Abstract
INTRODUCTION:
Venous malformations are the most frequent vascular malformation. Deep venous malformations are located in subcutaneous tissue or in the muscles. Percutaneous sclerotherapy is the treatment of choice, and the use of ethanol at low doses has not yet been described.
OBJECTIVE:
To analyze the results of treating Deep venous malformations patients with low doses of ethanol.
METHODS:
Thirty‐nine patients treated between July 1995 and June 2007 were followed up prospectively over a median period of 18 months. Twenty‐nine were female (74.4%) and 10 were male (25.6%), with ages ranging from 11 to 59 years (median of 24 years). All of the lesions affected limbs, and the main symptom reported was pain (97.4%). Each patient underwent fortnightly alcohol application sessions under local anesthesia on an outpatient basis. The lesions were classified into three groups according to size using nuclear magnetic resonance imaging: small, up to 3 cm (4 patients); medium, between 3 and 15 cm (27 patients); and large, greater than 15 cm (8 patients).
RESULTS:
The symptoms completely disappeared in 14 patients (35.9%) and improved in 24 (61.5%). The lesion size reduced to zero in 6 patients (15.4%) and decreased in 32 (82%). The median number of sessions was 7. There were no complications in 32 patients (82%), while 3 presented local paresthesia (7.7%), 2 superficial trombophlebites (5.1%), 1 skin ulcer (2.6%), and 1 case of hyperpigmentation (2.6%).
CONCLUSION:
Outpatient treatment for Deep venous malformations patients using ethanol at low doses was effective, with a low complication rate.
Keywords: Venous malformations, Percutaneous, Sclerotherapy, Ethanol, Local anesthesia
INTRODUCTION
Venous malformations are the most common symptomatic vascular malformations.1 They consist of spongy tangles of veins of varying sizes, formed by venular capillaries, venules, and veins.2 They may be located in any portion of the body in a diffusely or delimited form.
The superficial vascular malformations are associated with skin color modifications, which may present a bluish color and lead to aesthetic manifestations and, frequently, to a social phobia.3,4 Deep venous malformations (DVMs) are located deeply in subcutaneous tissues or in the muscles and generally cause local pain.2,3
Surgical excision does not produce good results because of its functional and aesthetic sequelae and because of the high recurrence rate.5,6 Percutaneous injection of sclerosing agents into the lesion has become the mainstay of treatment.7,8,9 Ethanol is considered the most powerful and effective sclerosing agent,10 but its use at high doses may cause complications, thus making this a high‐risk treatment.11,12
The use of ethanol at low doses administered in consecutive sessions on an outpatient basis has not yet been described in the literature. This may produce good clinical results with lower risk for patients. The goal of this study was to analyze the results of treating patients with DVMs with low doses of ethanol under local anesthesia, with a median follow‐up of 18 months.
MATERIAL AND METHODS
Thirty‐nine patients with DVMs were treated on an outpatient basis by means of percutaneous sclerosis using low doses of ethanol under local anesthesia between July 1995 and June 2007 and were followed up prospectively. Twenty‐nine were female (74.4%) and 10 were male (25.6%), with ages ranging from 11 to 59 years (median of 24 years). The length of follow‐up ranged from six to 72 months, with a median of 18 months.
The patients' clinical characteristics are presented in Table 1:
Table 1.
Clinical characteristics.
| Lesion location | Lower limb | 28 (71,8 %) |
| Upper limb | 11 (28,2 %) | |
| Clinical manifestation | Pain | 38 (97,4 %) |
| Other symptoms | 1 (2,6 %) | |
| Previous treatment | None | 31 (79,5 %) |
| Surgery,biopsy or embolization | 8 (20,5 %) |
Most of the lesions affected the lower limbs. The main symptom reported was pain, and most of the patients had not undergone any type of previous treatment.
The lesions were classified according to their size. Using nuclear magnetic resonance imaging (MRI), we measured them along their longer axis and grouped them into 3 categories: small, for lesions measuring up to 3 cm (4 patients); medium, for lesions between 3 and 15 cm (27 patients); and large, for lesions greater than 15 cm (8 patients).
Each patient underwent fortnightly therapeutic sessions of ethanol application. The most appropriate puncture site was chosen by means of palpation of the lesion to locate the point with the greatest volume (after MRI analysis). The ethanol application technique consisted of percutaneous puncture of the lesion13 using a 21 scalpel or a 30×7 mm needle. This was directed perpendicularly to the skin until it reached the anomalous venous space, which was confirmed by the presence of blood reflux. Following this, the radiological contrast medium sodium meglumine ioxaglate (Hexabrix 320) was injected manually in a slow and gradual manner. Under fluoroscopic control, the opacification of the anomalous space was monitored, with quantification of the conditions and volume of drainage into the venous system. Approximately 2 ml of 2% lidocaine hydrochloride was then injected, without vasoconstrictor. Next, the sclerosing agent was injected and the volume was calculated to be equal to the contrast used during the angiographic evaluation by respecting the maximum dose of 1 ml/kg. Immediately before removing the needle, another small quantity of anesthetic was injected with the aim of flushing out any ethanol residue still in the needle. Finally, the needle was removed from the puncture site and a slightly compressive dressing was applied for 24 hours. The patient remained under observation for around 30 minutes and was then discharged.
The final evaluation of the venous lesions was based on clinical (milimetric measurement of the circumference at the site of largest volume of the lesion) and radiological (MRI) parameters, ascertained before and after treatment. The clinical evolution following alcohol application, the reduction in the size of the lesions, the number of alcohol application sessions, and the complications related to the procedure were evaluated.
STATISTICAL ANALYSIS
Frequency distribution was used to describe the categorical variables, and central trend and variability measurements were used for the numerical variables. To investigate the relationship between the results before and after the embolization treatment, the McNemar test was applied. The Mann‐Whitney U test was used to compare the number of sessions for variables with 2 categories, and the Kruskal‐Wallis test was used for variables with 3 categories. A significance level of 5% was used for all of the statistical tests.
RESULTS
After the treatment, the symptoms completely disappeared in 14 patients (35.9%) and improved in 24 (61.5%). They remained unchanged in only 1 patient (2.6%). The lesion size reduced to zero in 6 patients (15.4%) and decreased in 32 (82%). It remained unchanged in only 1 patient (2.6%). The evolution of DVM size compared with the initial size of the lesion is presented in Table 2:
Table 2.
Evolution of lesion size.
| Group | Decrease n (%) | Unchanged n (%) |
| Small | 4 (100,0) | 0 |
| Medium | 26 (96,3) | 1 (3,7) |
| Large | 8 (100,0) | 0 |
n = number of patients
The treatment was effective, independent of the lesion size.
The number of sessions held for treatment of DVMs is presented in Table 3:
Table 3.
Number of sessions (n).
| Group | Range (n) | Median (n) | Mean (n) | SD | p‐value |
| Small | 3 ‐ 6 | 5 | 4,8 | 1,5 | |
| Medium | 3 ‐ 34 | 7 | 11,3 | 9,4 | 0,1168 |
| Large | 4 ‐ 23 | 12 | 12,6 | 6,7 |
n = number of sessions SD = standard deviation
p ‐ value obtained from Kruskal‐Wallis test
The number of sessions ranged from 3 to 34, with a median of 7. The number was statistically the same independently the size of the DVM.
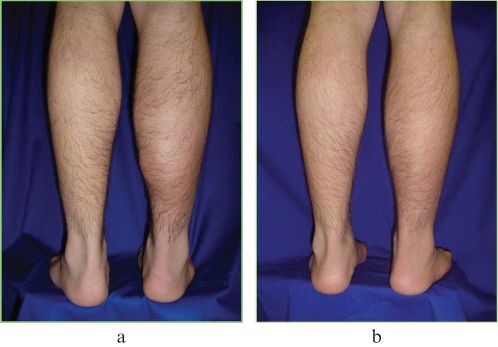
Figures 1a (before treatment) and 1b (after treatment) show a patient with a muscular lesion in his calf:
Figure 1.
Muscular lesion before treatment (a) and after treatment (b).‐ Muscular lesion before treatment (a) and after treatment (b).
There were no complications in 32 patients (82%), while 3 presented local paresthesia (7.7%), 2 superficial thrombophlebitis (5.1%), 1 skin ulcer (2.6%) and 1 case of hyperpigmentation (2.6%). All of the complications were treated conservatively, with good evolution.
DISCUSSION
DVMs are characterized by venous tumor formation with soft consistency that can be depressed by palpation, without any arterial tremor, murmur, or beat involving the subcutaneous cellular tissue.3 They are present from the time of birth and present slow growth as the individual develops. They do not undergo spontaneous involution, unlike hemangiomas, which present rapid growth followed by stabilization or spontaneous involution.14
They may be located in any region of the body, but most of them affect the limbs,3 as seen in the present sample. Pain is the main complaint, and it is directly related to the size of the lesion (because of compression of the neighboring tissue) as well as to inflammatory processes resulting from phlebitis.
In most cases, DVMs do not cause trophic skin lesions or cardiovascular manifestations. Occurrences of thrombophlebitis are common and usually caused by stagnant blood flow. If such conditions occur repeatedly, it leads to the formation of calcifications known as phleboliths, which can be seen on simple radiographs.2
The diagnosis of venous malformations is primarily clinical. If there is a suspicion that deep structures may be involved, especially in extensive lesions, complementary imaging examinations should be performed.15 The best imaging examination is magnetic resonance imaging.16 On T2‐weighted or inversion‐recovery sequences, DVMs consist of hyperintense channels or areas containing septation. Small fluid levels may be visible. Phleboliths may be evident as areas of signal void. Direct percutaneous cannulation of the malformation with contrast injection typically shows best the interconnecting spaces and draining veins.12
Ultrasonography may be used to distinguish between lesions of high and low flow rates and to assist in locating them. Recently, it has been used as a tool for guiding percutaneous punctures during sclerotherapy sessions.17,18 The percutaneous route for treating DVMs began to be used because it allows direct contact between the sclerosing agent and the vascular endothelium. Many different sclerosing agents have been used, including ehtibloc, sodium tetradecil sulphate, polydocanol, and ethanol.19
Sodium tetradecyl sulphate (sotradecol) and polidocanol present detergent action that causes endothelial damage by interfering with the lipids on the cell surface.20,21 However, the efficacy of these agents decreases in the presence of greater volume of blood that frequently occurs inside these anomalous cavities. In the same way as ethanol, these sclerosing agents cause endothelial lesions resulting in thrombosis and fibrosis, but there is a great tendency toward recanalization.10,22 Furthermore, the use of sotradecol to treat extensive localized venous anomalies in the cephalic segment causes significant complications such as anaphylactic shock and loss of vision.7,23
Ethibloc24 is a highly viscous sclerosing agent with a poorly understood mechanism of action. The efficacy of the agent is related to the presence of 60% ethanol in its composition.9 Ethanol deserves attention because of its potent fibrosing action, known and controllable side effects, low cost, and ease of availability.8,25,26 Direct contact between ethanol and the vascular endothelium promotes the denaturing of blood proteins, necrosis of the vessel wall, breakup of erythrocytes, and subsequent thrombosis, which then leads to fibrosis of the intima, with regression of the malformation.27
The ethanol dose used during intervention procedures is strongly correlated with the serum level of ethanol in these patients. Because of the toxic action of ethanol, occurrences of side effects are related directly to the quantity of the agent injected into the organism. Doses greater than 1.0 ml/kg may lead to respiratory depression, cardiac arrhythmia, rhabdomyolysis, and hypoglycemia.10,28
The great many studies have described the use of large volumes of ethanol under general anesthesia. However, many types of complications have been found, including the following: immediate thrombosis inside the lesion and blockage of the venous circulation, thereby leading to severe edema;29 superficial thrombophlebitis; deep vein thrombosis alone or complicated with pulmonary embolism;9,11 cardiac arrest;10 trophic skin scars or lesions;30 and even death secondary to cardiovascular collapse.31,32
All patients tolerated the consecutive procedures well, though there was a little local discomfort, in some cases, at the end of the sclerosing agent injection. The use of low‐dose ethanol minimizes the occurrence of side effects and local complications such as thrombophlebitis in the drainage veins. Moreover, with local anesthesia, the patients were able to return to their activities the day after the procedure was performed.
A disadvantage in this casuistic was the utilization of radioscopy during the procedure, although of short duration. More recently, we have been using ultrasonography33,34 during the procedure, which – besides not requiring ionizing radiation – allows precise localization of the puncture site and the quantification of the volume of the sclerosing agent injected.
The number of ethanol sessions was similar for all sizes of lesions, but the treatment was effective independent of the lesion size, given that partial or complete reduction of the lesions occurred in all of the groups. Percutaneous treatment for DVMs on an outpatient basis was effective since the symptoms disappeared or improved in 97.4% of the patients.
CONCLUSION
Outpatient treatment for DVM patients using ethanol at low doses was effective, with a low complication rate.
REFERENCES
- 1.Loose DA. Surgical management of venous malformations. Phlebology. 2007;22:276–82. doi: 10.1177/026835550702200608. 10.1258/026835507782655254 [DOI] [PubMed] [Google Scholar]
- 2.Paes E, Vollmar J. Diagnosis and surgical aspectes of congenital venous angiodysplasia in the extremities. Phlebology. 1995;10:160–64. [Google Scholar]
- 3.Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg. 1983;18:894–900. doi: 10.1016/s0022-3468(83)80043-8. 10.1016/S0022‐3468(83)80043‐8 [DOI] [PubMed] [Google Scholar]
- 4.Rivas S, López‐Gutiérrez JC, Díaz M, Andrés AM, Ros Z. Venous malformations. Diagnosis and treatment during the childhood. Cir Pediatr. 2006;19:77–80. [PubMed] [Google Scholar]
- 5.Szilagyi DE, Smith RF, Elliott JD, Hageerman JH. Congenital arteriovenous anomalies of the limbs. Arch Surg. 1976;111:423–9. doi: 10.1001/archsurg.1976.01360220119020. [DOI] [PubMed] [Google Scholar]
- 6.Laurian C. Malformations Vasculares Des Membres: Place de la chirurgie vasculaire et réparatrice. J Mal Vas. 1992;17:57–60. [PubMed] [Google Scholar]
- 7.de Lorimier AA. Sclerotherapy for venous malformations. J Pediatr Surg. 1995;30:188–93. doi: 10.1016/0022-3468(95)90558-8. 10.1016/0022‐3468(95)90558‐8 [DOI] [PubMed] [Google Scholar]
- 8.Shireman PK, Mccarthy WJ, Yao JST, Vogelzang RL. Treatment of venous malformation by direct injection with ethanol. J Vasc Surg. 1997;26:838–44. doi: 10.1016/s0741-5214(97)70098-3. 10.1016/S0741‐5214(97)70098‐3 [DOI] [PubMed] [Google Scholar]
- 9.Yakes WF, Luethke JM, Parker SH, Stavros AT, Rak KM, Hopper KD, et al. Ethanol embolization of vascular malformations. Radiographics. 1990;10:787–96. doi: 10.1148/radiographics.10.5.2217971. [DOI] [PubMed] [Google Scholar]
- 10.Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15:431–45. doi: 10.1097/01.rvi.0000124949.24134.cf. [DOI] [PubMed] [Google Scholar]
- 11.Lee BB, Do YS, Byun HS, Choo IW, Kim DI, Huh SH. Advanced management of venous malformation with ethanol sclerotherapy: mid‐term results. J Vasc Surg. 2003;37:533–8. doi: 10.1067/mva.2003.91. 10.1067/mva.2003.91 [DOI] [PubMed] [Google Scholar]
- 12.Mason KP, Michna E, Zurakowski D, Koka BV, Burrows PE. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000;217:127–32. doi: 10.1148/radiology.217.1.r00se30127. [DOI] [PubMed] [Google Scholar]
- 13.Boxt Ml, Levin DC, Felows KE. Diretc puncture angiography in congenital venous malformations. AJR Am J Roentgenol. 1983;140:135–6. doi: 10.2214/ajr.140.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: A classification based on endotelial characteristics. Plast Reconstr Surg. 1982;69:412–22. doi: 10.1097/00006534-198203000-00002. 10.1097/00006534‐198203000‐00002 [DOI] [PubMed] [Google Scholar]
- 15.Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, Therasse E. Soft‐tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics. 2001;21:1519–31. doi: 10.1148/radiographics.21.6.g01nv031519. [DOI] [PubMed] [Google Scholar]
- 16.Memis A, Arkun R, Ustun EE, Kandiloglu G. Magnetic resonance imaging of intramuscular haemangiomas with emphasis on contrast enhancement patterns. Clin Radiol. 1996;51:198–204. doi: 10.1016/s0009-9260(96)80323-0. 10.1016/S0009‐9260(96)80323‐0 [DOI] [PubMed] [Google Scholar]
- 17.Dubois J, Patriquin HB, Garel L, Powell J, Filiatrault D, David M, et al. Soft‐tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR Am J Roentgenol. 1998;171:247–52. doi: 10.2214/ajr.171.1.9648798. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly LF, Bissett GS, 3rd, Adams DM. Combined sonographic and fluoroscopic guidance: a modified technique for percutaneous sclerosis of low‐flow vascular malformations. AJR Am J Roentgenol. 1999;173:655–7. doi: 10.2214/ajr.173.3.10470897. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Lin X, Li W, Hu X, Ma G, Wang W. Sclerotherapy after embolization of draining vein: a safe treatment method for venous malformations, J Vasc Surg. 2008;47:1292–9. doi: 10.1016/j.jvs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Pascarella L, Bergan JJ, Yamada C, Mekenas L. Venous angiomata: treatment with sclerosant foam. Ann Vasc Surg. 2005;19:457–64. doi: 10.1007/s10016-005-4656-z. 10.1007/s10016‐005‐4656‐z [DOI] [PubMed] [Google Scholar]
- 21.Cabrera J, Cabrera J, Jr, Garcia‐Olmedo MA. Sclerosants in microfoam. A new approach in angiology. Int Angiol. 2001;20:322–9. [PubMed] [Google Scholar]
- 22.Mimura H, Kanazawa S, Yasui K, Fujiwara H, Hyodo T, Mukai T, et al. Percutaneous sclerotherapy for venous malformations using polidocanol under fluoroscopy. Acta Med Okayama. 2003;57:227–34. doi: 10.18926/AMO/51909. [DOI] [PubMed] [Google Scholar]
- 23.Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edström S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol) Scand J Plast Reconstr Surg Hand Surg. 1997;31:145–50. doi: 10.3109/02844319709085481. 10.3109/02844319709085481 [DOI] [PubMed] [Google Scholar]
- 24.Herbreteau D, Riche O, Enjolras F, Lemarchand C, Laurian C, Merland JJ. Les malformations vasculaires veineuses et leur traitement par ethibloc. J Mal Vas. 1992;17:50–3. [PubMed] [Google Scholar]
- 25.Ellman BA, Green CE, Eigenbrodt E, Garriott JC, Curry TS. Renal infarction with absolute ethanol. Invest Radiol. 1980;15:318–22. doi: 10.1097/00004424-198007000-00008. 10.1097/00004424‐198007000‐00008 [DOI] [PubMed] [Google Scholar]
- 26.Monzani F, Lippi F, Goletti O, Del Guerra P, Caraccio N, Lippolis PV, et al. Percutaneous aspiration and ethanol sclerotherapy for thyroid cysts. J Clin Endocrinol Metab. 1994;78:800–2. doi: 10.1210/jcem.78.3.8126160. 10.1210/jc.78.3.800 [DOI] [PubMed] [Google Scholar]
- 27.Berthelsen B, Fogdestam I, Svendsen P. Venous malformations in the face and neck: radiologic diagnosis and treatment with absolute ethanol. Acta Radiol Diagn. 1986;27:149–55. doi: 10.1177/028418518602700204. [DOI] [PubMed] [Google Scholar]
- 28.Mason KP, Michna E, Zurakowski D, Koka BV, Burrows PE. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000;217:127–32. doi: 10.1148/radiology.217.1.r00se30127. [DOI] [PubMed] [Google Scholar]
- 29.Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104:1–11. 10.1097/00006534‐199907000‐00001 [PubMed] [Google Scholar]
- 30.Lee KB, Kim DI, Oh SK, Do YS, Kim KH, Kim YW. Incidence of soft tissue injury and neuropathy after embolo/sclerotherapy for congenital vascular malformation. J Vasc Surg. 2008;48:1286–91. doi: 10.1016/j.jvs.2008.06.058. 10.1016/j.jvs.2008.06.058 [DOI] [PubMed] [Google Scholar]
- 31.Yun WS, Kim YW, Lee KB, Kim DI, Park KB, Kim KH, et al. Predictors of response to percutaneous ethanol sclerotherapy (PES) in patients with venous malformations: analysis of patient self‐assessment and imaging. J Vasc Surg. 2009;50:581–9. doi: 10.1016/j.jvs.2009.03.058. 10.1016/j.jvs.2009.03.058 [DOI] [PubMed] [Google Scholar]
- 32.Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008;46:545–97. doi: 10.1016/j.rcl.2008.02.008. 10.1016/j.rcl.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 33.Redodo P, Bastarrika G, Sierra A, Martínez‐Cuesta A, Cabrera J. Efficacy and safety of microfoam sclerotherapy in a patient with Klippel‐Trenaunay syndrome and a patent foramen ovale. Arch Dermatol. 2009;145:1147–51. doi: 10.1001/archdermatol.2009.210. 10.1001/archdermatol.2009.210 [DOI] [PubMed] [Google Scholar]
- 34.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound‐guided foam sclerotherapy compared with ultrasound‐guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 2008;47:578–84. doi: 10.1016/j.jvs.2007.11.026. 10.1016/j.jvs.2007.11.026 [DOI] [PubMed] [Google Scholar]