Abstract
Cellular and molecular mechanisms underlying pulsatile GnRH release are not well understood. In the present study, we examined the developmental changes in intracellular calcium dynamics, peptide release, gene expression, and DNA methylation in cultured GnRH neurons derived from the nasal placode of rhesus monkeys. We found that GnRH neurons were functionally immature, exhibiting little fluctuation in intracellular calcium ([Ca2+]i) and sparse pulses of GnRH peptide release in the first 12 d in vitro (div). By 14–18 div, GnRH neurons exhibited periodic [Ca2+]i oscillations, synchronizing at approximately 60-min intervals and GnRH pulses occurred at approximately 60-min intervals. Interestingly, the total GnRH peptide release further increased after 18 div. Measurement of GnRH mRNA and gene CpG methylation status at 0, 14, and 20 div indicated that mRNA levels significantly (P < 0.05) increased between 14 and 20 div, just as maximal decapeptide release was observed. By bisulfite sequencing across a 5′ CpG island of the GnRH gene, we further found that methylation at eight of 14 CpG sites significantly (P < 0.05) decreased between 0 and 20 div. These data indicate that epigenetic differentiation occurs during GnRH neuronal development and suggest that increased GnRH gene expression and decreased CpG methylation status are molecular phenotypes of mature GnRH neurons. To our knowledge, this is the first report that developmental DNA demethylation occurs in postmitotic neurons toward a stable neuronal phenotype.
Primate GnRH neuronal maturation is marked by age-dependent development of spontaneous synchronizing [Ca2+]i oscillations, pulsatile peptide release, increased GnRH gene expression and demethylation of a 5’ CpG-rich region of the GnRH gene.
GnRH is a decapeptide released into the hypothalamic portal circulation, through which it stimulates the synthesis and release of LH and FSH from the anterior pituitary gland. The pulsatile release of GnRH is essential for sexual maturation and maintenance of the ovulatory cycle (1). Consequently, mechanisms governing pulsatile GnRH release are central to reproductive function. However, the investigation of cellular and molecular mechanisms regulating GnRH release is very challenging because the primate brain contains only approximately 2000 GnRH neurons that are widely scattered among other neurons and neuroglia in the preoptic area (POA) and medial basal hypothalamus (MBH) (2,3).
Importantly, the unique origin of GnRH neurons provides a means to investigate the developmental events leading to fully functional neurons. GnRH neurons originate in the nasal epithelium in the mouse (4,5), rhesus monkey (6,7), and human (8). In the rhesus monkey (gestational period 165–168 d), GnRH neurons are found in the nasal pit as early as embryonic day (E) 32 and commonly at E34-E36 (6,7). The GnRH neurons then migrate along the nasal septum and terminal nerve, enter the forebrain at E38 (7), and subsequently migrate into the MBH of the rhesus monkey by E47 (6). While neurons continue to migrate on this tract until the third trimester, the basic distribution pattern of GnRH neurons is already established at E55 (7,9).
Cultured GnRH neurons derived from the nasal pit develop characteristics of functionally mature neurons. Remarkably, the time frame of in vitro maturation of GnRH neuronal function [i.e. spontaneous and synchronizing intracellular calcium ([Ca2+]i) oscillations and pulsatile GnRH release] is similar to the 2- to 3-wk in vivo migratory period outlined above. We previously observed that only few spontaneous [Ca2+]i oscillations occurred in primate GnRH neurons during the first 8–9 d in vitro (div), but by 14 div, [Ca2+]i oscillations appeared in most GnRH neurons (10). Likewise, perifusion experiments with cultured embryonic neurons indicate that GnRH was barely detectable until at least 14 div (11). Recently similar observations were reported in mouse GnRH neurons derived from the nasal placode, i.e. pulsatile GnRH release was detectable at 3 div and the amplitude of GnRH pulses and the secretory rate gradually increased with culture days, reaching the maximum at 14 div (12). These authors also found that synchronization of [Ca2+]i oscillations among GnRH neurons were sparse when cells were young in culture, but they became more frequent when cells matured for 14 div. Although the maturation of intracellular calcium dynamics and pulsatile GnRH release has been the subject of intense investigation in these culture models, the molecular mechanisms of GnRH neuronal maturation remain unclear.
The control of GnRH gene expression during embryonic development appears to be intricately related to mature neuronal function. It has been reported that parallel increases in GnRH mRNA expression and peptide biosynthesis occur along with the development of GnRH peptide release patterns in the mouse (13,14). In GT1 cells, which carry the rat GnRH gene, oscillatory GnRH gene promoter activity and gene expression have been shown in association with pulsatile GnRH release (15). Moreover, a distal 5′ enhancer region of the rat gene, which has high sequence similarity to a distal 5′ portion of the human gene (16), appears to regulate GnRH gene expression (17,18,19).
A question arises as to whether maturational changes occur at a distal portion of the GnRH gene during neuronal maturation. The distal 5′ region of the rhesus monkey GnRH gene contains a 243-bp segment (−2126 to −1863 bases from the transcription start site) that has 60% GC content and a CpG dinucleotide observed to expected content ratio of 0.65. These characteristics define the region as a CpG island (CGI) (20). CGIs, when associated with gene promoters, are often related to the epigenetic regulation (DNA methylation) of gene expression. Therefore, we hypothesized that epigenetic differentiation of the GnRH gene occurs during neuronal maturation. In the present study, to determine the temporal relationship between functional maturation and GnRH gene expression, we first examined the maturational pattern of primate GnRH neurons monitoring in vitro culture day-related changes in intracellular calcium dynamics and GnRH peptide release. Subsequently we measured GnRH mRNA expression and the methylation status of a 5′ CGI associated with the rhesus monkey GnRH gene at three time points during neuronal maturation.
Materials and Methods
General
All animals used in this study were born and raised in the Wisconsin National Primate Research Center. They were housed in pairs (cages 172 × 86 × 86 cm) in rooms with 12 h light (0600–1800 h) and 12 h dark (1800–0600 h) and controlled temperature (22 C). The animals were fed a standard diet from Harlan Teklad (20% protein primate diet; Madison, WI) twice daily, supplemented with fresh fruit and snacks daily. Water was available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the National Institutes of Health and U.S. Department of Agriculture.
Breeding methods and pregnancy determination
For the time-mated pregnancy, daily sex-skin color changes and menstrual records of adult female monkeys were obtained. Between d 9 and d 17 of the menstrual cycle, blood samples were obtained for assessment of LH levels. A few days before maximum sex-skin color change, female rhesus monkeys were placed with a fertile male until the breakdown of sex-skin color. Pregnancy was determined by ultrasound examination, uterine palpation, and RIA measurement of monkey chorionic gonadotropin. The day after the LH surge was designated as d 0 of gestation (E0). All fetectomies were conducted on either E36 or E37.
Tissue preparations and culture conditions
Before dissection of fetuses, the crown-rump length was measured, the developmental characteristics were observed under stereomicroscope, and the developmental stage was determined as described previously (7). Nasal placode tissues were dissected (a total of 15 fetuses were used in these studies) out into chilled sterile imidazole-buffered L15 medium (Invitrogen, Carlsbad, CA) using a stereomicroscope, under a plexiglas enclosure, with a very fine watchmakers’ forceps, a scalpel, and a fine iris scissors. Tissues were then cut into very small pieces (<0.5 mm3), which were plated onto 2-mm-diameter round glass coverslips (for Ca2+ imaging, gene expression and methylation analysis studies; Bellco Glass, Vineland, NJ) or plastic Thermanox coverslips (for perifusion; Nalgene Nunc International, Rochester, NY). Before cell plating, all coverslips were coated with a layer of dried rat-tail collagen and sterilized under a UV light. Cultures on coverslips were maintained in 35-mm tissue culture dishes (Dow Corning, Corning, NY) containing Medium 199 (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 0.6% glucose, 50 mg/ml gentamicin, and l-glutamine, and incubated at 37 C with 1.5% CO2 and 98.5% air. Serum was stripped with dextran-coated charcoal to remove steroids and other small molecules. On the third day of culture, we exposed cells to the antimitotic agent 5-fluoro-5-deoxyuridine (40 μm) for 72 h to eliminate mitotic cell proliferation. Medium was replaced every 3–4 d at the beginning of cultures and every 1–2 d after the cultures were established. Cultures were maintained up to 4 wk.
Ca2+ imaging
[Ca2+]i levels were assessed as previously described (21,22). Cells cultured on grid-etched round glass coverslips were exposed to the Ca2+ indicator fura-2 (Texas Fluorescence Labs Inc., Austin, TX). The coverslips with cells were mounted in a Dvorak-Stotler chamber. Fluorescence imaging of the dye-loaded cultured cells was achieved with a Nikon inverted microscope and superfluor objective lens (Nikon, Melville, NY). Cultured cells were continuously perifused with culture medium under 95% O2 and 5% CO2 at a rate of 50 μl/min. All experiments were conducted at room temperature (22–23 C). Under the microscope, GnRH neuron-like cells were chosen. GnRH cells are identifiable by their size, morphology, and unique appearance, forming neuronal bundles. Approximately 50–100 cells, including GnRH neurons, GnRH progenitor cells in the placode, nonneuronal cells, and in rare cases non-GnRH neurons were seen on each coverslip. Using a xenon lamp and ultrahigh speed wavelength changer (DG-4; Sutter Instruments, Navato, CA), cells were excited successively with UV light of 340 and 380 nm wavelengths, and an emission light of 510 nm was captured every 10 sec by a charge-coupled device camera (Photometrics, Tucson, AZ) attached to the microscope. The ratio of the emission from 340 nm excitation to 380 nm excitation, which is proportional to [Ca2+]i concentrations, was calculated by MetaFluor imaging software (Molecular Devices Corp., Downingtown, PA). After the experiment, the recorded region was photographed, and the photoengraved grid location was determined for later histological analysis. Subsequently cells were fixed with 2% paraformaldehyde (pH 7.6) and immunostained for GnRH and neuron-specific enolase as described below. In this way we could confirm that the Ca2+ recordings were from GnRH neurons.
Perifusion (GnRH release) experiments
Methods have been previously described in detail (11,22,23). Cells were analyzed at specified time points after the initiation of culture after embryonic tissue dissection. Cultures containing GnRH cells were mounted in Sykes-Moore chambers as described by Martinez de la Escalera et al. (24), i.e. two coverslips were placed, face to face, separated by a rubber O-ring, forming a chamber with a volume of 200 μl. Cells were then perifused with artificial cerebrospinal fluid containing 0.1% glucose (pH 7.4) under 95% O2 and 5% CO2 at 37 C. Perifusates were collected at 25 μl/min in 10-min fractions for 6 h using the ACUSYST (Endotronics, Minneapolis, MN) perifusion system. To test the viability of cells, 56 mm K+ was perifused for 20 min during the last hour (h 6) of the experiment for most cases including all early cultures when spontaneous GnRH pulsing did not occur. Samples were stored at −80 C until they were assayed for GnRH. After the perifusion experiment, cells were immunostained for GnRH as described below.
Immunostaining
All cultures were immunostained after [Ca2+]i imaging or perifusion experiments, as described previously (22). Briefly, cultured cells were gently rinsed with PBS and fixed with 2% paraformaldehyde in PBS for 30 min at room temperature. They were rinsed thoroughly with PBS and then placed in 10% normal goat serum in PBS for 2 h at room temperature. Cells were exposed to primary antibody for 48 h at 4 C. For GnRH staining, a cocktail of the polyclonal antiserum LR-1, which primarily recognizes mammalian GnRH (a gift of Dr. R. Benoit, University of Montréal, Montréal, Quebec, Canada), and GF6, which recognizes several forms of GnRH (a gift of Dr. N. Sherwood, University of British Victoria, Victoria, British Columbia, Canada), was used at a dilution of 1:20,000 and 1:9,000, respectively. Cultured cells were again rinsed thoroughly with PBS and were incubated with biotinylated antirabbit IgG for 1.5 h at room temperature. After rinsing in PBS, avidin-biotinylated peroxidase complex (Vector Laboratories, Burlingame, CA) in PBS was applied for 1.5 h at room temperature, followed by rinses in Tris-buffered saline. For visualization, 3,3′-diaminobenzidine and H2O2, in Tris-buffer, were applied. As a positive control, GnRH neurons in the hypothalamus of rhesus monkeys, at various ages, were also stained. To stain neuron-specific protein, cells were further exposed to antibody against neuron-specific enolase (INCSTAR Corp., Stillwater, MN) at a dilution of 1:1000. Double staining was accomplished using a similar procedure, except for a different chromagen (Vector SG complex; Vector Laboratories).
GnRH RIA
GnRH concentrations from perifusion experiments were measured in 200-μl samples with RIA using antiserum R-1245 (a gift from Dr. T. Nett, Colorado State University, Fort Collins, CO) as described previously (25). Synthetic GnRH (Richelieu Laboratory, Inc., Montréal, Canada) was used for both the trace and the reference standard. The sensitivity of the assay, at 95% binding, was 0.05 pg/tube. Intra- and interassay coefficients of variation were 8.3 and 11.4%, respectively.
Nucleic acid and protein isolation
Total RNA and genomic DNA were isolated from cultured cells using the AllPrep RNA/DNA/protein minikit (catalog no. 80004; QIAGEN, Valencia, CA). These samples were stored at −80 C and were used for the analysis of DNA methylation and mRNA expression.
Quantitative real-time PCR
Measurements were made using techniques that we have recently published (26). Briefly, concentrations of total RNA isolated from cultured cells was determined using a BioMate 3 spectrophotometer (Thermo Spectronic, Lanham, MD), and cDNA was generated with the StrataScript first-strand synthesis system kit (Stratagene, Cedar Creek, TX). Samples were stored at −80 C until used in quantitative real-time PCR. All reactions were multiplexed with GnRH and the housekeeping gene 18s amplified together. Amplifications were done with platinum quantitative real-time PCR SuperMix-UDG (Invitrogen) and ROX as a reference dye. 18s primers were JOE (6-carboxy-40, 50-dichloro-20, 70-dimethyoxyfluorescein) labeled, whereas GnRH primers were FAM (6-carboxy-fluorescein) labeled. JOE, FAM, and ROX fluorescence was simultaneously measured in each well with optimal absorbance between 535 and 555, 492 and 516, and 584 nm, respectively. Primer and sample concentrations were optimized to ensure linearity of amplification near 15 cycles for 18s and 25 cycles for GnRH. Primer sequences were as follows. GnRH: forward, CGGCTGGAGGAAAGAGAGATGCCG; reverse, GCCAGTTGACCAACCTCTTTGA. 18s primers were supplied by Invitrogen and sequences are proprietary, although they are known to amplify in the 3′ region of the transcript (catalog no. 115HM-02; Invitrogen).
Sodium bisulfite sequencing (DNA methylation analysis)
Genomic DNA isolated from snap-frozen sections was sodium bisulfite treated with the Imprint DNA modification kit (Sigma Aldrich, St. Louis, MO, catalog no. MOD50-1KT). The GnRH 5′ region (accession NW_001122890) was amplified using the following primers: forward, 5′-GAGTTTTGTTTTGTTATTTAGG-3′, and reverse, 5′-CAATAACTCAAACCTATAATCC-3′. This generated a 264-bp product that was then gel purified and ligated into the pCR 4-TOPO vector, which is part of the TOPO TA cloning kit (catalog no. 45-0030; Invitrogen). Ligated vectors were transfected into GC10 competent cells (catalog no. G2794; Sigma Aldrich) by heat shock, and cells were then grown in superoptimal catabolite. Cells were then spread on agar plates containing 100 μg/ml ampicillin and allowed to grow overnight at 37 C. The next day the individual, well-isolated colonies were picked from the plate and allowed to grow further by shaking in 3 ml of Luria-Bertani broth containing 100 μg/ml ampicillin at 37 C for 8 h. Cells were harvested by centrifugation, and cloned vectors were isolated using the Purelink quick plasmid miniprep kit (catalog no. K2100-11; Invitrogen). Vector inserts were sequenced with the supplied reverse sequencing primer in the vector kit. The Big Dye sequencing reactions were cleaned and sequenced at the University of Wisconsin Biotechnology Center Sequencing facility using an 3730xl automated DNA sequencing instrument (Applied Biosystems, Carlsbad, CA).
Data and statistical analyses
Fetuses
A total of 15 fetuses were used. Some of the fetuses contributed to all four experiments (calcium dynamics, peptide release, mRNA expression, and CpG methylation analysis). Each fetus provided cultures for each age group (≤12, 14–18, and ≥ 20 div) studied.
[Ca2+]i imaging experiments
All Ca2+ dynamics data were analyzed with the PULSAR algorithm (27) as described previously (22). Intervals between [Ca2+]i oscillations [interpulse interval (IPI)], pulse duration (ascending phase plus descending phase), and pulse amplitude (difference between the baseline and the peak) for each cell were obtained from PULSAR analysis. Group mean (±sem) from all cells was calculated from individual data. Synchronization of [Ca2+]i oscillations were defined as previously described (22). The mean (±sem) interval between the synchronizations was calculated among the synchronized cases. Six cultures for each age group from a total of nine fetuses were studied.
GnRH peptide release/perifusion experiments
GnRH pulse characteristics (pulse number, pulse amplitude, and pulse duration) were determined using the PULSAR algorithm from samples during h 1–5 due to instability of cultures during the first hour of perifusion experiments. The parameters used to characterize the GnRH pulses were similar to those described previously (25). GnRH peaks at less than 0.4 pg/ml, revealed by PULSAR algorithm, were eliminated because they were not reliable due to the proximity to the assay sensitivity of 0.25 pg/ml. The mean release per 4 h was calculated from sum of all values during the 4-h sampling period in each culture. IPI was calculated from the pulse number during that 4-h period. Four to seven cultures for each age group from a total of three fetuses were studied.
Quantitative PCR
Data were compared using the relative expression change in cycle threshold value (ΔCT) method (28) [n = 4 for each developmental time point (0, 14, and 20 div)]. Each of the four samples was a pool of two dishes from a total of three different fetuses.
CpG methylation
Big Dye sequencing reaction data were analyzed using PE Biosystems’ version 3.7 of sequencing analysis. Twenty clones containing the expected insert were sequenced for each time point. CpG methylation was averaged across the clones for each of the 14 CpG sites. For each time point, four culture dishes were pooled from two fetuses.
All group differences were compared using Student’s t tests. Differences between groups were considered to be significant when P ≤ 0.05.
Results
Developmental changes in [Ca2+]i oscillations
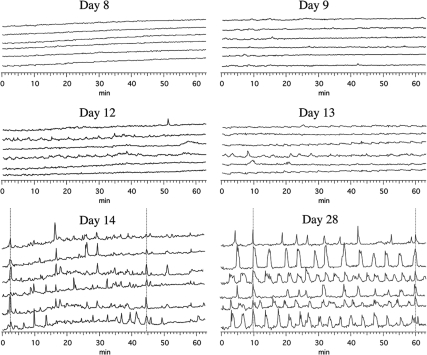
We assessed changes in [Ca2+]i in GnRH neurons using the Ca2+ dye fura 2 starting at 8 div though 28 div (Table 1). In young cultures such as 8–9 div, [Ca2+]i levels were mostly flat (Fig. 1). In young cultures through 12 div, there were infrequent, low-amplitude (2.53 ± 0.48 ΔF/F0; mean ± sem) [Ca2+]i oscillations. In contrast, by 14 div, [Ca2+]i oscillations were more regular with a higher amplitude (Fig. 1). In fact, the amplitude of [Ca2+]i oscillations increased significantly to 7.18 ± 0.93 ΔF/F0 (P < 0.001, Table 1). Average IPIs were long with large se (45.3 ± 21.4 min) in young cultures (Table 1) but developed into shorter and more regular IPIs in 14- to 18-div cultures (5.7 ± 1.0 min), although mean values were not statistically significant. These dynamics remained stable through 28 div (6.0 ± 1.3 min, Table 1).
Table 1.
Developmental changes in [Ca2+]i oscillations
| Div | Number of cultures, n (cells, n) | Pulse amplitude (ΔF/F0) | Pulse interval (min) | Pulse duration (min) | Synchronization interval (min) |
|---|---|---|---|---|---|
| ≤12 | 6 (184) | 2.53 ± 0.48 | 45.3 ± 21.4 | 1.4 ± 0.4 | 111.7 ± 22.5 |
| 14–18 | 6 (248) | 7.18 ± 0.93a | 5.7 ± 1.0 | 1.3 ± 0.2 | 61.1 ± 7.0b |
| ≥20 | 6 (256) | 5.99 ± 0.75c | 6.0 ± 1.3 | 1.1 ± 0.2 | 55.2 ± 4.3b |
P < 0.001 vs. 12 div or less.
P < 0.05 vs. 12 div or less.
P < 0.01 vs. 12 div or less.
Figure 1.
Developmental pattern of [Ca2+]i dynamics in monkey GnRH neurons. Representative [Ca2+]i traces from individual GnRH neurons at 8, 9, 12, 13, 14, and 28 div are shown. Note that there is little fluctuation of [Ca2+]i levels in young cultures (8 and 9 div, top panels). At 12–13 div, small fluctuations of [Ca2+]i start to appear (middle panels). After 14 div, most of GnRH neurons exhibit prominent oscillatory patterns of [Ca2+]i. Periodic synchronizations of [Ca2+]i oscillations are seen in older cultures. Synchronization of [Ca2+]i oscillations in the majority GnRH neurons are indicated by vertical lines.
Synchronization of [Ca2+]i oscillations was not often observed through 12 div. As a consequence, average pulse synchronization intervals decreased significantly between 12 div or less and 14–18 div (≤12 div: 111.7 ± 22.5 min vs. 14–18 div: 61.1 ± 7.0 min; P < 0.05, Table 1). Once synchronization of [Ca2+]i oscillations started to appear by 14–18 div, they remained similar, i.e. the [Ca2+]i synchronization interval about 60 min in 14- to 18-div cultures (Fig. 1) was similar to that in cultures of 20 div or greater.
Developmental changes in GnRH peptide release
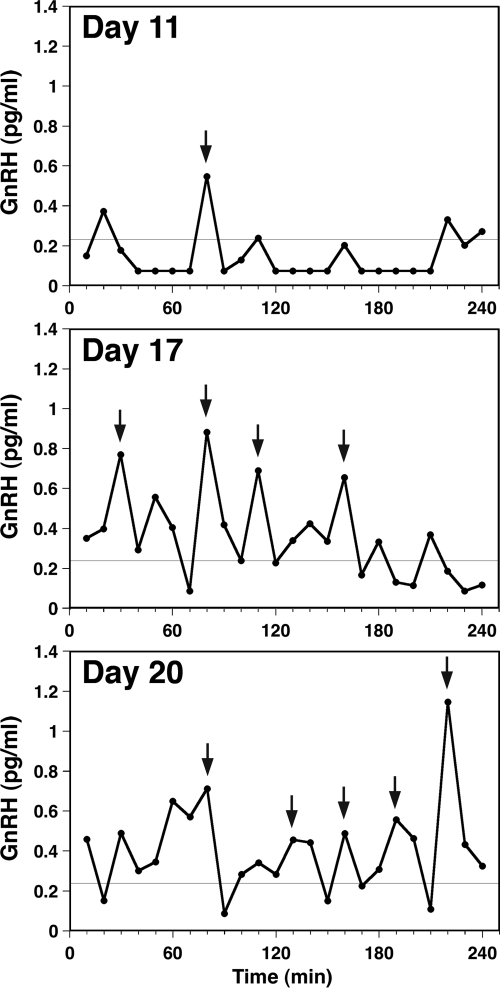
Using a perifusion system, we examined the developmental pattern of pulsatile GnRH release, starting at 8 div though 28 div (Table 2). As shown in an example of a culture at 11 div, GnRH pulses were rarely seen in young cultures (Fig. 2). However, pulse patterns gradually developed during the first 2–3 wk in vitro (Fig. 2). Peptide release calculated from total release during the 4-h sampling period was low (3.62 ± 0.39 pg/ml per 4 h) in 12 div or less (Table 2). By 14–18 div, total peptide release increased significantly (7.98 ± 0.95 pg/ml per 4 h; P < 0.01) as did pulse duration (≤12 div: 13.3 ± 2.9 min vs. 14–18 div: 21.7 ± 1.9 min; P < 0.05) and number of pulses over 4 h (≤12 div: 1.0 ± 0.4 vs. 14–18 div: 3.6 ± 0.6; P < 0.01). Although pulse amplitude, duration, and number did not change significantly beyond 2 wk in vitro, total release over 4 h continued to rise (≥20 div: 12.76 ± 1.54 pg/ml per 4 h) after 14–18 div (7.98 ± 0.95; P < 0.05). This continued increase in total release is intriguing, considering the time frame for increased GnRH mRNA levels, which is described below.
Table 2.
Developmental changes in GnRH pulsatility
| Div | Cultures, n | Release/4 h (pg/ml) | Pulse amplitude (pg/ml) | Pulse durations (min) | Pulse no./4 h | IPI (min)a |
|---|---|---|---|---|---|---|
| ≤12 | 4 | 3.62 ± 0.39 | 0.38 ± 0.04 | 13.3 ± 2.9 | 1.0 ± 0.4 | 210.0 ± 30.0 |
| 14–18 | 7 | 7.98 ± 0.95b | 0.54 ± 0.12 | 21.7 ± 1.9c | 3.6 ± 0.6b | 66.7 ± 10.3b |
| ≥20 | 6 | 12.76 ± 1.54b,d | 0.85 ± 0.25 | 27.2 ± 2.7b | 5.2 ± 0.5b | 49.7 ± 6.5b |
Calculated from pulse number per 4 h.
P < 0.01 vs. 12 div or less.
P < 0.05 vs. 12 div or less.
P < 0.05 vs. 14–18 div.
Figure 2.
Developmental pattern of GnRH peptide release in monkey GnRH neurons. Representative cases of in vitro GnRH release from cultures at 11, 17, and 20 div, obtained using a perifusion system, are shown. GnRH release was low with infrequent, low-amplitude pulses in young cultures (top panel). After 2 wk in culture (middle panel), pulse duration and frequency increased significantly, reaching maximal release levels by 20 div (bottom panel). GnRH peaks (indicated by arrows) were identified by the PULSAR algorithm. Fine lines at 0.25 pg/ml indicate the assay sensitivity.
Developmental changes in GnRH mRNA expression and GnRH gene methylation status
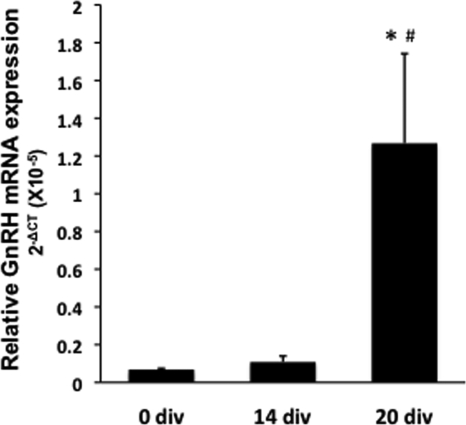
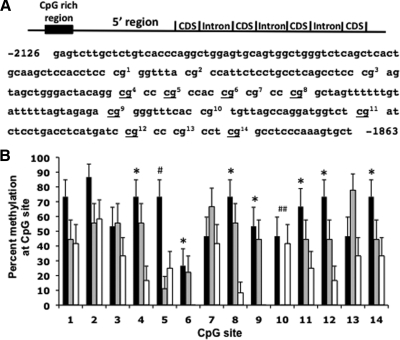
Total RNA and DNA from cells plated on the coverslips were extracted at 0, 14, and 20 div. Using quantitative RT-PCR, GnRH and 18s mRNA levels were measured from total RNA. Relative GnRH mRNA expression (mean ± sem) was calculated using the ΔCT method (28) (Fig. 3). We found that expression was low at the beginning of cultures (0 div: 6.84 × 10−7 ± 6.29 × 10−8) and remained low through 14 div (1.08 × 10−6 ± 3.18 × 10−7), but by 20 div, GnRH mRNA levels rose significantly (1.26 × 10−5 ± 4.75 × 10−6, P ≤ 0.05 vs. 0 and 14 div). Remarkably, CpG methylation status in a 5′ region of the GnRH gene reciprocally mirrored the mRNA expression pattern. Using bisulfite sequencing, we analyzed the CpG methylation status at 14 CpG sites. We found that methylation at eight of these sites significantly (P < 0.05) decreased during development, with the lowest CpG methylation status found at 20 div (Fig. 4).
Figure 3.
Changes in GnRH mRNA levels during GnRH neuronal development. Total RNA was extracted from cultures at 0, 14, and 20 div (n = 4 in all age groups). GnRH mRNA levels started to increase after 14 div, reaching the highest level at 20 div. *, P < 0.05 vs. 0 div; #, P = 0.05 vs. 14 div. GnRH mRNA levels are relative to 18s in each sample and analyzed using the ΔCT method (28).
Figure 4.
GnRH gene methylation during GnRH neuronal development. A, A schematic representation of the rhesus monkey GnRH gene depicting the location of the 5′ CGI and nucleotide sequence of this region. CpG sites are indicated with numbers corresponding to the CpG sites in the bar chart below. Underlined CpG sites exhibit higher methylation status at 0 compared with 20 div. B, GnRH neurons dissected from the nasal placode region of two rhesus monkey embryos at E36 and E37 were plated and then harvested on 0, 14, or 20 div. DNA extracted from pooled samples (four cultures) at each time point was bisulfite sequenced. Percent changes in methylation at each CpG site on 0 div (black bars), 14 div (gray bars), and 20 div (white bars) are shown. CpG methylation status was significantly higher at 0 div compared with 20 div at sites 4, 5, 6, 8, 9, 11, 12, and 14. *, P ≤ 0.01). CpG methylation status was significantly higher at 0 div compared with 14 div at site 5 (#, P < 0.05) and at 0 div compared with 14 div but not 20 div at site 10 (##, P < 0.05).
Discussion
The ontogeny of GnRH neurons in mammalian species is unique. They differentiate from progenitor cells in the embryonic nasal pit and migrate over a long distance to permanently reside in the POA-MBH during the first 2 wk after differentiation. The results of the current studies with cultured monkey GnRH neurons derived from the nasal placode indicate that characteristics of mature GnRH neurons develop gradually in vitro during the first 2 wk in culture. Furthermore, we found that patterns of spontaneous [Ca2+]i oscillations and pulsatile GnRH release developed along a tightly coordinated time frame during this period. Specifically, the amplitude of spontaneous [Ca2+]i oscillations and synchronization interval of [Ca2+]i oscillations reach mature levels by 14–18 div. Similarly, the duration and IPI of GnRH pulses also reached a mature state by 14–18 div. In fact, by 14–18 div, both the synchronization of spontaneous [Ca2+]i oscillations and GnRH pulse intervals were similar to the IPI of in vivo pulsatile GnRH release observed in adult monkeys (25). In contrast, GnRH mRNA expression remained low through 14 div, suggesting functional maturation of GnRH neurons in terms of ability to exhibit mature [Ca2+]i dynamics and capacity to release the decapeptide is independent of a significant increase in GnRH mRNA expression. However, mRNA did increase significantly between 14 and 20 div, when the maximum peptide release was observed. Remarkably, the DNA methylation status in a 5′ region of the GnRH gene reciprocally mirrored GnRH mRNA expression, In fact, decreasing DNA methylation status coincided with increasing GnRH mRNA levels during neuronal maturation.
Similar to our observations in these studies, using cultured mouse embryonic placode cells, Constantin et al. (12) reported that GnRH pulses became detectable at 3 div (cultures started E11.5), and the amplitude of GnRH pulses and the secretory rate gradually increased with culture days, reaching the maximum at 14 div (12). These authors also found that synchronization of [Ca2+]i oscillations among GnRH neurons were sparse when cells were young in culture but became more frequent and occurred in parallel with GnRH release in static cultures after 14 div. Additionally, at 1 wk in culture, the interval between spontaneous [Ca2+]i spikes in mouse embryonic GnRH neurons is approximately 13 min but decreases to approximately 7 min by 3 wk in culture (29). This shorter interval is similar to the approximately 8-min interval between spontaneous [Ca2+]i spikes reported for adult mouse pericam GnRH neurons (30). Although there are subtle differences in monkey and mouse studies, the results are consistent with a concept that GnRH neurons undergo functional maturation during the first 2 wk after differentiation from their progenitor cells with a close coincidence of GnRH release and oscillatory [Ca2+]i events.
The 2-wk period for functional maturation is not likely an artifact because of in vitro cultures. It has been consistently shown that in vitro maturation recapitulates in vivo neuronal maturation. For example, protein expression of metabotropic glutamate receptors in cultured rat hypothalamic neurons started at birth, increased with culture days in parallel to the postnatal (P) ages (31), and γ-aminobutyric acid neurotransmission was reversed from excitatory to inhibitory between 4 and 18 div in cultured cells, similar to those observed in slice preparations obtained from the hypothalami of P4 and P18 rats (32). In mouse GnRH neurons, Moore and Wray (14) reported that GnRH peptide contents gradually increase with a similar developmental time course in vivo and in vitro: GnRH contents in mouse nasal placode cultures (started at E11.5) significantly increase between 7 and 10 div, which parallels the in vivo increase between E14.5 and P1, and the GnRH peptide contents at 10 div are indistinguishable from peptide contents at a comparable time point in the mouse brain at P1.
The pulsatile release of GnRH is indispensable for normal reproductive function and is assumed to be due to the synchronous activity among widely scattered GnRH neurons in the POA/MBH. Although the concept that multiple GnRH neurons release the decapeptide synchronously in vivo is yet to be proven, findings of dendrodendritic bundling and shared synapses between two mouse GnRH neurons (33), and the presence of gap junctions in the median eminence (34) indicate interactions among GnRH neurons and/or their neuroterminals, which would enable synchronous activity. In vitro studies in cultured GnRH neurons derived from the embryonic nasal placode provide additional evidence for coordinated activity among GnRH neurons. For example, GnRH neurons exhibit synchronization of spontaneous [Ca2+]i oscillations with species-specific intervals, such as approximately 60 min in rhesus monkeys (10) and approximately 20 min in mice (29). Moreover, direct measurements from mature GnRH neurons in vitro indicate that GnRH is released with intervals at approximately 60 min in monkeys (11), approximately 20 min in mice (12), and approximately 40 min in sheep (35). Not surprising, the species-specific pulse intervals of GnRH release in vitro are consistent with IPI of in vivo GnRH/LH release in adult monkeys (25,36), mice (37), and sheep (38). Importantly, synchronization of [Ca2+]i oscillations was often seen as a calcium wave in monkey GnRH neurons (39), and synchronization of [Ca2+]i oscillations among 30–50% of GnRH neurons was associated with increases in GnRH peptide release in mouse neurons (12).
Although young (≤12 div) GnRH neurons released a small amount of GnRH and mature patterns of [Ca2+]i oscillations and GnRH release were observed by 14–18 div, the highest amount of GnRH release during the 4-h period did not occur until 20 div. Interestingly, this maximal peptide release was observed when GnRH mRNA levels significantly increased. These results indicate that low GnRH mRNA expression is sufficient for peptide release and that maximal peptide release from GnRH neurons requires increased gene expression. Similar observations were reported in mouse GnRH neurons, in which total peptide contents increased between 14 and 21 div, well after pulsatile GnRH release was first detected at 3 div (12). Taken together, the neurosecretory process does not require a high level of GnRH biosynthesis.
In the present study, we found a tight relationship between GnRH mRNA levels and CpG methylation status in a 5′ region of the GnRH gene. Perhaps it is reasonable to speculate that transcriptional efficiency, influenced by DNA methylation status, is responsible for differences in GnRH mRNA. It is, however, possible that methylation status is not a determinant of mRNA levels at this stage of neuronal development because GnRH biosynthesis requires several steps, including posttranscriptional processing of the primary GnRH gene transcript to a mature mRNA (40). Although, presently, no information is available for posttranscriptional control of GnRH mRNA levels in primates, especially during development, measurements of mRNA levels during mouse embryonic development (E16 to P0) indicate that mRNA levels are correlated with levels of transcription rather than differences in posttranscriptional processing (40). Given the close resemblances of GnRH neuronal developmental patterns in mouse and monkey discussed above, it is likely that posttranscriptional events are not primarily responsible for developmental changes in mRNA levels in the monkey. Rather, different levels of transcription likely control GnRH mRNA levels at this point of development.
Reviewing literature of studies conducted in GT1, GN, and NLT cells, it became clear that sequences within the 5′ region of the GnRH gene and trans-acting factors that associate with these sequences are well characterized for the rat but not humans. Specifically, the rat gene contains proximal and distal 5′ regions that are responsible for neuron specific (41,42,43) and pulsatile gene expression (44). The trans-acting factors that associate with these regions and appear to be responsible for episodic gene expression include octamer transcription factor-1 (15,18,45,46), GATA-4, and GATA-5 (19,47,48). Other factors including the homeobox protein Otx-2 (49), Msh homeobox 1 (50,51), and CCAAT/enhancer-binding protein-β (17) also interact with these regions, and corepressors from the Groucho-related-gene family (51,52) and GnRH enhancer region GATA-B site binding factor (48) are known to reduce gene expression through direct interactions with activators or competitive binding at cis sequences in the 5′ region of the GnRH gene. In contrast, few reports on human GnRH gene structure (53) and function are available. Investigations have identified 5′ flanking regions of the human gene that are necessary for migration into the brain (54), and other 5′ flanking regions are necessary for gene expression (16) or tissue-specific expression (55). Kepa et al. (16) also identified sequence similarities between rat and human genes in the 5′ region. Although it is currently not known whether methylation in the region we analyzed directly alters the activity of transcription factors listed above, it is clear that CpG methylation can indirectly inhibit trans-activating factor activity across a wide range of promoter sequences (at least 2 kb) through interaction with methyl-CpG-binding proteins (56). Because the region we analyzed contains the highest CpG density in the 5′ region of the rhesus GnRH gene and resides within 2 kb of all the known regulatory portions of the human GnRH gene, it is reasonable to speculate that CpG methylation in this region is associated with the epigenetic regulation of GnRH gene expression.
The apparent GnRH gene demethylation during neuronal development is quite remarkable, even though a causative relationship between CpG methylation status and mRNA expression is yet to be clarified. Until recently CpG demethylation was generally considered a passive process related to loss of methylation during DNA replication. Consequently, DNA demethylation would not be expected in nondividing cells, such as neurons. Recent studies, however, found that CpG methylation status decreases in neurons during context dependent memory consolidation (57) and electroconvulsive treatment induced neurogenesis (58), suggesting a demethylase is present and responsive to activity or context in mature neurons. The results of this study also suggest active demethylation occurs in postmitotic neurons. To our knowledge, our studies are the first to report developmental changes in DNA demethylation in a postmitotic stable neuronal phenotype. Perhaps DNA demethylation is a mechanism common to maturing peptide hormone secreting cells. Quite recently demethylation of the Ins2 gene promoter during development was reported in association with increased insulin gene expression in murine pancreatic β-cells (59).
In summary, we have found that GnRH neuronal development is characterized by an intricate coordination of Ca2+ events, peptide release, mRNA expression, and gene demethylation. Future studies remain to investigate the causal relationship between the DNA demethylation and GnRH neuronal maturation and the functional role of GpG demethylation in mature GnRH neuronal activity. Nonetheless, the results from these studies suggest that GnRH neurons derived from the nasal placode will be an important model for investigating the mechanisms of epigenetic regulation in neuroendocrine neurons.
Footnotes
This work was supported by National Institutes of Health Grants HD15433 and HD11355 and was possible to perform by National Institutes of Health supports (Grants P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Summary: The authors have no conflicts to disclose.
First Published Online September 22, 2010
Abbreviations: [Ca2+]i, Intracellular calcium; CGI, CpG island; ΔCT, change in cycle threshold value; div, days in vitro; E, embryonic day; IPI, interpulse interval; MBH, medial basal hypothalamus; P, postnatal; POA, preoptic area.
References
- Knobil E, Hotchkiss J 1988 The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill J, eds. The physiology of reproduction. New York: Raven Press; 1971–1994 [Google Scholar]
- Goldsmith PC, Lamberts R, Brezina LR 1993 Gonadotropin-releasing hormone neurons and pathways in the primate hypothalamus and forebrain. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 7–45 [Google Scholar]
- Silverman AJ, Antunes JL, Abrams GM, Nilaver G, Thau R, Robinson JA, Ferin M, Krey LC 1982 The luteinizing hormone-releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol 211:309–317 [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H 1989 Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW 1989 Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Resko JA 1990 Ontogeny of gonadotropinreleasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology 126:498–511 [DOI] [PubMed] [Google Scholar]
- Quanbeck C, Sherwood NM, Millar RP, Terasawa E 1997 Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol 380:293–309 [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PM, Hardelin JP, Petit C 1996 Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol 366:547–557 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Busser BW, Luchansky LL, Sherwood NM, Jennes L, Millar RP, Glucksman MJ, Roberts JL 2001 Presence of luteinizing hormone-releasing hormone fragments in the rhesus monkey forebrain. J Comp Neurol 439:491–504 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky LL 1999 Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone (LHRH) cells derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Constantin S, Caraty A, Wray S, Duittoz AH 2009 Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology 150:3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JA, Wray S 1997 Luteinizing hormone-releasing hormone (LHRH) neurons maintained in hypothalamic slice explant cultures exhibit a rapid LHRH mRNA turnover rate. J Neurosci 17:9481–9491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Jr JP, Wray S 2000 Luteinizing hormone-releasing hormone biosynthesis and secretion in embryonic LHRH neurons. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- Vazquez-Martinez R, Shorte SL, Faught WJ, Leaumont DC, Frawley LS, Boockfor FR 2001 Pulsatile exocytosis is functionally associated with GnRH gene expression in immortalized GnRH-expressing cells. Endocrinology 142:5364–5370 [DOI] [PubMed] [Google Scholar]
- Kepa JK, Spaulding AJ, Jacobsen BM, Fang Z, Xiong X, Radovick S, Wierman ME 1996 Structure of the distal human gonadotropin releasing hormone (hGnrh) gene promoter and functional analysis in Gt1–7 neuronal cells. Nucleic Acids Res 24:3614–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham DD, Mellon PL 2000 Transcription factors Oct-1 and C/EBPβ (CCAAT/enhancer-binding protein-β) are involved in the glutamate/nitric oxide/cyclic-guanosine 5′-monophosphate-mediated repression of mediated repression of gonadotropin-releasing hormone gene expression. Mol Endocrinol 14:212–228 [DOI] [PubMed] [Google Scholar]
- Leclerc GM, Boockfor FR 2005 Identification of a novel OCT1 binding site that is necessary for the elaboration of pulses of rat GnRH promoter activity. Mol Cell Endocrinol 245:86–92 [DOI] [PubMed] [Google Scholar]
- Leclerc GM, Bose SK, Boockfor FR 2008 Specific GATA-binding elements in the GnRH promoter are required for gene expression pulse activity: role of GATA-4 and GATA-5 in this intermittent process. Neuroendocrinology 88:1–16 [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M, Frommer M 1987 CpG islands in vertebrate genomes. J Mol Biol 196:261–282 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone releasing hormone (LHRH) neurons derived from fetal olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E 2008 Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 149:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E 2009 Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de la Escalera G, Choi AL, Weiner RI 1992 Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci USA 89:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Terasawa E 1988 Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E 2009 Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ 2008 Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- Moore Jr JP, Shang E, Wray S 2002 In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Strumia MM, Herbison AE 2007 Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci 27:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Kogelman L, Ghosh P, Liljelund P, Blackstone C 1994 Developmental regulation of the hypothalamic metabotropic glutamate receptor mGluR1. J Neurosci 14:3816–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN 1995 GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci 15:5065–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han SK, Herbison AE 2009 Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA 106:10835–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Maekawa F, Tsukamura H, Hirunagi K, Maeda K 1999 Morphological characterization of relationship between gap junctions and gonadotropin releasing hormone nerve terminals in the rat median eminence. Neurosci Lett 261:105–108 [DOI] [PubMed] [Google Scholar]
- Duittoz AH, Batailler M 2000 Pulsatile LHRH secretion from primary culture of sheep (Ovis aries) olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- Terasawa E, Krook C, Hei DL, Gearing M, Schultz NJ, Davis GA 1988 Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology 123:1808–1816 [DOI] [PubMed] [Google Scholar]
- Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ 1988 Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hyg) male mice. Neuroendocrinology 48:45–52 [DOI] [PubMed] [Google Scholar]
- Levine JE, Pau KY, Ramirez VD, Jackson GL 1982 Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 [DOI] [PubMed] [Google Scholar]
- Richter TA, Keen KL, Terasawa E 2002 Synchronization of Ca2+ oscillations among primate LHRH neurons and non-neuronal cells in vitro. J Neurophysiol 88:1559–1567 [DOI] [PubMed] [Google Scholar]
- Gore AC, Roberts JL 1997 Regulation of gonadotropin-releasing hormone gene expression in vivo and in vitro. Front Neuroendocrinol 18:209–245 [DOI] [PubMed] [Google Scholar]
- Kepa JK, Wang C, Neeley CI, Raynolds MV, Gordon DF, Wood WM, Wierman ME 1992 Structure of the rat gonadotropin releasing hormone (rGnRH) gene promoter and the functional analysis in hypothalamic cells. Nucleic Acids Res 20:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte DB, Lawson MA, Belsham DD, Eraly SA, Bond CT, Adelman JP, Mellon PL 1995 A neuron-specific enhancer targets the expression of the gonadotropin-releasing hormone gene to hypothalamic neurosecretory neurons. Mol Endocrinol 9:467–477 [DOI] [PubMed] [Google Scholar]
- Nelson SB, Eraly SA, Mellon PL 1998 The GnRH promoter: target of transcription factors, hormones, and signaling pathways. Mol Cell Endocrinol 140:151–155 [DOI] [PubMed] [Google Scholar]
- Nuñez L, Faught WJ, Frawley LS 1998 Episodic gonadotropin-releasing hormone gene expression revealed by dynamic monitoring of luciferase reporter activity in single, living neurons. Proc Natl Acad Sci USA 95:9648–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ME, Mellon PL 1995 The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol 15:6169–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Nelson SB, Huang KM, Mellon PL 1998 Oct-1 binds promoter elements required for transcription of the GnRH gene. Mol Endocrinol 12:469–481 [DOI] [PubMed] [Google Scholar]
- Lawson MA, Whyte DB, Mellon PL 1996 GATA factors are essential for activity of the neuron-specific enhancer of the gonadotropin-releasing hormone gene. Mol Cell Biol 16:3596–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Buhain AR, Jovenal JC, Mellon PL 1998 Multiple factors interacting at the GATA sites of the gonadotropin-releasing hormone neuron-specific enhancer regulate gene expression. Mol Endocrinol 12:364–377 [DOI] [PubMed] [Google Scholar]
- Kelley CG, Lavorgna G, Clark ME, Boncinelli E, Mellon PL 2000 The Otx2 homeoprotein regulates expression from the gonadotropin-releasing hormone proximal promoter. Mol Endocrinol 14:1246–1256 [DOI] [PubMed] [Google Scholar]
- Givens ML, Rave-Harel N, Goonewardena VD, Kurotani R, Berdy SE, Swan CH, Rubenstein JL, Robert B, Mellon PL 2005 Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J Biol Chem 280:19156–19165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave-Harel N, Miller NL, Givens ML, Mellon PL 2005 The Groucho-related gene family regulates the gonadotropin-releasing hormone gene through interaction with the homeodomain proteins MSX1 and OCT1. J Biol Chem 280:30975–30983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder R, Mellon PL 2009 Otx2 induction of the gonadotropin-releasing hormone promoter is modulated by direct interactions with Grg co-repressors. J Biol Chem 284:16966–16978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovick S, Wondisford FE, Nakayama Y, Yamada M, Cutler Jr GB, Weintraub BD 1990 Isolation and characterization of the human gonadotropin-releasing hormone gene in the hypothalamus and placenta. Mol Endocrinol 4:476–480 [DOI] [PubMed] [Google Scholar]
- Wolfe AM, Wray S, Westphal H, Radovick S 1996 Cell-specific expression of the human gonadotropin-releasing hormone gene in transgenic animals. J Biol Chem 271:20018–20023 [DOI] [PubMed] [Google Scholar]
- Dong KW, Yu KL, Chen ZG, Chen YD, Roberts JL 1997 Characterization of multiple promoters directing tissue-specific expression of the human gonadotropin-releasing hormone gene. Endocrinology 138:2754–2762 [DOI] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A 1997 MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88:471–481 [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD 2007 Covalent modification of DNA regulates memory formation. Neuron 53:857–869 [DOI] [PubMed] [Google Scholar]
- Ma DK, Guo JU, Ming GL, Song H 2009 DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 8:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, Mullen Y, Pfeifer GP, Ferreri K 2009 Insulin gene expression is regulated by DNA methylation. PLoS One 4:e6953 [DOI] [PMC free article] [PubMed] [Google Scholar]