens characteristic of this disorder reduce sensitivity of the hypothalamic-pituitary axis to P-mediated negative feedback (16,17).
The underlying neurobiological mechanisms for these steroid effects cannot be approached in patients. However, mice appear to be a good model with regard to steroid feedback effects. In adult female mice treated with P, GnRH neuron firing activity is suppressed (18). Addition of dihydrotestosterone (DHT) at a dose that is incapable of restoring seminal vesicle mass in castrated male mice (i.e. below normal male levels) (19) countered these effects of P. In the absence of P, DHT treatment increased GnRH neuron activity and LH release; this is important with regard to the typical steroid milieu in women with hyperandrogenemic disorders because P is rarely elevated due to oligoanovulation. In mechanistic studies, these same animal model steroid treatments had similar effects on GABAergic transmission to GnRH neurons, which can be excitatory to these cells (20,21,22), implying engagement of steroid-sensitive afferents in the response to these steroids. The effects of P or an androgen on intrinsic GnRH neuronal properties, however, are unknown.
Voltage-gated calcium channels (VGCCs) mediate Ca2+ influx, thereby regulating Ca2+-dependent cellular processes such as contraction, secretion, excitability, and gene expression (23,24,25,26). VGCCs are classified into low-voltage-activated (LVA) T-type channels and high-voltage-activated (HVA) L-, N-, P/Q-, and R-type channels. GnRH neurons express all five types of VGCCs (27,28,29). VGCCs in GnRH neurons are modified by estradiol feedback, and some of these changes are further dependent on time of day (29,30). In other systems, testosterone, DHT or P treatment can modulate whole-cell calcium currents (31,32,33,34,35). Whether or not GnRH neuron calcium currents are also altered by P and DHT treatment is not known.
To better understand the effects of steroid milieu on GnRH release, we studied how P and DHT treatment modulate HVA VGCCs in GnRH neurons using the whole-cell patch-clamp technique. The data suggest specific subtypes of these channels are targets of differential modulation by P and DHT treatment and are thus poised to be a contributing mechanism to the regulation of GnRH neuronal activity by these steroids.
Materials and Methods
Animals
Adult female GnRH-green fluorescent protein mice (36) (2–3 months) were ovariectomized (OVX) under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia to remove ovarian steroid feedback and received steroid implants as described previously (37). Briefly, some OVX mice received a SILASTIC brand capsule (Dow Corning, Midland, MI) containing 0.625 μg estradiol (OVXE) or 400 μg of the nonaromatizable androgen DHT (OVXD) or both (OVXED) in sesame oil; other OVX mice received 0.625 μg estradiol capsule and 2.5 mg P time-release pellet (Innovative Research of America, Sarasota, FL; OVXEP) or with DHT (OVXEDP). Postoperative analgesia was provided by a long-acting local anesthetic delivered to the surgical sites (0.25% bupivicaine; 7.5 μl/site; Abbott Laboratories). The levels of estradiol and P produced by these implants are physiological (14,38); the DHT implant produces an elevation above female androgen levels but below an effective male dose (19). All hormones were administered in vivo and were not present in any recording solutions. Recordings were made between 8 and 12 d after surgery and steroid replacement. No difference was noted in any parameter as a function of day after surgery, an observation that corresponds well with our previous experience with these models examining GABAergic transmission (37) and GnRH neuron firing rate (18). The treatment duration is longer than the P elevation of the mouse estrous cycle. It was chosen because it is similar to the duration of the P rise during pseudopregnancy in rodents (39) and is also similar to the luteal-phase rise in P that occurs in species that do not exhibit the abbreviated reproductive cycle of small rodents, facilitating comparisons with other species. All procedures were approved by the University of Virginia Animal Care and Use Committee.
Brain slice preparation
Brain slices were prepared as previously described (40,41). Briefly, mice were euthanized at times that corresponded to negative feedback (0900–1030 h, referred to as morning) or surge peak (positive feedback, 1430–1500 h, referred to as afternoon) in mice treated only with estradiol for shorter durations for the purpose of inducing daily surges, which persist for about 5 d after OVXE treatment (42). The brain was rapidly removed and placed in ice-cold high-sucrose saline solution containing the following (in mm): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 2.5 MgCl2. Coronal (300 μm) slices were cut with a Vibratome 3000 (Technical Products International, Inc., St. Louis, MO). Slices were incubated for 30 min at 30–32 C in 50% high-sucrose saline and 50% artificial cerebrospinal fluid (ACSF) solution, containing the following (in mm): 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 2.5 CaCl2 (pH 7.4). Slices were then transferred to 100% ACSF solution at room temperature (∼21–23 C) for 0.5–2.5 h. For recording, slices were placed in a recording chamber on the stage of an Olympus BX51WI upright fluorescent microscope and continuously superfused at 5–6 ml/min with oxygenated ACSF at room temperature. Slices were stabilized in the chamber for at least 5 min before recording.
Electrophysiological recording
Green fluorescent protein-GnRH neurons in the preoptic area were identified by brief illumination at 470 nm. Macroscopic Ca2+ currents from GnRH neurons were recorded using the whole-cell configuration of the patch-clamp technique. Patch pipettes (2.5–3.5 mΩ) were drawn from borosilicate glass capillaries (1.65 mm outer diameter, 1.12 mm inner diameter; World Precision Instruments, Sarasota, FL) using a Sutter P97 pipette puller (Sutter Instrument Co., Novato, CA). Electrode capacitance was electronically compensated. Liquid junction potential (<−4 mV) was not corrected (43). Currents were recorded with an Axopatch-700B amplifier (Molecular Devices, Sunnyvale, CA) and filtered at 10 kHz. Voltage command pulses were generated using pCLAMP9.2 software (Molecular Devices). Neuron membrane potential was held at −60 mV between protocols during voltage-clamp recordings. During whole-cell recording, input resistance (Rin), series resistance (Rs), and membrane capacitance (Cm) were continually monitored. Only recordings with Rin higher than 500 mΩ, Rs lower than 20 mΩ, and Cm higher than 10 pF and holding current between 0 and −50 pA were included for analysis. There were no differences among groups in any passive recording properties or series resistance attributable to steroid treatment or time of day. Cells with bad clamping (identified as sluggish, incomplete current response to pulse protocol and/or 10–90% rise time >2.5 msec) were discarded. Recordings were performed from 1–3 h after preparation of brain slices was complete. No more than four cells per animal were recorded. Recorded cells were mapped to an atlas (44) to determine whether any trends based upon anatomical location emerged; no such trends were apparent in these data sets (not shown).
Drugs and solutions
All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO) unless noted. For voltage-clamp recording of calcium current (ICa), the bath solution consisted of (in mm): 120 NaCl, 10 glucose, 26 NaHCO3, 1.25 Na2HPO4, 1.2 MgSO4, 2.5 CaCl2, 5 4-aminopyridine, 5 CsCl, 10 tetraethylammonium chloride, and 0.0005 tetrodotoxin (pH 7.4) (gassed with 95% O2 and 5% CO2). The pipette solution contained (in mm): 120 Cs-gluconate, 10 HEPES, 10 EGTA, 0.5 CaCl2, 4 Mg-ATP, 0.4 NaGTP, and 20 tetraethylammonium chloride (pH 7.3) (titrated with CsOH, 310 mOsm). Nitrendipine (50 μm), agatoxin IVA (200 nm), and conotoxin GVIA (1 μm) were bath applied; SNX-482 (1 μm) was locally applied by pressure micropipette. Pressure artifact from this local application sometimes delayed the peak of the current but had no effect on amplitude; rise time is thus compared only for total ICa because there is no local drug application during those measurements.
Voltage-clamp protocols
All currents were corrected for leak and capacitive currents online by a P/−6 protocol. To generate Ca2+ channel current-voltage (I-V) activation curves, currents were elicited by a voltage protocol of a 250-msec prepulse at −120 mV to remove inactivation, followed by current measurement at test potentials (250 msec) from −80 mV to +60 mV at 10-mV increments. To determine the steady-state inactivation of ICa, the membrane potential was initially hyperpolarized to −100 mV for 500 msec to remove inactivation, followed by a 1-sec prepulse of −100 to 10 mV in 10-mV increments, then a test pulse of 10 mV for 500 msec; current was quantified during the test pulse. To isolate LVA currents by selective activation, a 1-sec prepulse at −100 mV was followed by a 250-msec test pulse at −50 mV. The voltage protocol for activation of different subtypes of HVA VGCCs is a 250-msec prepulse at −90 mV, followed by current measurement at test potentials (250 msec) from −80 mV to +60 mV at 10-mV increments. Cocktails of specific inhibitors were used to isolate specific HVA subtypes. To isolate L-type channels, agatoxin IVA, conotoxin GVIA, and SNX-482 were used; to isolate N-type channels, nitrendipine, agatoxin IVA, and SNX-482 were used; to isolate P/Q-type channels, nitrendipine, agatoxin IVA, and SNX-482 were used; and to isolate R-type channels, nitrendipine, agatoxin IVA, and conotoxin GVIA were used.
Analysis
The peak current amplitude and sustained current 200 msec after the beginning of test potential were calculated. Current density was calculated by dividing the peak or sustained current amplitude by Cm. Current waveforms were fitted with the Clampfit program (Molecular Devices) or GraphPad Prism program (GraphPad Software, Inc., La Jolla, CA). The voltage dependencies of activation and steady-state inactivation were described with a single Boltzmann distribution: I (V) = Imax/(1 + exp[(V1/2 − V)/k]), where Imax is the maximal current elicited, V1/2 is the half-maximal voltage, and k is the voltage dependence (slope) of the distribution. For all I-V curves and steady-state inactivation curves, fitted values are reported with 95% linear confidence limits. To isolate different HVA subtypes, slices were incubated with other subtype channel blockers for 15 min in the recording chamber before current was recorded. To isolate LVA currents, the protocol was repeated 50 times at 30-sec intervals and averaged to reduce noise. Parametric or nonparametric analyses were performed in Prism as dictated by data distribution. Statistical comparisons of in vivo treatments were done with ANOVA followed by Bonferroni post hoc test. Statistical significance was set at P < 0.05. Data are shown as mean ± sem.
Results
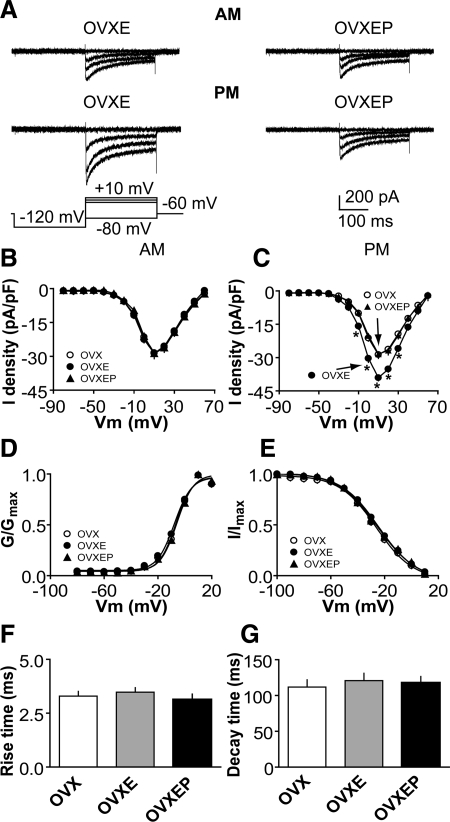
P treatment inhibits ICa in GnRH neurons
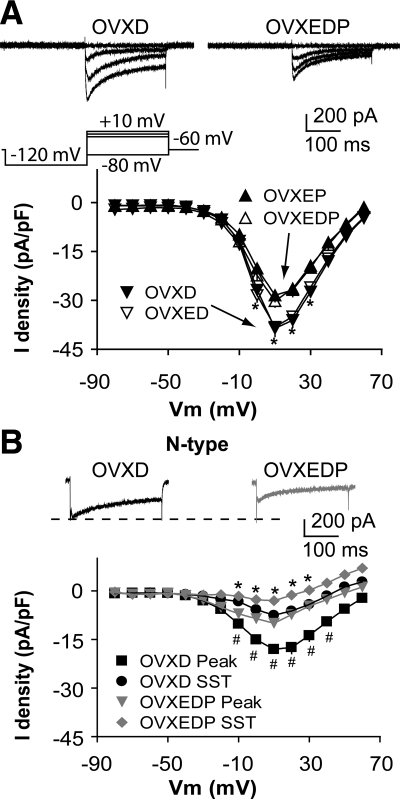
To study the effect of P on ICa, we compared the current among GnRH neurons from OVX, OVXE, and OVXEP mice. Because a shorter duration of in vivo estradiol treatment had a time-of-day-dependent effect on GnRH neuron ICa (29), cells were recorded at two times of day, morning and afternoon, to determine whether diurnal changes persist with longer estradiol treatment and in the presence of other gonadal steroids. Representative traces at selected potentials are shown from steroid-treated mice in Fig. 1A; I-V relationships for all groups are shown in Fig. 1, B and C. ICa in GnRH neurons was activated at potentials positive to −40 mV. In OVXE mice, ICa exhibited diurnal changes consistent with previous work examining these currents 2–4 d after estradiol treatment. Specifically, on d 8–12 after estradiol treatment, ICa were not different from those in OVX mice in the morning (n = 28 cells from eight OVX mice; n = 20 cells from five OVXE mice) but were increased in the afternoon (n = 30 cells from eight OVX mice; n = 23 cells from six OVXE mice; Fig. 1, A–C; P < 0.05, OVXE vs. OVX, and OVXEP vs. OVXE). In contrast, in OVXEP mice, there was no difference between ICa in the morning and afternoon (n = 15 cells from four mice). Rather, all measures were similar to morning values from OVXE mice and all values from OVX mice. There were no differences in rise or decay time, activation or steady-state inactivation among OVX, OVXE, and OVXEP mice at different times of day (Fig. 1, D–G; P > 0.6). The membrane potential at which the current was half-activated (V1/2act) was −6.3 ± 0.6 mV in OVX mice (n = 30 cells from eight mice), −6.3 ± 0.5 mV in OVXE mice (n = 43 cells from 11 mice), and −6.5 ± 0.8 mV in OVXEP mice (n = 15 cells from four mice; P > 0.3). The membrane potential at which the current was half-inactivated (V1/2inact) was −26.4 ± 1.0 mV in OVX mice (n = 30), −26.12 ± 1.2 mV in OVXE mice (n = 34), and −26.7 ± 2.1 mV in OVXEP mice (n = 15; P > 0.4). These data suggest the inhibitory action of P on GnRH neuron activity is in part due to its ability to block the estradiol-induced afternoon diurnal increase in ICa without altering current kinetics.
Figure 1.
P treatment inhibits the diurnal estradiol-mediated increase in ICa in GnRH neurons. A, Representative recordings showing activation of ICa in GnRH neurons from OVXE (left) and OVXEP (right) mice in the morning (top) and afternoon (bottom). For clarity, only four voltage steps during the test pulse (−80, −10, 0, and 10 mV) are shown (bottom left). B and C, Average I-V curves of peak current from GnRH neurons in the morning (B) and afternoon (C). *, P < 0.05, OVXE vs. OVXEP, and OVX vs. OVXE. D and E, Activation (D) and steady-state inactivation (E) curves in the morning and afternoon. F and G, Rise and decay time.
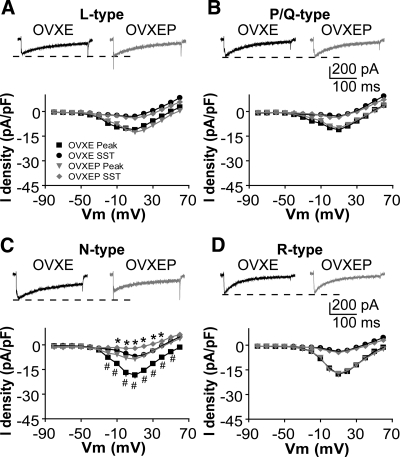
P treatment inhibits N-type HVA ICa
Next, we examined which subtype of HVA-mediated currents are affected by P in the afternoon. Different subtypes of HVA-mediated current were isolated with combinations of specific HVA blockers as indicated in the methods. Compared with GnRH neurons from OVXE mice, P decreased the amplitude and density of N-type currents in OVXEP mice [Fig. 2C, membrane potentials from −20 to +50 mV; n = 8 cells from three OVXE mice; n = 11 cells from three OVXEP mice; peak P < 0.01; sustained current (SST) P < 0.05] ICa subtype. The I-V relation, activation, and inactivation curves did not change (data not shown), consistent with above results. There was no difference in L-type (Fig. 2A; n = 8 OVXE cells from three mice; n = 8 OVXEP cells from three mice; P > 0.8), P/Q-type (Fig. 2B; n = 9 OVXE cells from three mice; n = 7 OVXEP cells from three mice; P > 0.6), or R-type (Fig. 2D; n = 9 OVXE cells from three mice; n = 8 OVXD cells from three mice; P > 1.1) HVA-mediated currents between OVXE and OVXEP mice.
Figure 2.
P treatment inhibits specifically N-type currents. A–D, Representative ICa of each subtype of HVA in response to a step from −90 to +10 mV (top) and average I-V plot for that subtype (below). The notch on the rising phase of the current in B and C is due to pressure artifact from application of SNX-482; amplitude is not affected by this artifact, and this cell was most representative of mean current amplitude and density. *, P < 0.05 SST; #, P < 0.01 peak OVXEP vs. OVXE.
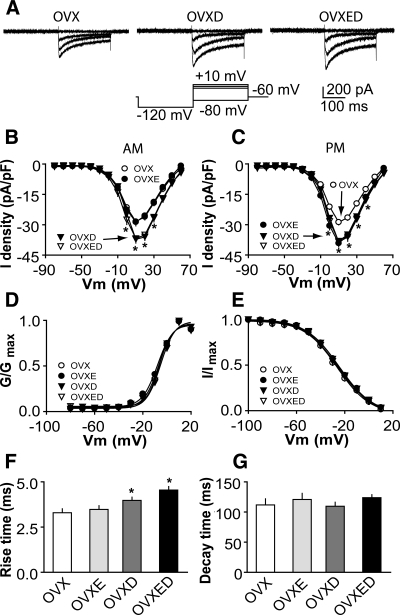
DHT treatment potentiates calcium currents in GnRH neuron
To study the effects of androgens on ICa, we compared the current among GnRH neurons from OVX, OVXD, and OVXED mice recorded at different times of day. ICa amplitude and density (Fig. 3, A–C) in cells from OVXD mice was greater than that in cells from OVX mice at most membrane potentials examined regardless of time of day (morning, P < 0.05 at membrane potentials 0 to +30 mV, n = 28 cells from eight OVX mice, n = 38 cells from 10 OVXD mice; afternoon, P < 0.05 at membrane potentials 0 to +40 mV, n = 30 cells from eight OVX mice, n = 40 cells from 11 OVXD mice). No diurnal variation in ICa was observed between cells from OVXD mice recorded in the morning vs. afternoon (Fig. 3, B and C; morning vs. afternoon P > 0.05). ICa in OVXD mice was not different from that in OVXED and OVXE mice in the afternoon (OVXED n = 10 cells from three mice; OVXE n = 23 from six mice), suggesting the augmenting effects of DHT and estradiol in the afternoon are not cumulative. However in the morning, ICa amplitude and density were greater in OVXED mice than in OVXE mice, suggesting DHT overcomes the diurnal suppression of ICa by estradiol. DHT had no effect on the activation or steady-state inactivation curves of ICa in GnRH neurons (Fig. 3, D and E). The V1/2 act was −6.3 ± 0.6 mV in OVX mice (n = 30 cells from eight mice) and −6.4 ± 0.5 mV in OVXD mice (n = 40 cells from 11 mice; P > 0.3). The V1/2 inact was −26.4 ± 1.0 mV in OVX mice (n = 30) and −26.2 ± 0.9 mV in OVXD mice (n = 40; P > 0.4). DHT slightly increased rise time but had no effect on decay time (Fig. 3, F and G; OVX n = 30 cells from eight mice; OVXD n = 40 cells from 11 mice; P < 0.05). These data suggest DHT treatment increases GnRH neuron ICa in a manner that, in contrast to estradiol, does not depend upon time of day and is thus persistent.
Figure 3.
DHT treatment increases ICa in GnRH neurons. A, Representative recordings showing activation of ICa in GnRH neurons from OVX (left), OVXD (middle), and OVXED (right) mice. For clarity, only four voltage steps during the test pulse (−80, −10, 0, and 10 mV) are shown (bottom left). B and C, Average I-V curves of peak current from GnRH neurons in the morning (B) and afternoon; data from OVXE mice are repeated for comparison (C). D and E, Activation (D) and steady-state inactivation (E) curves. F and G, Rise and decay time. *, P < 0.05, OVX vs. OVXD, and OVX vs. OVXED).
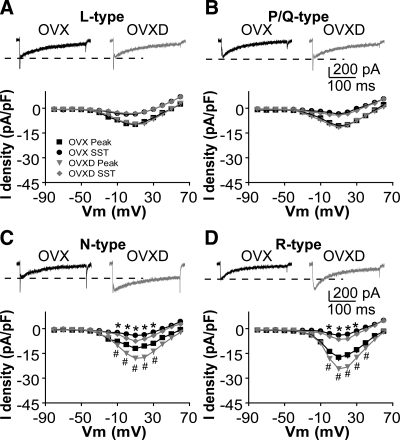
DHT treatment potentiates N- and R-type HVA ICa
We next examined which subtypes of HVA-mediated currents are affected by DHT (Fig. 4). Different subtypes were isolated with blockers as above. Compared with GnRH neurons from OVX mice, DHT increased the amplitude and density of N-type (Fig. 4C, membrane potentials from −10 to +30 mV; n = 10 cells from three OVX mice; n = 12 cells from three OVXD mice; P < 0.05) and R-type (Fig. 4D, membrane potentials from 0 to +40 mV; n = 12 cells from three OVX mice; n = 11 cells from three OVXD mice; peak P < 0.01; SST P < 0.05) ICa subtypes. The I-V relation, activation, and inactivation curves did not change (data not shown), consistent with above results. There was no difference in L-type (Fig. 4A; n = 12 OVX cells from three mice; n = 11 OVXD cells from three mice; P > 0.2) or P/Q-type (Fig. 4B; n = 8 OVX cells from three mice; n = 9 OVXD cells from three mice; P > 0.9) HVA-mediated currents between OVX and OVXD mice.
Figure 4.
DHT treatment increases specifically N-type and R-type currents. A–D, Representative ICa of each subtype of HVA in response to a step from −90 to +10 mV (top) and average I-V plot for that subtype (below). *, P < 0.05 SST; #, P < 0.05 peak in C; #, P < 0.01 peak in D, OVX vs. OVXD.
P treatment blocks the augmentation of N-type current by DHT treatment
In women with PCOS (16,17) and in previous work with these animal models (18,37), androgens interfere with the efficacy of P negative feedback. To determine how progestin and DHT treatments interact to regulate ICa in GnRH neurons, we examined currents in cells from OVXEDP mice. Whole-cell ICa amplitude and density in OVXEDP mice was less than in OVXD or OVXED mice and was not different from OVXEP mice (Fig. 5A; n = 10 cells from three mice; OVXED vs. OVXEDP P < 0.05; OVXD vs. OVXEDP P < 0.05). P cotreatment blocked the augmentation of N-type calcium channels by DHT treatment (Fig. 5B, membrane potentials from −10 to +40 mV; n = 12 cells from three OVXD mice; n = 8 cells from three OVXEDP mice; peak P < 0.01; SST P < 0.05).
Figure 5.
Cotreatment with P blocks the DHT-induced augmentation of ICa in GnRH neurons. A (top), Representative traces showing activation of ICa in GnRH neurons from OVXD (left) and OVXEDP (right) mice. For clarity, only four voltage steps during the test pulse (−80, −10, 0, and 10 mV) are shown; bottom, average I-V plot. B, Representative N-type current in response to a step from −90 to +10 mV (top) and average I-V plot (bottom). Data from OVXD and OVXED mice are repeated for comparison. *, P < 0.05 SST; #, P < 0.01 peak, OVXEP vs. OVXE.
DHT treatment has no effect on T-type calcium current
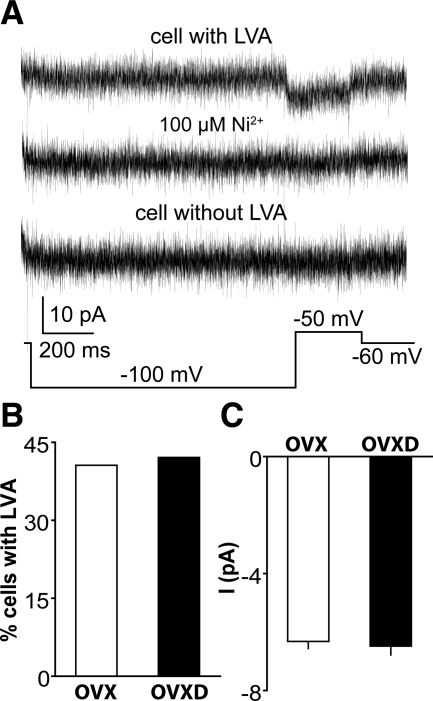
LVA T-type ICa are present in GnRH neurons but in our experience are not adequately revealed by the above protocol (29). Because activation of LVA currents can lead to action potential initiation and DHT is associated with increased GnRH neuron activity, we hypothesized that androgens increase LVA currents. We examined the effects of DHT on LVA currents using a prolonged (1 sec) prepulse at −100 mV to provide a strong signal to remove inactivation, followed by depolarization of the membrane potential to −50 mV to activate specifically LVA currents; this protocol was repeated 50 times at 30-sec intervals and the resulting current traces averaged (29). Consistent with previous work, approximately 42% of GnRH neurons exhibited a small-amplitude LVA-mediated current that could be blocked by 100 μm Ni2+ (Fig. 6A). Contrary to our hypothesis, neither the percentage of cells with LVA nor the peak amplitude of LVA current were different in OVX and OVXD mice (Fig. 6, B and C; n = 11 OVX cells from four animals; n = 8 OVXD cells from three animals; P > 0.5). These data suggest the DHT treatment used in these studies has no effect on T-type ICa in GnRH neurons.
Figure 6.
DHT treatment does not alter LVA-mediated current. A, Representative average of 50 repeats of a voltage protocol (bottom) to reveal LVA current (top trace) and its blockade by Ni2+ (middle trace); a cell without LVA is also shown (bottom trace). B and C, Percentage of GnRH neurons with LVA (B) and the peak current amplitude of LVA (C).
Discussion
Steroid feedback regulates GnRH pulse frequency, and alterations in the efficacy of this feedback are associated with infertility. Here we show that one mechanism by which steroids may alter GnRH neuron physiology is by inducing functional changes in the VGCCs in these cells. Specifically, P treatment reduces VGCC function in these cells, whereas DHT treatment increases VGCC function. In previous work with these same animal models, DHT treatment increased GnRH neuron firing, but P treatment inhibited GnRH neurons. The present changes in VGCC function are consistent with the previous changes in activity. However, P appears to be a dominant influence on VGCC function in these cells because it abolished the ability of DHT to increase ICa. Of note, in women with hyperandrogenemic fertility disorders, a steroid milieu that is androgen rich but P poor is typical due to oligoanovulation and lack of corpora lutea; thus, the activating effects of androgens on ICa may be expected to be predominant.
During the normal reproductive cycle, the primary action of P is to provide negative feedback that reduces the frequency of episodic GnRH release. P receptors are estrogen dependent (45); thus, we tested the effects of P in the presence of estradiol to ensure expression of these receptors. Because a shorter duration of estradiol treatment alters VGCCs in a time-of-day-dependent manner (29), we examined VGCCs at two times of day. The longer duration of estradiol (8–12 d) used in the present study had similar effects to those observed previously; specifically, estradiol increased VGCCs during the afternoon hours, which corresponds to the time of positive feedback actions of this steroid. Although the diurnal changes in LH induced by estradiol are more marked 2–4 d after OVXE treatment, there is still an approximately 6-fold increase in LH during the afternoon hours on d 10 and 12 after OVX plus estradiol treatment (42). The present data suggest estradiol-induced increases in GnRH neuron VGCCs during the afternoon may help mediate this. The addition of a physiological dose of P obliterated the afternoon estradiol-induced increases in GnRH neuron VGCCs. Progesterone specifically inhibited N-type calcium currents, which is one of the subtypes augmented by in vivo estradiol treatment (29). Interestingly, P did not inhibit L-type currents, which were also increased by shorter-duration estradiol treatment. It is possible that the effect of estradiol on L-type current is transient. The ability of P to block an estradiol-induced surge is one basis for daily oral steroid contraceptives (46,47). The present data suggest that one mechanism for this may be through P inhibition of estradiol-induced changes in VGCCs in GnRH neurons.
The reduction of GnRH pulse frequency that occurs as a result of P-mediated negative feedback is critical for setting the stage for the early follicular phase of the subsequent cycle. Specifically, after regression of the corpora lutea, the low-frequency GnRH signal preferentially stimulates FSH synthesis and release, allowing relative domination of this gonadotropin during the early follicular phase when follicular development is essential (48,49). In some forms of hyperandrogenemic infertility, such as the common disorder of PCOS (12), there is persistent high-frequency LH (and presumably GnRH) release, leading to a suppression of the FSH to LH ratio, which contributes to the oligoanovulation characteristic of that disorder (50). Elevated androgen levels reduce sensitivity to P feedback in women with PCOS (12). Here we show that an androgen, in the form of DHT, increases HVA VGCCs in GnRH neurons. Specifically, current via N- and R-type subunits was increased in DHT-treated mice. This effect was observed at both times of day examined and was similar in the presence and absence of estradiol, suggesting it is androgen receptor mediated.
Previous work in a similar mouse model indicates that DHT treatment increases GnRH neuron activity and LH release and also increases GABAergic transmission, which can be excitatory to GnRH neurons (18,20,37). In contrast, P reduced both GnRH neuronal activity and GABAergic transmission to these cells. Collectively, these data were interpreted as possible mechanisms for the apparent opposing actions of DHT and P in the control of GnRH neurons and also as possible mechanisms for independent excitatory effects of androgens in hyperandrogenic, nonovulating (i.e. low P) women. The present data support and extend these findings to a new mechanism, specifically a change in the intrinsic properties of GnRH neurons. Increased HVA calcium current in the presence of elevated androgens and absence of P, the typical steroid milieu of women with PCOS, may be associated with greater vesicle release per action potential; thus, these changes could increase output of this neuroendocrine system.
LVA calcium channels play an important role in generation of repetitive activity in several cell types, including cardiac pacemaker cells (51) and thalamic neurons (52). Hence we hypothesized that one mechanism by which DHT treatment increases GnRH neuron activity is to increase LVA-mediated current. Consistent with this hypothesis, the expression of T-type subunits and LVA-mediated currents in GnRH neurons appears to depend on developmental stage and species (28,29,30,53). No changes were observed, however, in the size of this current or in the percentage of cells expressing LVA-mediated current with and without DHT treatment, suggesting changes in this current do not underlie DHT-mediated increases in GnRH neuron activity.
The present data and previous work provide clear evidence for the regulation of GnRH neuron VGCCs by all three classes of sex steroids. Of interest in this regard, steroid receptor expression by mammalian GnRH neurons appears quite limited. Androgen receptor has not been detected in mammalian GnRH neurons (54,55). Likewise, no colocalization of P receptor and GnRH was detected in ewes (56) or monkeys (57), whereas approximately 20% of guinea pig GnRH neurons (58) express P receptor. Finally, GnRH neurons in vivo appear to express only the β-isoform of the estradiol receptor (59). The above observations suggest there are intermediate steroid-sensitive neurons that release neurotransmitters and/or neuromodulators to mediate the feedback of DHT and P on GnRH neuron intrinsic properties. In this regard, kisspeptin expression is testosterone regulated (60,61), as is GnRH release from medial basal hypothalamic fragments in response to neuropeptide Y (62). DHT also regulates GABAergic transmission to GnRH neurons (37); this could alter gene expression via effects on the GABAB receptor. Likewise, kisspeptin (63), opioid, GABAergic (64), dopamine, and neuropeptide Y neurons (65) have been proposed to mediate the effects of P on these cells. These neuromodulators have been demonstrated to regulate HVA ICa in other systems (66,67,68,69,70,71,72). Changes in macroscopic current can be via several mechanisms including changes in subunit gene expression, posttranslational modification of channel proteins, and trafficking in and out of the membrane (73,74,75). Together these data suggest DHT and P may indirectly alter GnRH neuron HVA-mediated calcium currents through these neuromodulators.
In conclusion, the present study shows a novel mechanism by which androgens and P feed back to modulate the function of GnRH neurons. The androgen DHT and P can change GnRH neuron intrinsic properties in addition to synaptic transmission to these cells to regulate GnRH neuron activity. Determining the underlying neural circuits engaged by DHT and P to modulate GnRH neuron VGCCs will be important to understand the central neural control of reproduction.
Acknowledgments
We thank Debra Fisher for excellent technical assistance and Justyna Pielecka-Fortuna, Jessica Kennett, Katarzyna Glanowska, and Pei-San Tsai for useful editorial comments.
Footnotes
This work was supported by NIH U54HD 28934.
Portions of this work were presented in abstract form at the 2009 Society for Neuroscience Meeting.
Disclosure Summary: J.S. and S.M.M. have nothing to disclose.
First Published Online August 25, 2010
Abbreviations: ACSF, Artificial cerebrospinal fluid; DHT, dihydrotestosterone; HVA, high-voltage activated; ICa, calcium current; I-V, current-voltage; LVA, low-voltage activated; OVX, ovariectomized; OVXD, OVX with DHT; OVXE, OVX with estradiol; P, progesterone; PCOS, polycystic ovary syndrome; SST, sustained current; VGCC; voltage-gated calcium channels.
References
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- Marshall JC, Dalkin AC, Haisenleder DJ, Griffin ML, Kelch RP 1993 GnRH pulses: the regulators of human reproduction. Trans Am Clin Climatol Assoc 104:31–46 [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E 1981 Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- Marshall JC, Griffin ML 1993 The role of changing pulse frequency in the regulation of ovulation. Hum Reprod 8:57–61 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Bittman EL, Foster DL, Karsch FJ 1981 The endocrine basis of the synergistic suppression of luteinizing hormone by estradiol and progesterone. Endocrinology 109:1414–1417 [DOI] [PubMed] [Google Scholar]
- Leipheimer RE, Bona-Gallo A, Gallo RV 1984 The influence of progesterone and estradiol on the acute changes in pulsatile luteinizing hormone release induced by ovariectomy on diestrus day 1 in the rat. Endocrinology 114:1605–1612 [DOI] [PubMed] [Google Scholar]
- Nippoldt TB, Reame NE, Kelch RP, Marshall JC 1989 The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab 69:67–76 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J 1996 Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the folliucular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137:5463–5467 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Hall JE, Boepple PA, Crowley Jr WF 1998 Differential Control of Gonadotropin Secretion in the Human: Endocrine Role of Inhibin. J Clin Endocrinol Metab 83:1835–1841 [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Hayes FJ, Crowley Jr WF 1998 Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest 21:602–611. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Eagleson CA 1999 Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am 28:295–324 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Goy RW 1997 Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinzining hormone levels in adulthood. Fertil Steril 67:155–163 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE 2003 Prenatal reprogramming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 144:1426–1434 [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM 2006 Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM 2006 Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 74:931–937 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite GnRH neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M 2008 Activation of A-type γ-amino butyric acid receptors excites gonadotrophin-releasing hormone neurons isolated from adult rats. J Neuroendocrinol 20:566–575 [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW 2007 Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol 19:474–482 [DOI] [PubMed] [Google Scholar]
- Batra S 1987 Increase by oestrogen of calcium entry and calcium channel density in uterine smooth muscle. Br J Pharmacol 92:389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E 2003 Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83:117–161 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J 2005 International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425 [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma 2003 Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 144:5118–5125 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2003 Calcium current subtypes in GnRH neurons. Biol Reprod 69:1914–1922 [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM 2010 Diurnal in vivo and rapid in vitroo effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 30:3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK 2009 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendt JM, Toro L, Stefani E, Erulkar SD 1992 Progesterone increases Ca2+ currents in myometrial cells from immature and nonpregnant adult rats. Am J Physiol 262:C293–C301 [DOI] [PubMed] [Google Scholar]
- Zhang M, Benishin CG, Pang PK 2002 Rapid inhibition of the contraction of rat tail artery by progesterone is mediated by inhibition of calcium currents. J Pharm Pharmacol 54:1667–1674 [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Dominguez LJ, Licata G, Shan J, Bing L, Karpinski E, Pang PK, Resnick LM 2001 Vascular effects of progesterone: role of cellular calcium regulation. Hypertension 37:142–147 [DOI] [PubMed] [Google Scholar]
- Nudler SI, Pagani MR, Urbano FJ, McEnery MW, Uchitel OD 2005 Testosterone modulates Ca(v2.2) calcium channels’ functional expression at rat levator ani neuromuscular junction. Neuroscience 134:817–826 [DOI] [PubMed] [Google Scholar]
- Er F, Gassanov N, Brandt MC, Madershahian N, Hoppe UC 2009 Impact of dihydrotestosterone on L-type calcium channels in human ventricular cardiomyocytes. Endocr Res 34:59–67 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2005 GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 72:33–41 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Rugh R 1968 The mouse: its reproduction and development. Minneapolis: Burgess Publishing [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH 1994 JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–116 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K 2001 The mouse brain in stereotaxic coordinates. 2nd ed. New York: Academic Press [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP 1999 Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology 140:827–834 [DOI] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Dahl GE, Evans NP, Thrun LA, Moenter SM, Padmanabhan V, Karsch FJ 1992 Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology 131:208–212 [DOI] [PubMed] [Google Scholar]
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 [DOI] [PubMed] [Google Scholar]
- Pohl CR, Richardson DW, Marshall G, Knobil E 1982 Mode of action of progesterone in the blockade of gonadotropin surges in the rhesus monkey. Endocrinology 110:1454–1455 [DOI] [PubMed] [Google Scholar]
- Hall JE 1993 Polycystic ovarian disease as a neuroendocrine disorder of the female reproductive axis. Endocrinol Metab Clin North Am 22:75–92 [PubMed] [Google Scholar]
- Ono K, Iijima T 2010 Cardiac T-type Ca2+ channels in the heart. J Mol Cell Cardiol 48:65–70 [DOI] [PubMed] [Google Scholar]
- Huguenard JR 1996 Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 58:329–348 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Ishii H, Yin C, Koyama M, Sakuma Y, Kato M 2010 Voltage-gated Ca2+ channel mRNAs and T-type Ca2+ currents in rat gonadotropin-releasing hormone neurons. J Physiol Sci 60:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Skinner DC, Robinson JE, King IS 1996 Androgen receptor-immunoreative cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology 63:120–131 [DOI] [PubMed] [Google Scholar]
- Huang X, Harlan RE 1993 Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res 624:309–311 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Allingham R 2001 Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- Leranth C, MacLusky NJ, Brown TJ, Chen EC, Redmond Jr DE, Naftolin F 1992 Transmitter content and afferent connections of estrogen-sensitive progestin receptor-containing neurons in the primate hypothalamus. Neuroendocrinology 55:667–682 [DOI] [PubMed] [Google Scholar]
- King JC, Tai DW, Hanna IK, Pfeiffer A, Haas P, Ronsheim PM, Mitchell SC, Turcotte JC, Blaustein JD 1995 A subgroup of LHRH neurons in guinea pigs with progestin receptors is centrally positioned within the total population of LHRH neurons. Neuroendocrinology 61:265–275 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL 2000 Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- Greives TJ, Humber SA, Goldstein AN, Scotti MA, Demas GE, Kriegsfeld LJ 2008 Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters Phodopus sungorus. J Neuroendocrinol 20:1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Urban JH, Das I, Levine JE 1996 Steroid modulation of neuropeptide Y-induced luteinizing hormone releasing hormone release from median eminence fragments from male rats. Neuroendocrinology 63:112–119 [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Gaytan F, García-Galiano D, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M 2008 Follicle-stimulating hormone responses to kisspeptin in the female rat at the preovulatory period: modulation by estrogen and progesterone receptors. Endocrinology 149:5783–5790 [DOI] [PubMed] [Google Scholar]
- Thind KK, Goldsmith PC 1997 Expression of estrogen and progesterone receptors in glutamate and GABA neurons of the pubertal female monkey hypothalamus. Neuroendocrinology 65:314–324 [DOI] [PubMed] [Google Scholar]
- Dufourny L, Caraty A, Clarke IJ, Robinson JE, Skinner DC 2005 Progesterone-receptive dopaminergic and neuropeptide Y neurons project from the arcuate nucleus to gonadotropin-releasing hormone-rich regions of the ovine preoptic area. Neuroendocrinology 82:21–31 [DOI] [PubMed] [Google Scholar]
- Soldo BL, Moises HC 1998 μ-Opioid receptor activation inhibits N- and P-type Ca2+ channel currents in magnocellular neurones of the rat supraoptic nucleus. J Physiol 513:787–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Miranda S, Dayanithi G, Custer E, Treistman SN, Lemos JR 2005 μ-Opioid receptor preferentially inhibits oxytocin release from neurohypophysial terminals by blocking R-type Ca2+ channels. J Neuroendocrinol 17:583–590 [DOI] [PubMed] [Google Scholar]
- Wang SJ 2005 Activation of neuropeptide Y Y1 receptors inhibits glutamate release through reduction of voltage-dependent Ca2+ entry in the rat cerebral cortex nerve terminals: suppression of this inhibitory effect by the protein kinase C-dependent facilitatory pathway. Neuroscience 134:987–1000 [DOI] [PubMed] [Google Scholar]
- Silva AP, Carvalho AP, Carvalho CM, Malva JO 2003 Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology 44:282–292 [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA 2001 Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J Physiol 531:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama N, Shibuya I, Tanaka K, Kabashima N, Ueta Y, Yamashita H 1998 Inhibition of N- and P/Q-type calcium channels by postsynaptic GABAB receptor activation in rat supraoptic neurones. J Physiol 509:371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, van den Pol AN 1998 Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J Neurosci 18:1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL 2004 Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol 287:H2091–H2098 [DOI] [PubMed] [Google Scholar]
- Golden KL, Marsh JD, Jiang Y 2002 Castration reduces mRNA levels for calcium regulatory proteins in rat heart. Endocrine 19:339–344 [DOI] [PubMed] [Google Scholar]
- Wang HG, George MS, Kim J, Wang C, Pitt GS 2007 Ca2+/calmodulin regulates trafficking of CaV1.2 Ca2+ channels in cultured hippocampal neurons. J Neurosci 27:9086–9093 [DOI] [PMC free article] [PubMed] [Google Scholar]